Highlights
-
•
Rpt2-RNAi expression impairs the 26S proteasome.
-
•
Reduction of Rpt2 in the Drosophila eye induces necrosis.
-
•
Rpt2 reduction causes specific dopaminergic cluster neurodegeneration.
-
•
Loss of Rpt2 induce hyperactive motor activity and sleep reduction.
Keywords: Drosophila melanogaster, Parkinson's disease, Proteasome, Gene knockout, Sleep disorders, Dopaminergic neurodegeneration
Abstract
The dysfunction of the proteasome-ubiquitin system is commonly reported in several neurodegenerative diseases. Post mortem samples of brains of patients with Parkinson´s disease present cytoplasmic inclusions that are rich in proteins such as ubiquitin, Tau, and α-synuclein. In Parkinson´s disease, a specific reduction of some of the proteasome subunits has also been reported. However, the specific role of the different proteasome subunits in dopaminergic neuron degeneration has not been thoroughly explored. In this work, we used the Gal4/UAS system to test fourteen Drosophila melanogaster RNAi lines from the Bloomington Drosophila Stock Center. Each of these lines targets a different proteasome subunit. To identify the strains that were able to induce neurodegeneration, we drove the expression of these lines to the eye and cataloged them as a function of the extent of neurodegeneration that they induced. The targeted proteasomal subunits are conserved in mammals and therefore may be relevant to study proteasome related diseases. The RNAi line among the regulatory subunits with the most penetrant phenotype targeted the proteasomal subunit Rpt2 and we decided to further characterize its phenotypes. Rpt2 knockdown in the Drosophila central nervous system reduced the activity of the proteasome, augmented the amount of insoluble ubiquitinated protein, and elicited motor and non-motor phenotypes that were similar to the ones found in Drosophila and other models for Parkinson’s disease. When Rpt2 is silenced pan-neurally, third instar larvae have locomotion dysfunctions and die during pupation. Larval lethality was avoided using the Gal80-Gal4 system to induce the expression of the Rpt2 RNAi to dopaminergic neurons only after pupation. The reduction of Rpt2 in adult dopaminergic neurons causes reduced survival, hyperactivity, neurodegeneration, and sleep loss; probably recapitulating some of the sleep disorders that Parkinson’s disease patients have before the appearance of locomotion disorders.
1. Introduction
Parkinson´s disease (PD) is the second most common neurodegenerative disease, affecting 1% of the population over 65; it is characterized by the loss of the dopaminergic neurons of the substantia nigra pars compacta. 90 % of PD cases are sporadic and the risk increases with age (Damier et al., 1999; Lang and Lozano, 1998; Moore et al., 2005). Parkinson´s disease patients may have sleep disorders before presenting motor problems (Ziemssen and Reichmann, 2007; Lee and Koh, 2015). In classical Parkinson´s disease, intracellular cytoplasmic aggregates known as Lewy bodies that contain highly ubiquitinated proteins are found in the surviving neurons of the substantia nigra. The principal component of these inclusions is ubiquitinated α-synuclein that maps to the gene PARK1, the first gene to be unambiguously associated with Parkinson´s disease (Forno, 1996; Polymeropoulos et al., 1997; Krüger et al., 1998; Tofaris et al., 2003; Nonaka et al., 2005; Rott et al., 2014). It has been discovered that mutations that affect the ubiquitin-proteasome system (UPS) such as Parkin (a Ubiquitin E3 ligase) and UCHL1 (a protein deubiquitinase) may also be the cause of familial Parkinson´s disease (Kitada et al., 1998; Leroy et al., 1998).
Most intracellular protein degradation happens in the UPS. In the canonical proteasome pathway, protein degradation is initiated when the target protein is labeled by the addition of at least four ubiquitin molecules. Ubiquitination is a highly evolutionarily conserved molecular pathway that includes the ATP dependent activation of ubiquitin by the ubiquitin-activating enzyme E1, one of several E2 enzymes or ubiquitin-conjugating enzymes (UBCs) and one in hundreds of substrate-specific ubiquitin-ligase enzymes or E3. However, experiments where the endogenous ubiquitin is silenced and a lysine less ubiquitin that prevents ubiquitin polymerization is expressed, have shown that there is an alternative pathway for monoubiquitinated protein degradation that preferentially degrades smaller proteins (Glickman and Ciechanover, 2002; Sorokin et al., 2010; Braten et al., 2016). The 26S proteasome is a multiprotein complex that includes the 20S catalytic core particle and the 19S regulatory particle. The 20S consist of four heptameric rings comprised by 7 different alpha subunits and 7 different beta subunits in an α7β7β7α7 arrange. Three beta subunits have proteolytic activity: β1 has caspase-like, β2 has trypsin-like and β5 has chymotrypsin-like activity. The human and the fly 19S share 18 individual homologous proteins that are part of two different sub-complexes. The base that binds both to the α-ring of the 20S and the lid; the base consists of six subunits that are ATPases of the AAA-superfamily (Rpt1−6) and four non-ATPases (Rpn1, Rpn2, Rpn10, and Rpn13). The lid consists of eight different subunits that are not ATPases (Rpn3, Rpn5−9, and Rpn11−12) (Sorokin et al., 2010). It has previously been reported that some of the proteasomal subunits are reduced or affected in post mortem samples of the substantia nigra of Parkinson´s patients, where it has been demonstrated that there is a selective loss of 20S proteasome α-subunits and a reduction in each of the three proteasomal enzymatic activities, a reduction of the expression of SKP1A, a member of the SCF (E3) ligase complex, in dopaminergic neurons of the substantia nigra in sporadic PD (McNaught et al., 2002; St. P. McNaught et al., 2003; Grünblatt et al., 2004; Chu et al., 2009; Bukhatwa et al., 2010). The physical interaction of the 26S proteasome with α-synuclein or Parkin points to a relevant role of the proteasome in PD development (Papaevgeniou and Chondrogianni, 2016). Drosophila melanogaster has been extensively used to identify genetic and environmental factors related to PD. Importantly, most human motor and non-motor phenotypes are recapitulated in this model (Hernández-Vargas et al., 2011; Yeh et al., 2011; Lessing and Bonini, 2009; Konsolaki, 2013; Ito et al., 2017).
In this study, we screened the effect of the in vivo reduction of 14 proteasome subunits using RNA interference (RNAi). Specific silencing of the Rpt2 subunit, also named Yhs4/Yta5 in yeast, S4 in mice, and PSMC1/P56/S4 in humans (Im and Chul Chung (2016)). The expression of an Rpt2-RNAi in the eye, strongly induced neurodegeneration in comparison with the effect of the reduction of the other 13 RNAi lines tested. To date, there is only one in vivo study where the Rpt2 subunit of the 19S proteasome regulatory particle has been knocked out and recapitulates several of the neuronal and motor symptoms of PD (Bedford et al., 2008). However, little behavioral analysis has been done in this context. Our results show that in vivo Rpt2 silencing in dopaminergic neurons of adult Drosophila, shortens their half-life, induces hyperactivity, sleep reduction, and neurodegeneration in most dopaminergic neuron clusters.
2. Experimental procedures
2.1. Drosophila culture, stocks and crosses
Fly stocks and crosses were raised on standard yeast medium at 25 °C. All UAS-RNAi lines used in this work were the third chromosome and are available at Bloomington Drosophila stock center. In experiments where proteasome subunits were silenced in the dopaminergic neurons, experimental (Tubulin-Gal80ts /+; TH-Gal4/UAS-RNAi) and control (Tubulin-Gal80ts /+; TH-Gal4/+) animals were produced and reared using the following protocol: Tubulin-Gal80ts/Tubulin-Gal80ts; TH-Gal4/ TH-Gal4; females were crossed at 25 °C with either UAS-RNAi-Rpt2 or w1118 males. Parents were removed and progeny was incubated at 18 °C until eclosion. One day old males were then transferred to a 28 °C incubator to allow RNAi expression and aged accordingly to the experimental design. In all cases, animals were transferred to fresh standard yeast medium every 3 days.
Transgenic lines were obtained from the Bloomington Drosophila stock center: P{Gal4-ninaE.GMR} (#1104), P{Gal4-elav.L} (#8760), P{TH-Gal4} (#8848), P{tubP-GAL80ts} (#7019), Rpt2-RNAi (#34,795), RNAi-Rpt3 (#34,917), RNAi-Rpt5 (#53,886), RNAi-α3 (#55,217), RNAi-α4 (#36,063), RNAi-α5 (#34,786), RNAi-α7 (#33,660), RNAi-β1 (#34,824), RNAi-β5 (#34,810), RNAi-β6 (#34,801), RNAi-Rpn1 (#34,824), RNAi-Rpn7 (#34,787), RNAi-Rpn8 (#31,567),RNAi-Rpn10 (#34,566).
2.2. Western blot analysis
For SDS PAGE, total protein from 30 Drosophila heads were extracted in lysis buffer (250 mM sucrose, 50 mM Tris−HCl pH 7.5, 5 mM EDTA, 25 mM KCl, 5 mM MgCl2, 100 mM PMSF and 1% SDS supplemented with 1X cOmpletetm Roche laboratories). 60 μg of the total extract was subjected to 12 % SDS-PAGE in a Bio-Rad MiniPROTEAN® Tetra Cell System. Protein was then transferred to nitrocellulose (Bio-Rad) in a Mighty Small Transphor (Amersham Pharmacia Biotech) at 300 mA for 4 h in transfer buffer (25 mM Tris, 192 mM Glycine, 20 % (v/v), methanol, pH = 8.3).
Native PAGE western blots were performed according to (Szlanka et al., 2003). Briefly: total protein from 30 Drosophila heads were extracted native homogenization buffer (20 mM Tris−HCl pH 7.5, 100 mM NaCl, 5 mM MgCl2, 1 mM ATP, 1 mM DTT, 250 mM Sucrose). Continuous 4 % poly-Acrylamide native gels (37.5:1) were prepared with 1 X TBE supplemented with 5 mM MgCl2, 1 mM ATP and 1 mM DTT. Protein was then transferred to a PVDF membrane(Bio-Rad) in a Mighty Small Transphor (Amersham Pharmacia Biotech) at 300 mA for 4 h in transfer buffer (25 mM Tris, 192 mM Glycine, 20 % (v/v), methanol, pH = 8.3).
Protein was quantified using Bradford Quick Start protein assay (Bio-Rad) according to the manufacturer’s protocol.
Membranes were blocked overnight with PBST (PBS, 0.1 % Tween-20) supplemented with 20 % instant non-fat milk (Carnation). Blots were then incubated for 2 h at room temperature in 1:2,000 anti-Rpt2 (Abcam 21,882) or 1:1,000 anti-Ubiquitin (Santa Cruz technologies Ub Antibody (P4D1), sc-8017) or 1:10,000 Anti-Proteasome alpha + beta antibody (Abcam 22,673) in PBST with 5% milk, washed 3 times in PBST 5% milk and then incubated for two hours at room temperature in 1:3000 HRP anti-mouse (Jackson Immuno Research) and washed another 3 times in PBST. Signal was detected using Kodak® BioMax® MS film and the Supersignal West Pico Chemiluminescent Kit (Thermo Scientific) accordingly to manufacturer’s instructions. Membranes were then stripped at 55 °C in 62 mM TRIS−HCL, pH = 6.8, 2% SDS and 0.1 M β-mercaptoethanol, and developed again using the procedure described above with 1: 3000 anti-actin (Developmental Hybridoma Bank). Densitometry was performed using the Gel Quantification utility of the FIJI application (ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/).
2.3. Analysis of insoluble ubiquitinated protein by western blot analysis
Western blot analysis of insoluble ubiquitinated proteins was done according to (Zhou et al., 2015). Briefly: Drosophila heads were homogenized in homogenization buffer (25 mM Tris−HCl, pH 7.4, 150 mM NaCl, 0.1 % (w/v) SDS) containing phenylmethylsulfonyl fluoride and protease inhibitors (Roche Applied Science) and incubated on ice for 30 min. The homogenized tissue was then sonicated and then centrifuged at 13 200 rpm for 30 min to obtain the soluble (supernatant) and insoluble (pellet) fractions. The pellet was solubilized using 30 μL of 4 X SDS BIO-RAD Laemmli sample buffer (Bio-Rad 161−0737,) at 4 °C overnight with continuous shaking, boiled for 5 min and loaded for gel electrophoresis, SDS-PAGE and western blot analysis.
2.4. qRT-PCR analysis
Quantitative Real-Time PCR was performed using SYBR™ Green following the manufacturer's instructions. Total RNA was extracted from Drosophila heads using TRizol™ Reagent. 1 μg of RNA was converted to cDNA using Reverse Transcriptase. Quantization of cncC transcripts was done using the primers and conditions reported by (Tsakiri et al., 2013). rp49 was used as an internal control of gene expression. Double delta ct were obtained and compared between groups.
2.5. Compound eye image acquisition
Eye images from flies anesthetized with CO2 were captured with a Zeiss Stemi 2000 stereoscopic microscope with an industrial 5.1 M P digital camera.
2.6. Larval locomotion assays
Individual third instar larvae were deposited in the center of a 14 cm petri dish with a layer of 2% agarose over a 2 mm printed grid with a black background. Larval locomotion was video recorded for one minute and the number of lines crossed by each larva was quantified and expressed in mm/min. For each genotype, 40 individuals were assayed. Significance was tested using ordinary one-way ANOVA with Tukey’s correction for multiple comparisons
2.7. Life expectancy and survival assays
Twenty food vials with 20 males of each genotype were placed at the beginning of the experiment. Animals were transferred to fresh vials every 3 days and the number of dead, trapped, and escaped individuals was registered. Differences between survival curves were analyzed using the Log-rank test (Mantel-Cox) and plotted with GraphPad Prism 6.0.
2.8. Negative geotaxis
Negative geotaxis was assayed essentially as reported by Ali (Ali et al., 2011). Briefly, 10 males of the same age range and genotype were anesthetized with CO2 and deposited in a polystyrene vial with an 8 cm mark from the bottom and were allowed to recover for one hour. Once recovered, flies were thrown to the bottom of the vial with 3 gentle taps and the number of individuals that were able to climb over the 8 cm mark within 10 s was recorded. Animals were allowed to rest for 1 min and the assay was repeated 10 times with the same population. In all cases, N ≥ 50 individuals all assays were performed between 9 a.m. and noon. Significance was tested using a two-sided unpaired t-test.
2.9. Spontaneous activity and sleep quantification
The Drosophila activity monitor “TriKinetics” was used accordingly to Pfeiffenberger (Pfeiffenberger et al., 2010). For each analysis period, 32 males were individually introduced in the TriKinetics polycarbonate tubes that had food on one side and were plugged with cotton on the other. Measurements were performed at 28 °C in a light and dark cycle of 12 h each. Animals were kept in the TriKinetics for a maximum of six days. The first 24 four hours were used to allow the flies to adapt to the environment and the 12 h light/dark cycles, activity data of the adaptation period was not used in the analysis. The activity was registered every minute using the “DAMSystem3Data” software. For spontaneous activity analysis, data for 30 min periods were analyzed and plotted. In Drosophila, sleep/rest is defined as periods of 5 min of inactivity, data from the monitor was analyzed and periods of 5 min or more of inactivity were quantified (Chiu et al., 2010). Actograms were plotted using GraphPad Prism 6.0. Significance between two populations was tested using a two-sided unpaired t-test.
2.10. Brain dissection and immuno-fluorescence
Brains were dissected in PBS, fixed with 4% paraformaldehyde in PBS for 15 min and washed 3 times for 15 min with PBS supplemented with 0.2 % Triton X-100 (PBST). Brains were blocked with 4% goat serum in PBST for 30 min and incubated overnight at 4 °C with anti-TH (Immuno Star 22,941) 1:1000 in PBST supplemented with 4% goat serum. After incubation, brains were washed 3 times for 15 min in PBST and incubated with goat anti-mouse Cy3 1:1000 in 4% goat serum PBST at 4 °C overnight. Washed 3 times for 15 min in PBST and mounted in Citifluor (Ted Pella Inc. Redding Ca). Brains were imaged in the Laboratory of Advanced Microscopy of the Instituto de Biotecnología (UNAM) with an inverted Olympus FV1000 confocal microscope using a 20X objective.
2.11. Brain dopaminergic neuronal cluster quantification
Confocal stacks were manually analyzed frame by frame using Image J. The number of neurons in each cluster was quantified and clusters were classified accordingly to White (White et al., 2010). In all phenotypes N = 12 brain hemispheres. Each cluster of the corresponding genotype age and treatment was compared with its corresponding controls for significance using a two sided unpaired t-test.
2.12. Statistical analysis
Statistical analysis was made using GraphPad Prism 6.0. All quantifications were comparisons of two different genotypes (experimental and controls) t Student test was used to compare means. In all locomotion parameters (speed, geotaxis, spontaneous activity, sleep time) and neuron number quantifications, significance was reported when p ≤ 0.05 with a confidence interval of 95 %. Significance is coded as follows p: * ≤ 0.05; ** ≤ 0.01; *** ≤ 0.001 and **** ≤ 0.0001.
3. Results
3.1. Systematic knockdown of proteasome subunits with RNAi identified Rpt2 as a strong inducer of eye degeneration
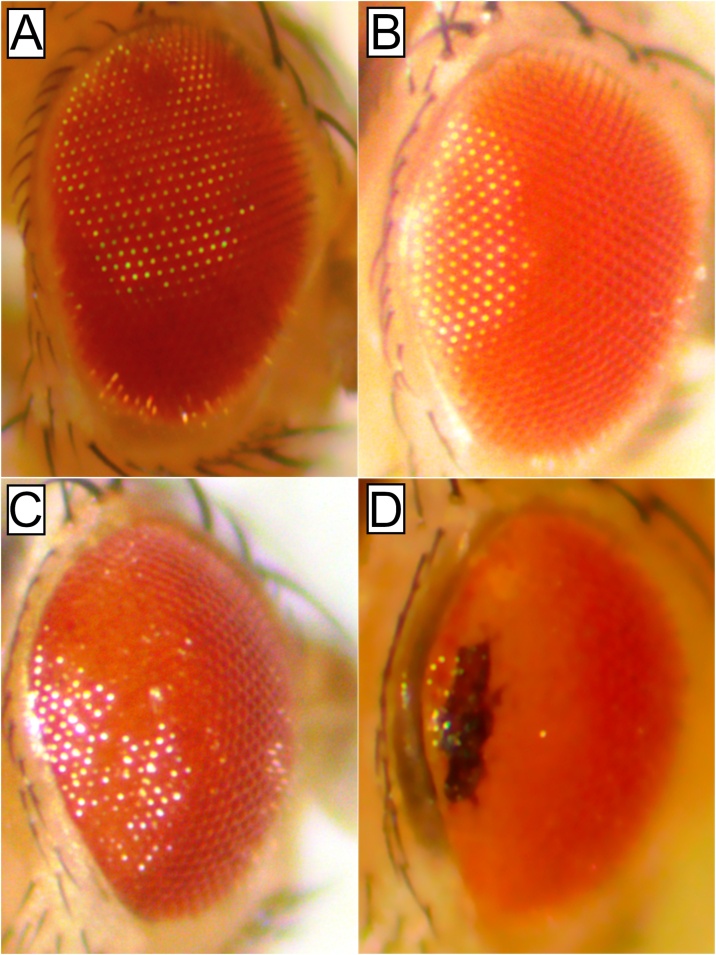
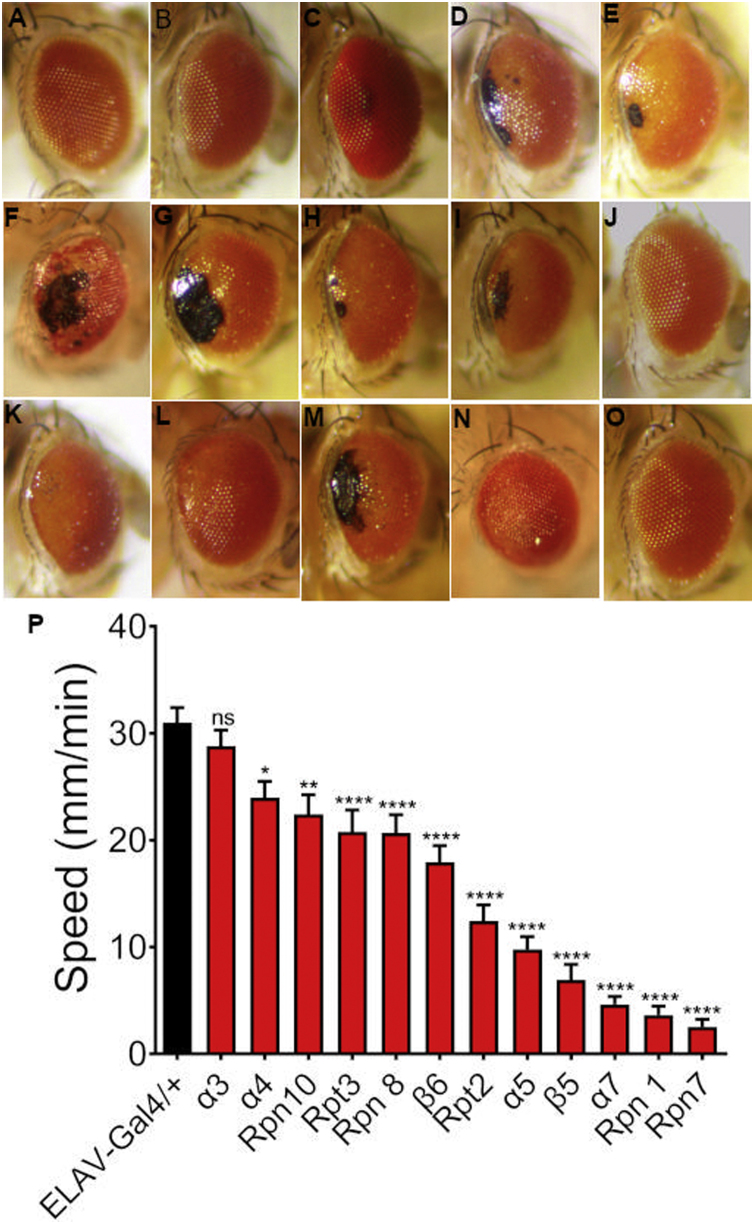
The Drosophila compound eye is an excellent model for the study of neurodegeneration (Rincon-Limas et al. (2012)). We took a systematic approach to characterize the effect of knocking-down the different proteasome subunits. Using the eye-specific ninaE.GMR-Gal4 driver, we tested the effect of the expression of 14 RNAis against proteasome subunits in this tissue. Ten out of the fourteen RNAis tested had different neurodegeneration expressivity that ranged from depigmentation to necrosis (sup Fig. 1). Out of the fourteen subunits tested, three were ATPases (Rpt2, Rpt3, and Rpt5). The induction of the RNAi-Rpt3 line with the ninaE.GMR-Gal4 had no evident phenotype; ninaE.GMR-Gal4 induction of the RNAi-Rpt5 caused slight depigmentation while the eye expression of the RNAi-Rpt2 had the most extreme phenotype showing depigmentation and necrosis, for this reason, we decided to further characterize its effect in neurodegeneration (Figure 1).
Fig. 1.
Different phenotypes induced by the knockdown of three ATPases subunits of the 19S proteasome regulatory particle. In all cases eyes are from flies one day after eclosion. A) Control only expressing Gal4 under the ninaE.GMR driver, no degeneration, or depigmentation can be observed. B) RNAi-Rpt3 expression in the eye has no obvious phenotype. C) RNAi-Rpt5 expression in the eye has slight depigmentation and a rough eye phenotype. D) RNAi-Rpt2 in the eye induces strong depigmentation rough eye phenotype and an area of necrotic tissue.
3.2. RNAi-Rpt2 is sufficient to significantly reduce the Rpt2 protein level
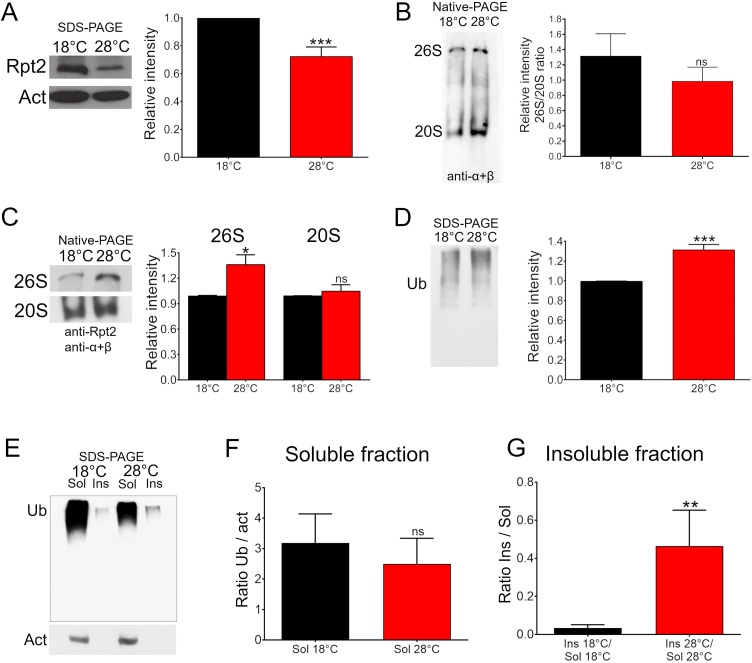
To demonstrate that the RNAi-Rpt2 can abate Rpt2 protein levels, we expressed it pan-neurally using an Elav-Gal4 driver repressed by a ubiquitous expressed thermosensitive Gal80 (tubulin-Gal80ts). In this genotype (tubulin-Gal80ts/Elav-Gal4 > UAS-RNAi-Rpt2), RNAi expression is repressed while the flies are kept at 18 °C, transferring them to 28 °C de-represses RNAi-Rpt2 and its expression becomes pan-neural. After induction of RNAi-Rpt2 expression for 48 h, Rpt2 protein levels became significantly reduced (t(6) = 8.01, p ≤ 0.0002) (Fig. 2 panel A). Native PAGE analysis shows that even though there is a slight tendency towards a reduction, the 26S/20S ratio does not change when both types of proteasome are simultaneously detected with an anti-α +β antibody when Rpt2 is knocked down (Fig. 2 panel B). Native page analysis showed that the independent detection of the 26S using an anti-Rpt2 antibody and the 20S using an anti-α+β antibody in native conditions there is an increase in the 26S proteasome (t(6) = 3.17, p ≤ 0.02) when Rpt2 is knocked down. While there is no apparent increase in the 20S proteasome (t(4) = 0.74, p ≤ 0.5) (Fig. 2 panel C). The reduction of Rpt2 significantly increased (t(4) = 9.84, p ≤ 0.0006) the amount of high molecular weight ubiquitinated proteins (Fig. 2 panel D). To further investigate if a reduction in Rpt2 compromises proteasome activity we analyzed if there is a change in the ratio of soluble and insoluble ubiquitinated proteins in fly head extracts when Rpt2 is pan-neuronally knocked down. A significant increase of ubiquitinated proteins was detected in the Rpt2 knocked down in the insoluble fraction (t(6) = 2.27, p = 0.0025), while the soluble fraction remained equal (t(6) = 0.54, p = 0.8330) (Fig. 2 panel E, F and G). These data are consistent with proteasome impairment and defective assembly or inactivation.
Fig. 2.
Rpt2 reduction and proteasome dysfunction in Rpt2 Knockdown flies. A) Western blot of an SDS-PAGE showing that the expression of RNAi-Rpt2 in the adult nervous system reduces total Rpt2 protein levels. The bar graph shows densitometric quantitation of Rpt2 expression (Unpaired t-test, n = 4 independent biological replicates). B) Western blot of a native-PAGE of the 26S and 20S proteasome detected with an anti-α+β antibody under this detection conditions no significant differences between the 26S/20S ratio can be observed (Unpaired t-test, n = 4 independent biological replicates). C) Independent detection of the 26S using an anti-Rpt2 antibody and the 20S using an anti-α+β antibody in native conditions shows an increase in the 26S proteasome when Rpt2 is knocked down. There is no apparent increase in the 20S proteasome. D) Western blot of an SDS-PAGE showing that the expression of RNAi-Rpt2 in the adult nervous system augments the amount of higher molecular weight ubiquitinated proteins. The bar graph shows densitometric quantitation of high molecular weight ubiquitinated protein (Unpaired t-test, n = 3 independent biological replicates, *** = p ≤ 0.001). Bar graph = mean with SEM (* = p ≤ 0.05, *** = p ≤ 0.001). E) Western blot of an SDS-PAGE showing that the expression of RNAi-Rpt2 in the adult nervous system augments the amount of insoluble ubiquitinated proteins. F) Densitometric quantitation demonstrating that the amount of soluble ubiquitinated protein does not change when the RNAi-Rpt2 is expressed. G) Densitometric quantitation demonstrating that the ratio of insoluble/soluble ubiquitinated protein significantly changes when the RNAi-Rpt2 is expressed (Unpaired t-test, n = 3 independent biological replicates, ** = p ≤ 0.01).
3.3. Larval spontaneous locomotion is impaired when Rpt2 is knocked-down
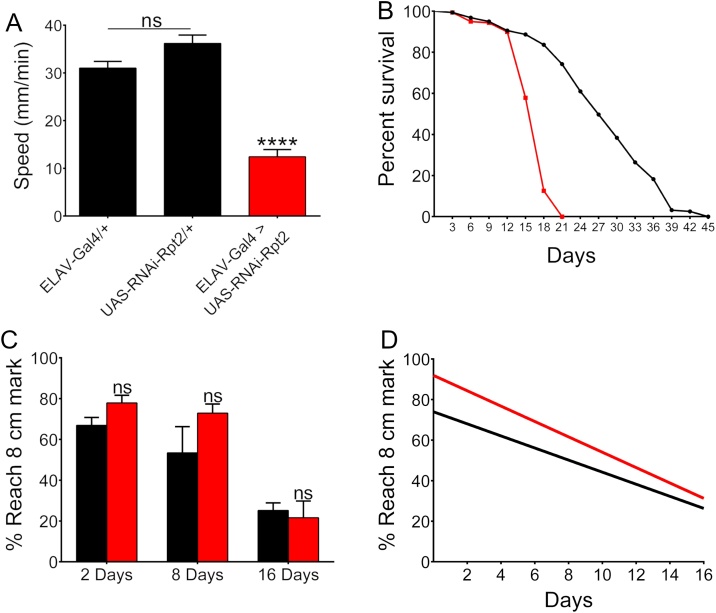
Motor dysfunction is one of the most characteristic symptoms of PD. The control lines Elav-GAL4 or the UAS-RNAi had no significant differences between them in larval speed (F(2, 117) = 3.3, p ≤ 0.06). The expression of RNAi-Rpt2 in the whole larval nervous system caused a significant reduction in larval speed when compared to both the Elav-Gal4 line (F(2, 117) = 11.93, p ≤ 0.0001) and the UAS-RNAi line (F(2, 117) = 15.25, p ≤ 0.0001) (Fig. 3 panel A) suggesting neuronal damage that eventually causes death before pupation. This larval locomotion defect was also reproduced when other proteasomes subunits were independently downregulated (sup Fig. 1).
Fig. 3.
Locomotion ability and survival are affected in Rpt2 Knockdown flies. A) Larval speed locomotion is severely affected when Rpt2 is knocked-down, n = 40. Significance was tested using ordinary one way ANOVA using Tukey’s correction for multiple comparisons. Bar graph = mean with SEM B) Survival curves for control (Tub-Gal80ts/+;TH-Gal4/+, black symbols) and experimental (Tub-Gal80ts/+;TH-Gal4/UAS-RNAi-Rpt2, red symbols) flies. Animals that expressed the RNAi-Rpt2 after eclosion had a median half-life of 18 days while controls had a median half-life of 27 days, n = 160. Significance was tested using the Log-rank (Mantel-Cox) test. C) Negative geotaxis (climbing ability), experimental (red symbols) and control flies (black symbols) do not present a significant difference in climbing ability, n ≥ 50 divided in groups of ten flies per trial, significance was tested with the unpaired t-test for each age. Bar graph = mean with SEM. D) Linear regression analysis of negative geotaxis. Climbing ability reduction represented by the regression was not significant although slope was more negative in experimental animals (red line). However, in experimental animals Y interception of best fit values at X = 0 is significantly different from the control´s (Yexp=-3.792*X+91.93 vs Ycont=-2.979*X+73.99), n ≥ 50, p = 0.045 suggesting hyperactivity. Significance was tested using unpaired t-test analysis. (**** = P ≤ 0.0001).
3.4. Adults with reduced Rpt2 in the dopaminergic neurons have severely reduced survival and hyperactive induced motor activity
RNAi-Rpt2 specifically expressed in the dopaminergic neurons with a tyrosine hydroxylase driver (TH-Gal4) had a first instar larval lethal phenotype. It has been reported that loss of tyrosine hydroxylase is lethal during early development because it is crucial for cuticle maturation (Friggi-Grelin et al., 2003a, 2003b). The reduction of Rpt2 during development probably impairs cuticle tyrosine-hydroxylase expressing cells thus explaining the larval lethal phenotype. To overcome the cuticle phenotype, expression of the RNAi-Rpt2 was temporally regulated using the ubiquitous expression of thermosensitive Gal80. One day after eclosion, experimental (tubulin-Gal80ts/TH-Gal4 > UAS-RNAi-Rpt2) and control (tubulin-Gal80ts/+; TH-Gal4/+) adult flies reared at 18 °C were transferred to 28 °C for the remainder of their lives. Importantly, the individuals with the reduced expression of Rpt2 in the dopaminergic neurons have a significantly shortened half-life when compared to controls (18 vs 27 days respectively, χ2(1), N = 318 = 189.4, p ≤ 0.0001) (Fig. 3 panel B). In flies, as in most animals, motor activity naturally declines during aging. To evaluate the induced motor activity, climbing assays were performed at three different ages, we found that at 2 days after eclosion, the flies expressing the RNAi apparently climbed more, although not in a statistically significant manner (t(16) = 2.02, p ≤ 0.06), this trend did not change during aging (8 days, t(13) = 1.8, p ≤ 0.1 and 16 days post-eclosion, t(9) = 0.42, p ≤ 0.7)(Figure 3 panel C). As expected, age caused a decline in climbing ability which is represented by the curve slope, experimental animals had a more negative slope than control lines, suggesting a faster motor decline, however, it was not significantly different from controls (t(42) = 0.89, p ≤ 0.4). The basal climbing ability is represented by the point of interception of the regressions with the Y-axis. Rpt2 knockdown flies had a basal climbing ability significantly higher than controls (t(42) = 2.07, p ≤ 0.045) (Fig. 3 panel D).
3.5. Rpt2 reduction in dopaminergic neurons induces spontaneous hyperactivity
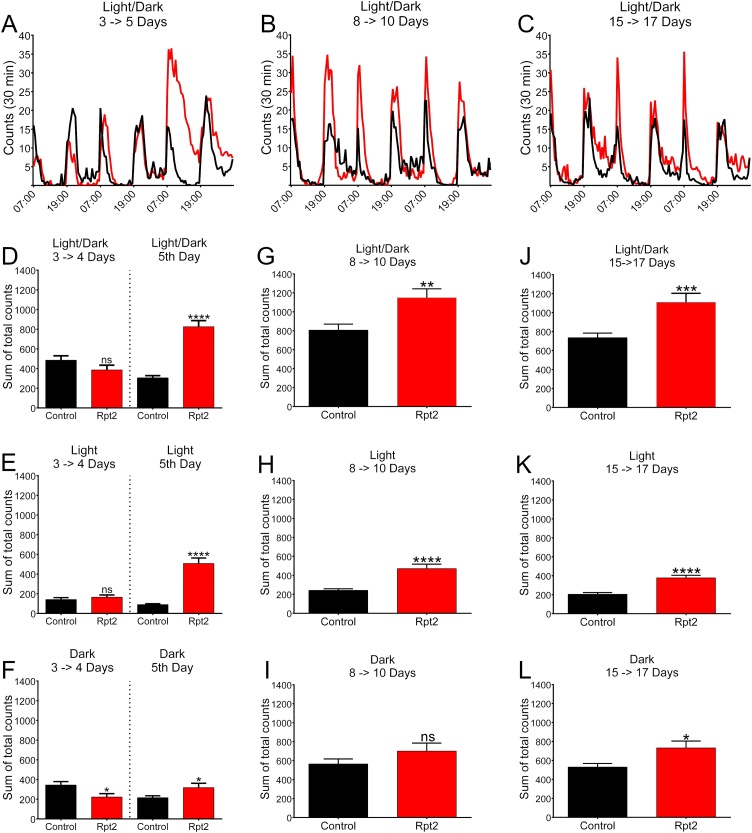
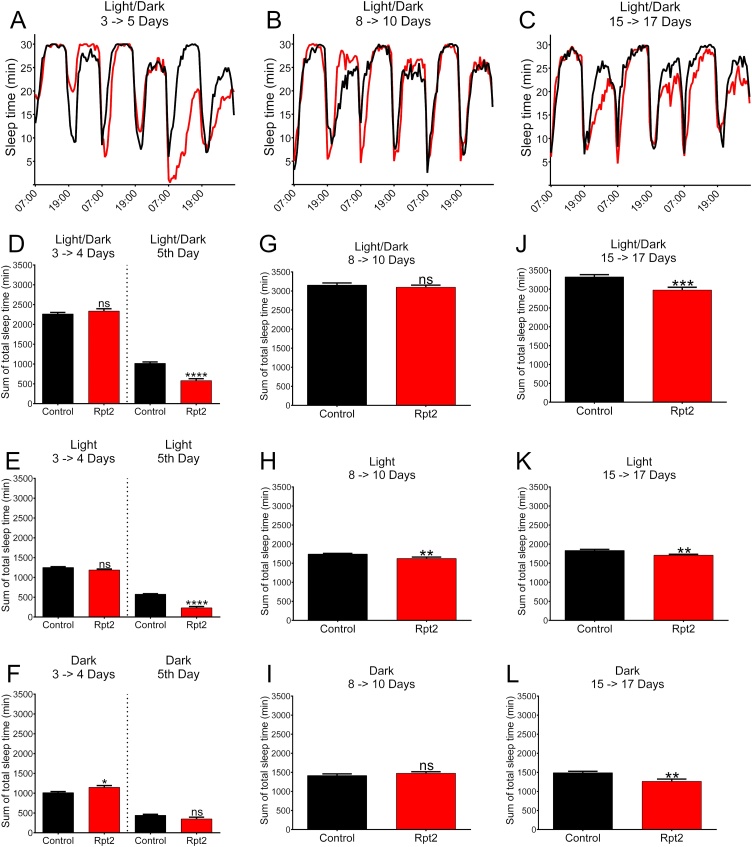
Dopamine is known to be important for the initiation and modulation of spontaneous activity (White et al., 2010). Spontaneous activity of Rpt2 knocked-down flies was measured using an activity monitor system (TriKinetics). Experimental flies and their respective controls were allowed to mature for 24 h after eclosion at 28 °C. Then transferred to the Trikinetics instrument (n = 32) that was kept in an incubator at 28 °C that had a 12 h light/dark cycle. The flies were allowed to adapt for 24 h and their activity was measured for the next 3 days (3–5 days after eclosion). A second and a third cohort was implemented respectively on day 8 (8–10 days after eclosion) and at day 15 (15–17 days after eclosion) and their activity was also monitored for 3 days after an adjustment period of 24 h. Actograms of total crosses every 30 min can be observed in Fig. 4 panels A-C. Interestingly, Rpt2 knocked-down flies’ activity was similar to the control for days 3 and 4 (t(51) = 1.72, p ≤ 0.091), but in day 5 they became significantly hyperactive (t(50) = 8.66, p ≤ 0.0001) (Fig. 4 panel D). Hyperactivity remained for the rest of the period studied (days 8–10, t(55) = 3.11, p ≤ 0.003 and for days 15–17, t(57) = 3.63, p ≤ 0.0006) (Figure 4 panel G and J). When the light (Figure 4 panels E, H and K) and dark (Figure 4 panels F, I and L) periods were analyzed separately, hyperactivity was most significant during the light period (days 3–4, t(50) = 0.81, p ≤ 0.42; day 5, t(50) = 8.62, p ≤ 0.0001; days 8–10, t(54) = 5.15, p ≤ 0.0001 and for days 15–17, t(57) = 5.27, p ≤ 0.0001); Significant hyperactivity during the dark period was also observed in day 5 and days 15–17 (days 3–4, t(50) = 2.41, p ≤ 0.02; day 5, t(50) = 2.35, p ≤ 0.02; days 8–10, t(54) = 1.43, p ≤ 0.16 and for days 15–17, t(57) = 5.27, p ≤ 0.001).
Fig. 4.
Spontaneous locomotor activity of Rpt2 knockdown flies. Actograms are shown on top of each column; quantization of the corresponding actograms and periods are shown in the lower panels. Panels A, B and C show actograms for days 3 to 5, 8 to 10, and 15 to 17 respectively. Hyperactivity in RNAi-Rpt2 (experimental) flies (red) begins to show only after the fifth day of aging. In every 3 day shown, experimental animals (red) accumulated significantly more activity than controls (black). D) Comparison of accumulated activity during the light and dark periods for days 3 and 4 was not significantly different between the control and RNAi-Rpt2, but at day 5 RNAi-Rpt2 group showed an increase in activity that is statistically different. Panels E (light period) and F (dark period) show accumulated activity for days 3 to 5, hyperactivity in RNAi-Rpt2 (experimental) flies (red) begins to show only after the fourth day of aging. G) Accumulated activity for days 8 to 10 quantified during the light and dark periods. Increased activity in RNAi-Rpt2 flies is significantly different from controls. Panels H (light period) and I (dark period) show accumulated activity for days 8 to 10; RNAi-Rpt2 flies have more accumulated activity than controls only in the light period. J) Accumulated activity for days 15 to 17 quantified during the light and dark periods. RNAi-Rpt2 flies have more accumulated activity than controls. Panels K (light period) and L (dark period) show accumulated activity for days 15 to 17 quantified during the light and dark periods respectively; in both periods RNAi-Rpt2 has more accumulated activity that is significantly different than controls. Significance was tested for each independent period using unpaired t-test, n ≥ 23, Bar graph = mean with SEM (* = P ≤ 0.05, ** = P ≤ 0.01, *** = P ≤ 0.001, **** = P ≤ 0.0001).
Knockdown of the Rpt2 proteasome subunit induces sleep reduction.
Sleep disorders are a common non-motor symptoms in Parkinson´s disease (Comella, 2007). Several research groups have found that in Drosophila, homologous mutations in genes associated with PD such as: LRRK2 (Sun et al., 2016), parkin (Julienne et al., 2017) and PINK1 (Julienne et al., 2017) and expression of human α-synuclein (Gajula Balija et al., 2011) also induce sleep disorders. In Drosophila, sleep is defined as any 5 min period without spontaneous activity (Chiu et al., 2010). Actograms (Fig. 5 panels A-C) show how many minutes of sleep time are accumulated in our experimental and control strains at any given 30 min period. A reduction of Rpt2 in dopaminergic neurons induces a reduced sleep cycle in the light/dark period in five day old flies and in 15–17 day old flies (days 3–4, t(52) = 1.04, p ≤ 0.3; day 5, t(52) = 7.50, p ≤ 0.0001; days 8–10, t(54) = 0.67, p ≤ 0.51; days 15–17, t(57) = 3.63, p ≤ 0.0006) (Fig. 5 panels D, G and J). Interestingly, the sleep alteration also begins by the 5th day after eclosion and most of this reduction occurs during the light cycle (days 3–4, t(52) = 1.99, p ≤ 0.052; day 5, t(52) = 10.28, p ≤ 0.0001; days 8–10, t(54) = 2.91, p ≤ 0.005 and for days 15–17, t(57) = 3.18, p ≤ 0.002) (Fig. 5 panels E, H and K). During the dark period, most of sleep reduction can only be observed in aged flies (>15 days) (days 3–4, t(52) = 2.60, p ≤ 0.01; day 5, t(52) = 1.93, p ≤ 0.06; days 8–10, t(54) = 0.97, p ≤ 0.34 and for days 15–17, t(57) = 3.24, p ≤ 0.002) (Fig. 5 panels F, I and L).
Fig. 5.
Sleep behavior of Rpt2 knockdown flies. Actograms are shown on top of each column; quantization of the corresponding actograms and periods are shown in the lower panels. Panels A, B, and C show overall accumulated sleep-time during light and dark periods for days 3 to 5, 8 to 10, and 15 to 17 respectively. Sleep-time in RNAi-Rpt2 (experimental) flies (red) is reduced only after the fourth day of aging. In every 3 day period shown, RNAi-Rpt2 animals (red) accumulated significantly less sleep-time than controls (black). D) Comparison of accumulated sleep-time during the light and dark periods for days 3 and 4, and day 5 (black control, red RNAi-Rpt2 experimental) show that at day 5, RNAi-Rpt2 flies have significantly reduced sleep-time accumulation compared to controls. Panels E (light period) and F (dark period) show accumulated sleep-time for days 3 to 5, a significant sleep-time decrease in RNAi-Rpt2 flies (red) can only be observed after the fourth day of aging and only in the light period. G) Accumulated sleep-time for days 8 to 10 quantified during the light and dark period. No significant differences were found in this period in accumulated sleep-time between RNAi-Rpt2 and control. Panels H (light period) and I (dark period) show accumulated sleep-time for days 8 to 10. A significant decrease in sleep-time accumulation was observed only during the light period in RNAi-Rpt2 animals. J) Accumulated sleep-time for days 15 to 17 quantified during the light and dark period showed a significant decrease in sleep-time in RNAi-Rpt2 flies. Panels K (light period) and L (dark period) show accumulated sleep-time for days 15 to 17. In both periods RNAi-Rpt2 flies had a significant decrease in sleep-time accumulation. Significance was tested for each independent period using unpaired t-test, n ≥23, Bar graph = mean with SEM (* = P < 0.05, ** = P < 0.01, *** = P < 0.001, **** = P < 0.0001).
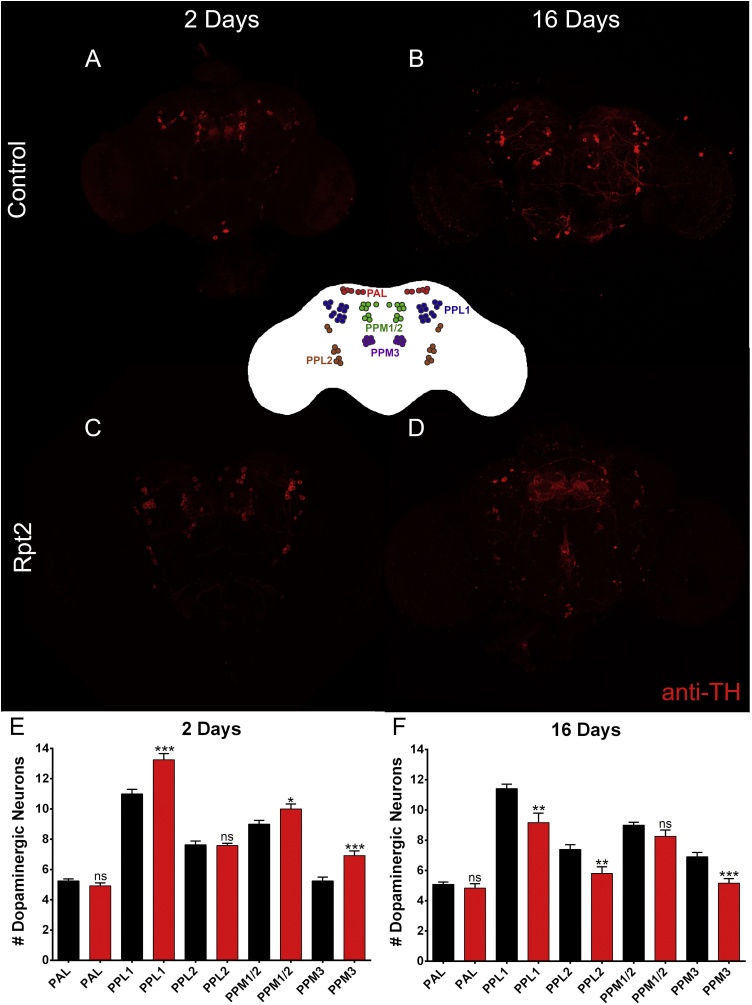
3.6. The reduction of the Rpt2 subunit in dopaminergic neurons induces neurodegeneration in specific dopaminergic clusters
As mentioned above, a reduction of the Rpt2 subunit in the dopaminergic neurons induces hyperactivity and sleep disorders. To characterize at the cellular level the effect of Rpt2 reduction, whole-mount anti-TH immunofluorescence experiments were performed in young and aged Drosophila adult brains (Fig. 6). The Drosophila brain has 8 different clusters of dopaminergic neurons, the ones that are best characterized and whose number of neurons are most reproducible are PAL, PPL1, PPL2, PPM1/2, PPM3 (Mao and Davis, 2009; White et al., 2010; and this work). We counted neurons of each of the mentioned clusters in brains from flies 2 and 16 days old. As expected, when RNAi-Rpt2 is expressed under the control of TH-Gal4, three of these dopaminergic clusters have higher neurodegeneration in 16 day old experimental flies compared to aged paired controls (PPL1 cluster, t(22) = 3.27, p ≤ 0.004 ; for PPL2 cluster, t(18) = 2.98, p ≤ 0.008 and the PPM3 cluster t(21) = 4.22, p ≤ 0.0004). The dopaminergic clusters that did not show augmented degeneration were the PAL and PPM1/2 clusters (PAL cluster, t(22) = 0.75, p ≤ 0.46 and the PPM1/2 cluster t(21) = 1.55, p ≤ 0.14).
Fig. 6.
Neuronal degeneration induced by the Rpt2 knockdown in dopaminergic neurons. Panels A (2 days after eclosion) and B (16 days after eclosion) show representative anti-TH immunofluorescence maximum intensity z-projections of confocal images of control adult Drosophila brains. Panels C (2 days after eclosion) and D (16 days after eclosion) show representative anti-TH immunofluorescence maximum intensity z-projections of confocal images of experimental (Rpt2 knockdown) Drosophila brains. Panels E and F show quantization of the different dopaminergic neuron clusters revealed by anti-TH immunofluorescence. Dopaminergic neuron degeneration can be observed in all clusters but PAL and PPM1/2. Dopaminergic neurons were quantified from each hemisphere independently. n = 12 hemispheres, * = P < 0.05, ** = P < 0.01, *** = P < 0.001, **** = P < 0.0001, Bar graph = mean with SEM. Significance was tested using unpaired t-test. Inlay between confocal images is a map of the stereotypical dopaminergic neuron clusters (modified from Kasture et al., 2018).
4. Discussion
Independent evidence from several authors suggests that components of the UPS are involved in most neurodegenerative diseases (Im and Chul Chung (2016)). In PD there is post mortem data from sporadic patients that shows that expression of the 26S proteasome subunits is reduced (St. P. McNaught et al., 2003; Grünblatt et al., 2004); despite this knowledge, little effort has been made to characterize if the reduction of regulatory proteasome subunits is enough to cause PD symptoms. In this work, we characterized the Parkinson’s disease-like phenotypes caused by a diminished amount of the Rpt2 proteasome subunit. The expression of the RNAi against the Rpt2 subunit in the Drosophila eye causes strong neurodegeneration in comparison with the induction of the expression RNAis against Rpt3 or Rpt5. Therefore we decided to further characterize RNAi-Rpt2 line. When Rpt2 is knocked-down in the dopaminergic neurons after pupation, it generates hyperactivity, sleep disorders, a reduction in survival, and dopaminergic neuron loss.
Rpt2, Rpt3, and Rpt5 are part of the 19S proteasome. Rpt2 and Rpt5 are the subunits that activate the proteasome to induce protein degradation by the interaction of their C-termini with the N-termini of the α subunits of the 20S proteasome and by inducing the opening of the proteasome pore (Smith et al., 2007). Cell culture experiments with knocked-out Rpt5 found that loss of this subunit induced protein inclusions but not cell dead (Droggiti et al., 2011). Rpt2 knock-out mice have extensive neurodegeneration and Lewy-like inclusion bodies (Bedford et al., 2008). It is known that this subunit is important for the 26S proteasome assembly. Variations of the Rpt3 gene are associated with PD, and some Parkinson´s disease patients have reduced Rpt3 transcript levels (Kumar et al. (2010); Wahl et al., 2008; Molochnikov et al., 2012). In a post mortem brain study of patients with schizophrenia a significant reduction of all 19S regulatory Rpt subunits was found, but not in any of the subunits of the catalytic 20S proteasome, suggesting that a variety of abnormal brain functions are related with a decrease in regulatory proteasome subunits (Scott and Meador-Woodruf (2019)). Our results show that the knockdown of the triple-A ATPase Rpt2 has a strong neurodegenerative phenotype in the eye. This phenotype is probably caused by hypoactivation of the proteasome that leads to a toxic accumulation of ubiquitinated proteins.
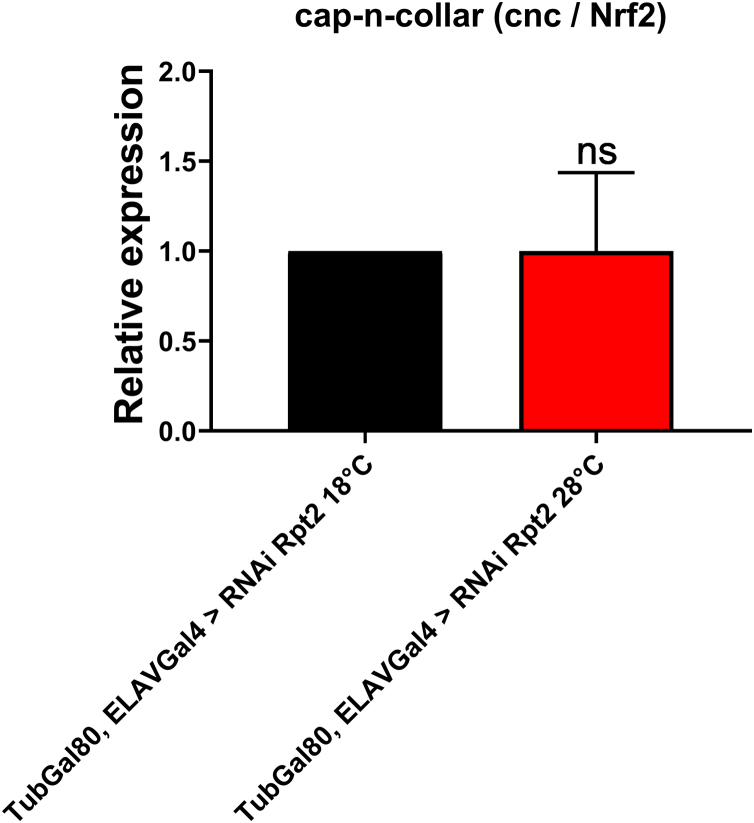
Third instar larvae with pan-neural reduction of the Rpt2 subunit using an Elav-Gal4 driver were less motile than controls. Similar results were obtained in larvae that express either wt or mutant A53 T α-synuclein pan-neurally (Varga et al., 2014). In adults, we show that the expression of RNAi-Rpt2 in the permissive conditions reduces the absolute amount of Rpt2 in the brain. Native gel electrophoresis and western blot analysis using an anti-α+β antibody that simultaneously detects the 26S and 20S proteasomes shows that their ratio (26S/20S) does not change significantly. The independent detection of the 26S using an anti-Rpt2 antibody and the 20S using an anti-α+β antibody showed that there is a relative increase in the 26S that contains Rpt2 when Rpt2 is knocked down; while there is no apparent increase in the amount of the 20S proteasome. These results correlate with an increase of insoluble and high molecular weight ubiquitinated proteins suggesting proteasome dysfunction. The knockdown of Rpt2 likely causes an alteration of proteasome assembly by changing subunit stoichiometry thus leading to an increase in the amount of defective proteasomes (Fig. 2). A similar phenomenon has already been described in Drosophila when Rpn10 is depleted. In the Rpn10 depletion experiments, an increase in 26S and 20S was observed as a consequence of a feedback circuit (Szlanka et al., 2003). In mammals, when the proteasome is inhibited, the proteasome upregulation elements NRF1 and NRF2 become activated thus inducing overexpression of the proteasome subunits (Rousseau and Bertolotti, 2018). It has been shown that in some conditions loss of proteasome activity in Drosophila somatic tissues and gonads also induce upregulation of the proteasome subunits through the Nrf2 stress response element (Tsakiri et al., 2013). We did explore the possibility that this pathway is also activated with reduced levels of Rpt2 but we were unable to find any evidence of induction or reduction of the Drosophila Nrf2 orthologue (cnc/Nrf2) in these conditions (Sup Fig. 2). The increased expression of the 26S proteasome containing Rpt2 does not implicate its correct assembly, conversely, the lack of Rpt2 may favor the accumulation of defective 26S particles as our data and as Szlanka’s suggests (Szlanka et al., 2003). The resulting sustained induction of proteasome subunits and defective assembly may be energetically costly to the cell thus causing constant stress that could lead to neuronal malfunction and death (Rousseau and Bertolotti, 2018). It could also be of interest to characterize if the reduction of Rpt2 affects differentially any of the three proteasome protease activities as it has been shown that they have differential sensitivities to different chemical challenges (Zhou and Lim, 2009). This would allow us to monitor the exact proteasome functions affected under Rpt2 knock down, however, at this moment this is beyond the scope of this work.
To specifically study the effect of Rpt2 reduction in adult dopaminergic neurons, Rpt2-RNAi was expressed using the tyrosine hydroxylase Gal4 driver (TH-Gal4) controlled with the thermosensitive Gal4 repressor, Gal80ts. Adults with a reduced amount of Rpt2 in the dopaminergic neurons have a very significant reduction of their half-life (18 days vs 27 days in controls). Importantly, a similar effect has been found using the same TH-Gal4 driver to reduce Parkin expression using an RNAi against Parkin or by overexpression of human α-synuclein in adults (Srivastav et al., 2015; Hernández-Vargas et al., 2011). Unlike the other phenotypes measured in this work, negative geotaxis decays similarly both in the experimental and control lines; however, it has been reported that induced locomotion is mostly controlled by the PAM dopaminergic cluster which incidentally, is where the expression of the TH-Gal4 driver is weakest (Friggi-Grelin et al., 2003a, 2003b). Interestingly, spontaneous locomotion and sleep were disrupted in knocked-down Rpt2 adult flies. Spontaneous locomotion and sleep were evaluated in young (3–5 days after eclosion), middle-aged (8–10 days after eclosion), and old flies (15–17 days after eclosion). Rpt2 knocked-down individuals became hyperactive after the 5th day. The hyperactivity remained during aging; and as flies aged (5–17 days old), their spontaneous hyperactivity during the light period was almost constant, while during the dark period, hyperactivity increased in a significant manner. It has been shown that in Drosophila, an increase in dopamine pools and release correlates with hyperactivity (Wang et al., 2011). An explanation for this hyperactivity could be that tyrosine hydroxylase is rapidly degraded, and impaired proteasome would not able to degrade it as efficiently and it could probably accumulate with other proteins inducing neuronal dysfunction and thus increasing anomalous dopamine liberation; there is at least one report that shows that proteasome inhibition increases dopaminergic neuron firing frequencies (Nakashima et al., 2018; Subramaniam et al., 2014; Rinetti and Schweizer, 2010; Subramaniam et al., 2014). The increase in dopamine and its degradation products could also contribute to neurodegeneration (Bazzini et al., 2008). Alternatively, the reduction of Rpt2 could impair the ability of the proteasome to degrade aged and misfolded proteins, which could, in turn, alter membrane permeability promoting calcium liberation from the mitochondrion or the endoplasmic reticulum thus inducing exacerbated dopamine liberation and neurodegeneration (Wu et al., 2009).
Sleep disorders in Parkinson´s patients are an early and very common non-movement symptom (Lee and Koh, 2015). These disorders affect 88 percent of patients and include insomnia, REM behavior disorder, sleep apnea, Restless Legs syndrome, vivid dreaming, excessive daytime sleepiness, and sleep attacks (Menza et al., 2010; Chahine et al., 2017). Relevantly, the study of the sleep-like state in flies and other insects has shown that these organisms share key features of vertebrate sleep: such as homeostatic rebound and circadian regulation, the similarities are also conserved at the molecular level as the key circadian clock system was discovered in Drosophila and shown to be conserved in mammals. Sleep in Drosophila is operationally defined as periods of locomotor inactivity of 5 min or longer and it is normal for wt Drosophila to sleep or rest during between two peaks of activity, one before the light period and the other before the dark period begins (Sitaraman et al., 2015; De Lazzari et al., 2018; Dubowy and Sehgal, 2017). Interestingly, Rpt2 knockdown individuals have a significant reduction in sleep during light hours, even when relatively young. During the dark period, the reduction is only observed in aged organisms. Our results show that significant neuronal degeneration occurs in the PPL1 and PPM3 dopaminergic neuronal clusters, these two clusters have been previously involved in sleep modulation (Kasture et al., 2018). Furthermore, a very similar sleep reduction pattern has been reported in flies that express oligomeric α-synuclein; and in a Drosophila PD model that has mutations in parkin and pink1 (Gajula Balija et al., 2011; Valadas et al., 2018). Our behavioral and neurodegeneration data seems to be analogous to sleep abnormalities observed in other PD models such as mice, and to what happens in humans as it has been reported that these abnormalities may appear years before the onset of Parkinson’s motor symptoms. (De Lazzari et al., 2018). The mechanism behind sleep dysregulation in PD, is still unknown but downregulation Rpt2 may be one of the pathways that should be explored in other animal models of the disease.
Pharmacological proteasome activation has been proposed as a therapy for many neurodegenerative diseases (Chondrogianni et al., 2015). Overexpression of Rpn11 in flies is sufficient to increase half-life, while its loss of function reduces it and cause neurodegenerative eye phenotype (Tonoki et al., 2009), our work shows that the reduction of Rpt2 in neuronal tissues causes similar phenotypes to the ones observed in Rpn11 loss of function. It has been demonstrated in in vitro and in animal models that overexpression of other proteasomal subunits may ameliorate the symptoms of several neurodegenerative diseases (Njomen and Tepe, 2019), and future studies should explore if the overexpression of Rpt2 could also have similar effects.
Human and Drosophila data suggest that protein accumulation and misfolding promotes early neuronal dysfunction and premature cell death. Our results appear to be analogous to the ones observed in post mortem brain tissue from patients with sporadic PD that have a reduction of several proteasome subunits. We also show that Rpt2 subunit reduction causes Parkinson’s disease-like symptoms in Drosophila.
Author contributions
Iván Fernández, Veronica Narváez, and Enrique Reynaud wrote the main manuscript text, Enrique Reynaud and Iván Fernández prepared figures; Iván Sánchez performed western blot and densitometric analysis. Veronica Narváez provided statistical analysis design, review, and critique. All authors reviewed the manuscript.
Financial statement
no authors have received any funding from any institution, including personal relationships, interests, grants, employment, affiliations, patents, inventions, honoraria, consultancies, royalties, stock options/ownership, or expert testimony for the last 12 months.
Funding
This study was supported by funds from Dirección General de Asuntos del Personal Académico de la Universidad Nacional Autónoma de México (DGAPA/UNAM) PAPIIT-IN204214, PAPIIT-IN206517 and Consejo Nacional de Ciencia y Tecnología, México (CONACyT) grant 255478. We also thank CONACyT for scholarships 342559, 404427 and 330155, 446128. We would also want to thank the National University of México (UNAM) and the Programa de Posgrado en Ciencias Bioquímicas UNAM.
Ethical statement
No humans or mammals were used in this study. All procedures performed in the study were in accordance with the ethical standards of the institutional (instituto de Biotecnología, UNAM) and/or the national bioethical research committee. Technical specifications for production, care and use of laboratory animals in Mexico can be found at: http://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF.
Conflict of Interest
We also declare no conflict of interests.
Acknowledgments
We thank René Hernández Vargas for technical support, Santiago Becerra for oligonucleotide synthesis and Jorge Yañez for DNA sequencing, Ing. Roberto Pablo Rodríguez and David Santiago Castañeda of the “Unidad de cómputo of the Instituto de Biotecnología” for computer maintenance and technical support. We also thank Andrés Saralegui Amaro and Jaime Arturo Pimentel Cabrera from the Laboratorio Nacional de Microscopía Avanzada (LNMA) for support in confocal imaging.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ibror.2020.07.001.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Ali Yousuf O., Escala Wilfredo, Ruan Kai, Grace Zhai R. Assaying Locomotor, Learning, and Memory Deficits in Drosophila Models of Neurodegeneration. J. Vis. Exp. 2011;49(March):1–6. doi: 10.3791/2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balija Gajula, Babu Madhu, Griesinger Christian, Herzig Alf, Zweckstetter Markus, Jäckle Herbert. Pre-fibrillar α-Synuclein mutants cause parkinson’s disease-like non-motor symptoms in Drosophila. Edited by ralf krahe. PLoS One. 2011;6(9):e24701. doi: 10.1371/journal.pone.0024701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzini Eleonora, Samuele Alberta, Granelli Marcella, Levandis Giovanna, Armentero Marie-Therese, Nappi Giuseppe, Blandini Fabio. Proteasomal inhibition and apoptosis regulatory changes in human isolated lymphocytes: the synergistic role of dopamine. J. Cell. Biochem. 2008;103(3):877–885. doi: 10.1002/jcb.21457. [DOI] [PubMed] [Google Scholar]
- Bedford Lynn, Hay David, Devoy Anny, Paine Simon, Powe Des G., Seth Rashmi, Gray Trevor. Depletion of 26S Proteasomes in Mouse Brain Neurons Causes Neurodegeneration and Lewy-like Inclusions Resembling Human Pale Bodies. J. Neurosci. 2008;28(33):8189–8198. doi: 10.1523/JNEUROSCI.2218-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braten Ori, Livneh Ido, Ziv Tamar, Admon Arie, Kehat Izhak, Caspi Lilac H., Gonen Hedva. Numerous Proteins with Unique Characteristics Are Degraded by the 26S Proteasome Following Monoubiquitination. Proc. Natl. Acad. Sci. U.S.A. 2016;113(32):E4639–E4647. doi: 10.1073/pnas.1608644113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhatwa Salma, Zeng Bai-Yun, Rose Sarah, Jenner Peter. A Comparison of Changes in Proteasomal Subunit Expression in the Substantia Nigra in Parkinson’s Disease, Multiple System Atrophy and Progressive Supranuclear Palsy. Brain Res. 2010;1326(April):174–183. doi: 10.1016/j.brainres.2010.02.045. [DOI] [PubMed] [Google Scholar]
- Chahine Lama M., Amara Amy W., Videnovic Aleksandar. A Systematic Review of the Literature on Disorders of Sleep and Wakefulness in Parkinson’s Disease from 2005 to 2015. Sleep Med. Rev. 2017;35:33–50. doi: 10.1016/j.smrv.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Joanna C., Low Kwang Huei, Pike Douglas H., Yildirim Evrim, Edery Isaac. Assaying locomotor activity to study circadian rhythms and sleep parameters in Drosophila. J. Visual. Exper. JoVE. 2010;43(September):1–8. doi: 10.3791/2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondrogianni Niki, Voutetakis Konstantinos, Kapetanou Marianna, Delitsikou Vasiliki, Papaevgeniou Nikoletta, Sakellari Marianthi, Lefaki Maria, Filippopoulou Konstantina, Gonos Efstathios S. proteasome activation: an innovative promising approach for delaying aging and retarding age-related diseases. Ageing Res. Rev. 2015;23(PA):37–55. doi: 10.1016/j.arr.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Chu Yaping, Dodiya Hemraj, Aebischer Patrick, Warren Olanow C., Kordower Jeffrey H. Alterations in lysosomal and proteasomal markers in parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol. Dis. 2009;35(3):385–398. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Comella Cynthia L. Sleep disorders in Parkinson’s disease: an overview. Mov. Disord. 2007;22(S17):S367–S373. doi: 10.1002/mds.21682. [DOI] [PubMed] [Google Scholar]
- Damier P., Hirsch E.C., Agid Y., Graybiel A.M. The Substantia Nigra of the human brain: II. patterns of loss of dopamine-containing neurons in Parkinson’s Disease. Brain. 1999;122(8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- Droggiti Anna, Ho Cherry Chen-Ying, Stefanis Leonidas, Dauer William T., Rideout Hardy J. targeted disruption of neuronal 19s proteasome subunits induces the formation of ubiquitinated inclusions in the absence of cell death. J. Neurochem. 2011;119(3):630–643. doi: 10.1111/j.1471-4159.2011.07444.x. [DOI] [PubMed] [Google Scholar]
- Dubowy Christine, Sehgal Amita. Circadian rhythms and sleep in Drosophila melanogaster. Genetics. 2017;205(4):1373–1397. doi: 10.1534/genetics.115.185157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forno Lysia S. Neuropathology of parkinsonʼs disease. J. Neuropathol. Exp. Neurol. 1996;55(3):259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin Florence, Coulom H.élène, Meller Margaret, Gomez Delphine, Hirsh Jay, Birman Serge. Targeted gene expression in Drosophila Dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 2003;54(4):618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin Florence, Iché Magali, Birman Serge. Tissue-specific developmental requirements of Drosophila tyrosine hydroxylase isoforms. Genesis. 2003;35(3):175–184. doi: 10.1002/gene.10178. [DOI] [PubMed] [Google Scholar]
- Glickman Michael H., Ciechanover Aaron. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 2002;82(2):373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Grünblatt E., Mandel S., Jacob-Hirsch J., Zeligson S., Amariglo N., Rechavi G., Li J. Gene expression profiling of parkinsonian substantia nigra pars compacta; alterations in Ubiquitin-Proteasome, heat shock protein, Iron and oxidative stress regulated proteins, cell Adhesion/Cellular matrix and vesicle trafficking genes. J. Neural Transm. 2004;111(12):1543–1573. doi: 10.1007/s00702-004-0212-1. [DOI] [PubMed] [Google Scholar]
- Hernández-Vargas René, Fonseca-Ornelas Luis, López-González Ignacio, Riesgo-Escovar Juan, Zurita Mario, Reynaud Enrique. Synphilin suppresses α-synuclein neurotoxicity in a Parkinson’s Disease Drosophila Model. Genesis New York, N.Y. 2000. 2011;49(5):392–402. doi: 10.1002/dvg.20740. [DOI] [PubMed] [Google Scholar]
- Im Eunju, Chul Chung Kwang. Precise assembly and regulation of 26s proteasome and correlation between proteasome dysfunction and Neurodegenerative Diseases. BMB Rep. 2016;49(9):459–473. doi: 10.5483/BMBRep.2016.49.9.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Kumpei, Kawasaki Haruhisa, Suzuki Takahiro, Takahara Tsubasa, Ishida Norio. Effects of Kamikihito and unkei-to on sleep behavior of wild type and parkinson model in Drosophila. Front. Psychiatry. 2017;8:132. doi: 10.3389/fpsyt.2017.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julienne Hannah, Buhl Edgar, Leslie David S., Hodge James J.L. Drosophila PINK1 and parkin loss-of-function mutants display a range of non-motor parkinson’s disease phenotypes. Neurobiol. Dis. 2017;104(August):15–23. doi: 10.1016/j.nbd.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasture Ameya Sanjay, Hummel Thomas, Sucic Sonja, Freissmuth Michael. Big lessons from tiny flies: drosophila melanogaster as a model to explore dysfunction of dopaminergic and serotonergic neurotransmitter systems. Int. J. Mol. Sci. 2018;19(6) doi: 10.3390/ijms19061788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada Tohru, Asakawa Shuichi, Hattori Nobutaka, Matsumine Hiroto, Yamamura Yasuhiro, Minoshima Shinsei, Yokochi Masayuki, Mizuno Yoshikuni, Shimizu Nobuyoshi. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Konsolaki Mary. Fruitful research: drug target discovery for neurodegenerative diseases in Drosophila. Expert Opin. Drug Discov. 2013;8(12):1503–1513. doi: 10.1517/17460441.2013.849691. [DOI] [PubMed] [Google Scholar]
- Krüger Rejko, Kuhn Wilfried, Müller Thomas, Woitalla Dirk, Graeber Manuel, Kösel Sigfried, Przuntek Horst, Epplen J.örg T., Schols Ludger, Riess Olaf. AlaSOPro Mutation in the Gene Encoding α-Synuclein in Parkinson’s Disease. Nat. Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kumar Brajesh, Chan Kim Young, DeMartino George N. The C Terminus of Rpt3, an ATPase Subunit of PA700 (19 S) Regulatory Complex, Is Essential for 26 S Proteasome Assembly but Not for Activation. J. Biol. Chem. 2010;285(50):39523–39535. doi: 10.1074/jbc.M110.153627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang Anthony E., Lozano Andres M. Parkinson’s Disease. First of Two Parts. N. Engl. J. Med. 1998;339(15):1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Lazzari Federica De, Bisaglia Marco, Agostino Zordan Mauro, Sandrelli Federica. Circadian Rhythm Abnormalities in Parkinson’s Disease from Humans to Flies and Back. Int. J. Mol. Sci. 2018;19(12):1–22. doi: 10.3390/ijms19123911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Hye Mi, Koh Seong-Beom. Many Faces of Parkinson’s Disease: Non-Motor Symptoms of Parkinson’s Disease. J. Mov. Disord. 2015;8(2):92–97. doi: 10.14802/jmd.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy E., Boyer R., Auburger G., Leube B., Ulm G., Mezey E., Harta G. The Ubiquitin Pathway in Parkinson’s Disease [6] Nature. 1998;395(6701):451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- Lessing Derek, Bonini Nancy M. Maintaining the brain: insight into human neurodegeneration from Drosophila melanogaster mutants. Nat. Rev. Genet. 2009;10(6):359–370. doi: 10.1038/nrg2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Zhengmei, Davis Ronald L. Eight Different Types of Dopaminergic Neurons Innervate the Drosophila Mushroom Body Neuropil: Anatomical and Physiological Heterogeneity. Front. Neural Circuits. 2009;3(July):1–17. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaught Kevin St.P., Belizaire Roger, Jenner Peter, Olanow C.Warren, Isacson Ole. Selective Loss of 20S Proteasome α-Subunits in the Substantia Nigra Pars Compacta in Parkinson’s Disease. Neurosci. Lett. 2002;326(3):155–158. doi: 10.1016/S0304-3940(02)00296-3. [DOI] [PubMed] [Google Scholar]
- McNaught P., Kevin St., Belizaire Roger, Isacson Ole, Jenner Peter, Olanow C.Warren. Altered proteasomal function in sporadic parkinson’s disease. Exp. Neurol. 2003;179(1):38–46. doi: 10.1006/exnr.2002.8050. [DOI] [PubMed] [Google Scholar]
- Menza Matthew, DeFronzo Dobkin Roseanne, Marin Humberto, Bienfait Karina. Sleep Disturbances in Parkinson’s Disease. Mov. Disord. 2010;25(S1):S117–S122. doi: 10.1002/mds.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molochnikov Leonid, Rabey Jose M., Dobronevsky Evgenya, Bonucelli Ubaldo, Ceravolo Roberto, Frosini Daniela, Grünblatt Edna. A Molecular Signature in Blood Identifies Early Parkinson’s Disease. Mol. Neurodegener. 2012;7(1):26. doi: 10.1186/1750-1326-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore Darren J., West Andrew B., Dawson Valina L., Dawson Ted M. Molecular pathophysiology of Parkinson’S disease. Annu. Rev. Neurosci. 2005;28(1):57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- Nakashima Akira, Kodani Yu, Kaneko Yoko S., Nagasaki Hiroshi, Akira Ota. Proteasome-mediated degradation of tyrosine hydroxylase triggered by its phosphorylation: a new question as to the intracellular location at which the degradation occurs. J. Neural Transm. 2018;125(1):9–15. doi: 10.1007/s00702-016-1653-z. [DOI] [PubMed] [Google Scholar]
- Njomen Evert, Tepe Jetze J. Proteasome Activation as a New Therapeutic Approach to Target Proteotoxic Disorders. Review-article. J. Med. Chem. 2019;62(14):6469–6481. doi: 10.1021/acs.jmedchem.9b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka Takashi, Iwatsubo Takeshi, Hasegawa Masato. Ubiquitination of alpha-synuclein. Biochemistry. 2005;44(1):361–368. doi: 10.1021/bi0485528. [DOI] [PubMed] [Google Scholar]
- Papaevgeniou Nikoletta, Chondrogianni Niki. UPS Activation in the Battle Against Aging and Aggregation-Related Diseases: An Extended Review. Ageing Res. Rev. 2016;23:1–70. doi: 10.1007/978-1-4939-3756-1_1. [DOI] [PubMed] [Google Scholar]
- Pfeiffenberger Cory, Lear Bridget C., Keegan Kevin P., Allada Ravi. Locomotor activity level monitoring using the Drosophila activity monitoring (DAM) system. Cold Spring Harb. Protoc. 2010;5(11) doi: 10.1101/pdb.prot5518. pdb.prot5518. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos M.H., Lavedan C., Leroy E., Ide S.E., Dehejia A., Dutra A., Pike B. Mutation in the Alpha-Synuclein Gene Identified in Families with Parkinson’s Disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/SCIENCE.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Rincon-Limas E., Diego Kurt Jensen, Fernandez-Funez Pedro. Drosophila models of proteinopathies: the little fly that could. Curr. Pharm. Des. 2012;18(8):1108–1122. doi: 10.2174/138161212799315894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinetti G.V., Schweizer F.E. Ubiquitination acutely regulates presynaptic neurotransmitter release in mammalian neurons. J. Neurosci. 2010;30(9):3157–3166. doi: 10.1523/JNEUROSCI.3712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott Ruth, Szargel Raymonde, Shani Vered, Bisharat Sleman, Engelender Simone. α-Synuclein Ubiquitination and Novel Therapeutic Targets for Parkinson’s Disease. Cns Neurol. Disord. - Drug Targets. 2014;13(4):630–637. doi: 10.2174/18715273113126660195. [DOI] [PubMed] [Google Scholar]
- Rousseau Adrien, Bertolotti Anne. Regulation of proteasome assembly and activity in health and disease. Nat. Rev. Mol. Cell Biol. 2018 doi: 10.1038/s41580-018-0040-z. [DOI] [PubMed] [Google Scholar]
- Scott Madeline R., Meador-Woodruf James H. Intracellular Compartment-Specific Proteasome Dysfunction in Postmortem Cortex in Schizophrenia Subjects. olecular Psychiatry. 2019;43(January) doi: 10.1038/s41380-019-0359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman Divya, Aso Yoshinori, Rubin Gerald M., Nitabach Michael N. Control of Sleep by Dopaminergic Inputs to the Drosophila Mushroom Body. Front. Neural Circuits. 2015;9:73. doi: 10.3389/fncir.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith David M., Chang Shih Chung, Park Soyeon, Finley Daniel, Cheng Yifan, Goldberg Alfred L. Docking of the Proteasomal ATPases’ Carboxyl Termini in the 20S Proteasome’s α Ring Opens the Gate for Substrate Entry. Mol. Cell. 2007;27(5):731–744. doi: 10.1016/j.molcel.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin A.V., Kim E.R., Ovchinnikov L.P. Proteasome system of protein degradation and processing. Biochem. Mosc. Suppl. Ser. A Membr. Cell Biol. (Mosc) 2010;74(13):1411–1442. doi: 10.1134/S000629790913001X. [DOI] [PubMed] [Google Scholar]
- Srivastav Saurabh, Singh Sandeep Kumar, Yadav Amarish Kumar, Srikrishna Saripella. Folic acid supplementation rescues anomalies associated with knockdown of parkin in dopaminergic and serotonergic neurons in Drosophila model of parkinson’s disease. Biochem. Biophys. Res. Commun. 2015;460(3):780–785. doi: 10.1016/j.bbrc.2015.03.106. [DOI] [PubMed] [Google Scholar]
- Subramaniam Mahalakshmi, Kern Beatrice, Vogel Simone, Klose Verena, Schneider Gaby, Roeper Jochen. Selective increase of in vivo firing frequencies in DA SN neurons after proteasome inhibition in the Ventral Midbrain. Eur. J. Neurosci. 2014;40(6):2898–2909. doi: 10.1111/ejn.12660. [DOI] [PubMed] [Google Scholar]
- Sun Xicui, Ran Dongzhi, Zhao Xiaofeng, Huang Yi, Long Simei, Liang Fengyin, Guo Wenyuan. Melatonin attenuates HLRRK2-Induced sleep disturbances and synaptic dysfunction in a Drosophila model of parkinson’s disease. Mol. Med. Rep. 2016;13(5):3936–3944. doi: 10.3892/mmr.2016.4991. [DOI] [PubMed] [Google Scholar]
- Szlanka Tamás, Haracska Lajos, Kiss István, Deák P.éter, Kurucz Eva, Andó István, Virágh Erika, Udvardy Andor. Deletion of Proteasomal Subunit S5a/Rpn10/P54 Causes Lethality, Multiple Mitotic Defects and Overexpression of Proteasomal Genes in Drosophila Melanogaster. J. Cell. Sci. 2003;116(Pt 6):1023–1033. doi: 10.1242/jcs.00332. [DOI] [PubMed] [Google Scholar]
- Tofaris George K., Razzaq Azam, Ghetti Bernardino, Lilley Kathryn S., Grazia Spillantini Maria. Ubiquitination of Alpha-Synuclein in lewy bodies is a pathological event not associated with impairment of proteasome function. J. Biol. Chem. 2003;278(45):44405–44411. doi: 10.1074/jbc.M308041200. [DOI] [PubMed] [Google Scholar]
- Tonoki Ayako, Kuranaga Erina, Tomioka Takeyasu, Hamazaki Jun, Murata Shigeo, Tanaka Keiji, Miura Masayuki. Genetic Evidence Linking Age-Dependent Attenuation of the 26S Proteasome with the Aging Process. Mol. Cell. Biol. 2009;29(4):1095–1106. doi: 10.1128/MCB.01227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiri Eleni N., Sykiotis Gerasimos P., Papassideri Issidora S., Terpos Evangelos, Dimopoulos Meletios A., Gorgoulis Vassilis G., Bohmann Dirk, Trougakos Ioannis P. Proteasome dysfunction in Drosophila signals to an Nrf2-Dependent regulatory circuit aiming to restore proteostasis and prevent premature aging. Aging Cell. 2013;12(5):802–813. doi: 10.1111/acel.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadas Jorge S., Esposito Giovanni, Vandekerkhove Dirk, Miskiewicz Katarzyna, Deaulmerie Liesbeth, Raitano Susanna, Seibler Philip, Klein Christine, Verstreken Patrik. ER Lipid Defects in Neuropeptidergic Neurons Impair Sleep Patterns in Parkinson’s Disease. Neuron. 2018;98(6):1155–1169. doi: 10.1016/j.neuron.2018.05.022. e6. [DOI] [PubMed] [Google Scholar]
- Varga Scott J., Qi Cheng, Podolsky Eric, Daewoo Lee. A New Drosophila Model to Study the Interaction between Genetic and Environmental Factors in Parkinson’s Disease. Brain Res. 2014;1583:277–286. doi: 10.1016/j.brainres.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Wahl Claudia, Kautzmann Sabine, Krebiehl Guido, Strauss Karsten, Woitalla Dirk, Müller Thomas, Bauer Peter, Riess Olaf, Krüger Rejko. A Comprehensive Genetic Study of the Proteasomal Subunit S6 ATPase in German Parkinson’s Disease Patients. J. Neural Transm. Vienna (Vienna) 2008;115(8):1141–1148. doi: 10.1007/s00702-008-0054-3. [DOI] [PubMed] [Google Scholar]
- Wang Zhe, Ferdousy Faiza, Lawal Hakeem, Huang Zhinong, Gavin Daigle J., Izevbaye Iyare, Doherty Olugbenga, Thomas Jerrad, Stathakis Dean G., O’Donnell Janis M. Catecholamines up integrates dopamine synthesis and synaptic trafficking. J. Neurochem. 2011;119(6):1294–1305. doi: 10.1111/j.1471-4159.2011.07517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White Katherine E., Humphrey Dickon M., Hirth Frank. The Dopaminergic System in the Aging Brain of Drosophila. Front. Neurosci. 2010;4(December):1–12. doi: 10.3389/fnins.2010.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Shengzhou, Hyrc Krzysztof L., Moulder Krista L., Lin Ying, Warmke Timothy, Joy Snider B. Cellular calcium deficiency plays a role in neuronal death caused by proteasome inhibitors. J. Neurochem. 2009;109(5):1225–1236. doi: 10.1111/j.1471-4159.2009.06037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Chun-Hung, Jansen Marlon, Schmidt-Glenewinkel Thomas. Role of the Proteasome in Fly Models of Neurodegeneration. Methods Mol. Biol. 2011;793(January):149–165. doi: 10.1007/978-1-61779-328-8_10. [DOI] [PubMed] [Google Scholar]
- Zhou Zhi Dong, Lim Tit Meng. Dopamine (DA) induced irreversible proteasome inhibition via DA derived quinones. Free Radic. Res. 2009;43(4):417–430. doi: 10.1080/10715760902801533. [DOI] [PubMed] [Google Scholar]
- Zhou Zhi Dong, Xie Shao Ping, Sathiyamoorthy Sushmitha, Saw Wuan Ting, Sing Tan Ye, Ng Shin Hui, Chua Heidi Pek Hup. F-Box Protein 7 Mutations Promote Protein Aggregation in Mitochondria and Inhibit Mitophagy. Hum. Mol. Genet. 2015;24(22):6314–6330. doi: 10.1093/hmg/ddv340. [DOI] [PubMed] [Google Scholar]
- Ziemssen Tjalf, Reichmann Heinz. Non-Motor Dysfunction in Parkinson’s Disease. Parkinsonism Relat. Disord. 2007;13(6):323–332. doi: 10.1016/j.parkreldis.2006.12.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.