Abstract
Heart failure with preserved ejection fraction (HFpEF) and pulmonary arterial hypertension (PAH) are two emerging diseases focusing the attention of numerous researchers. In the last PAH guideline, there is a crossroad between the two diseases and pulmonary hypertension (PH) due to heart failure (HF) is categorized as subtype 2. In order to assess the correct diagnosis and management, it should be better understood the points of convergence and divergence of two diseases. Although, risk factors, demographic characteristics and haemodynamics are different, we report several similarities regarding vascular alterations, some aspects of cardiac remodelling, and clinical presentation. This model suggests HFpEF and PAH as two comparable conditions, with different cardiac adaptation and trajectories, linked to the intrinsic properties of either right and left ventricles. In both diseases the early pathophysiological mechanisms appear to begin from peripheral vasculature and to be backward transmitted to the larger arterial vascular district, and eventually to the myocardial structure. In this paper we would propose a simple approach to recognize the concordances and, all at once, distinguish the peculiarities of the two diseases.
Keywords: Pulmonary arterial hypertension (PAH), heart failure with preserved ejection fraction (HFpEF), right heart (RH), pathophysiology
Introduction
One of the most important diagnostic challenges in clinical practice is the distinction between pulmonary hypertension (PH) due to primitive pulmonary arterial hypertension (PAH) and PH due to left heart diseases (LHD). Both diseases deliver some common characteristics and pathophysiological pathways, making the two processes similar for several aspects.
If the presence of LHD is clear in many cases such as heart failure with reduced ejection fraction (HFrEF), in case of heart failure with preserved ejection fraction (HFpEF), given the patient’s history and the clinical and echocardiographic findings, the LHD should be not evident.
HFpEF is a heterogeneous clinical syndrome, accounts about 50% of heart failure (HF) patients (1), and is characterized by the contemporary presence of several comorbidities, which often contribute to decompensate these patients (2). HFpEF patients, during an acute HF episode, experience an increase of left ventricle (LV) and left atrium (LA) filling pressures, with a passive backward transmission, often enhanced by a dynamic increase in mitral regurgitation and loss of LA compliance, leading to PH. PH due to HFpEF (HFpEF-PH) usually is an isolated post-capillary (Ipc) PH, although in several cases it could be combined, pre- and post-capillary PH (3). According to the previous findings, HFpEF patients may show increased systolic pulmonary artery pressure (PAPs) in compensated phases, and in the most recent PH classification these patients are included in the second group including both patients with HFrEF and HFpEF (4). PAH represents a multifactorial disease, comprising genetic and molecular mechanisms, which involve primary pulmonary arteries structure and function, with subsequent implication of pulmonary circulation vascular beds (5,6). This syndrome results in remodelling of pulmonary arteries and right heart (RH), that is the main responsible of clinical presentation and outcome (7).
This review will aim to point out the points of convergence and divergence existing between PAH and HFpEF regarding prevalence, pathophysiology, clinical characteristics and outcome.
Definitions
According to the European Society of Cardiology (ESC)/European Respiratory Society (ERS) guidelines, PH is defined by right heart catheterization (RHC) estimation as an increase in mean pulmonary artery pressure (PAPm) ≥25 mmHg at rest (4). In this definition either PAH (Group 1) and PH due to LHD (Group 2) are included. Both diseases are characterized by increased pulmonary artery pressures, thus involving the right ventricle (RV), and causing RH failure. Currently, RHC is the only one method able to distinguish PH subgroups through direct hemodynamic measurements. In PAH, together with PAPm ≥25 mmHg at rest, there are two mandatory criteria for diagnosis: pulmonary arterial wedge pressure (PAWP) ≤15 mmHg and pulmonary vascular resistance (PVR) >3 Woods unit (WU), in absence of chronic lung diseases, or chronic thromboembolic PH, or other rare diseases. This disorder involves pulmonary arteries with normal LA pressures. Otherwise, group 2 encompasses PH due to LHD (4). This group does not distinguish between two main HF phenotypes: PH in HFrEF and in HFpEF. Data from recent registries demonstrate that HFpEF is epidemiologically relevant, representing up to one half of HF population. Patients with HFpEF could develop PH as a result of increased LA pressure, and subsequent pulmonary venous congestion (8). This mechanism lacks of significant pulmonary vasoconstriction or remodelling and leads to development of Ipc PH-HFpEF. RHC shows PAPm ≥25 mmHg, PAWP >15 mmHg and PVR <3 WU, with clinical signs and symptoms of HF. In some case of HFpEF, a combined pre-capillary and post-capillary (Cpc) PH may be present because of chronic increased left-sided filling pressure and subsequent pulmonary arterial vasoconstriction and remodelling. In this subgroup, RHC shows PAPm ≥25 mmHg, PAWP >15 mmHg, and PVR >3 WU (3). Recently, the definitions have been changed including Ipc-PH and Cpc-PH (9).
Prevalence and outcome
Because of lack of randomization, confounding factors and selection bias, the registries evaluating the real incidence, prevalence and outcome of PAH in general population are extremely different. By literature only two registries in this setting have similar definitions and inclusion criteria: in the first French registry, prevalence and incidence of PAH in France were 15.0 cases/million of adult and 2.4 cases/million of adult for year. The same registry showed 1-year survival of these patients of 88% and 3-year survival about 60% (10,11). More recently, data from REVEAL registry showed that prevalence of PAH and idiopathic PAH were respectively 2.0–10.6 cases/million of adult and 0.9 cases/million of adult. Five-year survival rate of these patients ranged from 80% to 30% according to functional class I/II/III/IV and timing of diagnosis (newly versus previously diagnosis) (11-13).
The prevalence of PH-HFpEF, is still unclear. Most of the data are based on echocardiography examination and in particular on increased PAPs values. Data from TOPCAT trial demonstrated that percentage of patients with tricuspid regurgitant velocity >2.9 m/s (equivalent to estimated PAPs >35 mmHg) was 36% (14). In other studies, this percentage was higher ranging from 52% to 83% (15,16). This variability could be due to different PAPs cut-off values and non-invasive methodology employed to define PH. Similarly, the real prevalence of Cpc PH-HFpEF is under debate because lacking of reproducible hemodynamic findings. Several studies assessed the prevalence of Cpc PH-HFpEF from 7% to 12% (3,17). Although several studies demonstrated the strong relationship between increased PAPs and poor outcome, it appears plausible that Ipc PH-HFpEF has a better survival than Cpc PH-HFpEF (18,19). Therefore, the increased PAPs seems to be linearly related to outcome: the Danish multicentre study highlighted that a cut-off of 39 mmHg is able to discern subjects with worse prognosis (20). According to this data Lam et al. showed that for every 10% of PAPs rise correspond a risk elevation of 28% during 3-year follow-up (15).
Histology
Parenchymal and vascular alterations in PAH and HFpEF have specific characteristics: in PAH the dysfunction occurs at pre-capillary site and it is mainly due to endothelial dysfunction causing capillaries and arteriole vasoconstriction, vascular obliteration and pulmonary blood fluid redistribution from basal site to apical district. Pulmonary vessels dysfunction is due to an increase of parietal fibrosis, extracellular matrix deposition, and myocyte hypertrophy related to the reduced vasodilatation properties of the endothelium. The initial alteration begins at peripheral pulmonary vascular level but it is quickly transmitted to the medium and larger arterial lumen up to the involvement of the two main branches of pulmonary artery. In the larger pulmonary vessel and increase of media and adventitia layers is appreciable, nevertheless due to the inconspicuous muscular component, the progressive lumen enlargement became evident after short period of PAH occurrence (5). In HFpEF the main pulmonary vascular alteration occurs initially at venous level and they are due to an increased venous lumen with a partial attempt of parietal thickness and collagen deposition. Enlarged veins and chronic congestion, lead to an increased vessel permeability related to the left atrial pressure increase. The reduced vein capacitance and relative pressure elevation are backward transmitted to the capillary district in which oxygen exchange became impaired. Due to the augmented extracellular capillary composition, gas exchange is reduced and it causes a further vasoconstriction. Such modifications could involve also the pulmonary arteriole developing a mixed PH with double pre and post capillary etiology (21).
Pathophysiology
Points of convergence
PH occurrence is the final stage of PAH and HFpEF, and RH adaptation is the common consequence of both diseases. The pathophysiological common pathway is probably the nitric oxide (NO)—soluble guanylate cyclase (sGC)—cyclic guanosine monophosphate (cGMP) inhibition. The reduction in NO delivery and production is well demonstrated by the clinical efficacy of therapies aimed to restore this pathway in PAH, although in HFpEF current drugs have been poorly tested and not significative benefit is reported. NO activates sGC by binding its prosthetic heme group, thereby catalyzing cyclic cGMP synthesis. cGMP causes vasodilation and may inhibit smooth muscle cell proliferation and platelet aggregation. Intracellular cGMP is rapidly inactivated to GMP by the activity of phosphodiesterases-5 (PDE-5). Inhibition of the cGMP-specific PDE-5 leads to an accumulation of cGMP, enhancing the action of NO (22). In PAH PDE-5 is the most abundantly expressed isoform and appears to be up-regulated. Similarly, in HFpEF cGMP phosphorylation leads to NO release inhibition, that impairs endothelial and cardiac elastance, and it increases the activation of protein kinase G responsible for an upregulation of titin isoform. This process appears mediated by increased inflammatory status, oxidative stress and increased pro thrombotic mechanisms.
These common bio-molecular adaptations could explain the occurrence of increased PVR, pulmonary vascular lumen narrowing due to thickening of the vessel media, changes in functional parameters of the lung vasculature, and RV hypertrophy in both diseases (23). The processes inducing RH adaptation are probably analogous in both diseases. The first portion of RH which bears increased pulmonary pressure is the outflow tract, that begins to distend; this process is associated to a myocardial fibres remodelling, stretching and thickening in all RV districts, but particularly in the right region of interventricular septum. When the thickening is the prevalent mechanism, it will afford an adaptive remodelling. Oppositely, when distention and stretching are the leading processes, a maladaptive remodelling will occur. Adaptive remodelling is characterized by more concentric remodelling (higher mass-volume ratio), with preserved RV systolic and diastolic function. Maladaptive remodelling appears to be a consequence of continuous RV pressure overload, leading to RV wall stress and dilatation, secondary tricuspid regurgitation and subsequent systo-diastolic dysfunction and failure (7,8,18). These mechanisms also lead to RV dyssynchrony, which depends on RV myocytes that prolong their contraction time delaying systolic leftward septal movement. Some other items, such as neuro-hormonal activation, coronary perfusion and myocardial metabolism could influence the severity of PH and RV remodelling. Another causal factor of maladaptive remodelling is ventriculo-arterial uncoupling, which represents the lack of relationship between RV contractility and afterload (3,24,25). This mechanism depends on lacking of ventricular and arterial elastance, and discordance between cardiac contractility and vascular compliance in the decompensated phases of both diseases.
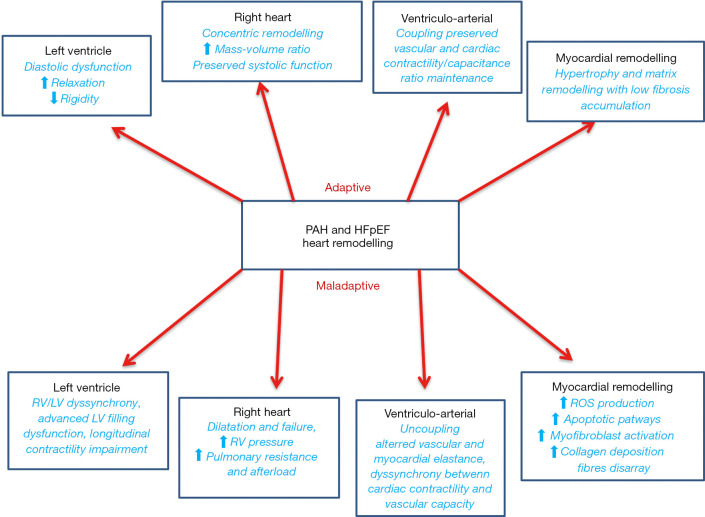
In HFpEF, vascular and endothelial dysfunction increasing systemic vascular stiffness could be transmitted to the pulmonary circulation leading to, increase in cardiac afterload and RV chamber stiffness and hypertrophy. Thus, in more advanced stages LV and RV morphological adaptations became haemodynamically relevant, inducing altered filling pressure, myocardial relaxation and reduced compliance. Current haemodynamic and structural changes result in increased end diastolic pressure, reduced atrio-ventricular diastolic blood flow, and consequent fluid accumulation in pulmonary vein district (Figure 1).
Figure 1.
Heart remodelling in PAH and HFpEF. PAH, pulmonary arterial hypertension; HFpEF, heart failure with preserved ejection fraction; LV, left ventricle; RV, right ventricle; ROS, reactive oxygen species.
Points of divergence
RH adaptive mechanisms are similar for both PAH and HFpEF, even if the reasons of increased pulmonary pressures are different. In PAH patients, PH is due to sustained vasoconstriction, pulmonary vascular remodelling, endothelial cell proliferation, and thus thrombosis in situ, which causes increased pulmonary arterial resistance. Inflammation and autoimmune mediators are involved in these mechanisms, as well as genetic factors (5). In particular, activation of the endothelin (ET) system is an exclusive determinant of PAH and the increased plasma and lung tissue levels appear one of the most important causative mechanisms. Indeed, ET blockade has become an important target for PAH treatment. The ET-1 exerts vasoconstrictor and mitogenic effects by binding two distinct receptor isoforms in the pulmonary vascular smooth muscle cells, ETA and ETB receptors. ETB receptors are also present in the endothelial cells and their activation leads, in physiological conditions, to the release of vasodilators and antiproliferative substances, such as NO and prostacyclin, that may counterbalance the deleterious effects of ET-1. In pathological condition, ETB receptors overexpression leads to vascular remodelling and narrowing (26,27). In HFpEF the ventriculo-arterial uncoupling has been recently described as substantial contributor identifying the lack of relationship between RV contractility and afterload. The main drivers of PH are impaired LV filling pressure due to diastolic dysfunction and impaired cardiac relaxation related to increased myocardial stiffness. Diastolic dysfunction occurs from increased type 1 collagen deposition in interstitial space and myocardial fibrosis. Increased stiffness is also related to cardiomyocyte hypertrophy structural disarray, cytoskeletal dysfunction, and titin alterations. All these alterations reduce cardiac elastance and increase myocardial rigidity, leading to a LV filling pressure increase (1,28). Thus, LV overload increases LA pressure, LA volume and remodelling, reducing fibres contractility and elastance. Permanent elevated LA pressure is transmitted backward to pulmonary veins and it promotes chronic pulmonary venous congestion, which in turns is responsible of pathologic changes in both arterial and venous districts. The elevated capillary pressure induces intimal fibrosis and medial hypertrophy, as well as luminal narrowing, with subsequent increased arterial resistance. The final product of all these factors is precapillary PH, defined as Cpc-PH in HFpEF. In this form, plexiform lesions pathognomonic of PAH are not found (3,8,9). Probably, the real causal factors of these microvascular and structural alterations are comorbidities, such as hypertension, diabetes and dyslipidemia. Ipc-PH and Cpc-PH represent two phases of the same process. During the early Ipc-PH phase, RV substantially maintains the systo-diastolic function, but undergoes adaptive remodelling (9).
RH characteristics
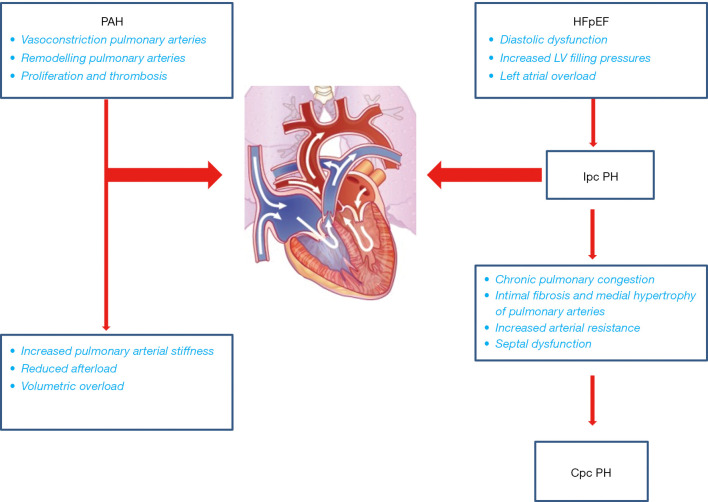
The initial RV dysfunction and subsequent maladaptive remodelling, are probably due to septal dysfunction. Indeed, a substantial component of RV contractility is due to LV, through shared short axis fibers and trans-septal contribution. In HFpEF patients, despite normal ejection fraction (EF), LV evidences some degrees of impaired contractility (due to reduced longitudinal systolic function or diastolic dysfunction) and consequently impaired RV/LV interactions (29). In this setting RV loses its ability to compensate the pressure overload, causing a ventricular-vascular uncoupling. This phenomenon is characterized by increased pulmonary arterial stiffness and increased afterload, leading to subsequent RV volumetric overload and irreversible failure (24,25) (Figure 2).
Figure 2.
Pulmonary hypertension pathophysiology in HFpEF and PAH. PAH, pulmonary arterial hypertension; HFpEF, heart failure with preserved ejection fraction; Cpc, capillary and post-capillary; Ipc, isolated post-capillary; PH, pulmonary hypertension.
In a recent position paper about RV evaluation in HFpEF a staging based on clinical signs and RV dysfunction has been introduced. Although this classification is not yet supported by cross sectional data, it reflects the pulmonary and systemic congestion together with clinical assessment (7). Conversely to the LV, the RV is more compliant to volume loading even if the function of one ventricle is strictly dependent of the other and impacts to the opposite side by the pressure gradient across interventricular septum. Since RV dysfunction is more strictly related to afterload with respect to LV, identification of RV dysfunction may be better described in relation to ventriculo-systolic coupling. On the basis of different patterns and adaptations we can distinguish between appropriate and disproportional RV remodeling and these adaptations could vary in acute and chronic conditions as well as in PAH and in HFpEF. Although traditional approach in PH due to left-side HF encompasses post-capillary PH, some studies have recently demonstrated the concomitant presence of pre capillary PH even in HF due to left side dysfunction in a certain percentage of patients. In this subset hemodynamic is characterized by significant increase of both PVR and wedge pressure in combination with diastolic pressure gradient. The recognition of these patients appears of clinical relevance because of worse outcome and much more deterioration of RV contractility associated with RV and RA larger dimension and further pulmonary pressure increase. This is confirmed by recent study comparing echo with haemodynamic data in which patients with combined pre and post capillary hypertension had impaired outcome compared with isolated post capillary hypertension (9).
In primitive PAH, RV maladaptation occurs over a short timing course because of poor adaptation of RV to the sudden pulmonary pressure increase and pre capillary overload. RV dilatation with specific enlargement of the outflow tract reflecting increased PVR is typical. Therefore, in a consistent percentage of PAH patients a dilatation of main pulmonary tract is appreciable. The systolic function in terms of RV EF and longitudinal function are both reduced and both pulmonary and tricuspid regurgitation became significative after early period. Due to the persistent post load increase, the tricuspid annulus tends to became enlarged with further increase of valve regurgitation and RA dilatation. Increased RA pressure leads to reduced systemic vein return and central vein pressure elevation.
The above described characteristics, are replaced by invasive haemodynamic analysis: the main difference between PAH and HFpEF patients is PAWP. This measurement is a mirror of increased LA pressures and should be recorded as the mean of three measurements. Some patients have PAWP =15 mmHg and are initially classified in PAH group (4,8,30,31). To elucidate this situation, it could be helpful a bolus administration of 500 mL of saline solution, which causes increase in LV filling pressures, LA pressure overload, and subsequent increased PAWP (>15 mmHg) (32). Another invasive parameter able to discriminate PAH from HFpEF patients is PVR. This value is >3 WU in patients with pre-capillary PH, but lower in HFpEF (33). The measurements capable to differentiate HFpEF patients in Ipc-PH or Cpc-PH are trans-pulmonary gradient (TPG = PAPm − PAWP) and diastolic pulmonary gradient (DPG = diastolic PAP − PAWP). In patients with Ipc PH-HFpEF, TPG is <12 mmHg, DPG <7 mmHg, PAWP >15 mmHg and PVR <3 WU. Patients with Cpc PH-HFpEF due to vascular remodelling and subsequent precapillary PH, display PAWP >15 mmHg, DPG ≥7 mmHg and PVR >3 WU (9,16,32,34-36). Finally, in PAH patients who underwent RHC, a vasoreactivity test is usually also done. This test consists of administration of intravenous (IV) adenosine, IV prostacyclin, in order to assess pulmonary artery pressures reduction. If PAPm reduction is >10 mmHg, with an absolute value of PAPm <40 mmHg, patients have a positive response, which permits the treatment with high dose of calcium channel blockers (4). By now, RHC remains the universal method able to discern patients with precapillary PH (Table 1).
Table 1. Echocardiographic and hemodynamic difference between PAH and HFpEF.
| Variables | PAH | HFpEF |
|---|---|---|
| Echocardiographic variables | ||
| LV ejection fraction (%) | ≥50 | ≥50 |
| Left atrial enlargement | Rare | Present |
| Right atrial enlargement | Present | Rare |
| RV hypertrophy | Common | Rare |
| LV mass index | Normal | Increased |
| LV hypertrophy | Absent | Present |
| Lateral mitral E/E’ | <8 | ≥12 |
| Diastolic dysfunction degree | Grade I | Grade II and III |
| RVOT mid-systolic notching | More frequent | Less frequent |
| Mitral flow DT (ms) | >200 | <200 |
| Hemodynamic variables | ||
| LA pressures (mmHg) | Normal | Increased |
| PAPs (mmHg) | ≥40 | ≥40 |
| PAPm (mmHg) | ≥25 | ≥25 |
| PAWP (mmHg) | ≤15 | >15 |
| PVR (WU) | >3 | ≤3 |
PAH, pulmonary arterial hypertension; HFpEF, heart failure with preserved ejection fraction; LV, left ventricle; RV, right ventricle; E/E’, ratio of peak early Doppler mitral valve flow velocity and early diastolic mitral valve flow velocity; RVOT, right ventricular outflow tract; DT, deceleration time; LA, left atrium; PAPs, systolic pulmonary artery pressure; PAPm, mean pulmonary artery pressure; PAWP, pulmonary arterial wedge pressure; PVR, pulmonary vascular resistance.
Clinical characteristics
Points of convergence
The first common point between PAH and HFpEF is gender. In both PAH and HFpEF females are more often affected, with a particularly high percentage in PAH, ranging from 60% to 80% (10,12,13,30). Similarly, many studies confirmed in HFpEF the female gender prevalence, ranging from 50% to 70% (30,31,37-43). Clinical presentation of both diseases is comparable. The most common symptoms are shortness of breath, fatigue, weakness, angina and syncope. These symptoms are not specific and mainly related to increased RV pressures (3,4). During the early stage, both HFpEF and PAH patients complain of shortness of breath during exercise. In PAH this symptom is sometimes associated with nausea and dyspepsia (4). In more advanced stages, a sustained increase of pulmonary pressure could lead to RV failure and central venous pressure increase. During end-stages of both diseases, independently from etiology, patients show symptoms and signs of congestion: dyspnea at rest, jugular venous dilation, hepato-splenomegaly, ascites, peripheral oedema and cool extremities (4,44). The untreatable systemic congestion is the final clinical picture of both diseases, although in subjects with HFpEF an arrhythmic adverse event could occur earlier.
Points of divergences
PAH and HFpEF are quite distinct respect to epidemiological and demographic findings. Firstly, patients with diagnosis of HFpEF are older (about 60–70 years) than patients affected by PAH (35–50 years), even if most recent registries of idiopathic PAH show a trend towards an increased mean age (up to 70 years) (10-13,37-43). HFpEF patients demonstrate a higher rate of traditional risk factors and comorbidities in comparison with PAH patients. HFpEF subjects have, respect to PAH patients, higher prevalence of atrial fibrillation (AF), hypertension, LV hypertrophy, and diabetes. Several studies confirmed the high comorbidities burden of HFpEF. The MAGGIC registry showed an elevated prevalence of hypertension, AF, diabetes and coronary artery disease (40). The I-PRESERVE trial evidenced in hospitalized patients an even higher prevalence of hypertension (86%), AF (34%) and diabetes (31%) (41). Furthermore, the OPTIMIZE-HF registry, comparing HFpEF to HFrEF, showed that patients with preserved EF were more likely to be older, female, and to have non-ischemic etiology (42). Bhatia et al., in a population-based study reported an increased rate of hypertension (51% vs. 49%), AF (32% vs. 23%) and diabetes (32% vs. 38%) in HFpEF respect to HFrEF (43). The only cross-sectional study comparing PAH and HFpEF showed that HFpEF patients presented higher mean age (69 vs. 47 years), body mass index (30 vs. 26 kg/m2), and rate of diabetes (57% vs. 19%) (37). Thenappan et al. studied the comorbidities burden in HFpEF patients, and found that indeed these patients appear more frequently affected than PAH patients by obesity (46% vs. 15%), coronary artery disease (27% vs. 4%), hypertension (79% vs. 29%), diabetes (37% vs. 8%), and renal dysfunction (30) (Table 2).
Table 2. Demographic and clinical characteristics in PAH and HFpEF patients.
| Variables | PAH | HFpEF |
|---|---|---|
| Demographic characteristics | ||
| Age (years) | 40–65 | 60–70 |
| Female gender (%) | 60–80 | 50–70 |
| WHO functional class | III/IV | III/IV |
| BMI (kg/m2) | ~26 | ~30 |
| Comorbidities | ||
| Hypertension (%) | 20–30 | 50–85 |
| Diabetes mellitus (%) | 5–10 | 30–50 |
| Obesity (%) | 10–15 | 30–40 |
| Coronary artery disease (%) | 4–7 | 20–35 |
| Renal dysfunction | Absent | Common |
| Atrial fibrillation | Rare | Common |
PAH, pulmonary arterial hypertension; HFpEF, heart failure with preserved ejection fraction; BMI, body mass index.
Non-invasive diagnostic tools
Points of convergence
Echocardiography is the most usual imaging tool to assess LV and RV morphology and function. In either PAH and HFpEF, the common point in between is the normal range of LV systolic function. Both diseases show preserved LV EF, defined as EF ≥50%. Moreover, all these patients experience an increased PAPs value ≥35 mmHg in the first phases. In more advanced stages with severe RV dysfunction, echocardiographic findings remain similar: both diseases are characterized by reduced tricuspid annular plane systolic excursion (TAPSE) <16 mm, RV outflow tract enlargement and RV dysfunction (45-47). An emerging technique for RV study is cardiac magnetic resonance (CMR). Cine CMR, can precisely measure RV EF, stroke volume, and segmental and global parietal kinesis, as well as eventual dyssynchrony between the RV and LV chambers. The RV outflow tract and pulmonary valve morphology, difficult to visualize by traditional ultrasound, can also be detected by this technique. CMR also provides information on pulmonary arteries dimensions, distensibility, and lung blood flow distribution, that can be reduced in the apical segments (48,49). Oppositely, other diagnostic methods, such as electrocardiogram (ECG), chest X-ray and natriuretic peptide measurement are discordant in PAH and HFpEF.
Points of divergence
Different ECG patterns characterize PAH and HFpEF. Most patients with PAH are in sinus rhythm with QRS right axis deviation and RV hypertrophy. Oppositely, in HFpEF, there is often AF, with LV hypertrophy and left axis deviation. Chest X-ray pattern is often different: it is possible to observe enlarged RH profile with dilated pulmonary artery in PAH; lung-heart arc, pulmonary congestion and Kerley B-lines appear more pronounced in HFpEF (4,8,29,44). On echocardiography patients with HFpEF present LV hypertrophy and LA dilatation, absent in PAH. The LA enlargement is the real echocardiographic sign to discriminate both diseases. In PAH LA is into normal range and in advanced phases, it is possible to observe RA enlargement with pressure overload (4,8,50). Echocardiography could also assess various diastolic dysfunction degrees. Isovolumic LV relaxation time (IVRT), ratio of peak early (E) and peak atrial (A) Doppler mitral valve flow velocity (E/A), deceleration time (DT), and ratio of E and early diastolic mitral valve flow velocity (E’) (E/E’) are all measures of diastolic function. In HFpEF patients there are low E/A, prolonged DT and increased E/E’, providing diagnostic evidence of diastolic dysfunction. In particular, an elevated E/E’ ratio >15 is an unmistakable sign of raised LV filling pressure. In this sense, HFpEF shows a typical pattern defined by increased E/E’ (≥12), increased E/A (>0.8) and reduced DT (<200 ms), with pseudonormal or restrictive filling trans-mitral patterns. In PAH patients it is possible to observe mild diastolic dysfunction with abnormal relaxation pattern (grade I), with E/E’ <8 and DT >200 ms (8,30,33,51). RV outflow tract mid-systolic notching pattern, evaluable on pulse-wave Doppler echocardiography, could assist in characterization of PVRs and hemodynamic proprieties of PH (8,31). Finally, laboratory parameters used in HF diagnosis, as B-type natriuretic peptide (BNP) or N-terminal pro-BNP (NTproBNP), are elevated in both diseases; however, HFpEF patients show higher serum levels of natriuretic peptides respect to PAH patients (4,44,52) (Table 1).
Conclusions
Although HFpEF and PAH are two distinct diseases, with peculiar pathophysiological and causal factors, for some aspects they appear similar. Both illnesses seem to arise at peripheral level, at systemic and pulmonary vascular districts respectively, with comparable alterations into arterial and pulmonary capillary structures (53). At vascular systemic level, endothelial dysfunction and vessel rigidity are induced by reduced NO availability, increased oxidative stress and rigidity, probably mediated from several external factors, such as elevated comorbidity burden and metabolic diseases. Similarly, at pulmonary level the increased pressure results first in vasoconstriction and reduced compliance, mediated by cGMP reduction and abnormal ET levels. In both diseases vascular modifications are characterized by collagen deposition, vascular narrowing, smooth muscle cells migration and over expression. The different central cardiac adaptations depend on intrinsic characteristics of the LV and RV. Thus, the two processes tend to distinguish each other because of different adaptation mechanisms and different capacity to respond to increased afterload. From functional and morphological points of view, they have further similarities, due to preserved LV EF and dilated RH, with common increase in pulmonary pressure values. The only typical divergences are the precapillary pressure and PVR values, which in turns are due to different vascular and cardiac compensation mechanisms in either pulmonary and systemic districts. Similarly, tailored therapy really effective on outcome in both diseases is still lacking. Most clinical studies found some improvement in exercise capacity, quality of life, and pulmonary pressure, but they failed to demonstrate a significant benefit in terms of mortality. Because of the above cited biomolecular convergences, current and novel treatments proposed in PAH should be tempted also in HFpEF, and vice versa. A new agenda, clarifying the precise vascular dysfunction mechanisms in PAH and HFpEF, appears mandatory to optimize management and ameliorate the life expectancy in both conditions.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure forms (available at: http://dx.doi.org/10.21037/cdt-19-405). The authors have no conflicts of interest to declare.
References
- 1.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 2011;32:670-9. 10.1093/eurheartj/ehq426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263-71. 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 3.Dixon DD, Trivedi A, Shah SJ. Combined post- and pre-capillary pulmonary hypertension in heart failure with preserved ejection fraction. Heart Fail Rev 2016;21:285-97. 10.1007/s10741-015-9523-6 [DOI] [PubMed] [Google Scholar]
- 4.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119. 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 5.Tuder RM, Archer SL, Dorfmüller P, et al. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol 2013;62:D4-12. 10.1016/j.jacc.2013.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soubrier F, Chung WK, Machado R, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 2013;62:D13-21. 10.1016/j.jacc.2013.10.035 [DOI] [PubMed] [Google Scholar]
- 7.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013;62:D22-33. 10.1016/j.jacc.2013.10.027 [DOI] [PubMed] [Google Scholar]
- 8.Thenappan T, Prins KW, Cogswell R, et al. Pulmonary hypertension secondary to heart failure with preserved ejection fraction. Can J Cardiol 2015;31:430-9. 10.1016/j.cjca.2014.12.028 [DOI] [PubMed] [Google Scholar]
- 9.Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J 2019;53:1801897. 10.1183/13993003.01897-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023-30. 10.1164/rccm.200510-1668OC [DOI] [PubMed] [Google Scholar]
- 11.McGoon MD, Benza RL, Escribano-Subias P, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol 2013;62:D51-9. 10.1016/j.jacc.2013.10.023 [DOI] [PubMed] [Google Scholar]
- 12.McGoon MD, Krichman A, Farber HW, et al. Design of the REVEAL registry for US patients with pulmonary arterial hypertension. Mayo Clin Proc 2008;83:923-31. 10.1016/S0025-6196(11)60769-6 [DOI] [PubMed] [Google Scholar]
- 13.Farber HW, Miller DP, Poms AD, et al. Five-Year outcomes of patients enrolled in the REVEAL Registry. Chest 2015;148:1043-54. 10.1378/chest.15-0300 [DOI] [PubMed] [Google Scholar]
- 14.Shah AM, Shah SJ, Anand IS, et al. TOPCAT Investigators Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail 2014;7:104-15. 10.1161/CIRCHEARTFAILURE.113.000887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam CS, Roger VL, Rodeheffer RJ, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 2009;53:1119-26. 10.1016/j.jacc.2008.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung CC, Moondra V, Catherwood E, et al. Prevalence and risk factors of pulmonary hypertension in patients with elevated pulmonary venous pressure and preserved ejection fraction. Am J Cardiol 2010;106:284-6. 10.1016/j.amjcard.2010.02.039 [DOI] [PubMed] [Google Scholar]
- 17.Gerges M, Gerges C, Pistritto AM, et al. Pulmonary Hypertension in Heart Failure. Epidemiology, Right Ventricular Function, and Survival. Am J Respir Crit Care Med 2015;192:1234-46. 10.1164/rccm.201503-0529OC [DOI] [PubMed] [Google Scholar]
- 18.Melenovsky V, Hwang SJ, Lin G, et al. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014;35:3452-62. 10.1093/eurheartj/ehu193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam CS, Borlaug BA, Kane GC, et al. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009; 119:2663-70. 10.1161/CIRCULATIONAHA.108.838698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kjaergaard J, Akkan D, Iversen KK, et al. Prognostic importance of pulmonary hypertension in patients with heart failure. Am J Cardiol 2007;99:1146-50. 10.1016/j.amjcard.2006.11.052 [DOI] [PubMed] [Google Scholar]
- 21.Fayyaz AU, Edwards WD, Maleszewski JJ, et al. Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation 2018;137:1796-810. 10.1161/CIRCULATIONAHA.117.031608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CN, Watson G, Zhao L. Cyclic guanosine monophosphate signalling pathway in pulmonary arterial hypertension. Vascul Pharmacol 2013;58:211-8. 10.1016/j.vph.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 23.Montani D, Chaumais MC, Savale L, et al. Phosphodiesterase type 5 inhibitors in pulmonary arterial hypertension. Adv Ther 2009;26:813-25. 10.1007/s12325-009-0064-z [DOI] [PubMed] [Google Scholar]
- 24.Voelkel NF, Quaife RA, Leinwand LA, et al. National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 2006;114:1883-91. 10.1161/CIRCULATIONAHA.106.632208 [DOI] [PubMed] [Google Scholar]
- 25.Harrison A, Hatton N, Ryan JJ. The right ventricle under pressure: evaluating the adaptive and maladaptive changes in the right ventricle in pulmonary arterial hypertension using echocardiography (2013 Grover Conference series). Pulm Circ 2015;5:29-47. 10.1086/679699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biasin V, Chwalek K, Wilhelm J, et al. Endothelin-1 driven proliferation of pulmonary arterial smooth muscle cells is c-fos dependent. Int J Biochem Cell Biol 2014;54:137-48. 10.1016/j.biocel.2014.06.020 [DOI] [PubMed] [Google Scholar]
- 27.Benza RL, Gomberg-Maitland M, Demarco T, et al. Endothelin-1 Pathway Polymorphisms and Outcomes in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2015;192:1345-54. 10.1164/rccm.201501-0196OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2014;11:507-15. 10.1038/nrcardio.2014.83 [DOI] [PubMed] [Google Scholar]
- 29.Kanwar M, Tedford RJ, Agarwal R, et al. Management of pulmonary hypertension due to heart failure with preserved ejection fraction. Curr Hypertens Rep 2014;16:501. 10.1007/s11906-014-0501-5 [DOI] [PubMed] [Google Scholar]
- 30.Thenappan T, Shah SJ, Gomberg-Maitland M, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail 2011;4:257-65. 10.1161/CIRCHEARTFAILURE.110.958801 [DOI] [PubMed] [Google Scholar]
- 31.Arkles JS, Opotowsky AR, Ojeda J, et al. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am J Respir Crit Care Med 2011;183:268-76. 10.1164/rccm.201004-0601OC [DOI] [PubMed] [Google Scholar]
- 32.Robbins IM, Hemnes AR, Pugh ME, et al. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail 2014;7:116-22. 10.1161/CIRCHEARTFAILURE.113.000468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta DK, Solomon SD. Imaging in heart failure with preserved ejection fraction. Heart Fail Clin 2014;10:419-34. 10.1016/j.hfc.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 34.Naeije R, Vachiery JL, Yerly P, et al. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J 2013;41:217-23. 10.1183/09031936.00074312 [DOI] [PubMed] [Google Scholar]
- 35.Benza RL, Raina A, Abraham WT, et al. Pulmonary hypertension related to left heart disease: insight from a wireless implantable hemodynamic monitor. J Heart Lung Transplant 2015;34:329-37. 10.1016/j.healun.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 36.Adir Y, Humbert M, Sitbon O, et al. Out-of-proportion pulmonary hypertension and heart failure with preserved ejection fraction. Respiration 2013;85:471-7. 10.1159/000339595 [DOI] [PubMed] [Google Scholar]
- 37.Olsson KM, Sommer L, Fuge J, et al. Capillary pCO2 helps distinguishing idiopathic pulmonary arterial hypertension from pulmonary hypertension due to heart failure with preserved ejection fraction. Respir Res 2015;16:34. 10.1186/s12931-015-0194-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoeper MM, Meyer K, Rademacher J, et al. Diffusion Capacity and Mortality in Patients With Pulmonary Hypertension Due to Heart Failure With Preserved Ejection Fraction. JACC Heart Fail 2016;4:441-9. 10.1016/j.jchf.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 39.Guazzi M. Pulmonary hypertension in heart failure preserved ejection fraction: prevalence, pathophysiology, and clinical perspectives. Circ Heart Fail 2014;7:367-77. 10.1161/CIRCHEARTFAILURE.113.000823 [DOI] [PubMed] [Google Scholar]
- 40.Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J 2012;33:1750-7. 10.1093/eurheartj/ehr254 [DOI] [PubMed] [Google Scholar]
- 41.Badar AA, Perez-Moreno AC, Hawkins NM, et al. Clinical Characteristics and Outcomes of Patients With Coronary Artery Disease and Angina: Analysis of the Irbesartan in Patients With Heart Failure and Preserved Systolic Function Trial. Circ Heart Fail 2015;8:717-24. 10.1161/CIRCHEARTFAILURE.114.002024 [DOI] [PubMed] [Google Scholar]
- 42.Fonarow GC, Stough WG, Abraham WT, et al. OPTIMIZE-HF Investigators and Hospitals. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol 2007;50:768-77. 10.1016/j.jacc.2007.04.064 [DOI] [PubMed] [Google Scholar]
- 43.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006;355:260-9. 10.1056/NEJMoa051530 [DOI] [PubMed] [Google Scholar]
- 44.Ponikowski P, Voors AA, Anker SD, et al. Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 45.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685-713. 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 46.Bossone E, Bodini BD, Mazza A, et al. Pulmonary arterial hypertension: the key role of echocardiography. Chest 2005;127:1836-43. 10.1378/chest.127.5.1836 [DOI] [PubMed] [Google Scholar]
- 47.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 2006;174:1034-41. 10.1164/rccm.200604-547OC [DOI] [PubMed] [Google Scholar]
- 48.Vonk-Noordegraaf A, Souza R. Cardiac magnetic resonance imaging: what can it add to our knowledge of the right ventricle in pulmonary arterial hypertension? Am J Cardiol 2012;110:25S-31S. 10.1016/j.amjcard.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 49.Ruocco G, Palazzuoli A. Early detection of pulmonary arterial hypertension: do not forget the right ventricle. Nat Rev Cardiol 2015;12:134. 10.1038/nrcardio.2014.191-c1 [DOI] [PubMed] [Google Scholar]
- 50.Richter SE, Roberts KE, Preston IR, et al. A Simple Derived Prediction Score for the Identification of an Elevated Pulmonary Artery Wedge Pressure Using Precatheterization Clinical Data in Patients Referred to a Pulmonary Hypertension Center. Chest 2016;149:1261-8. 10.1378/chest.15-0819 [DOI] [PubMed] [Google Scholar]
- 51.Flachskampf FA, Biering-Sørensen T, Solomon SD, et al. Cardiac Imaging to Evaluate Left Ventricular Diastolic Function. JACC Cardiovasc Imaging 2015;8:1071-93. 10.1016/j.jcmg.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 52.Anjan VY, Loftus TM, Burke MA, et al. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol 2012;110:870-6. 10.1016/j.amjcard.2012.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenkranz S, Gibbs JS, Wachter R, et al. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016;37:942-54. 10.1093/eurheartj/ehv512 [DOI] [PMC free article] [PubMed] [Google Scholar]