Spontaneous coronary artery dissection (SCAD) is an important cause of acute coronary syndrome (ACS) particularly in women (1,2). Coronary angiography is the gold-standard for establishing a diagnosis and intracoronary imaging should be performed when the diagnosis is unclear (3). However, this disease remains under-diagnosed as the angiographic findings can be subtle. We present a case series of cardiac magnetic resonance imaging (CMR) after the acute SCAD event, with the aim to report CMR findings and characteristics that could assist diagnosis when angiography is not pathognomonic.
Fourteen SCAD patients from our overall cohort of 410 patients in the Vancouver SCAD registries from 2009 to 2017 underwent CMR. Decision to perform CMR was at the discretion of the treating cardiologist and was performed as per the institutional policy. T1-weighted imaging with late gadolinium enhancement (LGE) and T2-weighted CMR images were reviewed independently, blinded to clinical data. All patients were referred to our specialised SCAD clinic, and consented/enrolled and prospectively followed in our SCAD registries approved by the University of British Columbia research ethics board.
All 14 patients were women, with mean age of 52.5±11.9 years. All presented with a troponin-positive myocardial infarction (MI) (28.6% ST-elevation MI, and 71.4% non-ST-elevation MI); 28.6% presented with ventricular arrhythmias. Emotional stressors were reported in 50%, physical stressors in 42.9% and 14.3% were receiving hormonal treatment when presenting with SCAD. Mean body mass index was 25.2±4.9 kg/m2 and had few traditional cardiovascular risk factors; 7.1% current smokers, 14.3% diabetes mellitus, 28.6% dyslipidemia and 35.7% hypertension. Depression was reported in 21.4%, and 28% had prior diagnosis of connective tissue or autoimmune disorder. These women had history of 2.7±1.5 pregnancies and 2/14 were grand multiparas.
Angiographically, there were 1.3±2.0 SCAD lesions with a mean vessel diameter of 2.19±0.50 mm and diameter stenosis of 75.6%±21.9%. Fifty percent had thrombolysis in myocardial infarction (TIMI) flow <3 and the mean left ventricular ejection fraction was 50.2%±15.6%. Fibromuscular dysplasia was present in 35.7% on screening. Only 1 patient underwent percutaneous coronary intervention (92.8% were conservatively managed).
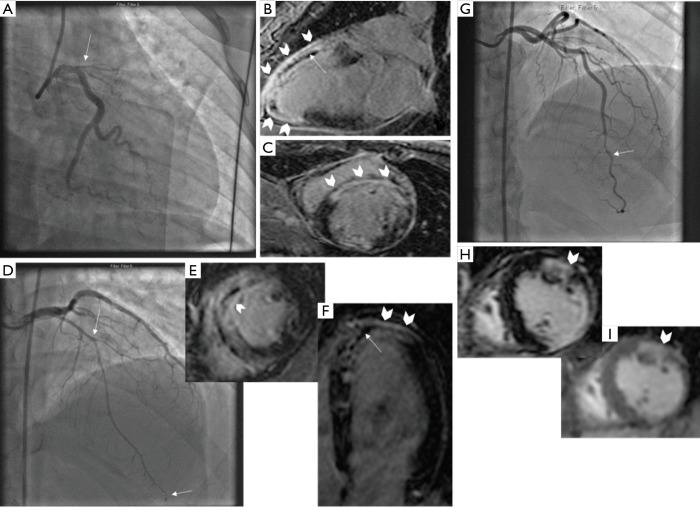
Ten patients presenting with the first SCAD event underwent early CMR testing at a mean of 6.8±6.0 days. Four patients had recurrent SCAD and underwent CMR at a mean of 263±164 days after the first SCAD event. CMR was performed because the underlying diagnosis of SCAD was not initially recognized on angiography. CMR demonstrated LGE in all 14 patients (Figure 1): 8/14 (57.1%) were transmural, 2/14 (14.3%) affected 75% of myocardium, 3/14 (21.4%) were subendocardial, and 1/14 (7.1%) had patchy enhancement. Microvascular obstruction (MVO) was present in 5/14 (35.7%), and 1/14 had intramural hemorrhage (7.1%). These changes coincided with the angiographic territory of SCAD involvement.
Figure 1.
CMR findings in patients with SCAD. Patient 1: (A) coronary angiogram showing ostial LAD SCAD involving large territory, (B,C) CMR findings of LGE (block arrows) and MVO (straight arrow). Patient 2: (D) Coronary angiogram showing diffuse mid-apical LAD SCAD (between straight arrows), (E,F) CMR findings of LGE (block arrows) and MVO (straight arrow). Patient 3: (G) Coronary angiogram showing distal LAD SCAD, (H) T1-weighted CMR showing LGE (block arrow), and (I) T2-weighted CMR showing myocardial edema (block arrow). CMR, cardiac magnetic resonance imaging; SCAD, spontaneous coronary artery dissection; MVO, microvascular obstruction; LAD, left anterior descending; LGE, late gadolinium enhancement.
Findings on CMR in ACS can be helpful to delineate underlying etiology in patients where angiographic diagnosis of SCAD is unclear. SCAD infarct appearance on CMR are distinct from non-SCAD entities such as myocarditis and Takotsubo syndrome. Myocarditis can have LGE involving the mid endocardial rather than subendocardium with SCAD. Takotsubo syndrome tend to have diffuse involvement compared to the focal involvement in SCAD, and also typically lacks LGE. Coronary vasospasm can result in ischemia or infarction (depending on intensity and duration of spasm), which can result in no CMR abnormality, or subendocardial LGE, respectively. In one patient with recurrent SCAD in another territory from the first SCAD event, we noted LGE in the acute territory, and scar in the previous SCAD territory where angiographic healing occurred. As previously reported, SCAD is associated with high rates of spontaneous healing (4).
There was no novel SCAD-specific lesion characteristic per se on CMR and the site of the coronary tear was not identified on MR angiography, which may be a function of the time delay from the acute event and lower spatial resolution. CMR can also determine myocardial viability and the effect of revascularisation if performed for SCAD. Although recent expert consensus documents highlight the role of coronary CT angiography in potential recurrent SCAD cases, or assess for healing in stable patients (5), MRI may have a role in stable pregnant patients to avoid radiation risk. SCAD is an important cause for ACS and patient and public education is crucial. In patients where the cause for ACS is unclear on coronary angiography, CMR can be considered to assess for acute and long-term effects of SCAD. However, despite its clinical utility, we do not recommend routine CMR for SCAD patients due to costs and resources involved.
In conclusion, CMR in patients with acute or recurrent SCAD demonstrated acute infarction with LGE in all patients of varying degrees. Other changes include MVO and myocardial edema. CMR is useful to confirm SCAD infarct diagnosis and should be considered in patients with unclear etiology of ACS, to differentiate from other causes such as myocarditis and Takotsubo syndrome.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Conflicts of Interest: The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. Dr. Saw has received unrestricted research grant supports (from the Canadian Institutes of Health Research, Heart & Stroke Foundation of Canada, National Institutes of Health, University of British Columbia Division of Cardiology, AstraZeneca, Abbott Vascular, St Jude Medical, Boston Scientific, and Servier), salary support (Michael Smith Foundation of Health Research), speaker honoraria (AstraZeneca, Abbott Vascular, Boston Scientific, and Sunovion), consultancy and advisory board honoraria (AstraZeneca, St Jude Medical, Abbott Vascular, Boston Scientific, Baylis, Gore, FEops), and proctorship honoraria (Abbott Vascular, St Jude Medical and Boston Scientific).
References
- 1.Saw J, Humphries K, Aymong E, et al. Spontaneous Coronary Artery Dissection: Clinical Outcomes and Risk of Recurrence. J Am Coll Cardiol 2017;70:1148-58. 10.1016/j.jacc.2017.06.053 [DOI] [PubMed] [Google Scholar]
- 2.Saw J, Starovoytov A, Humphries K, et al. Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J 2019;40:1188-97. 10.1093/eurheartj/ehz007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv 2014;84:1115-22. 10.1002/ccd.25293 [DOI] [PubMed] [Google Scholar]
- 4.Hassan S, Prakash R, Starovoytov A, et al. Natural History of Spontaneous Coronary Artery Dissection With Spontaneous Angiographic Healing. JACC Cardiovasc Interv 2019;12:518-27. 10.1016/j.jcin.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 5.Hayes SN, Kim ESH, Saw J, et al. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement From the American Heart Association. Circulation 2018;137:e523-57. 10.1161/CIR.0000000000000564 [DOI] [PMC free article] [PubMed] [Google Scholar]