Abstract
Purpose
To assess clinical characteristics and device satisfaction of patients with chronic obstructive pulmonary disease (COPD) treated with glycopyrrolate/eFlow® Closed System (CS) nebulizer (further referred to as eFlow) under real-world conditions.
Participants and Methods
Patients with COPD currently using eFlow were identified by the study sponsor. Consenting patients who met study inclusion criteria completed a cross-sectional survey that included a device satisfaction questionnaire. Means, medians, and standard deviations were calculated.
Results
Sixty-six patients met inclusion criteria and completed the survey. Participants’ mean ± standard deviation age was 64.9 ± 11.9 years and the majority were white (86.4%) and female (59.1%). Almost two-thirds were former smokers. Thirty-nine (59.1%) reported their COPD to be severe/very severe and 38 (57.6%) reported a COPD exacerbation resulting in a hospitalization, ER visit, or medication modification over the past 12 months. Among 55 participants who had previously used another type of nebulizer, 44 (80%) were overall “much more”/“somewhat more” satisfied with the eFlow compared with their previous nebulizer(s). Regardless of prior nebulizer use, 60 (90.9%) participants were “satisfied”/“very satisfied” overall with the eFlow. Assembly and disassembly, operation, and cleaning were perceived as being “easy”/“very easy” by at least 65% of participants. Among all participants, 57 (86.4%) were “confident”/“very confident” of glycopyrrolate administration. On a Likert scale of 1 (“I don’t like it”) to 7 (“I like it a lot”), mean scores were at least 5.9 for portability, ease of cleaning, size, weight, short administration time, and relative silence of the device. Over 80% of participants said they “probably”/"definitely" would continue to use eFlow.
Conclusion
Based on this real-world study, the majority of patients were highly satisfied with, and confident in, using eFlow.
Keywords: device satisfaction, cross-sectional, survey, nebulizer, COPD
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive, debilitating disease characterized by persistent respiratory symptoms and airflow limitation.1 The main symptoms of COPD are breathlessness, chronic cough, and excessive sputum production.1
COPD is a leading cause of morbidity and mortality in the United States and is responsible for a substantial healthcare cost. In the United States, COPD is estimated to affect over 27 million people2 and is the fourth leading cause of death.3 Direct medical costs of COPD are projected to be $49 billion in 2020 with hospitalizations accounting for up to 70% of the cost.4
Inhaled bronchodilators are the cornerstone of pharmacologic therapy in COPD. Lonhala® Magnair® (glycopyrrolate/eFlow® Closed System [CS] nebulizer, further referred to as eFlow; Sunovion Pharmaceuticals Inc., Marlborough, MA, USA) Inhalation Solution 25 µg/mL glycopyrrolate is a twice-daily, long-acting muscarinic antagonist (LAMA), indicated for the long-term maintenance treatment of airflow obstruction in COPD.5–7 The Magnair device was developed by PARI Pharma GmbH (Starnberg, Germany) and is based on eFlow CS technology, consisting of a vibrating, perforated membrane designed to administer a nebulized fine mist of consistent particle size.8 The unit is small, light in weight, and portable. In operation, the device is virtually silent, and can deliver nebulized glycopyrrolate in 2–3 minutes, with proper assembly and cleaning.6,7
The safety and efficacy of glycopyrrolate/eFlow CS have been previously reported.9,10 In addition, patient satisfaction and confidence with eFlow have been reported in the GOLDEN-5 Phase 3 randomized, active-controlled, open-label study.11 The purpose of the present study was to assess the clinical characteristics and device satisfaction of patients with COPD using glycopyrrolate/eFlow CS under real-world conditions, and compare findings to those from the GOLDEN-5 clinical trial.11
Participants and Methods
Study Design and Participants
This study was a cross-sectional survey of patients with COPD who were currently using glycopyrrolate/eFlow. Patients were invited to participate in the study if they had: 1) participated in an early experience program, which provided a limited number of patients and healthcare providers the opportunity to use glycopyrrolate/eFlow before it became commercially available; 2) registered on the sponsor’s website (www.lonhalamagnair.com), or 3) participated in a copayment card assistance program. Participants were included in the study if they reported a COPD diagnosis from a physician/healthcare professional and were currently using glycopyrrolate/eFlow.
Survey
The survey was developed to be administered by telephone or online and required approximately 30 minutes to complete. In addition to demographic and clinical questions, the survey included questions related to COPD symptomatology and severity, eFlow characteristics, and a 19-item device satisfaction questionnaire, the details of which are provided in Table 4. The questionnaire in the current study was identical to the one used in the GOLDEN-5 Phase 3 study, with the exception of one question that was eliminated due to lack of relevance in the real-world setting.
Table 4.
Device Satisfaction Questionnaire Responses for Participants Using the Glycopyrrolate/eFlow CS Device
| Questions | Response | Participants n (%) |
|
|---|---|---|---|
| Previous device use | Q1. Before you started using Lonhala Magnair, what device(s) did you use to treat your COPD? | Metered-dose inhaler | 48 (72.7) |
| Dry powder inhaler | 38 (57.6) | ||
| Soft-mist inhaler | 34 (51.5) | ||
| Nebulizer | 55 (83.3) | ||
| Q2. Please indicate how the metered dose inhaler, dry powder inhaler, and/or soft-mist inhaler were used to treat your COPD? Were they used as … | n=60 | ||
| Rescue medication | 5 (8.3) | ||
| Maintenance therapy | 11 (18.3) | ||
| Both | 44 (73.3) | ||
| Q3. Please indicate how the nebulizer was used to treat your COPD? Was it used as … | n=55 | ||
| Rescue medication | 3 (5.5) | ||
| Maintenance therapy | 21 (38.2) | ||
| Both | 31 (56.4) | ||
| Comparison with previously used nebulizers | Q4. Compared with your previous nebulizer, how easy or difficult is it to assemble and disassemble the Lonhala Magnair nebulizer? Is it … | n=55 | |
| Much more difficult | 2 (3.6) | ||
| Somewhat more difficult | 4 (7.3) | ||
| About the same | 19 (34.5) | ||
| Somewhat easier | 9 (16.4) | ||
| Much easier | 21 (38.2) | ||
| Q5. Compared with your previous nebulizer, how easy or difficult is it to operate the Lonhala Magnair nebulizer? Is it … | n=55 | ||
| Much more difficult | 0 (0) | ||
| Somewhat more difficult | 3 (5.5) | ||
| About the same | 19 (34.5) | ||
| Somewhat easier | 13 (23.6) | ||
| Much easier | 20 (36.4) | ||
| Q6. Compared with your previous nebulizer, how easy or difficult is it to clean and disinfect the Lonhala Magnair nebulizer? Is it … | n=55 | ||
| Much more difficult | 0 (0) | ||
| Somewhat more difficult | 7 (12.7) | ||
| About the same | 21 (38.2) | ||
| Somewhat easier | 10 (18.2) | ||
| Much easier | 17 (30.9) | ||
| Q7. Compared with your previous nebulizer, how confident are you the COPD medicine is being delivered into your lungs using the Lonhala Magnair nebulizer? Are you … | n=55 | ||
| Not confident | 0 (0) | ||
| Somewhat less confident | 2 (3.6) | ||
| About the same | 13 (23.6) | ||
| Somewhat more confident | 15 (27.3) | ||
| Much more confident | 25 (45.5) | ||
| Q8. Compared with your previous nebulizer, how would you rate your overall satisfaction with using the Lonhala Magnair nebulizer? Are you … | n=55 | ||
| Not satisfied | 0 (0) | ||
| Somewhat less satisfied | 2 (3.6) | ||
| About the same | 9 (16.4) | ||
| Somewhat more satisfied | 16 (29.1) | ||
| Much more satisfied | 28 (50.9) | ||
| Ease of use | Q9. How easy or difficult is it to assemble and disassemble the Lonhala Magnair nebulizer? Is it … | n=66 | |
| Very difficult | 0 (0) | ||
| Somewhat difficult | 4 (6.1) | ||
| Acceptable | 15 (22.7) | ||
| Easy | 14 (21.2) | ||
| Very easy | 33 (50.0) | ||
| Q10. How easy or difficult is it to operate the Lonhala Magnair nebulizer? Is it … | n=66 | ||
| Very difficult | 0 (0) | ||
| Somewhat difficult | 1 (1.5) | ||
| Acceptable | 10 (15.2) | ||
| Easy | 20 (30.3) | ||
| Very easy | 35 (53.0) | ||
| Q11. How easy or difficult is it to clean the Lonhala Magnair nebulizer? Is it … | n=66 | ||
| Very difficult | 0 (0) | ||
| Somewhat difficult | 5 (7.6) | ||
| Acceptable | 18 (27.3) | ||
| Easy | 17 (25.8) | ||
| Very easy | 26 (39.4) | ||
| Confidence of drug delivery | Q12. When using the Lonhala Magnair nebulizer, how confident are you the COPD medicine is being delivered into your lungs? Are you … | n=66 | |
| Not confident | 0 (0) | ||
| Somewhat confident | 9 (13.6) | ||
| Confident | 28 (42.4) | ||
| Very confident | 29 (43.9) | ||
| Overall satisfaction | Q13. How would you rate your overall satisfaction with using the Lonhala Magnair nebulizer? Are you … | n=66 | |
| Not satisfied | 2 (3.0) | ||
| Somewhat satisfied | 4 (6.1) | ||
| Satisfied | 25 (37.9) | ||
| Very satisfied | 35 (53.0) | ||
| Design and instruction for use | Q14. Do you think the Lonhala Magnair device design supports intuitive use (easy to understand and use)? Would you say … | n=66 | |
| Very much | 40 (60.6) | ||
| Neutral | 24 (36.4) | ||
| Not Much | 2 (3.0) | ||
| Q15. How would you rate the weight of the Lonhala Magnair device? Would you say it is … | n=66 | ||
| Light | 62 (93.9) | ||
| Medium weight | 4 (6.1) | ||
| Heavy | 0 (0) | ||
| Q16. How would you rate the size of the Lonhala Magnair device? Would you say it is … | n=66 | ||
| Too small | 0 (0) | ||
| Ideal size | 63 (95.5) | ||
| Too large | 3 (4.5) | ||
| Q17. How helpful are the Instructions for Use? Would you say they are … | n=66 | ||
| Very helpful | 35 (53.0) | ||
| Helpful | 28 (42.4) | ||
| Not helpful (too complex) | 2 (3.0) | ||
| Do not know/not sure | 1 (1.5) | ||
| Continued use | Q18. How likely are you to continue using the Lonhala Magnair nebulizer? Would you say … | n=66 | |
| Definitely not | 0 (0) | ||
| Probably not | 2 (3.0) | ||
| Maybe | 10 (15.2) | ||
| Probably will | 20 (30.3) | ||
| Definitely will | 34 (51.5) | ||
| Confidence in everyday use | Q19 How confident do you feel using the Lonhala Magnair nebulizer every day? Would you say … | n=66 | |
| Not confident | 1 (1.5) | ||
| Somewhat confident | 6 (9.1) | ||
| Confident | 28 (42.4) | ||
| Very confident | 31 (47.0) | ||
Survey Recruitment
Patients were invited to participate in the survey through a pre-notification letter, email, or telephone outreach depending on the patient’s available contact information. The pre-notification letter and email contained similar information and included both a telephone number for completing the survey by phone and a hyperlink for completing the survey via the internet. If no response was received after approximately 10 days, patients were called if a phone number was available. Patients who responded and subsequently agreed to participate were required to provide verbal or electronic informed consent prior to starting the survey. The targeted number of completed surveys was at least 50. Participants who completed the survey were compensated for their time. The survey recruitment and fielding began in February 2019 and ended in April 2019.
As protected health information (PHI) was required in the conduct of this study, a Health Insurance Portability and Accountability Act of 1996 (HIPAA) Waiver of Authorization was applied for and obtained from the New England Institutional Review Board (NEIRB) prior to any PHI being identified. NEIRB reviewed and approved all study-related procedures and materials (study protocol, pre-notification letter/email, telephone recruiting script, and patient survey), deeming the study to meet ethical standards for human subjects research. Patient-level data were handled in compliance with HIPAA regulations. Any information that could uniquely identify individual patients was removed from the survey data prior to analysis and reporting.
Survey Measures
Demographic and Clinical Characteristics
Patient-reported clinical and demographics information was collected from all participants. Clinical information included age at COPD diagnosis, patient-reported overall health and COPD severity, number of COPD exacerbations in the past 12 months that resulted in an inpatient hospitalization, emergency room (ER) visit, or modification of usual medications, current smoking status, body mass index (BMI) calculated from self-reported height and weight, and comorbidities. Demographic information included sex, current age, race/ethnicity, US region of residence, marital status, education, employment status, household income, and insurance type.
Device Characteristics and Satisfaction
The survey included six questions related to eFlow characteristics. Participants rated eFlow’s portability, perceived ease of cleaning, size, weight, medication administration time, and relative silence during operation on a 7-point Likert scale of 1 (“I don’t like it”) to 7 (“I like it a lot”). In addition, they completed a 19-item device satisfaction questionnaire, which was modified from the 20-item device satisfaction questionnaire used in the GOLDEN-5 clinical trial to be relevant for real-world participants.11 This questionnaire assessed participants’ overall experience with eFlow, and for those with previous nebulizer experience, compared the use and satisfaction of eFlow with their previous nebulizer. Questions also covered the perceived ease of use of eFlow in terms of assembly, operation, and cleaning, whether participants were satisfied that the medication was being administered efficiently, and how satisfied they were overall. Aspects of the device design and instructions for use were also assessed, along with the likelihood of participants continuing to use the device, and their confidence in its everyday use.
Patient-Reported Outcomes
The survey also included two patient-reported outcomes questionnaires: the COPD Assessment Test™ (CAT)12,13 and the modified Medical Research Council (mMRC) dyspnea scale.14 The CAT is a validated, 8-item questionnaire, which quantifies the impact of COPD on a patient’s wellbeing and daily life. Each of the 8-items is scored on a scale that ranges from 0 to 5, where 0 = “Not affected” to 5 = “Very much affected”. The individual item scores are summed to give a total score that ranges from 0 to 40. Total scores may be categorized into four categories: <10, “low COPD impact”; 10–20, “medium COPD impact”; >20–<30, “high COPD impact”; and ≥30, “very high COPD impact”. The mMRC dyspnea scale consists of a single item with five responses describing the degree or grade of breathlessness associated with various physical activities, such as walking or dressing. It is used as a proxy measure of COPD severity, with higher scores indicating more-severe dyspnea. The responses range from 0 = “I only get breathless with strenuous exercise” (no dyspnea) to 4 = “I am too breathless to leave the house or I am breathless when dressing” (severe dyspnea). A response of 0 or 1 is considered a low degree of dyspnea or breathlessness and a response of 2 or higher is considered a high degree of dyspnea or breathlessness.14
Statistical Analyses
A descriptive analysis of the survey data was conducted. Categorical variables were presented as frequencies and percentages, and continuous measures as mean ± standard deviation (SD) and medians. Participants’ responses to the device satisfaction questionnaire were compared with the responses of participants in the GOLDEN-5 clinical trial. Subgroup analyses were also conducted for the current study. Differences in level of satisfaction were assessed using Fisher’s exact test between subgroups by age (<65, 65–74, ≥75), number of co-morbid conditions (ie, psychiatric disease, musculoskeletal disease, and cardiovascular or heart disease) that could favor nebulized medication use (0–1, ≥2), CAT score (<20, ≥20), and mMRC (0–1, ≥2). Analyses were performed using IBM SPSS Statistics version 23.
Results
Sample Identification and Patient Disposition
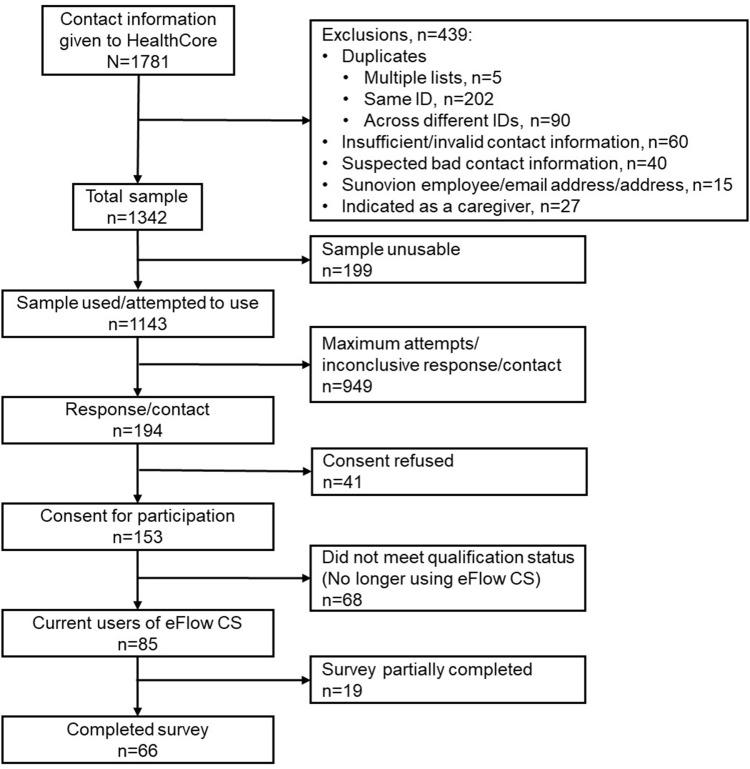
Contact information for 1781 potential participants was identified and transferred from the sponsor’s database to HealthCore via secure file transfer protocol (sFTP). Of these potential participants, 439 were excluded due to duplicate information, inadequate contact information, employment with the sponsor, or contact details of caregivers rather than patients, leaving a sample list of 1342 potential participants. The contact information of a further 199 potential participants was found to be unusable resulting in a sample list of 1143 names.
A total of 194 patients responded to the recruitment letter or email, or were contacted and recruited by telephone. Of these participants, 78.9% gave electronic or verbal consent to participate. Among the 153 patients who gave consent to participate, 66 completed the survey and 68 were excluded due to not meeting all study inclusion criteria; the remaining 19 started but did not complete the survey. The primary reason for study exclusion was that the patient was not currently using glycopyrrolate/eFlow CS. Full details of the survey sample disposition are shown in Figure 1.
Figure 1.
Survey sample disposition.
The survey response rate (ie, participants that responded/participants with attempted contact) was 17.0% (194/1143), the cooperation rate (ie, completed surveys/[responded – excluded]) was 52.4% (66/[194–68]), and the list completion rate (ie, completed surveys/sample used) was 5.8% (66/1143). The median survey time was 23 minutes; 16 (24.2%) surveys were conducted by telephone and 50 (75.8%) via the internet.
Demographic and Clinical Characteristics
Participants’ demographics and clinical characteristics are shown in Tables 1 and 2, respectively. Participants were geographically distributed across the United States. In brief, the mean ± SD age of the participants was 64.9 ± 11.9 years, 39 (59.1%) were female, and 57 (86.4%) were white, non-Hispanic. Seven of the 66 participants received help completing the survey (data not shown). Participants mean ± SD BMI was 29.2 ± 7.4 kg/m2, with 41 (62.1%) being either overweight or obese (3 participants had missing data for this question). In total, 56 (84.8%) participants were either current or former smokers. At some time, 42 (63.6%) participants had been told they had hypertension, 36 (54.5%) asthma, and 31 (47.0%) a musculoskeletal disorder. At the time of the survey, participants had been using glycopyrrolate/eFlow for a mean ± SD time of 5.7 ± 4.82 months (median, 4 months).
Table 1.
Participants’ Demographic Characteristics at the Time of the Survey
| Parameters | Participants (N=66) |
|---|---|
| Age, years, mean ± SD (median) | 64.9 ± 11.91 (64.0) |
| Age Categories, years, n (%) | |
| ≤54 | 12 (18.2) |
| 55–64 | 22 (33.3) |
| 65–74 | 18 (27.3) |
| ≥75 | 14 (21.2) |
| Female, n (%) | 39 (59.1) |
| Race/ethnicity, n (%) | |
| White, non-Hispanic | 57 (86.4) |
| Other | 8 (12.1) |
| Missing | 1 (1.5) |
| Marital status, n (%) | |
| Single | 9 (13.6) |
| Married | 40 (60.6) |
| Separated/divorced/widowed | 16 (24.2) |
| Other | 1 (1.5) |
| Insurance type, n (%)a | |
| Private insuranceb | 48 (72.7) |
| Traditional Medicare | 31 (47.0) |
| Supplemental coverage or Medigap policy | 12 (18.2) |
| Medicare Advantage | 13 (19.7) |
| Medicaid | 3 (4.5) |
| VA benefits, TRICARE, or other coverage due to military | 3 (4.5) |
| Some other type of coverage | 3 (4.5) |
| No medical insurance | 2 (3.0) |
| Missing | 1 (1.5) |
| Number of medical insurance types, n (%) | |
| None | 2 (3.0) |
| 1 type of insurance | 30 (45.5) |
| ≥2 types of insurance | 33 (50.0) |
| Missing | 1 (1.5) |
| Education, n (%) | |
| High school or less | 22 (33.3) |
| Some college, no degree | 21 (31.8) |
| Associate’s degree | 12 (18.2) |
| Bachelor’s degree, graduate degree or higher | 11 (16.7) |
| Employment status, n (%) | |
| Working full- or part-time | 18 (27.3) |
| Homemaker/disabled/retired | 48 (72.7) |
Notes: aTypes of insurance categories are not mutually exclusive. bPrivate insurance from employer, union, or purchased on own.
Abbreviations: SD, standard deviation; VA, Veterans Affairs.
Table 2.
Participants’ Clinical Characteristics
| Parameters | Participants (N=66) |
|---|---|
| Smoking status, n (%) | |
| Current smoker | 9 (13.6) |
| Former smoker | 47 (71.2) |
| Never smoked | 10 (15.2) |
| Smokes e-cigarettes, n (%) | 2 (3.0) |
| Body mass index, kg/m2, mean ± SD (median) | 29.2 ± 7.38 (28.7) |
| Physical activity compared to people of similar age, n (%) | |
| A lot less active | 24 (36.4) |
| Less active | 25 (37.9) |
| About as active | 14 (21.2) |
| More active | 3 (4.5) |
| Ever told of following conditions, n (%) | |
| Hypertension | 42 (63.6) |
| Asthma | 36 (54.5) |
| Musculoskeletal disordersa | 31 (47.0) |
| Hypercholesterolemia | 26 (39.4) |
| Psychiatric disordersb | 21 (31.8) |
| Diabetes and/or diabetes complications | 18 (27.3) |
| Heart diseasec | 14 (21.2) |
| Cardiovascular disease | 13 (19.7) |
| Peripheral vascular disease | 10 (15.2) |
Notes: aRheumatoid arthritis, osteoarthritis, muscle wasting/weakness, chronic fatigue, physical weakness, or chest muscle weakness. bDepression, anxiety, schizophrenia, or bipolar disorder. cTransient ischemic attack, atrial fibrillation, or heart failure.
Abbreviation: SD, standard deviation.
Participants’ overall health status, and COPD history and severity are shown in Table 3. A total of 47 (71.2%) participants reported their overall health to be fair or poor and 39 (59.1%) reported their COPD to be severe or very severe. Thirty-eight (57.6%) participants reported one or more COPD exacerbations resulting in a hospitalization, ER visit, or medication modification during the previous 12 months.
Table 3.
Participants’ COPD Symptoms and COPD History
| Parameters | Participants (N=66) |
|---|---|
| Age at first COPD diagnosis, n (%) | |
| <45 years | 7 (10.6) |
| 45–54 years | 20 (30.3) |
| 55–64 years | 14 (21.2) |
| 65–74 years | 10 (15.2) |
| ≥75 years | 4 (6.1) |
| Unknown | 11 (16.7) |
| Mean ± SD (median), years | 55.9 ± 14.27 (55.0) |
| Current overall health status, n (%) | |
| Excellent/Very good | 1 (1.5) |
| Good | 18 (27.3) |
| Fair | 35 (53.0) |
| Poor | 12 (18.2) |
| COPD severity, n (%) | |
| Mild | 3 (4.5) |
| Moderate | 24 (36.4) |
| Severe | 31 (47.0) |
| Very severe | 8 (12.1) |
| Participants with ≥1 COPD exacerbation leading to a hospitalization, ER visit, or medication modifications in past 12 months, n (%) | 38 (57.6) |
| Number of COPD exacerbations/participant, n (%) | |
| 0 | 28 (42.4) |
| 1 | 10 (15.2) |
| 2 | 12 (18.2) |
| ≥3 | 14 (21.2) |
| Unknown | 2 (3.0) |
Abbreviations: ER, emergency room; SD, standard deviation.
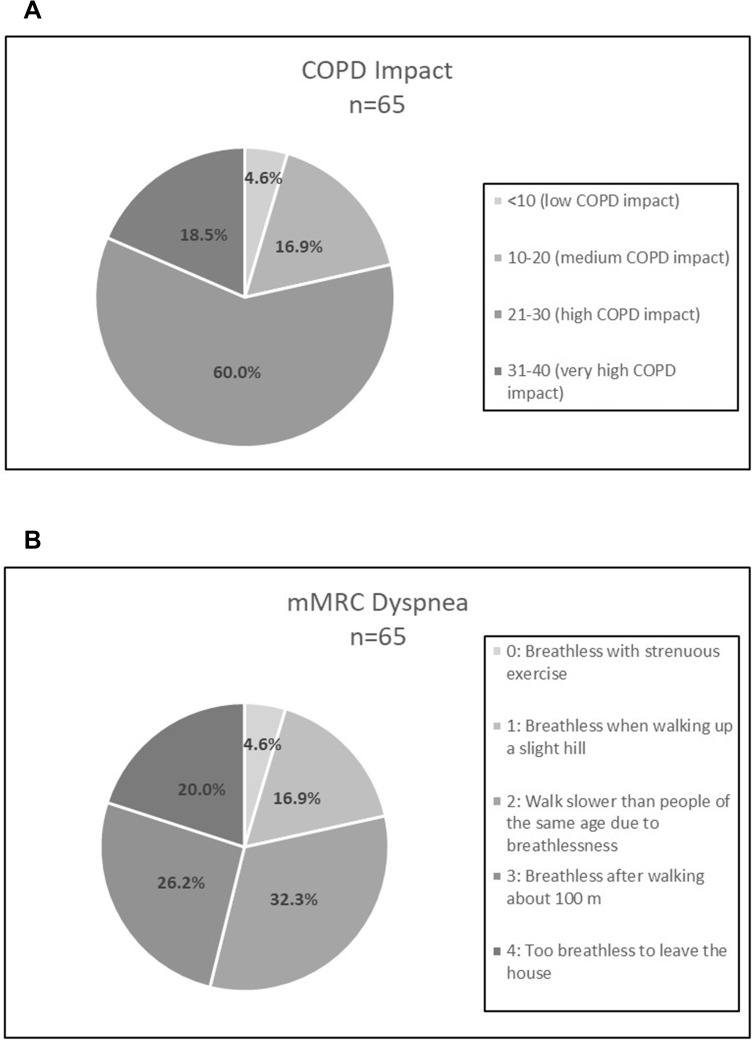
Results for the CAT and mMRC dyspnea questionnaires are shown in Figure 2. Over 90% of participants scored ≥10 on CAT with almost 20% scoring 31–40. For mMRC dyspnea, 78.5% of participants scored ≥2 with 20% reporting they were too breathless to leave the house.
Figure 2.
COPD symptomatology scores from the (A) CAT and (B) mMRC Dyspnea Scale.
Abbreviations: CAT, COPD Assessment Test; mMRC, modified Medical Research Council.
Device Characteristics
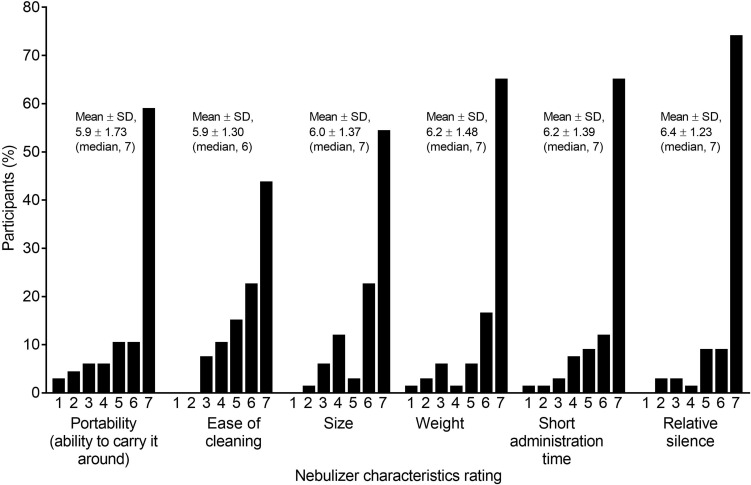
Participants’ ratings for the eFlow characteristics are shown in Figure 3. Mean ratings for each of the characteristics ranged from 5.9 for both portability and ease of cleaning to 6.4 for the relative silence of operation. The median favorability response of participants was 7 out of 7 for five questions, and 6 out of 7 for one question (ease of cleaning) for these e-Flow characteristics.
Figure 3.
eFlow CS characteristics ratings.
Note: Scale: ‘1’ =“I don’t like it” and ‘7’ = “I like it a lot”.
Abbreviation: SD, standard deviation.
Device Satisfaction
Responses for the device satisfaction questionnaire are shown in Table 4. Prior to starting eFlow, 60 (90.9%) participants had used one or more type of inhaler and 55 (83.3%) had previously used a nebulizer.
Participants with Prior Nebulizer Experience
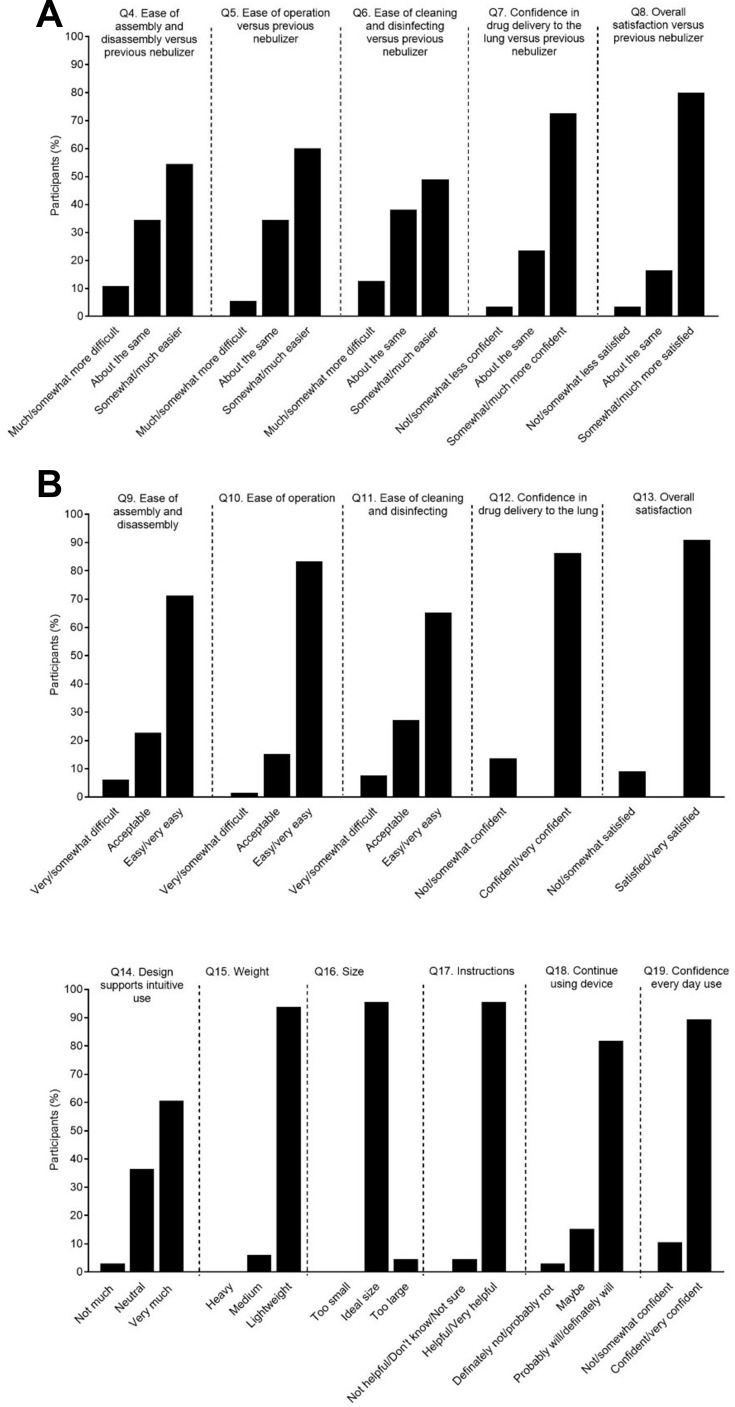
Among participants who had previously used a nebulizer, 44 (80%) were “much more” or “somewhat more” satisfied with eFlow compared with their previous nebulizer(s) (Figure 4A). Assembly and disassembly, operation, and cleaning and disinfection of eFlow were rated as “much more” or “somewhat more” easier compared with their previous nebulizer(s) by 30 (54.5%), 33 (60%), and 27 (49.1%) participants, respectively (Figure 4A). Among prior nebulizer users, 40 (72.7%) were “somewhat” or “much more” confident in glycopyrrolate administration with eFlow compared with their prior nebulizer(s) (Figure 4A)
Figure 4.
Participants’ rating of the eFlow CS device (A) compared with previously used nebulizer(s) (B) regardless of previous nebulizer use.
All Participants (Regardless of Prior Nebulizer Use)
In the overall population, 60 (90.9%) participants were “satisfied” or “very satisfied” overall with eFlow, and assembly and disassembly, operation, and cleaning were perceived as “easy” or “very easy” by 47 (71.2%), 55 (83.3%), and 43 (65.2%) participants, respectively (Figure 4B). Overall, 57 (86.4%) participants were “confident” or “very confident” of glycopyrrolate administration (Figure 4B). A total of 40 (60.6%) participants considered “very much” that eFlow had an intuitive design, 62 (93.9%) that it was “light” in weight, 63 (95.5%) that it was an “ideal size”, and 63 (95.5%) that the instructions were “helpful” or “very helpful” (Figure 4B). When participants were asked how likely they would be to continue using eFlow, 54 (81.8%) said they “probably” or “definitely” will (20 [30.3%] and 34 [51.1%], respectively; Figure 4B).
In sub-group analyses, a higher proportion of patients (25 [49.0%]) with more symptom burden (mMRC ≥2) were "very confident" that the COPD medicine was administered using eFlow, compared to those with mMRC <2 (3 [21.4%]); the difference was not statistically significant. The majority of patients were "satisfied" (25 [37.9%]) or "very satisfied" (35 [53.0%]) with glycopyrrolate/eFlow, regardless of the number of comorbidities. More patients (22 [100%]) with a higher number of comorbid conditions (≥2 conditions) were "satisfied" or "very satisfied" with glycopyrrolate/eFlow as compared to patients (38 [86.4%]) with fewer comorbid conditions (0 or 1 condition).
Comparison with GOLDEN-5 Device Satisfaction Questionnaire
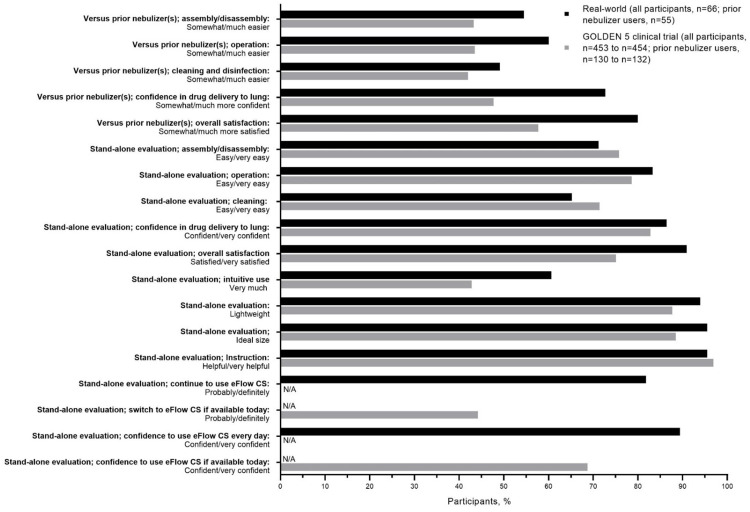
The baseline (mean ± SD) age of the patients was similar for the two studies (real-world study, 64.9 ± 11.9 years vs GOLDEN-5,11 63.3 ± 8.5 years). In the GOLDEN-5 study, 38.3% of patients had prior nebulizer use compared with over 80% in the current study. Comparisons of positive responses from participants using eFlow who completed the device satisfaction questionnaire in the current real-world study and the GOLDEN-5 clinical trial11 are shown in Figure 5.
Figure 5.
eFlow CS device satisfaction questionnaire: comparison of positive responses from participants in the current real-world study versus the Phase 3 GOLDEN 5 clinical trial.11
Abbreviation: N/A, not applicable.
Previous Nebulizer Users
For prior nebulizer users, slightly higher proportions of participants in the real-world study compared with patients in GOLDEN-5 perceived that eFlow was “somewhat easier” or “much easier” to assemble and disassemble (55% [30/55] vs 43% [57/132]), operate (60% [33/55] vs 44% [57/131]), and clean and disinfect (49% [27/55] vs 42% [55/131]). A higher proportion of prior nebulizer users in the real-world study responded that they were “somewhat more” or “much more” confident that glycopyrrolate was being administered as designed with eFlow compared with their prior nebulizer(s) (real-world study, 73% [40/55] vs GOLDEN-5, 48% [62/130]). Compared with their previous nebulizer(s), 80% (44/55) of real-world participants said they were “somewhat more” or “much more” satisfied with eFlow, while in GOLDEN-5 the proportion was lower (58% [75/130]).
Overall Participants (Regardless of Previous Nebulizer Use)
In the overall population (ie, regardless of prior nebulizer use), similar proportions of participants in the real-world study compared with patients in GOLDEN-5 found that eFlow was “easy” or “very easy” to assemble and disassemble (71% [47/66] vs 76% [344/454]), operate (83% [55/66] vs 79% [357/454]), and clean (65% [43/66] vs 71% [324/454]). Confidence in glycopyrrolate administration via eFlow (“confident” or “very confident”) was similar for the two studies (real-world study, 86% [57/66] vs GOLDEN-5, 83% [376/454]). In the overall evaluation, a high proportion of participants (91% [60/66]) in the real-world study expressed that, overall, they were “satisfied” or “very satisfied” with using eFlow; in GOLDEN-5 the proportion was lower (75% [340/453]).
A higher proportion of real-world participants (61% [40/66]) reported that the eFlow design “very much” supported intuitive use; the proportion was lower in GOLDEN-5 (43% [194/453]). The proportions of positive responses for rating the weight and size of the eFlow were similar and high for the two studies (“light” weight: real-world study, 94% [62/66]; GOLDEN-5, 88% [398/454], and “ideal” size: real-world study, 95% [63/66]; GOLDEN-5, 89% [402/454]). A high proportion of participants in both studies found that the instructions were “helpful” or “very helpful” (real-world study, 95% [63/66]; GOLDEN-5, 97% [440/454]). Among GOLDEN-5 patients, 44% (201/454) said they would “probably” or “definitely” switch to glycopyrrolate/eFlow if it were available outside the clinical trial setting, whereas 82% (54/66) of current users in the real-world study responded that they would “probably” or “definitely” continue using glycopyrrolate/eFlow. Confidence (“confident” or “very confident”) using glycopyrrolate/eFlow everyday was high in the real-world (89% [59/66]) and slightly lower in GOLDEN-5 (69% [312/454]).
Discussion
This is the first study to report real-world data on patients with COPD using glycopyrrolate/eFlow. Participants in the real-world study reported high satisfaction and confidence using glycopyrrolate/eFlow. Overall, results from the current study support the patient satisfaction findings from the Phase 3 clinical long-term safety study.
The 2020 GOLD report states that the choice of inhaler device should be individually tailored and will depend on access, cost, prescriber, and, most importantly, the patient’s ability and preference.1 A previous patient preference study reported that ease and perceived convenience of use, portability, shorter administration time, and device efficacy are among the most important inhalation device attributes to COPD patients.15 The eFlow is relatively lightweight, small, portable, and is designed to administer the medication in 2–3 minutes, with proper assembly and cleaning. Patients in the current study reported high satisfaction with these device attributes. In addition, eFlow is virtually silent, which was also viewed favorably among study participants.
To our knowledge, this is the first real-world study to report COPD patient satisfaction with a nebulized inhalation device. Previous studies in handheld inhalation devices found patient satisfaction and confidence to be associated with better treatment compliance and better outcomes.16,17 A cross-sectional survey of over 1400 patients with COPD reported a significant association between overall satisfaction with inhalers and treatment compliance. Further, the study found a direct association between inhaler satisfaction and fewer exacerbations.16 Another point-in-time survey of over 370 US patients with COPD reported an association between greater device confidence and higher self-reported adherence to inhaler usage.17 Low confidence in inhaler usage was associated with lower adherence and poor COPD-related health status.
Over 70% of participants in this real-world study reported their overall health to be fair or poor and almost 60% reported having severe or very severe COPD. Further, CAT and mMRC results indicated a high COPD symptom burden among study participants. Studies have found that patients who are elderly or have cognitive or physical disabilities may have challenges using handheld inhalers.1,18–20 Patients using eFlow can breathe normally without the need for hand–breath coordination, as the eFlow nebulizer delivers a fine mist through natural breathing.21
Results from the current real-world study were slightly more positive than those from the GOLDEN-5 open-label clinical trial. A possible explanation is that the GOLDEN-5 study was a 48-week trial and required daily e-diary completion and regular periodic clinical visits. By contrast, there were no such requirements in this real-world study. The higher burden of tasks associated with the clinical trial may partly explain the somewhat lower satisfaction scores. Another potential explanation is that more patients in the real-world study reported previous nebulizer use. This familiarity with nebulizers may have been a factor in the greater satisfaction in this real-world study compared to the clinical trial.
Study Limitations
This study had several limitations. The device satisfaction questionnaire was developed by the study sponsor and was not formally validated. In addition, the results of this real-world study are based on participants’ self-reports and, therefore, could not be independently verified through clinical documentation. Some study participants had participated in an early experience program prior to study enrollment, which gave them the opportunity to use glycopyrrolate/eFlow before it became commercially available; this may have introduced a potential bias into the study, since, by participating in the program, their user technique and adherence may have been enhanced above that which would have occurred outside the program. Nebulizers had been previously used by 83.3% of participants; this may potentially have affected their responses; however, it was not a main goal of the study to compare responses of patients who previously used different nebulizers. The study results may also be subject to recall bias, particularly in the questions related to prior nebulizer use. Lastly, this study had a relatively small sample size (n=66), which limited the statistical power to assess significant differences in sub-populations. The small sample size may also limit the generalizability of the results.
Conclusion
Consistent with findings from the GOLDEN-5 clinical study, patients in this real-world setting reported a high satisfaction with, and high confidence in, using the glycopyrrolate/eFlow CS nebulizer every day.
Acknowledgments
This study was supported by a contract from Sunovion Pharmaceuticals, Inc. to HealthCore, Inc., Wilmington, DE, USA. Jane M. Gilbert, BSc, CMPP, of Elevate Medical Affairs, which was contracted by Sunovion to provide publication support service, provided writing and editorial assistance. The authors acknowledge the valuable contributions of all survey participants.
Data Sharing Statement
Sunovion Pharmaceuticals Inc. is part of a clinical trial data sharing consortium that facilitates access for qualified researchers to selected anonymized clinical trial data. For up-to-date information on data availability, please visit https://www.clinicalstudydatarequest.com/Study-Sponsorts.aspx and click on Sunovion.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. The authors received no honoraria related to the development of this publication.
Disclosure
Carole Dembek is an employee of Sunovion Pharmaceuticals Inc. Edward M Kerwin has received consultancy and advisory fees from Amphastar, AstraZeneca, Boehringer Ingelheim, Cipla, GlaxoSmithKline, Mylan, Novartis, Oriel, Pearl, Sunovion, Teva, and Theravance. The authors report no other conflicts of interest in this work.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease, 2020 report. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf. Accessed June 26, 2020.
- 2.Sullivan J, Pravosud V, Mannino DM, Siegel K, Choate R, Sullivan T. National and state estimates of COPD morbidity and mortality - United States, 2014–2015. Chronic Obstr Pulm Dis. 2018;5(4):324–333. doi: 10.15326/jcopdf.5.4.2018.0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention - National Center for Health Statistics. Chronic obstructive pulmonary disease (COPD) Includes: chronic bronchitis and emphysema. Available from: https://www.cdc.gov/nchs/fastats/copd.htm. Accessed December9, 2019.
- 4.Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥ 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31–45. doi: 10.1378/chest.14-0972 [DOI] [PubMed] [Google Scholar]
- 5.Braido F, Chrystyn H, Baiardini I, et al. “Trying, but failing” - the role of inhaler technique and mode of delivery in respiratory medication adherence. J Allergy Clin Immunol Pract. 2016;4(5):823–832. doi: 10.1016/j.jaip.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 6.Sunovion Pharmaceuticals Inc. LONHALA™ MAGNAIR™ (glycopyrrolate) inhalation solution for oral inhalation use. Prescribing information. December, 2018. Available from: https://www.lonhalamagnair.com/LonhalaMagnair-Prescribing-Information.pdf. Accessed May14, 2019.
- 7.Sunovion Pharmaceuticals Inc. MAGNAIR™ Nebulizer system instructions for use. December, 2017. Available from: https://www.lonhalamagnair.com/LonhalaMagnair-Instructions-for-Use.pdf. Accessed May14, 2019.
- 8.Pham S, Ferguson GT, Kerwin E, Goodin T, Wheeler A, Bauer A. In vitro characterization of the eFlow closed system nebulizer with glycopyrrolate inhalation solution. J Aerosol Med Pulm Drug Deliv. 2018;31(3):162–169. doi: 10.1089/jamp.2017.1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson GT, Goodin T, Tosiello R, Wheeler A, Kerwin E. Long-term safety of glycopyrrolate/eFlow® CS in moderate-to-very-severe COPD: Results from the Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) 5 randomized study. Respir Med. 2017;132:251–260. doi: 10.1016/j.rmed.2017.08.020 [DOI] [PubMed] [Google Scholar]
- 10.Kerwin E, Donohue JF, Goodin T, Tosiello R, Wheeler A, Ferguson GT. Efficacy and safety of glycopyrrolate/eFlow® CS (nebulized glycopyrrolate) in moderate-to-very-severe COPD: Results from the glycopyrrolate for obstructive lung disease via electronic nebulizer (GOLDEN) 3 and 4 randomized controlled trials. Respir Med. 2017;132:238–250. doi: 10.1016/j.rmed.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 11.Kerwin EM, Donohue JF, Ferguson GT, Ganapathy V, Ozol-Godfrey A, Rajagopalan K. Satisfaction with the use of eFlow closed-system nebulizer in patients with moderate-to-very severe chronic obstructive pulmonary disease: Findings from a long-term safety study. J Aerosol Med Pulm Drug Deliv. 2019;32(1):24–33. doi: 10.1089/jamp.2018.1477 [DOI] [PubMed] [Google Scholar]
- 12.Jones P, Jenkins C, Bauerle O, on behalf of the CAT Development Steering Group. COPD Assessment Test (CAT) healthcare professional user guide. Issue 3. February, 2012. Available from: https://www.lungemedicin.dk/fagligt/skemaer/142-cathcp-vejledning/file.html. Accessed May16, 2019.
- 13.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 14.Paladini L, Hodder R, Cecchini I, Bellia V, Incalzi RA. The MRC dyspnoea scale by telephone interview to monitor health status in elderly COPD patients. Respir Med. 2010;104(7):1027–1034. doi: 10.1016/j.rmed.2009.12.012 [DOI] [PubMed] [Google Scholar]
- 15.Hodder R, Price D. Patient preferences for inhaler devices in chronic obstructive pulmonary disease: experience with Respimat® Soft Mist™ Inhaler. Int J Chron Obstruct Pulmon Dis. 2009;4:381–390. doi: 10.2147/COPD.S3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chrystyn H, Small M, Milligan G, Higgins V, Gil EG, Estruch J. Impact of patients’ satisfaction with their inhalers on treatment compliance and health status in COPD. Respir Med. 2014;108(2):358–365. doi: 10.1016/j.rmed.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 17.Amin AN, Ganapathy V, Roughley A, Small M. Confidence in correct inhaler technique and its association with treatment adherence and health status among US patients with chronic obstructive pulmonary disease. Patient Prefer Adherence. 2017;11:1205–1212. doi: 10.2147/PPA.S140139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarad N. Chronic obstructive pulmonary disease (COPD) and old age? Chron Respir Dis. 2011;8(2):143–151. doi: 10.1177/1479972311407218 [DOI] [PubMed] [Google Scholar]
- 19.Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23–30. doi: 10.2147/CIA.S52999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogliani P, Calzetta L, Coppola A, et al. Optimizing drug delivery in COPD: The role of inhaler devices. Respir Med. 2017;124:6–14. doi: 10.1016/j.rmed.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 21.Dhand R, Dolovich M, Chipps B, Myers TR, Restrepo R, Farrar JR. The role of nebulized therapy in the management of COPD: Evidence and recommendations. COPD. 2012;9(1):58–72. doi: 10.3109/15412555.2011.630047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease, 2020 report. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf. Accessed June 26, 2020.
- Centers for Disease Control and Prevention - National Center for Health Statistics. Chronic obstructive pulmonary disease (COPD) Includes: chronic bronchitis and emphysema. Available from: https://www.cdc.gov/nchs/fastats/copd.htm. Accessed December9, 2019.
- Sunovion Pharmaceuticals Inc. LONHALA™ MAGNAIR™ (glycopyrrolate) inhalation solution for oral inhalation use. Prescribing information. December, 2018. Available from: https://www.lonhalamagnair.com/LonhalaMagnair-Prescribing-Information.pdf. Accessed May14, 2019.
- Sunovion Pharmaceuticals Inc. MAGNAIR™ Nebulizer system instructions for use. December, 2017. Available from: https://www.lonhalamagnair.com/LonhalaMagnair-Instructions-for-Use.pdf. Accessed May14, 2019.
- Jones P, Jenkins C, Bauerle O, on behalf of the CAT Development Steering Group. COPD Assessment Test (CAT) healthcare professional user guide. Issue 3. February, 2012. Available from: https://www.lungemedicin.dk/fagligt/skemaer/142-cathcp-vejledning/file.html. Accessed May16, 2019.