Abstract
Wnt/β-catenin and phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin complex 1 (PI3K/AKT/mTORC1) pathways both are critically involved in colorectal cancer (CRC) development, although they are implicated in the modulation of distinct oncogenic mechanisms. In homeostatic and pathologic conditions, these pathways show a fine regulation based mainly on feedback mechanisms, and are connected at multiple levels involving both upstream and downstream common effectors. The ability of the Wnt/β-catenin and PI3K/AKT/mTORC1 pathways to reciprocally control themselves represents one of the main resistance mechanisms to selective inhibitors in CRC, leading to the hypothesis that in specific settings, particularly in cancer driven by genetic alterations in Wnt/β-catenin signaling, the relationship between Wnt/β-catenin and PI3K/AKT/mTORC1 pathways could be so close that they should be considered as a unique therapeutic target. This review provides an update on the Wnt/β-catenin and PI3K/AKT/mTORC1 pathway interconnections in CRC, describing the main molecular players and the potential implications of combined inhibitors as an approach for CRC chemoprevention and treatment.
Keywords: Wnt/β-Catenin, PI3K/AKT/mTORC1, Colorectal Cancer, Crosstalk, Resistance
Abbreviations used in this manuscript: AKT, protein kinase B; APC, adenomatous polyposis coli; CRC, colorectal cancer; DEPTOR, DEP domain-containing mTOR-interacting protein; DVL, Dishevelled; eEF2K, elongation factor 2 kinase; EGFR, epidermal growth factor receptor; eIF4E, eukaryotic translation initiation factor 4E; FAP, familial adenomatous polyposis; FOXO3A, Forkhead box O3A; FZD, frizzled; GSK3, glycogen synthase kinase 3; GTPase, guanosine triphosphatase; LRP, low-density lipoprotein receptors 5 and 6; MEK, Mitogen-activated protein kinase kinase; MNK, Mitogen-activated Protein kinase interacting kinases; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin complex 1; PI3K, phosphatidylinositol-3-kinase; PORCN, Porcupine; RAS, Rat Sarcoma; Rheb, ras homolog enriched in brain; RNF43, ring finger protein 43; S6K, S6 Kinase; TNKS, tankyrase; TNKSi, tankyrase inhibitors; TSC, tuberous sclerosis; YAP, Yes-associated protein; ZNRF3, zinc/ring finger 3; 4E-BP1, 4E binding protein 1
Summary.
This article recapitulates the evidence on the interaction between Wnt/β-catenin and phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin complex 1 pathways in cancer, with a primary focus on intestinal tumorigenesis, and describes the main molecular mechanisms underlying this crosstalk and impact on the resistance to colorectal cancer target therapies.
Colorectal cancer (CRC) is the third most common malignancy in terms of both incidence and mortality worldwide.1 Although surgery combined with standard chemotherapy is effective for early stages, metastatic CRC often is resistant to conventional treatments.
The Wnt/β-catenin pathway and the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) axis frequently are deregulated in CRC, therefore representing an attractive target for chemoprevention and treatment.2,3
The Wnt/β-catenin pathway, also known as the canonical Wnt pathway, controls the self-renewal of intestinal stem cells and is crucial for preserving intestinal homeostasis.4,5 However, sustained Wnt/β-catenin signaling activation triggers hyperproliferation and oncogenic transformation of intestinal epithelial cells, leading to CRC onset.4
The PI3K/AKT/mTOR pathway regulates multiple cellular events including cell growth, proliferation, metabolism, protein and lipid synthesis, and autophagy.6 Moreover, the aberrant activation of the PI3K/AKT/mTOR pathway often has been reported in cancer settings.6
A close connection between Wnt/β-catenin and PI3K/AKT/mTOR signaling has been described in CRC. Indeed, these pathways share common effectors and are able to mutually regulate each other.
Over the years, numerous inhibitors of Wnt/β-catenin and PI3K/mTOR complex 1 (mTORC1) pathways have been developed and tested against CRC. These targeted therapies have shown clinical effectiveness in the treatment of advanced CRC, although the long-term effects frequently fail owing to the acquisition of resistance mechanisms.7,8 In particular, Wnt/β-catenin pathway inhibition has been associated with the up-regulation of the PI3K/AKT/mTORC1 pathway and, similarly, blocking the PI3K/AKT/mTORC1 cascade results in Wnt/β-catenin signaling hyperactivation as a compensatory mechanism.
This review comprehensively discusses the literature concerning the mechanisms linking the Wnt/β-catenin and PI3K/AKT/mTORC1 pathways in CRC development and elucidates the biological processes and components leading to pharmacologic resistance to selective inhibitors.
Role of Wnt/β-Catenin Signaling in Colorectal Cancer
β-Catenin Turnover Regulation
Since the critical role of Wnt/β-catenin signaling in the etiology of CRC has been established, many studies have been conducted to identify key molecular players that could represent concrete targets for CRC chemoprevention and therapy.
In the inactive state, β-catenin levels are kept stably low through the dynamic activity of a protein complex known as the destruction complex or degradosome.9 This complex includes casein kinase 1α, glycogen synthase kinase 3 (GSK3), Axis inhibition protein 1, adenomatous polyposis coli (APC), protein phosphatase 2A, and the E3-ubiquitin ligase β-transducin repeats–containing protein.9, 10, 11, 12, 13 APC and Axis inhibition protein 1 function as scaffold proteins in the destruction complex, while casein kinase Iα and GSK3 are the main serine/threonine kinases involved in β-catenin phosphorylation.14 β-transducin repeats–containing protein mediates the ubiquitination of phosphorylated β-catenin and targets it for degradation by the proteasome machinery.15,16 It is noteworthy that Axis inhibition protein 1 has been proposed as the rate-limiting factor for the degradosome assembly because its endogenous levels are finely regulated and kept low in the absence of Wnt stimulation.17
Wnt ligands are active molecules that bind the 7-transmembrane receptor frizzled (FZD) family and the co-receptors low-density lipoprotein receptors 5 and 6 (LRP5/6).18, 19, 20, 21 The formation of the ligand-receptor complex Wnt-FZD-LRP5/6 and the recruitment of the adaptor dishevelled (DVL) by FZD lead to LRP6 phosphorylation, Axis inhibition protein 1 translocation to the plasma membrane, its association with LRP6, and the dissociation of GSK3 from Axis inhibition protein 1 and APC with consequent dephosphorylation and stabilization of β-catenin.22,23 These events cause the assembly of the signalosome, a multiprotein complex able of transducing Wnt signals, as well as the degradosome disassembly with consequent cytosolic β-catenin accumulation and nuclear translocation. Finally, nuclear β-catenin acts as a transcriptional co-activator interacting with T-cell transcription factor,24 or lymphoid enhancer factor,25 and inducing the transcription of target genes, including AXIN2,26 c-MYC,27 CCND1,28,29 thus promoting cell proliferation and activation of oncogenic mechanisms.
Two main critical steps have been proposed to be responsible for the impairment of β-catenin degradation on Wnt stimulation, attributable to the inhibition of β-catenin phosphorylation or ubiquitination, respectively. Despite inhibition of β-catenin ubiquitination showing effects on the prevention of β-catenin degradation,30 most of the evidence supports the concept that the inhibition of β-catenin phosphorylation and the disassembly of the β-catenin destruction complex represent the crucial event for Wnt/β-catenin pathway activation. In particular, by studying the kinetics of β-catenin turnover at the steady-state level, Hernández et al31 showed that in the early phases after Wnt stimulation, the reduced levels of GSK3β-mediated β-catenin phosphorylation are responsible for β-catenin degradation inhibition, while a subsequent recovery of β-catenin phosphorylation would restore its degradation. Thus, according to this model, inhibition of β-catenin phosphorylation leads to β-catenin accumulation during Wnt stimulation.
Moreover, in the early phases after Wnt stimulation, Axis inhibition protein 1 protein stability is increased, thus enhancing the signalosome assembly and Wnt/β-catenin signaling initiation.32 On the other hand, on long-term Wnt stimulation, adenosine diphosphate–ribosylation by poly (adenosine diphosphate–ribose) polymerase enzyme tankyrase (TNKS) triggers Axis inhibition protein 1 proteolysis and degradation, resulting in the dissociation of the β-catenin destruction complex and sustained Wnt/β-catenin signaling activation.32 Thus, Axis inhibition protein 1 also functions as a fundamental switch for controlling both Wnt/β-catenin cascade activation and inhibition.
More comprehensive results about the mechanisms underlying Wnt/β-catenin signaling regulation and the dynamics of the β-catenin destruction complexes under the presence of Wnt signals have been described recently.33 Indeed, under Wnt stimulation, a fraction of the β-catenin destruction complexes is disassembled with a consequent initial accumulation of cytosolic β-catenin. At the same time, a portion of functional complexes do persist, capable of progressively restoring β-catenin phosphorylation and degradation rates.33
Moreover, emerging evidence has shown that ring finger protein 43 (RNF43), the transmembrane E3 ubiquitin ligase zinc and ring finger 3 (ZNRF3) and DVL play a critical role in controlling extrinsic Wnt/β-catenin pathway activation by regulating the levels of Wnt receptors on the cellular membrane. Indeed, RNF43 and ZNRF3, promoting the internalization and the subsequent degradation of Wnt receptors FZD and LRP6, act as crucial negative regulators of the signaling.34,35 Importantly, recent data have shown that DVL is required not only for Wnt/β-catenin signaling initiation but also for RNF43 and ZNRF3-mediated degradation of FZD and LRP6.36 In contrast, R-spondin, by inhibiting ZNRF3, operates as a Wnt/β-catenin signaling enhancer.35
In addition, an interaction between Hippo and the Wnt/β-catenin signaling pathway has been described. However, the literature evidence still is quite controversial and the mechanisms underlying this crosstalk are under investigation. Indeed, although some studies described the Hippo signaling transducers Yes-associated protein (YAP) and Transcriptional coactivator with PDZ-binding motif as components of the degradosome and transcriptional co-activators of β-catenin,37 other reports have indicated a possible involvement of the Hippo cascade in noncanonical Wnt signaling, showing that it acts as an inhibitor of the canonical Wnt signaling.38,39 Moreover, APC has been described as a dual upstream regulator of both Hippo YAP and β-catenin pathways, which independently contribute to the intestinal tumorigenesis in the absence of functional APC,40 although another study showed that Wnt/β-catenin signaling directly regulates YAP expression through the β-catenin/T-cell transcription factor 4 transcriptional complex.41
Overall, these extensive findings suggest that the regulation of this pathway is more complex than previously thought, and probably many mechanisms still need to be clarified.
Mutations in Wnt/β-Catenin Signaling Components
Genetic alterations in the Wnt/β-catenin pathway components, frequently observed in CRC, lead to the intrinsic aberrant canonical Wnt/β-catenin signaling activation. Inactivating germ-line mutations in the APC gene, encoding for the core scaffold element of the β-catenin destruction complex, are causative for the development of the rare hereditary CRC-predisposing syndrome familial adenomatous polyposis (FAP),42,43 while somatic mutations in the APC gene constitute the most frequent initiating event in sporadic CRC (approximately 80%–90% of cases).44 Recent data have shown that APC regulates β-catenin levels, also acting as a modulator of the Wnt receptor LRP6.45,46 Indeed, genetic alterations in the APC gene induce an autoassembly of the signalosome in the absence of Wnt ligands, thus resulting in uncontrolled pathway activation. Importantly, these new relevant data have raised the possibility of counteracting the functional effects induced by APC loss through the modulation of the upstream receptors.
Activating point mutations of the β-catenin encoding gene CTNNB1 have been observed in a small percentage of CRC cases with wild-type APC, 47 while somatic mutations in AXIN2 mainly have been associated with mismatch repair–deficient CRC cases.48 Somatic AXIN1 mutations, resulting in loss of function, also are found at low frequency in colorectal adenomas and CRC.49,50 Finally, further causative alterations have been described in RNF43, ZNRF3, and TCF4 genes. 35,51,52
The mTOR Pathway in CRC
PI3K/AKT/mTORC1 Axis
mTOR is a serine/threonine protein kinase made of 2 multiprotein complexes: mTORC1 and mTORC2.53 Mammalian lethal with SEC13 protein 8 (mLST8) (also known as G Protein beta Subunit-like),54 DEP domain-containing mTOR-interacting protein (DEPTOR),55 and the Tel-2 interacting protein 1/Telomere maintenance 2 factors56 are common to both protein complexes. Regulatory-associated protein of mTOR57 and Proline-Rich AKT Substrate of 40 KDa58 are distinctive of the mTORC1 complex, while Rapamycin-insensitive companion of mTOR,59 mammalian Stress-activated protein kinase-interacting protein 1,60 and Protein observed with RICTOR 1/261 belong to the mTORC2 complex. The 2 complexes act through different mechanisms and show a distinct sensitivity to stimuli, in particular to rapamycin, which is higher for mTORC1.62 Although the mTORC2 function still is not completely characterized, mTORC1 has been largely described.6 mTORC1 is activated on different stimuli, such as growth factors, nutrients, cellular stress, hypoxia, and DNA damage.6 The heterodimer complex constituted by tuberous sclerosis (TSC)1 and TSC2 plays a key role in the upstream regulation of the pathway and functions as a bridge to flow activating signals and molecules onto mTORC1. The TSC1/TSC2 complex works as a guanosine triphosphatase (GTPase) activating protein for Ras homolog enriched in brain (Rheb), a GTPase belonging to the Ras family.63 Rheb-GTP functions as a potent inducer of mTORC1 kinase activity. The TSC1/TSC2 complex, promoting the conversion from Rheb-GTP to Rheb–guanosine diphosphate, acts as a negative regulator of mTORC1 signaling.64
Although the mTORC1 cascade is induced through different mechanisms, the main activation route in response to mitogenic stimuli involves the upstream regulators PI3K and AKT.65 PI3K induces AKT by promoting the conversion of phosphatidylinositol (3,4)-bisphosphate to phosphatidylinositol (3,4,5)-trisphosphate, and triggering AKT phosphorylation at Thr308 via 3’phosphoinositide-dependent kinase 1.66 This event leads to mTORC1 activation by AKT-mediated phosphorylation and the consequent inactivation of Proline-Rich AKT Substrate of 40 KDa (PRAS40)58 and TSC2.67,68 Moreover, AKT also acts through mTORC1 phosphorylation at Ser2448.69 Once activated, mTORC1 mediates the phosphorylation of 2 main downstream targets: eukaryotic translation initiation factor 4E (eIF4E) binding protein 1 (4E-BP1), and p70S6 ribosomal kinase 1 (S6 Kinase 1 [S6K1] or p70S6 ribosomal kinase 1). Phosphorylated 4E-BP1 dissociates from the cap-binding protein eIF4E, thus promoting messenger RNA translation.70,71 In addition, activation of S6K1 protein leads to ribosomal protein S6 phosphorylation and consequent induction of translation initiation and elongation.72
Genetic Alterations in the PI3K/AKT/mTORC1 Pathway
The involvement of the mTORC1 pathway in cancer onset and promotion has been widely described. The aberrant activation of this pathway frequently depends on genetic alterations in upstream regulators rather than in mTOR genes. Gain-of-function mutations in the PIK3CA gene have been described in 12%–32% of CRC patients and have been associated with proximal CRC.73, 74, 75, 76, 77, 78, 79 Moreover, somatic mutations in components of the PI3K/AKT/mTORC1 pathway have been observed frequently in Lynch syndrome cases.80 Inactivating mutations or loss of heterozygosis in the PTEN gene, a negative regulator of PI3K activity, also have been found, particularly in tumors with microsatellite instability.79,81, 82, 83, 84, 85, 86 PTEN epigenetic silencing represents an additional mechanism for PI3K activity regulation in CRC.87 Furthermore, somatic mutations in the STK11/LKB1 gene, which encode for an mTORC1 repressor,88 have been observed in a small percentage of sporadic CRC cases.89,90 Finally, AKT mutations are a rare occurrence in CRC.91
PI3K/AKT/mTORC1 Signaling in Intestinal Tumorigenesis Driven by Apc Loss
The involvement of PI3K/AKT/mTORC1 signaling in the progression of Apc-driven CRC has been shown largely in murine models. Treatment with the mTORC1 inhibitor everolimus was found to reduce tumor burden in the ApcΔ716 mouse model of FAP that showed induced mTORC1 signaling in the intestinal polyps.92 Similar findings have been obtained in the ApcMin/+ mouse model of FAP,93,94 and in the Apc conditional knock-out mouse model with treatment with rapamycin.95 It should be noted that both β-catenin–dependent and β-catenin–independent mechanisms have been described to explain mTORC1 induction with Wnt pathway activation.92,96
Subsequent studies further clarified the involvement of mTORC1 signaling upon Apc inactivation. Indeed, although mTORC1 activation is crucial to ensure the survival and proliferation of murine enterocytes upon Apc gene deletion, its inhibition has no relevant effects on the intestines of Apc wild-type mice.97 This study described important mechanisms linking mTORC1 activation to the intestinal tumorigenesis driven by Apc loss. Upon Apc gene deletion, activated mTORC1 enhances the elongation translation rate by inhibiting the elongation factor 2 kinase (eEF2K) via S6K and activating the eEF2. Importantly, this mechanism involving the mTORC1-S6K-eEF2K-eEF2 axis seems crucial to sustain the growth and expansion of Apc-deficient enterocytes.97 Furthermore, the synergic effect of the Wnt/β-catenin and PI3K/AKT/mTORC1 pathways on the development of colonic lesions was shown in another study by crossing the ApcMin/+ and FC PIK3ca∗ mice. In this model, characterized by a germ-line mutation in the Apc gene and constitutive activation of PI3K in the intestine, the simultaneous activation of both pathways boosted tumor initiation and promotion.98
Recently, Brandt et al99 showed that although mTORC1 inhibition could represent an effective preventive strategy against Apc mutant CRC, it could promote carcinogenesis in long-standing inflammatory bowel diseases. Indeed, in a setting of chronic inflammation, mTORC1 signaling plays a critical protective role by promoting intestinal regeneration upon injury. Interestingly, given the existing link between protein intake and mTORC1 activation, the investigators have proposed that it would be possible to affect colonic tumor progression by adjusting the dietary protein intake, depending on the underlying mechanisms of CRC development (ie, low-protein intake in Wnt-sustained tumors or high-protein intake in a condition of persistent inflammation).99 This evidence suggests a new possible scenario in the prevention of CRC, coupling a pharmacologic approach with dietary strategies that could increase the effectiveness of targeted drugs substantially.
Common Effectors and Linking Elements Between Wnt/β-Catenin and PI3K/AKT/mTORC1 Pathways
GSK3β
Wnt/β-catenin and PI3K/AKT/mTORC1 pathways share some effectors critically involved in both signaling pathways. GSK3β represents a common key element, which independently participates in both signaling cascades regulating different cellular processes. GSK3 kinase is constitutively active and is regulated negatively through different mechanisms. Distinct intracellular pools of GSK3β have been implicated in the regulation of Wnt/β-catenin and PI3K/AKT/mTORC1.100 The activation of both signaling pathways results in the inhibition of GSK3β activity, although via different upstream events. The regulation of GSK3β activity during Wnt signaling occurs mainly through protein–protein interactions and intracellular sequestration.100 In the Wnt/β-catenin pathway, a fraction of AXIN-bound GSK3β has an important role in controlling β-catenin degradation, through the regulation of β-catenin phosphorylation.101 In particular, the GSK3β interaction with the scaffolding protein AXIN is crucial to allow an efficient GSK3β-mediated phosphorylation of β-catenin.10 Importantly, APC also regulates GSK3β. Indeed, the APC dissociation from AXIN, which is induced rapidly on Wnt stimulation, weakens GSK3β activity, with consequent β-catenin stabilization.102 In addition, the GSK3β-mediated LRP6 phosphorylation, which occurs on Wnt stimulation, leads to GSK3β inhibition.103
GSK3β also interacts with the PI3K/AKT/mTORC1 pathway through different mechanisms. 96,102,104 Upon PI3K activation, phosphorylated AKT is able to inhibit GSK3β kinase activity by inducing its phosphorylation at Ser9.105,106 In addition, in specific circumstances, such as reduced AKT activation, GSK3β can be phosphorylated and inactivated by S6K.107 Importantly, much evidence has shown that GSK3β inhibition (induced upon Wnt stimulation) does not occur through AKT-mediated phosphorylation at Ser9.108 Thus, AKT activation has no direct effects on the Wnt/β-catenin pathway.109,110 However, as suggested by Ding et al,109 it is possible that in conditions of AKT hyperactivation and active canonical Wnt signaling, the simultaneous inhibition of GSK3β activity induced by the 2 signaling pathways may result in enhanced β-catenin accumulation. Moreover, APC loss, leading to GSK3β dysregulation and reduction of its kinasic activity, has been associated with mTORC1 activation with a mechanism independent from β-catenin accumulation.102 Thus, APC inhibition represents a critical event for the simultaneous induction of both β-catenin and mTORC1 signaling through independent downstream mechanisms sharing the upstream inhibition of GSK3β.102
mTORC1 inhibition promotes GSK3β nuclear translocation,111 and, importantly, GSK3β expression seems crucial for cancer cell responsiveness to mTOR inhibitors.112 A new mechanism explaining the relationship between GSK3β and mTORC1 has been proposed recently.113,114 The investigators showed that GSK3β nuclear translocation induced by rapamycin was associated with increased phosphorylation of the nuclear proteins Forkhead Box K1 and General Transcription Factor IIF Subunit 1, slowing down cell proliferation.113,114
Although the role of the PI3K/AKT/mTORC1 pathway in the modulation of translation is widely described,115 the involvement of Wnt/β-catenin signaling in this process is poorly characterized. Preclinical studies have shown that both intrinsic and extrinsic Wnt pathway activation, leading to S6K and 4E-BP1 phosphorylation, may affect the protein synthesis turning on the mTORC1 cascade. Importantly, the Wnt-driven mTORC1 signaling activation seems to be independent from β-catenin and mediated by an axis involving APC-AXIN-GSK3β and TSC2 (Figure 1A).96
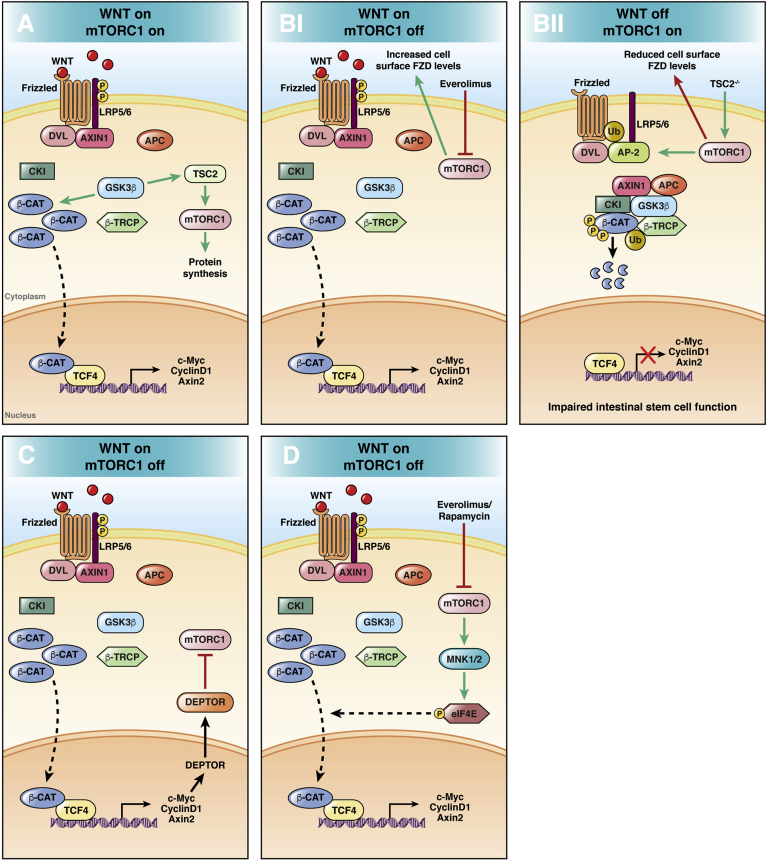
Figure 1.
Main mechanisms of Wnt/β-catenin and PI3K/AKT/mTORC1 pathway interconnections. (A) The Wnt/β-catenin pathway modulates transcription through β-catenin and translation via mTORC1 regulating GSK3β activity.96 (Bi) mTORC1 inhibition by the rapalog everolimus induces Wnt/β-catenin signaling activation by increasing the expression of FZD receptor levels with a mechanism dependent on DVL. (Bii) Activated mTORC1 promotes the association between DVL and the clathrin Adaptor protein 2 (AP-2) adaptor with a consequent reduction of FZD expression levels, with a negative regulation of Wnt/β-catenin signaling.116 (C) Wnt/β-catenin signaling switches off the mTORC1 cascade by inducing its negative regulator DEPTOR.117 (D) Inhibited PI3K/AKT/mTORC1 pathway leads to increased eIF4E phosphorylation via MNK.124 Phosphorylated eIF4E is associated with β-catenin nuclear translocation and signaling activation.127 β-CAT, β-catenin; β-TRCP, Beta-transducin repeat containing E3 ubiquitin protein ligase.
FZD, DVL, and Deptor
Recently, Zeng et al116 showed that mTORC1 negatively regulates Wnt/β-catenin signaling by modulating the extrinsic Wnt response both ex vivo and in vivo, and showed that mTORC1 would affect the expression of the Wnt receptor FZD with a mechanism dependent on the Wnt-positive regulator DVL (Figure 1B). Importantly, the mTORC1-driven inhibition of Wnt/β-catenin signaling resulted in a compromised stem cell function in intestinal organoids, highlighting the importance of mutual regulation for intestinal homeostasis maintenance.116 The role of DVL in the regulation of Wnt receptors LRP6 and FZD has been shown previously in vitro through a genome editing approach. Although DVL is critically involved in the signalosome assembly, it also modulates the cellular turnover of Wnt receptors, being implicated in a negative feedback loop controlled by ZNRF3 and RNF43.36
Wang et al117 recently described a mechanism linking the Wnt/β-catenin and mTORC1 pathways, showing that Wnt/β-catenin signaling induction, up-regulating the mTORC1-negative regulator DEPTOR, leads to DEPTOR-mediated mTORC1 suppression (Figure 1C).
eIF4E
An additional connection between the 2 signaling pathways involves the translation initiation factor eIF4E.118 The up-regulation of eIF4E has been described in multiple cancers,119 including CRC.120,121 The main oncogenic mechanism attributed to eIF4E is related to promoting the translation of genes critically involved in the malignant transformation of epithelial cells.122 The oncogenic activity of eIF4E is enhanced through its phosphorylation at Ser209, promoted by Mitogen-activated Protein kinase (MAPK) interacting kinases (MNK) 1 and MNK2.123 It is known that mTORC1 inhibition by rapamycin leads to increased eIF4E phosphorylation and activation via a MNK1- and PI3K-dependent mechanism in different types of cancer cells, including CRC cell lines.124 It should be noted that this feedback mechanism could be counteracted by using PI3K inhibitors, as shown in human lung cancer cells.125 Importantly, eIF4E phosphorylation also has been associated with Wnt/β-catenin signaling activation by promoting β-catenin nuclear translocation in different cancer settings (Figure 1D).126,127
Wnt/β-Catenin and PI3K/AKT/mTORC1 Pathways as Drivers of CRC Resistance to Treatment
Target cancer therapy resistance generally is driven by the development of additional mutations in downstream effectors of the same targeted pathway or activation of alternative signaling pathways as compensatory mechanisms.7
Rapalogs and Dual PI3K/mTOR Inhibitors
Everolimus and temsirolimus are 2 mTORC1 inhibitors whose efficacy have been evaluated in phase II clinical trials in metastatic CRC patients.128,129 However, a small retrospective analysis of tumors with activating PIK3CA mutations showed that treatment with the mTORC1 inhibitor everolimus had no relevant effect.130 In addition, it has been shown that mTORC1/2 inhibition could restore sensitivity to everolimus in PIK3CA mutant CRC in preclinical models.130 PKI-587,131 XL765,132 BEZ235,133 and LY3023414134 are dual PI3K/mTOR inhibitors that have completed phase I clinical trials in patients with advanced solid tumors, including CRC. It has been shown that the Wnt/β-catenin pathway activation induces resistance to dual PI3K/mTOR inhibitor PKI-587 in PIK3CA mutant CRC cells, while GSK3β inactivation restored the sensitivity to PKI-587.135 However, these counterintuitive results are not in agreement with the general view supporting the negative regulator role of GSK3β in the Wnt/β-catenin pathway, and contradict robust literature data showing that GSK3β inhibitors activate Wnt/β-catenin signaling. Importantly, endogenous levels of GSK3β are crucial in determining the sensitivity to mTORC1 inhibitors in CRC. Indeed, GSK3β down-regulation has been associated with mTORC1 inhibitor resistance.136 Moreover, high levels of the GSK3β nuclear exporter Frequently rearranged in advanced T-cell lymphomas 1/2, which interferes with the cytostatic effects of rapamycin, might be relevant to predict the response to rapalogs as shown in lung and breast cancer cell lines (Figure 2A).114 Recently, Foley et al137 showed that turning off both PI3K and mTOR by using the inhibitors BEZ235 and LY3023414 may represent an effective therapeutic strategy in CRC carrying concomitant APC and PIK3CA mutations.
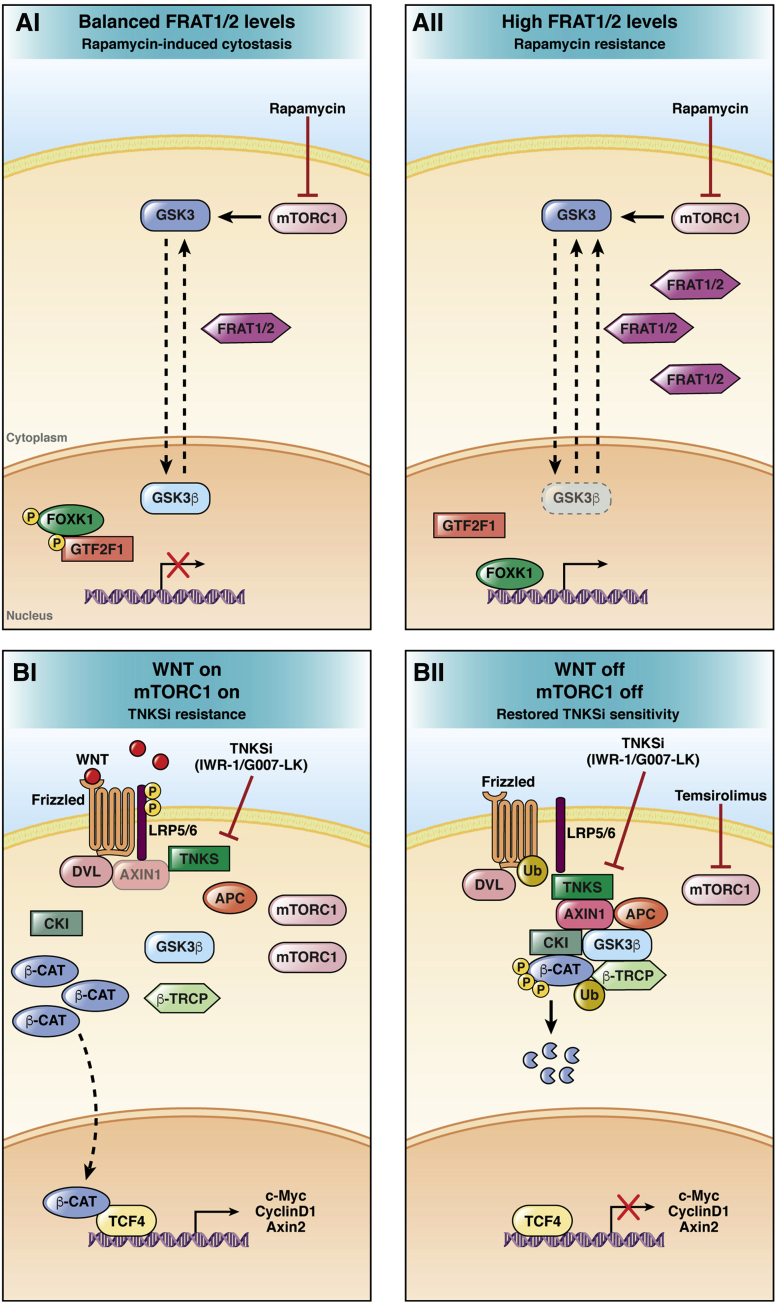
Figure 2.
Resistance mechanisms to rapamycin and TNKSi. (Ai) mTORC1 inhibition promotes GSK3β nuclear translocation. Nuclear GSK3β mediates rapamycin-induced cytostasis by increasing the phosphorylation of Forkhead Box K1 and General Transcription Factor IIF Subunit 1. (Aii) In conditions of high cellular levels of the GSK3β nuclear exporter Frequently rearranged in advanced T-cell lymphomas 1/2 (FRAT 1/2), upon mTORC1 inhibition, nuclear GSK3β levels are not sufficient to induce cytostasis leading to rapamycin resistance.113,114 (Bi) mTORC1 induction is associated with TNKSi resistance and persistent Wnt/β-catenin signaling activation. (Bii) mTORC1 activity reduction by temsirolimus restores the sensitivity to TNKSi, leading to Wnt/β-catenin down-regulation.144 β-CAT, β-catenin; β-TRCP, Beta-transducin repeat containing E3 ubiquitin protein ligase; CKI, Casein Kinase I.
Tankyrase Inhibitors
Tankyrase inhibitors (TNKSi) are small molecules that induce Axis inhibition protein 1/2 stabilization, abrogating Wnt/β-catenin signaling.4 Several TNKSi, including XAV939,138 IWR-1,139 JW74,140 and G007-LK,141 have been shown to impair Wnt/β-catenin signaling in vitro or in mouse models of CRC. However, Wnt/β-catenin inhibition induced by TNKSi has been associated with intestinal cytotoxicity and CRC cell lines have shown heterogeneous sensitivity to TNKSi.142,143 Mashima et al,144 by using the TNKSi-resistant cell line 320-IWR, provided evidence of mTORC1 activation as a resistance mechanism to TNKSi IWR-1 and G007-LK. Interestingly, inhibition of mTORC1 by temsirolimus restored sensitivity to TNKSi (Figure 2B). These data further show that mTORC1 activation might represent a survival mechanism used by cancer cells upon Wnt/β-catenin signaling inhibition. Recently, the combined effect of TNKSi inhibitor G007-LK, the PI3K inhibitor NVP-BKM120, and the epidermal growth factor receptor (EGFR) inhibitor erlotinib has been evaluated in CRC cell lines and xenografts. In both TNKSi-sensitive COLO320 DM and TNKSi-insensitive HCT-15 cell lines the combined inhibition of TNKSi, PI3K, and EGFR was able to reduce cell growth, as well as tumor size in vivo, by acting on multiple cancer-related pathways including Wnt/β-catenin, AKT/mTOR, EGFR, and Rat Sarcoma (RAS) signaling, with different effects depending on the genetic profile of the studied cell lines.145
Forkhead box O3a (FOXO3a) is a transcription factor that acts as a tumor suppressor, inducing cell-cycle arrest and apoptosis.146 Phosphorylation of FOXO3a by AKT induces its sequestration into the cytoplasm and inhibition of its transcriptional activity while PI3K or AKT inhibitors relocate FOXO3a into the nucleus, restoring its tumor-suppressor role. Importantly, high levels of nuclear β-catenin confer resistance to FOXO3a-induced apoptosis in metastatic CRC patient–derived cells treated with PI3K/AKT inhibitors.147 Notably, TNKSi XAV-939 reverted this resistance, sensitizing cells to FOXO3a-induced apoptosis.147 In addition, CRC patient–derived cells with high levels of nuclear β-catenin show reduced apoptosis upon treatment with the AKT or PI3K inhibitors API2 or NVP-BKM120.148 As suggested by the investigators, these data support the concept that Wnt/β-catenin hyperactivation leads to pharmacologic resistance to PI3K and AKT inhibitors in CRC. Importantly, the association of the TNKSi NVP-TNKS656 (a derivative of XAV939) with PI3K and AKT inhibitors improve the response to treatment both in vitro and in vivo, in particular in the presence of high nuclear β-catenin and FOXO3a levels.148
Taken together, these findings indicate that a valid strategy to overcome resistance to PI3K/AKT/mTOR inhibitors could be combining these inhibitors with TNKSi to block the Wnt/β-catenin pathway.
Targeting RAS/Rapidly Accelerated Fibrosarcoma/Mitogen-Activated Protein Kinase Kinase/Extracellular Regulated Kinase
EGFR is upstream of both mitogen-activated protein kinase and PI3K/AKT pathways. Much evidence has shown that, after the administration of the anti-EGFR therapies cetuximab or panitumumab, CRC acquires drug resistance through the activation of the downstream extracellular regulated kinase 1/2 signaling as a consequence of mutations in KRAS, NRAS, and Mitogen-activated protein kinase kinase (MAPKK or MEK) (MEK)1/2.149 MEK is downstream of Kirsten rat sarcoma 2 viral oncogene homolog in the RAS/Rapidly Accelerated Fibrosarcoma/MEK/extracellular regulated kinase pathway and inhibition of MEK blocks signal transduction independently from the upstream mutation. Moreover, it is known that PIK3CA mutations confer resistance to MEK inhibitors in KRAS mutant cancers.150 Recently, a study showed that β-catenin was responsible for the resistance of PIK3CA mutated tumors to MEK inhibitors.151 Notably, pharmacologic inhibition of β-catenin with TNKSi NVP-TNKS656 can resensitize PIK3CA mutant cells to MEK inhibitors.151
Porcupine Inhibitors
A further mechanism that turns off the Wnt/β-catenin signaling is through the impairment of the enzymatic activity of Porcupine (PORCN), which mediates the palmitoleation and subsequent secretion of Wnt ligands.152, 153, 154 Various PORCN inhibitors have been tested in preclinical models as a strategy against Wnt-driven cancers, some of which, including LGK974 or ETC-159, have been used in early phase clinical trials.155, 156, 157 However, as for the majority of Wnt inhibitors, PORCN inhibitors also impair intestinal homeostasis and are subjected to resistance.157 Interestingly, Axis inhibition protein 1 suppression recently has been described as a driver of resistance to the PORCN inhibitor LGK974 in CRC cell lines, carrying genomic rearrangements in the RSPO3 gene.158 It should be noted that the combination of PORCN inhibitor ETC-159 with PI3K/mTOR inhibitors has shown a synergistic effect against tumor growth in pancreatic cancer cells with RNF43 mutations, as well as in vivo models, indicating that the dual blockage might be effective in CRC.159
An overview of Wnt/β-catenin, rapalogs, and dual PI3K/mTOR inhibitors tested in clinical and preclinical CRC settings is shown in Table 1.
Table 1.
Overview of Wnt/β-Catenin, Rapalogs, and Dual PI3K/mTOR Inhibitors Discussed in This Review
| Inhibitors | Target | Tested settings | Findings | References |
|---|---|---|---|---|
| Rapamycin | mTORC1 | Murine model | Inhibition of intestinal neoplasia in Apc mutant models | 93,95 |
| Everolimus | mTORC1 | Murine model | Reduced tumor burden in the ApcΔ716 mouse model | 92 |
| Everolimus | mTORC1 | CRC (phase II study) | Limited efficacy in metastatic CRC | 128 |
| Temsirolimus | mTORC1 | CRC (phase II study) | Limited efficacy in KRAS mutant CRC | 129 |
| Temsirolimus | mTORC1 | CRC cell lines | Reversed resistance to TNKSi | 144 |
| TAK228 | mTORC1/2 | Murine models and spheroids | Overcomes resistance to everolimus and induces response in PIK3CA mutant CRCs | 130 |
| PKI-587 | PI3K/mTORC | Solid tumors (phase I study) | Antitumor activity in patients resistant to conventional therapies | 131 |
| PKI-587 | PI3K/mTORC | CRC cell lines | Resistance to PKI-587 in PIK3CA mutant CRC cells | 135 |
| XL765 | PI3K/mTORC | Solid tumors (phase I study) | Antitumor activity in patients resistant to conventional therapies | 132 |
| BEZ235 | PI3K/mTORC | Solid tumors (phase I study) | No effect in patients with advanced solid tumors | 133 |
| LY3023414 | PI3K/mTORC | Solid tumors (phase I study) | Efficacy in patients with advanced solid tumors | 134 |
| LY3023414 | PI3K/mTORC | Murine models and spheroids | Potential treatment strategy in PIK3CA mutant CRCs | 137 |
| XAV939 | TNKS | Cell lines, patient-derived cells, murine models | Reversed resistance in patient-derived primary cultures and in corresponding xenograft tumors in mice | 147 |
| JW74 | TNKS | Cell lines and murine models | Decreased cell growth in CRC xenograft and reduced polyp formation in ApcMin/+ mice | 140 |
| G007-LK | TNKS | Cell lines and murine models | Reduced CRC cell line growth; tumor growth inhibition in Apc mutant CRC xenograft and genetically engineered CRC models | 141 |
| G007-LK | TNKS | Cell lines and murine models | Enhanced effect of PI3K (BKM120) and EGFR (erlotinib) inhibition in CRC cells and reduced growth of CRC xenografts in vivo | 145 |
| NVP-TNKS656 | TNKS | Cell lines, patient-derived cells, murine models | Overcomes resistance to PI3K or AKT inhibitors in CRC patient-derived sphere cultures and represses tumor growth in CRC-PDX models | 148 |
| NVP-TNKS656 | TNKS | Cell lines and murine models | Overcomes resistance to MEK inhibitors in CRC with KRAS and PIK3CA mutations | 151 |
| ETC-159 | PORCN | Murine models | Effective for treatment of RSPO translocation in CRC xenografts | 156 |
| LGK974 | PORCN | Cell lines and murine models | Loss of AXIN1 mediates resistance to LGK974 in CRC cells carrying RSPO3 fusions | 158 |
AXIN1, Axis inhibition protein 1; PDX, Patient derived xenografts.
Conclusions
The interconnection between the Wnt/β-catenin and PI3K/AKT/mTORC1 pathways has been shown widely in different cancer settings, including CRC. Most of the studies elucidating the relationship between these pathways have been conducted in preclinical models. As discussed in this review, there are many connecting elements between these 2 pathways capable of interfering with key processes regulating the β-catenin turnover: (1) in particular, the regulation of β-catenin phosphorylation mediated by GSK3β and the degradosome assembly/disassembly; (2) the modulation of the Wnt receptor levels (extrinsic Wnt activation) in which DVL has a crucial role; (3) mechanisms affecting the β-catenin nuclear translocation, which involves many components including eIF4E; and (4) the regulation of the downstream effectors of the PI3K/AKT/mTORC1 pathway mediated by GSK3β. Moreover, clinical data in patients with advanced disease showed increased resistance to targeted therapies, highlighting the relevance of this interaction in predicting patient response. In this context, GSK3β levels seem crucial for predicting the response to mTORC1 inhibitors. In addition, defined cancer and precancerous subsets could benefit from the combination of PI3K/mTORC1 inhibitors with TNKSi. In particular, simultaneous inhibition of both the Wnt/β-catenin and PI3K/AKT/mTORC1 pathways may represent a valid chemopreventive strategy in FAP, in which the proliferative boost and oncogenic transformation of intestinal epithelial cells is supported by Wnt/β-catenin signaling overactivation. In conclusion, it is important to note 2 relevant concepts for future studies: the interdependence, which characterizes these pathways, constitutes a critical factor, for developing new drugs against CRC progression; and the dynamics that characterize these 2 signaling pathways, in the context of colorectal carcinogenesis, should be evaluated, taking into account the response and the fine regulation processes that distinguish these pathways. Future studies on this topic in human beings will be indispensable to better define unsolved mechanisms in this challenging scenario and to establish the translational impact of these crosstalk mechanisms.
Footnotes
Author contributions Anna Prossomariti, Giulia Piazzi, and Chiara Alquati searched and analyzed the literature for the article and wrote the manuscript; and Luigi Ricciardiello edited and reviewed the manuscript and critically contributed to the discussion of the content.
Conflicts of interest The authors disclose no conflicts.
Funding Supported by the Italian Foundation for Cancer Research AIRC grant IG 21723 (L.R.).
Contributor Information
Anna Prossomariti, Email: anna.prossomariti2@unibo.it.
Luigi Ricciardiello, Email: luigi.ricciardiello@unibo.it.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Cheng X., Xu X., Chen D., Zhao F., Wang W. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed Pharmacother. 2019;110:473–481. doi: 10.1016/j.biopha.2018.11.082. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J., Roberts T.M., Shivdasani R.A. Targeting PI3K signaling as a therapeutic approach for colorectal cancer. Gastroenterology. 2011;141:50–61. doi: 10.1053/j.gastro.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Clevers H., Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Krausova M., Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal. 2014;26:570–579. doi: 10.1016/j.cellsig.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 6.Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo M., Lamba S., Lorenzato A., Sogari A., Corti G., Rospo G., Mussolin B., Montone M., Lazzari L., Arena S., Oddo D., Linnebacher M., Sartore-Bianchi A., Pietrantonio F., Siena S., Di Nicolantonio F., Bardelli A. Reliance upon ancestral mutations is maintained in colorectal cancers that heterogeneously evolve during targeted therapies. Nat Commun. 2018;9:2287. doi: 10.1038/s41467-018-04506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis L.M., Hicklin D.J. Resistance to targeted therapies: refining anticancer therapy in the era of molecular oncology. Clin Cancer Res. 2009;15:7471–7478. doi: 10.1158/1078-0432.CCR-09-1070. [DOI] [PubMed] [Google Scholar]
- 9.Stamos J.L., Weis W.I. The β-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda S., Kishida S., Yamamoto H., Murai H., Koyama S., Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubinfeld B., Albert I., Porfiri E., Fiol C., Munemitsu S., Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 12.Hart M.J., de los Santos R., Albert I.N., Rubinfeld B., Polakis P. Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin and GSK3β. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 13.Rubinfeld B., Souza B., Albert I., Müller O., Chamberlain S.H., Masiarz F.R., Munemitsu S., Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- 14.Liu C., Li Y., Semenov M., Han C., Baeg G.-H., Tan Y., Zhang Z., Lin X., He X. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 15.Aberle H., Bauer A., Stappert J., Kispert A., Kemler R. β-catenin is a target for the ubiquitin–proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitagawa M., Hatakeyama S., Shirane M., Matsumoto M., Ishida N., Hattori K., Nakamichi I., Kikuchi A., Nakayama K., Nakayama K. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J. 1999;18:2401–2410. doi: 10.1093/emboj/18.9.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee E., Salic A., Krüger R., Heinrich R., Kirschner M.W. The roles of APC and axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:e10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhanot P., Brink M., Samos C.H., Hsieh J.-C., Wang Y., Macke J.P., Andrew D., Nathans J., Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 19.Dann C.E., Hsieh J.-C., Rattner A., Sharma D., Nathans J., Leahy D.J. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature. 2001;412:86–90. doi: 10.1038/35083601. [DOI] [PubMed] [Google Scholar]
- 20.Pinson K.I., Brennan J., Monkley S., Avery B.J., Skarnes W.C. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 21.Tamai K., Semenov M., Kato Y., Spokony R., Liu C., Katsuyama Y., Hess F., Saint-Jeannet J.-P., He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 22.Cong F., Schweizer L., Varmus H. Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors. Frizzled and LRP. Development. 2004;131:5103–5115. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- 23.Zeng X., Tamai K., Doble B., Li S., Huang H., Habas R., Okamura H., Woodgett J., He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molenaar M., van de Wetering M., Oosterwegel M., Peterson-Maduro J., Godsave S., Korinek V., Roose J., Destrée O., Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 25.Behrens J., von Kries J.P., Kühl M., Bruhn L., Wedlich D., Grosschedl R., Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 26.Leung J.Y., Kolligs F.T., Wu R., Zhai Y., Kuick R., Hanash S., Cho K.R., Fearon E.R. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657–21665. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- 27.He T.C., Sparks A.B., Rago C., Hermeking H., Zawel L., da Costa L.T., Morin P.J., Vogelstein B., Kinzler K.W. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 28.Tetsu O., McCormick F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 29.Shtutman M., Zhurinsky J., Simcha I., Albanese C., D’Amico M., Pestell R., Ben-Ze’ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li V.S.W., Ng S.S., Boersema P.J., Low T.Y., Karthaus W.R., Gerlach J.P., Mohammed S., Heck A.J.R., Maurice M.M., Mahmoudi T., Clevers H. Wnt signaling through inhibition of β-catenin degradation in an intact axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Hernández A.R., Klein A.M., Kirschner M.W. Kinetic responses of β-catenin specify the sites of Wnt control. Science. 2012;338:1337–1340. doi: 10.1126/science.1228734. [DOI] [PubMed] [Google Scholar]
- 32.Yang E., Tacchelly-Benites O., Wang Z., Randall M.P., Tian A., Benchabane H., Freemantle S., Pikielny C., Tolwinski N.S., Lee E., Ahmed Y. Wnt pathway activation by ADP-ribosylation. Nat Commun. 2016;7:11430. doi: 10.1038/ncomms11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee A., Dhar N., Stathos M., Schaffer D.V., Kane R.S. Understanding how Wnt influences destruction complex activity and β-catenin dynamics. iScience. 2018;6:13–21. doi: 10.1016/j.isci.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koo B.-K., Spit M., Jordens I., Low T.Y., Stange D.E., van de Wetering M., van Es J.H., Mohammed S., Heck A.J.R., Maurice M.M., Clevers H. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature. 2012;488:665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 35.Hao H.-X., Xie Y., Zhang Y., Charlat O., Oster E., Avello M., Lei H., Mickanin C., Liu D., Ruffner H., Mao X., Ma Q., Zamponi R., Bouwmeester T., Finan P.M., Kirschner M.W., Porter J.A., Serluca F.C., Cong F. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature. 2012;485:195–200. doi: 10.1038/nature11019. [DOI] [PubMed] [Google Scholar]
- 36.Jiang X., Charlat O., Zamponi R., Yang Y., Cong F. Dishevelled promotes Wnt receptor degradation through recruitment of ZNRF3/RNF43 E3 ubiquitin ligases. Mol Cell. 2015;58:522–533. doi: 10.1016/j.molcel.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., Bresolin S., Frasson C., Basso G., Guzzardo V., Fassina A., Cordenonsi M., Piccolo S. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Park H.W., Kim Y.C., Yu B., Moroishi T., Mo J.-S., Plouffe S.W., Meng Z., Lin K.C., Yu F.-X., Alexander C.M., Wang C.-Y., Guan K.-L. Alternative Wnt signaling activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varelas X., Miller B.W., Sopko R., Song S., Gregorieff A., Fellouse F.A., Sakuma R., Pawson T., Hunziker W., McNeill H., Wrana J.L., Attisano L. The hippo pathway regulates Wnt/β-catenin signaling. Dev Cell. 2010;18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Cai J., Maitra A., Anders R.A., Taketo M.M., Pan D. β-Catenin destruction complex-independent regulation of Hippo–YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 2015;29:1493–1506. doi: 10.1101/gad.264515.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konsavage W.M., Kyler S.L., Rennoll S.A., Jin G., Yochum G.S., Yochum G.S. Wnt/β-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730–11739. doi: 10.1074/jbc.M111.327767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinzler K.W., Nilbert M.C., Su L.K., Vogelstein B., Bryan T.M., Levy D.B., Smith K.J., Preisinger A.C., Hedge P., McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 43.Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., Horii A., Koyama K., Utsunomiya J., Baba S., Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 44.Kinzler K.W., Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 45.Saito-Diaz K., Benchabane H., Tiwari A., Tian A., Li B., Thompson J.J., Hyde A.S., Sawyer L.M., Jodoin J.N., Santos E., Lee L.A., Coffey R.J., Beauchamp R.D., Williams C.S., Kenworthy A.K., Robbins D.J., Ahmed Y., Lee E. APC inhibits ligand-independent Wnt signaling by the clathrin endocytic pathway. Dev Cell. 2018;44:566–581.e8. doi: 10.1016/j.devcel.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGough I.J., Vincent J.-P. APC moonlights to prevent wnt signalosome assembly. Dev Cell. 2018;44:535–537. doi: 10.1016/j.devcel.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim I.-J., Kang H.C., Park J.-H., Shin Y., Ku J.-L., Lim S.-B., Park S.Y., Jung S.-Y., Kim H.K., Park J.-G. Development and applications of a beta-catenin oligonucleotide microarray: beta-catenin mutations are dominantly found in the proximal colon cancers with microsatellite instability. Clin Cancer Res. 2003;9:2920–2925. [PubMed] [Google Scholar]
- 48.Lustig B., Jerchow B., Sachs M., Weiler S., Pietsch T., Karsten U., van de Wetering M., Clevers H., Schlag P.M., Birchmeier W., Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fearnhead N.S., Wilding J.L., Winney B., Tonks S., Bartlett S., Bicknell D.C., Tomlinson I.P.M., Mortensen N.J.M., Bodmer W.F. Multiple rare variants in different genes account for multifactorial inherited susceptibility to colorectal adenomas. Proc Natl Acad Sci U S A. 2004;101:15992–15997. doi: 10.1073/pnas.0407187101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin L.-H., Shao Q.-J., Luo W., Ye Z.-Y., Li Q., Lin S.-C. Detection of point mutations of the Axin1 gene in colorectal cancers. Int J Cancer. 2003;107:696–699. doi: 10.1002/ijc.11435. [DOI] [PubMed] [Google Scholar]
- 51.Duval A., Gayet J., Zhou X.P., Iacopetta B., Thomas G., Hamelin R. Frequent frameshift mutations of the TCF-4 gene in colorectal cancers with microsatellite instability. Cancer Res. 1999;59:4213–4215. [PubMed] [Google Scholar]
- 52.Ruckert S., Hiendlmeyer E., Brueckl W.M., Oswald U., Beyser K., Dietmaier W., Haynl A., Koch C., Rüschoff J., Brabletz T., Kirchner T., Jung A. T-cell factor-4 frameshift mutations occur frequently in human microsatellite instability-high colorectal carcinomas but do not contribute to carcinogenesis. Cancer Res. 2002;62:3009–3013. [PubMed] [Google Scholar]
- 53.Jhanwar-Uniyal M., Wainwright J.V., Mohan A.L., Tobias M.E., Murali R., Gandhi C.D., Schmidt M.H. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv Biol Regul. 2019;72:51–62. doi: 10.1016/j.jbior.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Kim D.-H., Sarbassov D.D., Ali S.M., Latek R.R., Guntur K.V.P., Erdjument-Bromage H., Tempst P., Sabatini D.M. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 55.Peterson T.R., Laplante M., Thoreen C.C., Sancak Y., Kang S.A., Kuehl W.M., Gray N.S., Sabatini D.M. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaizuka T., Hara T., Oshiro N., Kikkawa U., Yonezawa K., Takehana K., Iemura S., Natsume T., Mizushima N. Tti1 and Tel2 are critical factors in mammalian target of rapamycin complex assembly. J Biol Chem. 2010;285:20109–20116. doi: 10.1074/jbc.M110.121699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim D.-H., Sarbassov D.D., Ali S.M., King J.E., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 58.Haar E Vander, Lee S., Bandhakavi S., Griffin T.J., Kim D.-H. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol. 2007;9:316–323. doi: 10.1038/ncb1547. [DOI] [PubMed] [Google Scholar]
- 59.Sarbassov D.D., Ali S.M., Kim D.-H., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 60.Frias M.A., Thoreen C.C., Jaffe J.D., Schroder W., Sculley T., Carr S.A., Sabatini D.M. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Pearce L.R., Sommer E.M., Sakamoto K., Wullschleger S., Alessi D.R. Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem J. 2011;436:169–179. doi: 10.1042/BJ20102103. [DOI] [PubMed] [Google Scholar]
- 62.Zheng X.F., Florentino D., Chen J., Crabtree G.R., Schreiber S.L. TOR kinase domains are required for two distinct functions, only one of which is inhibited by rapamycin. Cell. 1995;82:121–130. doi: 10.1016/0092-8674(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 63.Inoki K., Li Y., Xu T., Guan K.-L. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tee A.R., Manning B.D., Roux P.P., Cantley L.C., Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 65.Memmott R.M., Dennis P.A. Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell Signal. 2009;21:656–664. doi: 10.1016/j.cellsig.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alessi D.R., James S.R., Downes C.P., Holmes A.B., Gaffney P.R., Reese C.B., Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 67.Inoki K., Li Y., Zhu T., Wu J., Guan K.-L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 68.Manning B.D., Tee A.R., Logsdon M.N., Blenis J., Cantley L.C. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 69.Acosta-Jaquez H.A., Keller J.A., Foster K.G., Ekim B., Soliman G.A., Feener E.P., Ballif B.A., Fingar D.C. Site-specific mTOR phosphorylation promotes mTORC1-mediated signaling and cell growth. Mol Cell Biol. 2009;29:4308–4324. doi: 10.1128/MCB.01665-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gingras A.C., Kennedy S.G., O’Leary M.A., Sonenberg N., Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beretta L., Gingras A.C., Svitkin Y.V., Hall M.N., Sonenberg N. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 1996;15:658–664. [PMC free article] [PubMed] [Google Scholar]
- 72.Holz M.K., Ballif B.A., Gygi S.P., Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 73.Liao X., Morikawa T., Lochhead P., Imamura Y., Kuchiba A., Yamauchi M., Nosho K., Qian Z.R., Nishihara R., Meyerhardt J.A., Fuchs C.S., Ogino S. Prognostic role of PIK3CA mutation in colorectal cancer: cohort study and literature review. Clin Cancer Res. 2012;18:2257–2268. doi: 10.1158/1078-0432.CCR-11-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barault L., Veyrie N., Jooste V., Lecorre D., Chapusot C., Ferraz J.-M., Lièvre A., Cortet M., Bouvier A.-M., Rat P., Roignot P., Faivre J., Laurent-Puig P., Piard F. Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int J Cancer. 2008;122:2255–2259. doi: 10.1002/ijc.23388. [DOI] [PubMed] [Google Scholar]
- 75.Rosty C., Young J.P., Walsh M.D., Clendenning M., Sanderson K., Walters R.J., Parry S., Jenkins M.A., Win A.K., Southey M.C., Hopper J.L., Giles G.G., Williamson E.J., English D.R., Buchanan D.D. PIK3CA activating mutation in colorectal carcinoma: associations with molecular features and survival. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Roock W., Claes B., Bernasconi D., De Schutter J., Biesmans B., Fountzilas G., Kalogeras K.T., Kotoula V., Papamichael D., Laurent-Puig P., Penault-Llorca F., Rougier P., Vincenzi B., Santini D., Tonini G., Cappuzzo F., Frattini M., Molinari F., Saletti P., De Dosso S., Martini M., Bardelli A., Siena S., Sartore-Bianchi A., Tabernero J., Macarulla T., Di Fiore F., Gangloff A.O., Ciardiello F., Pfeiffer P., Qvortrup C., Hansen T.P., Van Cutsem E., Piessevaux H., Lambrechts D., Delorenzi M., Tejpar S. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 77.Samuels Y., Wang Z., Bardelli A., Silliman N., Ptak J., Szabo S., Yan H., Gazdar A., Powell S.M., Riggins G.J., Willson J.K.V., Markowitz S., Kinzler K.W., Vogelstein B., Velculescu V.E. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 78.Gavin P.G., Colangelo L.H., Fumagalli D., Tanaka N., Remillard M.Y., Yothers G., Kim C., Taniyama Y., Kim S Il, Choi H.J., Blackmon N.L., Lipchik C., Petrelli N.J., O’Connell M.J., Wolmark N., Paik S., Pogue-Geile K.L. Mutation profiling and microsatellite instability in stage II and III colon cancer: an assessment of their prognostic and oxaliplatin predictive value. Clin Cancer Res. 2012;18:6531–6541. doi: 10.1158/1078-0432.CCR-12-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Day F.L., Jorissen R.N., Lipton L., Mouradov D., Sakthianandeswaren A., Christie M., Li S., Tsui C., Tie J., Desai J., Xu Z.-Z., Molloy P., Whitehall V., Leggett B.A., Jones I.T., McLaughlin S., Ward R.L., Hawkins N.J., Ruszkiewicz A.R., Moore J., Busam D., Zhao Q., Strausberg R.L., Gibbs P., Sieber O.M. PIK3CA and PTEN gene and exon mutation-specific clinicopathologic and molecular associations in colorectal cancer. Clin Cancer Res. 2013;19:3285–3296. doi: 10.1158/1078-0432.CCR-12-3614. [DOI] [PubMed] [Google Scholar]
- 80.Ekstrand A.I., Jönsson M., Lindblom A., Borg Å., Nilbert M. Frequent alterations of the PI3K/AKT/mTOR pathways in hereditary nonpolyposis colorectal cancer. Fam Cancer. 2010;9:125–129. doi: 10.1007/s10689-009-9293-1. [DOI] [PubMed] [Google Scholar]
- 81.Danielsen S.A., Lind G.E., Bjørnslett M., Meling G.I., Rognum T.O., Heim S., Lothe R.A. Novel mutations of the suppressor gene PTEN in colorectal carcinomas stratified by microsatellite instability- and TP53 mutation- status. Hum Mutat. 2008;29:E252–E262. doi: 10.1002/humu.20860. [DOI] [PubMed] [Google Scholar]
- 82.Berg M., Danielsen S.A., Ahlquist T., Merok M.A., Ågesen T.H., Vatn M.H., Mala T., Sjo O.H., Bakka A., Moberg I., Fetveit T., Mathisen Ø., Husby A., Sandvik O., Nesbakken A., Thiis-Evensen E., Lothe R.A. DNA sequence profiles of the colorectal cancer critical gene set KRAS-BRAF-PIK3CA-PTEN-TP53 related to age at disease onset. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dicuonzo G., Angeletti S., Garcia-Foncillas J., Brugarolas A., Okrouzhnov Y., Santini D., Tonini G., Lorino G., De Cesaris M., Baldi A. Colorectal carcinomas and PTEN/MMAC1 gene mutations. Clin Cancer Res. 2001;7:4049–4053. [PubMed] [Google Scholar]
- 84.Guanti G., Resta N., Simone C., Cariola F., Demma I., Fiorente P., Gentile M. Involvement of PTEN mutations in the genetic pathways of colorectal cancerogenesis. Hum Mol Genet. 2000;9:283–287. doi: 10.1093/hmg/9.2.283. [DOI] [PubMed] [Google Scholar]
- 85.Shin K.H., Park Y.J., Park J.G. PTEN gene mutations in colorectal cancers displaying microsatellite instability. Cancer Lett. 2001;174:189–194. doi: 10.1016/s0304-3835(01)00691-7. [DOI] [PubMed] [Google Scholar]
- 86.Zhou X.-P., Loukola A., Salovaara R., Nystrom-Lahti M., Peltomäki P., de la Chapelle A., Aaltonen L.A., Eng C. PTEN mutational spectra, expression levels, and subcellular localization in microsatellite stable and unstable colorectal cancers. Am J Pathol. 2002;161:439–447. doi: 10.1016/S0002-9440(10)64200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goel A., Arnold C.N., Niedzwiecki D., Carethers J.M., Dowell J.M., Wasserman L., Compton C., Mayer R.J., Bertagnolli M.M., Boland C.R. Frequent inactivation of PTEN by promoter hypermethylation in microsatellite instability-high sporadic colorectal cancers. Cancer Res. 2004;64:3014–3021. doi: 10.1158/0008-5472.can-2401-2. [DOI] [PubMed] [Google Scholar]
- 88.Shaw R.J., Bardeesy N., Manning B.D., Lopez L., Kosmatka M., DePinho R.A., Cantley L.C. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 89.Dong S.M., Kim K.M., Kim S.Y., Shin M.S., Na E.Y., Lee S.H., Park W.S., Yoo N.J., Jang J.J., Yoon C.Y., Kim J.W., Kim S.Y., Yang Y.M., Kim S.H., Kim C.S., Lee J.Y. Frequent somatic mutations in serine/threonine kinase 11/Peutz-Jeghers syndrome gene in left-sided colon cancer. Cancer Res. 1998;58:3787–3790. [PubMed] [Google Scholar]
- 90.Avizienyte E., Roth S., Loukola A., Hemminki A., Lothe R.A., Stenwig A.E., Fosså S.D., Salovaara R., Aaltonen L.A. Somatic mutations in LKB1 are rare in sporadic colorectal and testicular tumors. Cancer Res. 1998;58:2087–2090. [PubMed] [Google Scholar]
- 91.Carpten J.D., Faber A.L., Horn C., Donoho G.P., Briggs S.L., Robbins C.M., Hostetter G., Boguslawski S., Moses T.Y., Savage S., Uhlik M., Lin A., Du J., Qian Y.-W., Zeckner D.J., Tucker-Kellogg G., Touchman J., Patel K., Mousses S., Bittner M., Schevitz R., Lai M.-H.T., Blanchard K.L., Thomas J.E. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 92.Fujishita T., Aoki K., Lane H.A., Aoki M., Taketo M.M. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in Apc 716 mice. Proc Natl Acad Sci U S A. 2008;105:13544–13549. doi: 10.1073/pnas.0800041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koehl G.E., Spitzner M., Ousingsawat J., Schreiber R., Geissler E.K., Kunzelmann K. Rapamycin inhibits oncogenic intestinal ion channels and neoplasia in APCMin/+ mice. Oncogene. 2010;29:1553–1560. doi: 10.1038/onc.2009.435. [DOI] [PubMed] [Google Scholar]
- 94.Valvezan A.J., Huang J., Lengner C.J., Pack M., Klein P.S. Oncogenic mutations in adenomatous polyposis coli (Apc) activate mechanistic target of rapamycin complex 1 (mTORC1) in mice and zebrafish. Dis Model Mech. 2014;7:63–71. doi: 10.1242/dmm.012625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hardiman K.M., Liu J., Feng Y., Greenson J.K., Fearon E.R. Rapamycin inhibition of polyposis and progression to dysplasia in a mouse model. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Inoki K., Ouyang H., Zhu T., Lindvall C., Wang Y., Zhang X., Yang Q., Bennett C., Harada Y., Stankunas K., Wang C., He X., MacDougald O.A., You M., Williams B.O., Guan K.-L. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 97.Faller W.J., Jackson T.J., Knight J.R.P., Ridgway R.A., Jamieson T., Karim S.A., Jones C., Radulescu S., Huels D.J., Myant K.B., Dudek K.M., Casey H.A., Scopelliti A., Cordero J.B., Vidal M., Pende M., Ryazanov A.G., Sonenberg N., Meyuhas O., Hall M.N., Bushell M., Willis A.E., Sansom O.J. mTORC1-mediated translational elongation limits intestinal tumour initiation and growth. Nature. 2015;517:497–500. doi: 10.1038/nature13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Deming D.A., Leystra A.A., Nettekoven L., Sievers C., Miller D., Middlebrooks M., Clipson L., Albrecht D., Bacher J., Washington M.K., Weichert J., Halberg R.B. PIK3CA and APC mutations are synergistic in the development of intestinal cancers. Oncogene. 2014;33:2245–2254. doi: 10.1038/onc.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brandt M., Grazioso T.P., Fawal M.-A., Tummala K.S., Torres-Ruiz R., Rodriguez-Perales S., Perna C., Djouder N. mTORC1 inactivation promotes colitis-induced colorectal cancer but protects from APC loss-dependent tumorigenesis. Cell Metab. 2018;27:118–135.e8. doi: 10.1016/j.cmet.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 100.Kaidanovich-Beilin O., Woodgett J.R. GSK-3: functional insights from cell biology and animal models. Front Mol Neurosci. 2011;4:40. doi: 10.3389/fnmol.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hinoi T., Yamamoto H., Kishida M., Takada S., Kishida S., Kikuchi A. Complex formation of adenomatous polyposis coli gene product and axin facilitates glycogen synthase kinase-3β-dependent phosphorylation of β-catenin and down-regulates β-catenin. J Biol Chem. 2000;275:34399–34406. doi: 10.1074/jbc.M003997200. [DOI] [PubMed] [Google Scholar]
- 102.Valvezan A.J., Zhang F., Diehl J.A., Klein P.S. Adenomatous polyposis coli (APC) regulates multiple signaling pathways by enhancing glycogen synthase kinase-3 (GSK-3) activity. J Biol Chem. 2012;287:3823–3832. doi: 10.1074/jbc.M111.323337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Piao S., Lee S.-H., Kim H., Yum S., Stamos J.L., Xu Y., Lee S.-J., Lee J., Oh S., Han J.-K., Park B.-J., Weis W.I., Ha N.-C. Direct inhibition of GSK3β by the phosphorylated cytoplasmic domain of LRP6 in Wnt/β-catenin signaling. PLoS One. 2008;3:e4046. doi: 10.1371/journal.pone.0004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang J., Zhang Y., Bersenev A., O’Brien W.T., Tong W., Emerson S.G., Klein P.S. Pivotal role for glycogen synthase kinase–3 in hematopoietic stem cell homeostasis in mice. J Clin Invest. 2009;119:3519–3529. doi: 10.1172/JCI40572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cross D.A.E., Alessi D.R., Cohen P., Andjelkovich M., Hemmings B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 106.Frame S., Cohen P., Biondi R.M. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell. 2001;7:1321–1327. doi: 10.1016/s1097-2765(01)00253-2. [DOI] [PubMed] [Google Scholar]
- 107.Zhang H.H., Lipovsky A.I., Dibble C.C., Sahin M., Manning B.D. S6K1 Regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Mol Cell. 2006;24:185–197. doi: 10.1016/j.molcel.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McManus E.J., Sakamoto K., Armit L.J., Ronaldson L., Shpiro N., Marquez R., Alessi D.R. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ding V.W., Chen R.-H., McCormick F. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J Biol Chem. 2000;275:32475–32481. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- 110.Doble B.W., Patel S., Wood G.A., Kockeritz L.K., Woodgett J.R. Functional redundancy of GSK-3α and GSK-3β in Wnt/β-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bautista S.J., Boras I., Vissa A., Mecica N., Yip C.M., Kim P.K., Antonescu C.N. mTOR complex 1 controls the nuclear localization and function of glycogen synthase kinase 3β. J Biol Chem. 2018;293:14723–14739. doi: 10.1074/jbc.RA118.002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Koo J., Yue P., Gal A.A., Khuri F.R., Sun S.-Y. Maintaining glycogen synthase kinase-3 activity is critical for mTOR kinase inhibitors to inhibit cancer cell growth. Cancer Res. 2014;74:2555–2568. doi: 10.1158/0008-5472.CAN-13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He L., Gomes A.P., Wang X., Yoon S.O., Lee G., Nagiec M.J., Cho S., Chavez A., Islam T., Yu Y., Asara J.M., Kim B.Y., Blenis J. mTORC1 promotes metabolic reprogramming by the suppression of GSK3-dependent Foxk1 phosphorylation. Mol Cell. 2018;70:949–960.e4. doi: 10.1016/j.molcel.2018.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.He L., Fei D.L., Nagiec M.J., Mutvei A.P., Lamprakis A., Kim B.Y., Blenis J. Regulation of GSK3 cellular location by FRAT modulates mTORC1-dependent cell growth and sensitivity to rapamycin. Proc Natl Acad Sci U S A. 2019;116:19523–19529. doi: 10.1073/pnas.1902397116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zeng H., Lu B., Zamponi R., Yang Z., Wetzel K., Loureiro J., Mohammadi S., Beibel M., Bergling S., Reece-Hoyes J., Russ C., Roma G., Tchorz J.S., Capodieci P., Cong F. mTORC1 signaling suppresses Wnt/β-catenin signaling through DVL-dependent regulation of Wnt receptor FZD level. Proc Natl Acad Sci U S A. 2018;115:E10362–E10369. doi: 10.1073/pnas.1808575115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Q., Zhou Y., Rychahou P., Harris J.W., Zaytseva Y.Y., Liu J., Wang C., Weiss H.L., Liu C., Lee E.Y., Evers B.M. Deptor is a novel target of Wnt/β-catenin/c-Myc and contributes to colorectal cancer cell growth. Cancer Res. 2018;78 doi: 10.1158/0008-5472.CAN-17-3107. canres.3107.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gingras A.-C., Raught B., Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 119.Carroll M., Borden K.L.B. The oncogene eIF4E: using biochemical insights to target cancer. J Interf Cytokine Res. 2013;33:227–238. doi: 10.1089/jir.2012.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Berkel H.J., Turbat-Herrera E.A., Shi R., de Benedetti A. Expression of the translation initiation factor eIF4E in the polyp-cancer sequence in the colon. Cancer Epidemiol Biomarkers Prev. 2001;10:663–666. [PubMed] [Google Scholar]
- 121.Xu T., Zong Y., Peng L., Kong S., Zhou M., Zou J., Liu J., Miao R., Sun X., Li L. Overexpression of eIF4E in colorectal cancer patients is associated with liver metastasis. Onco Targets Ther. 2016;9:815–822. doi: 10.2147/OTT.S98330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Larsson O., Li S., Issaenko O.A., Avdulov S., Peterson M., Smith K., Bitterman P.B., Polunovsky V.A. Eukaryotic translation initiation factor 4E–induced progression of primary human mammary epithelial cells along the cancer pathway is associated with targeted translational deregulation of oncogenic drivers and inhibitors. Cancer Res. 2007;67:6814–6824. doi: 10.1158/0008-5472.CAN-07-0752. [DOI] [PubMed] [Google Scholar]
- 123.Topisirovic I., Ruiz-Gutierrez M., Borden K.L.B. Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res. 2004;64:8639–8642. doi: 10.1158/0008-5472.CAN-04-2677. [DOI] [PubMed] [Google Scholar]
- 124.Wang X., Yue P., Chan C.-B., Ye K., Ueda T., Watanabe-Fukunaga R., Fukunaga R., Fu H., Khuri F.R., Sun S.-Y. Inhibition of mammalian target of rapamycin induces phosphatidylinositol 3-kinase-dependent and Mnk-mediated eukaryotic translation initiation factor 4E phosphorylation. Mol Cell Biol. 2007;27:7405–7413. doi: 10.1128/MCB.00760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun S.-Y., Rosenberg L.M., Wang X., Zhou Z., Yue P., Fu H., Khuri F.R. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 126.Lim S., Saw T.Y., Zhang M., Janes M.R., Nacro K., Hill J., Lim A.Q., Chang C.-T., Fruman D.A., Rizzieri D.A., Tan S.Y., Fan H., Chuah C.T.H., Ong S.T. Targeting of the MNK-eIF4E axis in blast crisis chronic myeloid leukemia inhibits leukemia stem cell function. Proc Natl Acad Sci U S A. 2013;110:E2298–E2307. doi: 10.1073/pnas.1301838110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Z., Sun Y., Qu M., Wan H., Cai F., Zhang P. Inhibiting the MNK-eIF4E-β-catenin axis increases the responsiveness of aggressive breast cancer cells to chemotherapy. Oncotarget. 2017;8:2906–2915. doi: 10.18632/oncotarget.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ng K., Tabernero J., Hwang J., Bajetta E., Sharma S., Del Prete S.A., Arrowsmith E.R., Ryan D.P., Sedova M., Jin J., Malek K., Fuchs C.S. Phase II study of everolimus in patients with metastatic colorectal adenocarcinoma previously treated with bevacizumab-, fluoropyrimidine-, oxaliplatin-, and irinotecan-based regimens. Clin Cancer Res. 2013;19:3987–3995. doi: 10.1158/1078-0432.CCR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Spindler K.L., Sorensen M.M., Pallisgaard N., Andersen R.F., Havelund B.M., Ploen J., Lassen U., Jakobsen A.K.M. Phase II trial of temsirolimus alone and in combination with irinotecan for KRAS mutant metastatic colorectal cancer: outcome and results of KRAS mutational analysis in plasma. Acta Oncol. 2013;52:963–970. doi: 10.3109/0284186X.2013.776175. [DOI] [PubMed] [Google Scholar]
- 130.Fricke S.L., Payne S.N., Favreau P.F., Kratz J.D., Pasch C.A., Foley T.M., Yueh A.E., Van De Hey D.R., Depke M.G., Korkos D.P., Sha G.C., DeStefanis R.A., Clipson L., Burkard M.E., Lemmon K.K., Parsons B.M., Kenny P.A., Matkowskyj K.A., Newton M.A., Skala M.C., Deming D.A. MTORC1/2 inhibition as a therapeutic strategy for PIK3CA mutant cancers. Mol Cancer Ther. 2019;18:346–355. doi: 10.1158/1535-7163.MCT-18-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shapiro G.I., Bell-McGuinn K.M., Molina J.R., Bendell J., Spicer J., Kwak E.L., Pandya S.S., Millham R., Borzillo G., Pierce K.J., Han L., Houk B.E., Gallo J.D., Alsina M., Brana I., Tabernero J. First-in-human study of PF-05212384 (PKI-587), a small-molecule, intravenous, dual inhibitor of PI3K and mTOR in patients with advanced cancer. Clin Cancer Res. 2015;21:1888–1895. doi: 10.1158/1078-0432.CCR-14-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Papadopoulos K.P., Tabernero J., Markman B., Patnaik A., Tolcher A.W., Baselga J., Shi W., Egile C., Ruiz-Soto R., Laird A.D., Miles D., LoRusso P.M. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245409 (XL765), a novel, orally administered PI3K/mTOR inhibitor in patients with advanced solid tumors. Clin Cancer Res. 2014;20:2445–2456. doi: 10.1158/1078-0432.CCR-13-2403. [DOI] [PubMed] [Google Scholar]
- 133.Bendell J.C., Kurkjian C., Infante J.R., Bauer T.M., Burris H.A., Greco F.A., Shih K.C., Thompson D.S., Lane C.M., Finney L.H., Jones S.F. A phase 1 study of the sachet formulation of the oral dual PI3K/mTOR inhibitor BEZ235 given twice daily (BID) in patients with advanced solid tumors. Invest New Drugs. 2015;33:463–471. doi: 10.1007/s10637-015-0218-6. [DOI] [PubMed] [Google Scholar]
- 134.Bendell J.C., Varghese A.M., Hyman D.M., Bauer T.M., Pant S., Callies S., Lin J., Martinez R., Wickremsinhe E., Fink A., Wacheck V., Moore K.N. A first-in-human phase 1 study of LY3023414, an oral PI3K/mTOR dual inhibitor, in patients with advanced cancer. Clin Cancer Res. 2018;24:3253–3262. doi: 10.1158/1078-0432.CCR-17-3421. [DOI] [PubMed] [Google Scholar]
- 135.Park Y.-L., Kim H.-P., Cho Y.-W., Min D.-W., Cheon S.-K., Lim Y.J., Song S.-H., Kim S.J., Han S.-W., Park K.J., Kim T.-Y. Activation of WNT/β-catenin signaling results in resistance to a dual PI3K/mTOR inhibitor in colorectal cancer cells harboring PIK3CA mutations. Int J Cancer. 2019;144:389–401. doi: 10.1002/ijc.31662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thorne C.A., Wichaidit C., Coster A.D., Posner B.A., Wu L.F., Altschuler S.J. GSK-3 modulates cellular responses to a broad spectrum of kinase inhibitors. Nat Chem Biol. 2015;11:58–63. doi: 10.1038/nchembio.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Foley T.M., Payne S.N., Pasch C.A., Yueh A.E., Van De Hey D.R., Korkos D.P., Clipson L., Maher M.E., Matkowskyj K.A., Newton M.A., Deming D.A. Dual PI3K/mTOR inhibition in colorectal cancers with APC and PIK3CA mutations. Mol Cancer Res. 2017;15:317–327. doi: 10.1158/1541-7786.MCR-16-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang S.-M.A., Mishina Y.M., Liu S., Cheung A., Stegmeier F., Michaud G.A., Charlat O., Wiellette E., Zhang Y., Wiessner S., Hild M., Shi X., Wilson C.J., Mickanin C., Myer V., Fazal A., Tomlinson R., Serluca F., Shao W., Cheng H., Shultz M., Rau C., Schirle M., Schlegl J., Ghidelli S., Fawell S., Lu C., Curtis D., Kirschner M.W., Lengauer C., Finan P.M., Tallarico J.A., Bouwmeester T., Porter J.A., Bauer A., Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 139.Chen B., Dodge M.E., Tang W., Lu J., Ma Z., Fan C.-W., Wei S., Hao W., Kilgore J., Williams N.S., Roth M.G., Amatruda J.F., Chen C., Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Waaler J., Machon O., von Kries J.P., Wilson S.R., Lundenes E., Wedlich D., Gradl D., Paulsen J.E., Machonova O., Dembinski J.L., Dinh H., Krauss S. Novel synthetic antagonists of canonical Wnt signaling inhibit colorectal cancer cell growth. Cancer Res. 2011;71:197–205. doi: 10.1158/0008-5472.CAN-10-1282. [DOI] [PubMed] [Google Scholar]
- 141.Lau T., Chan E., Callow M., Waaler J., Boggs J., Blake R.A., Magnuson S., Sambrone A., Schutten M., Firestein R., Machon O., Korinek V., Choo E., Diaz D., Merchant M., Polakis P., Holsworth D.D., Krauss S., Costa M. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013;73:3132–3144. doi: 10.1158/0008-5472.CAN-12-4562. [DOI] [PubMed] [Google Scholar]
- 142.Zhong Y., Katavolos P., Nguyen T., Lau T., Boggs J., Sambrone A., Kan D., Merchant M., Harstad E., Diaz D., Costa M., Schutten M. Tankyrase inhibition causes reversible intestinal toxicity in mice with a therapeutic index < 1. Toxicol Pathol. 2016;44:267–278. doi: 10.1177/0192623315621192. [DOI] [PubMed] [Google Scholar]
- 143.Quackenbush K.S., Bagby S., Tai W.M., Messersmith W.A., Schreiber A., Greene J., Kim J., Wang G., Purkey A., Pitts T.M., Nguyen A., Gao D., Blatchford P., Capasso A., Schuller A.G., Eckhardt S.G., Arcaroli J.J. The novel tankyrase inhibitor (AZ1366) enhances irinotecan activity in tumors that exhibit elevated tankyrase and irinotecan resistance. Oncotarget. 2016;7:28273–28285. doi: 10.18632/oncotarget.8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mashima T., Taneda Y., Jang M.-K., Mizutani A., Muramatsu Y., Yoshida H., Sato A., Tanaka N., Sugimoto Y., Seimiya H. mTOR signaling mediates resistance to tankyrase inhibitors in Wnt-driven colorectal cancer. Oncotarget. 2017;8:47902–47915. doi: 10.18632/oncotarget.18146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Solberg N.T., Waaler J., Lund K., Mygland L., Olsen P.A., Krauss S. Tankyrase inhibition enhances the antiproliferative effect of PI3K and EGFR inhibition, mutually affecting β-catenin and AKT signaling in colorectal cancer. Mol Cancer Res. 2018;16:543–553. doi: 10.1158/1541-7786.MCR-17-0362. [DOI] [PubMed] [Google Scholar]
- 146.Myatt S.S., Lam E.W.-F. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 147.Tenbaum S.P., Ordóñez-Morán P., Puig I., Chicote I., Arqués O., Landolfi S., Fernández Y., Herance J.R., Gispert J.D., Mendizabal L., Aguilar S., Cajal SR y, Schwartz S., Vivancos A., Espín E., Rojas S., Baselga J., Tabernero J., Muñoz A., Palmer H.G. β-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med. 2012;18:892–901. doi: 10.1038/nm.2772. [DOI] [PubMed] [Google Scholar]
- 148.Arques O., Chicote I., Puig I., Tenbaum S.P., Argiles G., Dienstmann R., Fernandez N., Caratu G., Matito J., Silberschmidt D., Rodon J., Landolfi S., Prat A., Espin E., Charco R., Nuciforo P., Vivancos A., Shao W., Tabernero J., Palmer H.G. Tankyrase inhibition blocks Wnt/β-catenin pathway and reverts resistance to PI3K and AKT inhibitors in the treatment of colorectal cancer. Clin Cancer Res. 2016;22:644–656. doi: 10.1158/1078-0432.CCR-14-3081. [DOI] [PubMed] [Google Scholar]
- 149.Siravegna G., Mussolin B., Buscarino M., Corti G., Cassingena A., Crisafulli G., Ponzetti A., Cremolini C., Amatu A., Lauricella C., Lamba S., Hobor S., Avallone A., Valtorta E., Rospo G., Medico E., Motta V., Antoniotti C., Tatangelo F., Bellosillo B., Veronese S., Budillon A., Montagut C., Racca P., Marsoni S., Falcone A., Corcoran R.B., Di Nicolantonio F., Loupakis F., Siena S., Sartore-Bianchi A., Bardelli A. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21:795–801. doi: 10.1038/nm.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wee S., Jagani Z., Xiang K.X., Loo A., Dorsch M., Yao Y.-M., Sellers W.R., Lengauer C., Stegmeier F. PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res. 2009;69:4286–4293. doi: 10.1158/0008-5472.CAN-08-4765. [DOI] [PubMed] [Google Scholar]
- 151.Moon J.-H., Hong S.-W., Kim J.E., Shin J.-S., Kim J.-S., Jung S.-A., Ha S.H., Lee S., Kim J., Lee D.H., Park Y.S., Kim D.M., Park S.-S., Hong J.K., Kim D.Y., Kim E.H., Jung J., Kim M.J., Kim S.-M., Deming D.A., Kim K., Kim T.W., Jin D.-H. Targeting β-catenin overcomes MEK inhibition resistance in colon cancer with KRAS and PIK3CA mutations. Br J Cancer. 2019;120:941–951. doi: 10.1038/s41416-019-0434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Takada R., Satomi Y., Kurata T., Ueno N., Norioka S., Kondoh H., Takao T., Takada S. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 153.Coombs G.S., Yu J., Canning C.A., Veltri C.A., Covey T.M., Cheong J.K., Utomo V., Banerjee N., Zhang Z.H., Jadulco R.C., Concepcion G.P., Bugni T.S., Harper M.K., Mihalek I., Jones C.M., Ireland C.M., Virshup D.M. WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. J Cell Sci. 2010;123:3357–3367. doi: 10.1242/jcs.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Proffitt K.D., Virshup D.M. Precise regulation of porcupine activity is required for physiological Wnt signaling. J Biol Chem. 2012;287:34167–34178. doi: 10.1074/jbc.M112.381970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu J., Pan S., Hsieh M.H., Ng N., Sun F., Wang T., Kasibhatla S., Schuller A.G., Li A.G., Cheng D., Li J., Tompkins C., Pferdekamper A., Steffy A., Cheng J., Kowal C., Phung V., Guo G., Wang Y., Graham M.P., Flynn S., Brenner J.C., Li C., Villarroel M.C., Schultz P.G., Wu X., McNamara P., Sellers W.R., Petruzzelli L., Boral A.L., Seidel H.M., McLaughlin M.E., Che J., Carey T.E., Vanasse G., Harris J.L. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci U S A. 2013;110:20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Madan B., Ke Z., Harmston N., Ho S.Y., Frois A.O., Alam J., Jeyaraj D.A., Pendharkar V., Ghosh K., Virshup I.H., Manoharan V., Ong E.H.Q., Sangthongpitag K., Hill J., Petretto E., Keller T.H., Lee M.A., Matter A., Virshup D.M. Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene. 2016;35:2197–2207. doi: 10.1038/onc.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zimmerli D., Hausmann G., Cantù C., Basler K. Pharmacological interventions in the Wnt pathway: inhibition of Wnt secretion versus disrupting the protein-protein interfaces of nuclear factors. Br J Pharmacol. 2017;174:4600–4610. doi: 10.1111/bph.13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Picco G., Petti C., Centonze A., Torchiaro E., Crisafulli G., Novara L., Acquaviva A., Bardelli A., Medico E. Loss of AXIN1 drives acquired resistance to WNT pathway blockade in colorectal cancer cells carrying RSPO3 fusions. EMBO Mol Med. 2017;9:293–303. doi: 10.15252/emmm.201606773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhong Z., Sepramaniam S., Chew X.H., Wood K., Lee M.A., Madan B., Virshup D.M. PORCN inhibition synergizes with PI3K/mTOR inhibition in Wnt-addicted cancers. Oncogene. 2019;38:6662–6677. doi: 10.1038/s41388-019-0908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]