Abstract
Polymorphisms of methylenetetrahydrofolate reductase (MTHFR) in hormone metabolism pathways might cause metabolic disturbances and contribute to the development of polycystic ovary syndrome (PCOS) and ovarian cancer, but the published studies were inconsistent. The aim of the present study was to evaluate the MTHFR C677T (rs1801133) and A1298C (rs1801131) gene polymorphisms in the risk of PCOS and ovarian cancer by meta-analysis. A comprehensive electronic search was conducted in databases for studies published from 1995 to 2020. The pooled ORs were calculated by Revman 5.2 software. Twenty-nine articles including 45 case–control studies were included. We found that MTHFR C677T polymorphisms were correlated with elevated PCOS risk (TT vs. CT+CC: OR = 1.41, 95%CI = 1.20–1.67; TT+CT vs. CC: OR = 1.54, 95%CI = 1.07–2.22; CT vs. CC+TT: OR = 1.18, 95%CI 1.04–1.33; TT vs. CC: OR = 1.47, 95%CI = 1.03–2.11; T vs. C: OR = 1.25, 95%CI = 1.06–1.47), which were more obvious in Middle Eastern subgroup. MTHFR A1298C polymorphisms were also associated with overall PCOS susceptibility (CC vs. AC+AA: OR = 2.55, 95% CI = 1.61–4.03; CC+AC vs. AA: OR = 1.84, 95%CI = 1.04–3.28; CC vs. AA: OR = 2.66, 95%CI = 1.68–4.22; C vs. A: OR = 1.67, 95%CI = 1.03–2.71), which were mainly reflected in Asian subjects. For ovarian cancer, MTHFR C677T polymorphisms were only related with elevated ovarian cancer risk in Asian population, while no significant association was found for A1298C polymorphisms. This meta-analysis suggested that MTHFR C677T and MTHFR A1298C polymorphisms were correlated with elevated PCOS risk. MTHFR C667T only posed a higher risk for ovarian cancer in Asians instead of other populations, while MTHFR A1298C polymorphisms were not related to ovarian cancer risk. Further studies are needed to validate the conclusion.
Keywords: meta-analysis, methylenetetrahydrofolate reductase, ovarian cancer, polycystic ovary syndrome, polymorphism, variant
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine malfunctions, reportedly affecting 5–10% of reproductive age women [1]. According to the Rotterdam consensus in 2003, PCOS is featured with oligo- or anovulation, hyperandrogenism and polycystic ovaries [2]. Clinical manifestations of PCOS may include menstrual irregularities, signs of androgen excess, obesity and insulin resistance, with increased risk of Type 2 diabetes and cardiovascular events [3–5]. PCOS impairs female fertility to varying degrees, which remains to be the leading cause for medical assistance. The relationship between PCOS and ovarian cancer has long been controversial. PCOS has been hypothesized to increase ovarian cancer risk through increased androgen exposure in pre-clinical studies [6,7]. Several case–control studies also explored the association of PCOS with ovarian cancer risk, while a recent meta-analysis concluded that there was an increased ovarian cancer risk observed in PCOS population by pooling three individual studies (OR = 1.4; 95% CI = 0.9–2.2) [8]. However, later studies reported no association between self-reported PCOS and ovarian cancer risk. Meanwhile, several studies proposed that an elevation in cancer risk might be relevant to only certain histological subtypes [9,10].
With emerging genetic findings such as homologous repair deficiency in gynecologic malignancies, more and more attention was focused on the genetic root of both diseases. Existing clinical studies suggest that both genetic background and environmental factors with a cluster of metabolic disturbances might contribute to the development of PCOS and ovarian cancer [11,12]. It was also suggested that if the mutation of critical genes in the hormone metabolism pathways could contribute to both diseases individually. It might give us the hint on the pathophysiologic relationship between PCOS and ovarian malignancies. Take homocysteine (Hcy) as an example, it is a sulfur-containing amino acid derived from methionine metabolism and has been proved to be related with insulin resistance and increased risk of cardiovascular diseases in PCOS patients. Elevated plasma level of homocysteine is caused by its deficient transformation, including its transmethylation to methionine, which is regulated by methylenetetrahydrofolate reductase (MTHFR). The MTHFR is an enzyme involved in folate metabolism, and mutations of MTHFR gene would result in reduced activity of the enzyme, thus increasing total Hcy levels in plasma [13,14]. Several gene abnormalities of MTHFR have been explored and two types of SNPs have been found. One is at base position 677 (rs1801133), a C-to-T transition (an alanine to valine substitution). The other is at base position 1298 (rs1801131), an A-to-C transition (a glutamate-to-alanine substitution). It is estimated that approximately 10–15% of Caucasians are homozygous for the TT genotype at positions 677, which is more common in Hispanics (25%) and least common in individuals of African descent (6%) [15]. Another study reported that the overall frequency of the 677TT genotype and 1298CC genotype in the Chinese Han population was 23.2% and 3.9%, respectively [16].
In 1999, Gleuck first reported the association between MTHFR C677T polymorphisms and PCOS, ever since several similar researches have been conducted [17]. In 2014, two independent meta-analyses were published to explore the relationship between MTHFR C677T polymorphisms and PCOS risk but with controversial conclusions [18,19]. One reason of inconsistent conclusions is that neither of the two studies included all current published data. Also, one study categorized subjects from Middle Eastern countries as Caucasian, which might affect the subgroup analysis. Similarly, a certain amount of case–control studies explored the relationship between MTHFR polymorphisms and ovarian cancer. However, the results were conflicting and inconclusive, presumably due to small sample size in each published study while possible selection bias such as ethnicity was unignorable [20,21]. Therefore, in the present study, we conducted a comprehensive meta-analysis by collecting the existing published data to better clarify MTHFR C677T and A1298C polymorphisms in the risk of PCOS and ovarian cancer.
Materials and methods
Search for eligible literature
A comprehensive electronic search was performed using PubMed, Embase, Medline (Ovid), Weipu, CNKI and Wanfang databases for studies published from March 1995 to February 2020. The following subject terms and keywords were used: “methylenetetrahydrofolate reductase”, “MTHFR”, “PCOS”, “polycystic ovarian syndrome”, “ovarian cancer” “polymorphism”, “variant” and “mutation”. The search was updated every week until February 20, 2020.
Inclusion and exclusion criteria
Articles fulfilling the following criteria were included: (i) studied the MTHFR C677T and A1298C polymorphisms in PCOS or ovarian cancer patients, (ii) provided sufficient data in both case and control groups to calculate the odds ratios (ORs) and the corresponding 95% confidence intervals (95% CIs), (iii) mentioned specific diagnostic criteria for PCOS (the NIH criteria or the Rotterdam criteria) and described patients’ symptoms in details, (iv) case–control studies. When duplicate data were present in different articles, only the latest one would be taken into consideration. In addition, Newcastle–Ottawa Scale (NOS) was used to assess the quality of the observational studies included. Three aspects of selection, comparability, and exposure (nine scores in total) were carefully assessed. Studies of moderate or high quality were included (score of 5 or higher). As such, articles that didn’t fulfill the criteria mentioned above were excluded.
Data extraction
All potential studies were investigated by two independent viewers. The following items were extracted: first author, year of publication, ethnicity, matched parameters, target genotypes, genotyping methods, participant numbers and genotype distributions in cases and controls. Any discrepancies were resolved by discussion with a third reviewer until a consensus was reached.
Statistical analysis
Meta-analyses were undertaken using the Revman 5.2 softer ware (Cochrane Collaboration, Copenhagen) to calculate the pooled ORs and corresponding 95% CIs. SNPs of MTHFR C677T and A1298C were considered as binary variables. Different contrast models were judged: (i) homozygous mutants contrast, (ii) homozygous and heterozygous mutants contrast, (iii) heterozygous mutants contrast, (iv) homozygous mutants contrast in homozygotes, (v) mutant allelic contrast. Besides the overall comparisons, we also performed subgroup analyses stratified by ethnicities in consideration of population differences. Heterogeneity assumptions were tested using Higgins I2test. When the I2value was less than 50%, a fixed-effects model was used otherwise a random-effects model was applied. The Z test was performed to determine the significance of the pooled ORs where P less than 0.05 was considered statistically significant. The presence of publication bias was evaluated by visually inspecting the asymmetry in funnel plots and Egger’s test. All statistical analyses were performed using Revman 5.2 software (Cochrane Collaboration, Copenhagen, Denmark) except the Egger’s test, which was conducted using STATA 14.0 (StataCorp LP, College Station, TX, U.S.A.).
Results
Search results
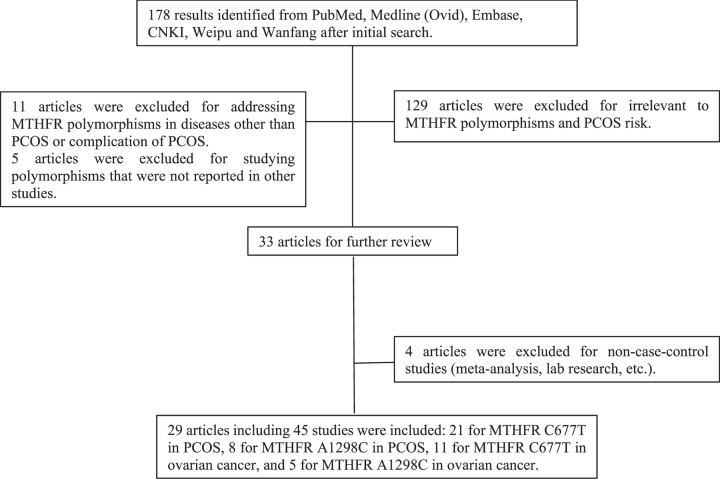
After primary search, 178 results were retrieved. In our further review, 129 articles were not related to MTHFR polymorphisms and PCOS risk by reading titles and abstracts and thus were excluded. 11 articles focused on MTHFR polymorphisms in different diseases or complication of PCOS like thrombophilia and pregnancy loss. Five studies estimated genetic variants other than C677T and A1298C. Four articles were excluded for non-case–control studies such as meta-analysis and lab research (Figure 1). Among the remaining 29 enrolled articles, 25 were of moderate quality (NOS score of 6 or 7) and 4 were of high quality (NOS score of 8 or 9) therefore were all included in this meta-analysis. By reviewing the genotype counts, 9 articles were found to focus on both C677T and A1298C thus were considered as 18 separate studies. Two articles discussed two different ethnicities thus separate ethnicities were considered as individual studies. One article genotyped subjects from three independent studies and were considered as three studies. Therefore, 45 studies were enrolled for this meta-analysis. We also summarized matched parameters in both case and control groups as shown in Table 1 [17,22–49].
Figure 1. The flow chart of study selection.
Table 1. Characteristics of included studies.
| First author | Year | Ethnicity | Study disease | MTHFR polymorphism | Genotyping method | Matched parameters | Study quality (NOS) |
|---|---|---|---|---|---|---|---|
| Carlus | 2016 | Asian | PCOS | C667T | PCR-RFLP | Age, height, weight, LH, glucose, BMI, LH/FSH | 8 |
| Choi | 2009 | Asian | PCOS | C667T | PCR-RFLP | BMI, weight, waist/hip ratio, FSH, estradiol, prolactin | 7 |
| Geng | 2016 | Asian | PCOS | C667T, A1298C | PCR-RFLP | Age, FSH, prolactin, estradiol | 6 |
| Glueck | 1999 | Caucasian | PCOS | C667T, A1298C | PCR-RFLP | Age, race | 7 |
| Idali | 2012 | Middle Eastern | PCOS | C667T | PCR-RFLP | Unknown | 6 |
| Jain | 2012 | Asian | PCOS | C667T | PCR-RFLP | Age, FSH, TSH, prolactin | 7 |
| Jiang | 2015 | Asian | PCOS | C667T, A1298C | PCR-RFLP | Age | 6 |
| Jiao | 2018 | Asian | PCOS | C667T | PCR-RFLP | Age, estradiol | 6 |
| Karadeniz | 2010 | Middle Eastern | PCOS | C667T | PCR-RFLP | Age, BMI, estradiol, DHEA-S, TSH, prolactin, total cholesterol, triglyceride, LDL-cholesterol | 8 |
| Lee | 2003 | Asian | PCOS | C667T | PCR-RFLP | Unknown | 6 |
| Naghavi | 2015 | Middle Eastern | PCOS | C667T | PCR-RFLP | Age, race | 6 |
| Orio | 2003 | Caucasian | PCOS | C667T | PCR-RFLP | Age, BMI, waist/hip ratio, FSH, prolactin, vitamin B12, folate, homocysteine, fasting glucose | 7 |
| Ozegowska | 2016 | Caucasian | PCOS | C667T | PCR-RFLP | Age, waist/hip ratio, fasting glucose, LDL-C, HDL-C, cholesterol/HDL, SBP, DBP | 7 |
| Palep-Singh | 2007 | Asian& Caucasian | PCOS | C667T, A1298C | PCR-RFLP | South Asian: age, FSH, insulin, cholesterol, LDL. Caucasian: birth weight, waist/hip ratio, right ovarian volume, FSH, testosterone, cholesterol, triglyceride, LDL | |
| Qi | 2015 | East Asian | PCOS | C667T, A1298C | PCR-RFLP | Vitamin B12, homocysteine | 6 |
| Sills | 2001 | Caucasian | PCOS | C667T | PCR-RFLP | Age, fasting glucose, androstenedione, DHEA-S, homocysteine | 7 |
| Szafarowska | 2016 | Caucasian | PCOS | C667T, A1298C | PCR-RFLP | Homocysteine, AMH | 6 |
| Tsanadis | 2002 | Caucasian | PCOS | C667T | PCR-RFLP | Age, BMI, DHEA-S, glucose | 7 |
| Wu | 2016 | Asian | PCOS | C667T, A1298C | PCR-RFLP | Age, prolactin, FSH, estradiol, triglyceride | 7 |
| Gao | 2012 | Asian | OC | C667T | PCR-RFLP | Age, BMI, tobacco smoking, alcohol use, menopausal status | 7 |
| Jakubowska | 2012 | Caucasian | OC | C667T | PCR-RFLP | Age, BMI | 6 |
| Ozkilic | 2016 | Middle Eastern | OC | C667T | PCR-RFLP | Age | 6 |
| Pawlik | 2011 | Caucasian | OC | C667T | PCR-RFLP | Age, BMI, FSH, LH, estradiol | 6 |
| Prasad | 2011 | Asian | OC | C667T | MassARRAY | Unknown | 6 |
| Song | 2012 | Asian | OC | A1298C | PCR-RFLP | Age, tobacco use, alcohol use, menopausal status | 7 |
| Terry | 2010 | Caucasian | OC | C667T, A1298C | TaqMan | Age, oral contraceptive use, liveborn number | 7 |
| Webb | 2011 | Caucasian | OC | C667T, A1298C | PCR-PFLP | Age, BMI, oral contraceptive use, energy intake | 7 |
| Wu | 2007 | Asian | OC | C667T | NA | Age, BMI | 6 |
| Zhang | 2012 | Asian | OC | C667T | PCR-RFLP | Tobacco use, alcohol use, menopausal status, hormone replacement therapy | 8 |
Abbreviations: AMH, Anti-Müllerian hormone; BMI, body mass index; DHEA-S, dehydroepiandrosterone sulfate; FSH, follicle-stimulating hormone; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; LH, luteinizing hormone; MTHFR, methylenetetrahydrofolate reductase; NOS, Newcastle–Ottawa scale; OC, ovarian cancer; PCOS, polycystic ovary syndrome; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism; TSH, thyroid-stimulating hormone.
MTHFR C677T polymorphisms in PCOS
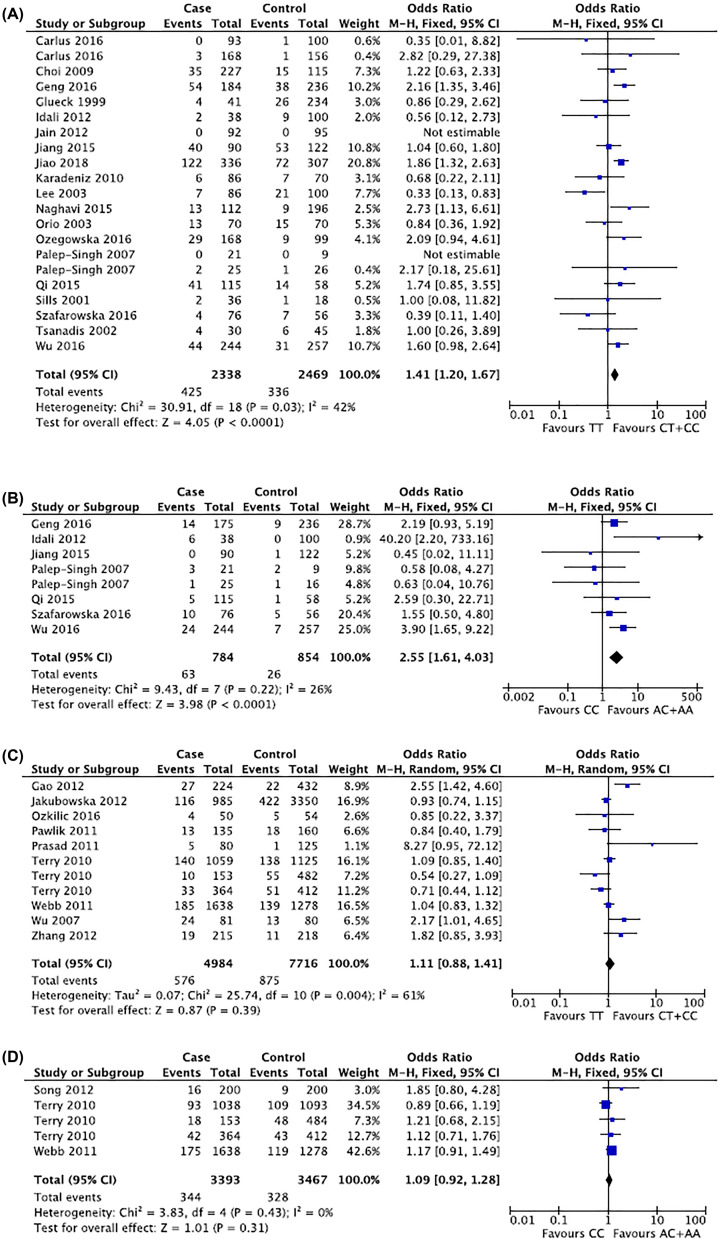
As shown in Tables 2 and 4, the variant allele T has a significant association with the risk of PCOS compared with allele C (T vs. C: OR = 1.25, 95%CI = 1.06–1.47) in random effect model (I2 = 64%) by pooling 9614 alleles together. In consistent with this, significant association was also found in T containing genotypes (TT+CT or TT) and TT genotypes alone (TT+CT vs. CC: OR = 1.54, 95%CI = 1.07–2.22; TT vs. CC: OR = 1.47, 95%CI = 1.03–2.11; TT vs. CT+CC: OR = 1.41, 95%CI = 1.20–1.67) among 4807 participants (Figure 2A). Interestingly, when comparing heterozygous CT with homozygous TT+CC, the pooled ORs again showed statistical significance (CT vs. CC+TT: OR = 1.18, 95%CI = 1.04–1.33). This indicates that C677T was correlated with elevated risk of PCOS, both in homozygous individuals and heterozygous mutants. Subgroup meta-analyses in stratified ethnicities showed that C677T mutant Middle Eastern population contained higher PCOS risk (TT+CT vs. CC: OR = 2.66, 95%CI = 1.54–4.58; CT vs. CC+TT: OR = 2.64, 95%CI = 1.27–5.49; TT vs. CC: OR = 2.21, 95%CI = 1.16–4.21; T vs. C: OR = 1.82, 95%CI = 1.39–2.37), though only 602 participants were included. No similar tendency was displayed in Asian or Caucasian population.
Table 2. Genotype distributions in cases and controls for MTHFR C677T and A1298C polymorphisms in PCOS.
| Author | Year | Country | Polymorphism | Case | Control | ||||
|---|---|---|---|---|---|---|---|---|---|
| AA∧ | Aa | aa | AA | Aa | aa | ||||
| Carlus# | 2016 | Asian | C677T | 77 | 16 | 0 | 83 | 16 | 1 |
| Carlus | 2016 | Asian | C677T | 132 | 33 | 3 | 126 | 29 | 1 |
| Choi | 2009 | Asian | C677T | 67 | 125 | 35 | 33 | 67 | 15 |
| Geng | 2016 | Asian | C677T | 51 | 79 | 54 | 102 | 96 | 38 |
| Glueck | 1999 | Caucasian | C677T | 14 | 23 | 4 | 119 | 89 | 26 |
| Idali | 2012 | Middle Eastern | C677T | 17 | 19 | 2 | 66 | 25 | 9 |
| Jain | 2012 | Asian | C677T | 76 | 16 | 0 | 82 | 13 | 0 |
| Jiang | 2015 | Asian | C677T | 13 | 37 | 40 | 13 | 56 | 53 |
| Jiao | 2018 | Asian | C677T | 52 | 162 | 122 | 96 | 139 | 72 |
| Karadeniz | 2010 | Eastern | C677T | 15 | 65 | 6 | 35 | 28 | 7 |
| Lee | 2003 | Asian | C677T | 33 | 46 | 7 | 27 | 52 | 21 |
| Naghavi | 2015 | Middle Eastern | C677T | 61 | 38 | 13 | 136 | 51 | 9 |
| Orio | 2003 | Caucasian | C677T | 16 | 41 | 13 | 17 | 38 | 15 |
| Ozegowska | 2016 | Caucasian | C677T | 87 | 52 | 29 | 53 | 37 | 9 |
| Palep-Singh* | 2007 | Asian | C677T | 14 | 7 | 0 | 9 | 0 | 0 |
| Palep-Singh | 2007 | Caucasian | C677T | 11 | 12 | 2 | 10 | 15 | 1 |
| Qi | 2015 | Asian | C677T | 14 | 60 | 41 | 21 | 23 | 14 |
| Sills | 2001 | Caucasian | C677T | 25 | 9 | 2 | 8 | 9 | 1 |
| Szafarowska | 2016 | Caucasian | C677T | 33 | 39 | 4 | 19 | 30 | 7 |
| Tsanadis | 2002 | Caucasian | C677T | 12 | 14 | 4 | 20 | 19 | 6 |
| Wu | 2016 | Asian | C677T | 94 | 106 | 44 | 122 | 104 | 31 |
| Geng | 2016 | Asian | A1298C | 104 | 57 | 14 | 157 | 70 | 9 |
| Idali | 2012 | Eastern | A1298C | 12 | 20 | 6 | 94 | 6 | 0 |
| Jiang | 2015 | Asian | A1298C | 66 | 24 | 0 | 98 | 23 | 1 |
| Palep-Singh | 2007 | Asian | A1298C | 9 | 9 | 3 | 0 | 7 | 2 |
| Palep-Singh* | 2007 | Caucasian | A1298C | 14 | 10 | 1 | 10 | 5 | 1 |
| Qi | 2015 | Asian | A1298C | 71 | 39 | 5 | 43 | 14 | 1 |
| Szafarowaska | 2016 | Caucasian | A1298C | 40 | 26 | 10 | 34 | 17 | 5 |
| Wu | 2016 | Asian | A1298C | 143 | 77 | 24 | 166 | 84 | 7 |
‘A’ represents wild-type allele and ‘a’ represents mutant allele.
Study separately enrolled two sub-populations of Indian and were thus considered as two studies.
Study separately enrolled Asian and Caucasian and were thus considered as two studies.
Table 4. Summary of different comparative results for MTHFR C677T polymorphisms in PCOS.
| Genotypes | Overall and subgroup | Participants | OR and 95%CI | Z value | P value | I2 (%) | Effect model# |
|---|---|---|---|---|---|---|---|
| TT vs. CT+ CC | Overall | 4,807 | 1.41 [1.20, 1.67] | 4.05 | 0.01 | 42 | F |
| Asian | 3,211 | 1.38 [0.99, 1.92] | 1.92 | 0.05 | 55 | R | |
| Caucasian | 994 | 1.09 [0.72, 1.65] | 0.39 | 0.7 | 0 | F | |
| Middle Eastern | 602 | 1.13 [0.39, 3.32] | 0.23 | 0.82 | 60 | R | |
| TT+ CT vs. CC | Overall | 4,807 | 1.54 [1.07, 2.22] | 2.31 | 0.02 | 85 | R |
| Asian | 3,211 | 1.76 [0.99, 3.13] | 1.62 | 0.05 | 90 | R | |
| Caucasian | 994 | 1.03 [0.78, 1.36] | 0.21 | 0.84 | 29 | F | |
| Middle Eastern | 602 | 2.66 [1.54, 4.58] | 3.52 | 0.01 | 53 | R | |
| CT vs. CC+ TT | Overall | 4,807 | 1.18 [1.04, 1.33] | 2.64 | 0.01 | 49 | F |
| Asian | 3,211 | 1.10 [0.95, 1.27] | 1.25 | 0.21 | 0 | F | |
| Caucasian | 994 | 0.99 [0.75, 1.31] | 0.05 | 0.96 | 40 | F | |
| Middle Eastern | 602 | 2.64 [1.27, 5.49] | 2.6 | 0.01 | 74 | R | |
| TT vs. CC | Overall | 2,872 | 1.47 [1.03, 2.11] | 2.1 | 0.04 | 59 | R |
| Asian | 1,929 | 1.58 [0.92, 2.72] | 1.67 | 0.09 | 75 | R | |
| Caucasian | 567 | 1.14 [0.72, 1.81] | 0.58 | 0.56 | 0 | F | |
| Middle Eastern | 376 | 2.21 [1.16, 4.21] | 2.41 | 0.02 | 0 | F | |
| T vs. C | Overall | 9,614 | 1.25 [1.06, 1.47] | 0.64 | 0.01 | 64 | R |
| Asian | 6,422 | 1.27 [1.01, 1.61] | 2.03 | 0.04 | 74 | R | |
| Caucasian | 1,988 | 1.04 [0.85, 1.27] | 0.34 | 0.73 | 19 | F | |
| Middle Eastern | 1,204 | 1.82 [1.39, 2.37] | 4.38 | 0.01 | 0 | F |
Effect model includes fixed-effect model (F) and random-effect model (R).
Figure 2. Representative forest plots.
(A) TT vs. CT+CC for MTHFR C667T polymorphisms in PCOS. (B) CC vs. AC+AA for A1298C polymorphisms in PCOS. (C) TT vs. CT+CC for MTHFR C667T polymorphisms in ovarian cancer. (D) CC vs. AC+AA for A1298C polymorphisms in ovarian cancer.
MTHFR A1298C polymorphisms in PCOS
The result of the association between MTHFR A1298C and PCOS risk was as followed. About 7 articles including 8 studies with 784 PCOS patients and 854 healthy controls were pooled. Except the heterozygous mutant comparison that presented an insignificant OR, others showed significant outcome between A1298C mutation and elevated PCOS risk (CC vs. AC+AA: OR = 2.55, 95% = 1.61–4.03; CC+AC vs. AA: OR = 1.84, 95%CI = 1.04–3.28; CC vs. AA: OR = 2.66, 95%CI = 1.68–4.22; C vs. A: OR = 1.67, 95%CI = 1.03–2.71) (Figure 2B). When stratified by ethnicity, only Asians shared consistent results with the overall population (CC vs. AC+AA: OR = 2.46, 95% = 1.44–4.22; CC+AC vs. AA: OR = 1.33, 95%CI = 1.05–1.67; CC vs. AA: OR = 2.43, 95%CI = 1.41–4.18; C vs. A: OR = 1.39, 95%CI = 1.14–1.69). Notably, fixed-effect model was conducted in both Asian and Caucasian comparisons but random-effect model was used for overall population. This might suggest the consistency among the same ethnicity but differences among different populations might exist. The above data indicated that MTHFR A1298C posed a higher risk for PCOS in overall population, particularly in Asians instead of other populations. The detailed results were summarized in Tables 2 and 5.
Table 5. Summary of different comparative results for MTHFR A1298C polymorphisms in PCOS.
| Genotypes | Overall and subgroup | Participants | OR and 95%CI | Z value | P value | I2 (%) | Effect model# |
|---|---|---|---|---|---|---|---|
| CC vs. AC+AA | Overall | 1,638 | 2.55 [1.61, 4.03] | 3.98 | 0.01 | 26 | F |
| Asian | 1,327 | 2.46 [1.44, 4.22] | 3.28 | 0.01 | 6 | F | |
| Caucasian | 173 | 1.37 [0.48, 3.90] | 0.59 | 0.55 | 0 | F | |
| CC+AC vs. AA | Overall | 1,638 | 1.84 [1.04, 3.28] | 2.09 | 0.04 | 81 | R |
| Asian | 1,327 | 1.33 [1.05, 1.67] | 2.4 | 0.02 | 14 | F | |
| Caucasian | 173 | 1.37 [0.74, 2.54] | 1.01 | 0.31 | 0 | F | |
| AC vs. AA+CC | Overall | 1,638 | 1.52 [0.89, 2.57] | 1.54 | 0.12 | 78 | R |
| Asian | 1,327 | 1.11 [0.88, 1.41] | 0.87 | 0.39 | 34 | F | |
| Caucasian | 173 | 1.25 [0.66, 2.39] | 0.69 | 0.49 | 0 | F | |
| CC vs. AA | Overall | 1,150 | 2.66 [1.68, 4.22] | 4.15 | 0.01 | 49 | F |
| Asian | 923 | 2.43 [1.41, 4.18] | 3.2 | 0.01 | 39 | F | |
| Caucasian | 115 | 1.51 [0.52, 4.42] | 0.75 | 0.45 | 0 | F | |
| C vs. A | Overall | 3,276 | 1.67 [1.03, 2.71] | 2.07 | 0.04 | 83 | R |
| Asian | 2,654 | 1.39 [1.14, 1.69] | 3.3 | 0.01 | 34 | F | |
| Caucasian | 346 | 1.31 [0.80, 2.14] | 1.08 | 0.28 | 0 | F |
Effect model includes fixed-effect model (F) and random-effect model (R).
MTHFR C677T and A1298C polymorphisms in ovarian cancer
Genetic distributions and pooled ORs were shown in Tables 3 and 6. Eleven studies including 12,700 participants were evaluated for C677T polymorphisms in ovarian cancer risk. While the overall group analysis presented no relationship between C-to-T mutation and ovarian cancer susceptibility (Figure 2C), the stratified group analysis of 1455 Asian subjects indicated an increased cancer risk (TT vs. CT+CC: OR = 2.35, 95%CI = 1.59–3.48; TT+CT vs. CC: OR = 1.49, 95%CI = 1.19–1.86; TT vs. CC: OR = 2.84, 95%CI = 1.88–4.31; T vs. C: OR = 1.49, 95%CI = 1.26–1.77). Since the Asian population only consisted of a minority among the overall group subjects, it should be noted that the overall group conclusion might be changed when sample sizes of different ethnicities vary. In general, MRHFR C677R polymorphisms was associated with increased cancer risk in Asian population.
Table 3. Genotype distributions in cases and controls for MTHFR C677T and A1298C polymorphisms in ovarian cancer.
| Author | Year | Country | Polymorphism | Case | Control | ||||
|---|---|---|---|---|---|---|---|---|---|
| AA∧ | Aa | aa | AA | Aa | aa | ||||
| Gao | 2012 | Asian | C677T | 97 | 100 | 27 | 232 | 178 | 22 |
| Jakubowska | 2012 | Caucasian | C677T | 423 | 446 | 116 | 1447 | 1481 | 422 |
| Ozkilic | 2016 | Middle Eastern | C677T | 18 | 28 | 4 | 19 | 30 | 5 |
| Pawlik | 2011 | Caucasian | C677T | 67 | 55 | 13 | 63 | 79 | 18 |
| Prasad | 2011 | Asian | C677T | 72 | 3 | 5 | 116 | 8 | 1 |
| Terry# | 2010 | Caucasian | C677T | 427 | 492 | 140 | 499 | 488 | 138 |
| Terry | 2010 | Caucasian | C677T | 71 | 72 | 10 | 210 | 217 | 55 |
| Terry | 2010 | Caucasian | C677T | 164 | 167 | 33 | 193 | 168 | 51 |
| Webb | 2011 | Caucasian | C677T | 744 | 709 | 185 | 571 | 568 | 139 |
| Wu | 2007 | Asian | C677T | 17 | 40 | 24 | 32 | 35 | 13 |
| Zhang | 2012 | Asian | C677T | 102 | 94 | 19 | 115 | 92 | 11 |
| Song | 2012 | Asian | A1298C | 107 | 77 | 16 | 112 | 79 | 9 |
| Terry | 2010 | Caucasian | A1298C | 515 | 430 | 93 | 534 | 450 | 109 |
| Terry | 2010 | Caucasian | A1298C | 68 | 67 | 18 | 236 | 200 | 48 |
| Terry | 2010 | Caucasian | A1298C | 173 | 149 | 42 | 189 | 180 | 43 |
| Webb | 2011 | Caucasian | A1298C | 770 | 693 | 175 | 598 | 561 | 119 |
‘A’ represents wild-type allele and ‘a’ represents mutant allele.
Study separately genotyped subjects from three studies, the New England Case Control Study (NEC), Nurses’ Health Study (NHS), and Mayo Clinic Ovarian Cancer Case Control Study (MAY), thus were considered as three studies.
Table 6. Summary of different comparative results for MTHFR C677T polymorphisms in ovarian cancer.
| Genotypes | Overall and subgroup | Participants | OR and 95%CI | Z value | P value | I2 (%) | Effect model# |
|---|---|---|---|---|---|---|---|
| TT vs. CT+ CC | Overall | 12,700 | 1.11 [0.88, 1.41] | 0.87 | 0.39 | 61 | R |
| Asian | 1,455 | 2.35 [1.59, 3.48] | 4.3 | 0.01 | 0 | F | |
| Caucasian | 11,141 | 1.82 [0.85, 3.93] | 0.72 | 0.47 | 16 | F | |
| TT+ CT vs. CC | Overall | 12,700 | 1.06 [0.99, 1.15] | 1.58 | 0.11 | 49 | F |
| Asian | 1,455 | 1.49 [1.19, 1.86] | 3.44 | 0.01 | 3 | F | |
| Caucasian | 11,141 | 1.02 [0.94, 1.10] | 0.46 | 0.64 | 32 | F | |
| CT vs. CC+ TT | Overall | 12,700 | 1.05 [0.97, 1.13] | 1.17 | 0.24 | 0 | F |
| Asian | 1,455 | 1.11 [0.88, 1.39] | 0.89 | 0.37 | 0 | F | |
| Caucasian | 11,141 | 1.04 [0.96, 1.13] | 0.92 | 0.36 | 22 | F | |
| TT vs. CC | Overall | 7,150 | 1.18 [0.89, 1.55] | 1.16 | 0.24 | 68 | R |
| Asian | 915 | 2.84 [1.88, 4.31] | 4.93 | 0.01 | 0 | F | |
| Caucasian | 6,199 | 0.97 [0.85, 1.11] | 0.41 | 0.68 | 25 | F | |
| T vs. C | Overall | 25,400 | 1.08 [0.96, 1.21] | 1.31 | 0.19 | 66 | R |
| Asian | 2,910 | 1.49 [1.26, 1.77] | 4.6 | 0.01 | 10 | F | |
| Caucasian | 22,282 | 1.00 [0.94, 1.06] | 0 | 1 | 29 | F |
Effect model includes fixed-effect model (F) and random-effect model (R).
The results of MTHFR A1298C polymorphisms in ovarian cancer were straightforward (Figure 2D). The meta-analysis failed to show significant association between variant genotypes (or alleles) and ovarian cancer susceptibility in corresponding effect models, neither in overall group analysis nor Caucasian subgroup analysis (Tables 3 and 7).
Table 7. Summary of different comparative results for MTHFR A1298C polymorphisms in ovarian cancer.
| Genotypes | Overall and subgroup | Participants | OR and 95%CI | Z value | P value | I2 (%) | Effect model# |
|---|---|---|---|---|---|---|---|
| CC vs. AC+AA | Overall | 6,860 | 1.09 [0.92, 1.28] | 1.01 | 0.31 | 0 | F |
| Caucasian | 6,460 | 1.06 [0.90, 1.26] | 0.74 | 0.46 | 0 | F | |
| CC+AC vs. AA | Overall | 6,860 | 1.00 [0.91, 1.10] | 0.06 | 0.95 | 0 | F |
| Caucasian | 6,460 | 0.99 [0.90, 1.09] | 0.19 | 0.85 | 0 | F | |
| AC vs. AA+CC | Overall | 6,860 | 0.97 [0.88, 1.07] | 0.67 | 0.5 | 0 | F |
| Caucasian | 6,460 | 0.97 [0.88, 1.07] | 0.64 | 0.52 | 0 | F | |
| CC vs. AA | Overall | 3,974 | 1.08 [0.91, 1.27] | 0.85 | 0.4 | 0 | F |
| Caucasian | 3,730 | 1.05 [0.88, 1.25] | 0.57 | 0.57 | 0 | F | |
| C vs. A | Overall | 13,720 | 1.02 [0.94, 1.09] | 0.41 | 0.68 | 0 | F |
| Caucasian | 12,920 | 1.01 [0.93, 1.09] | 0.19 | 0.85 | 0 | F |
Effect model includes fixed-effect model (F) and random-effect model (R).
Publication bias
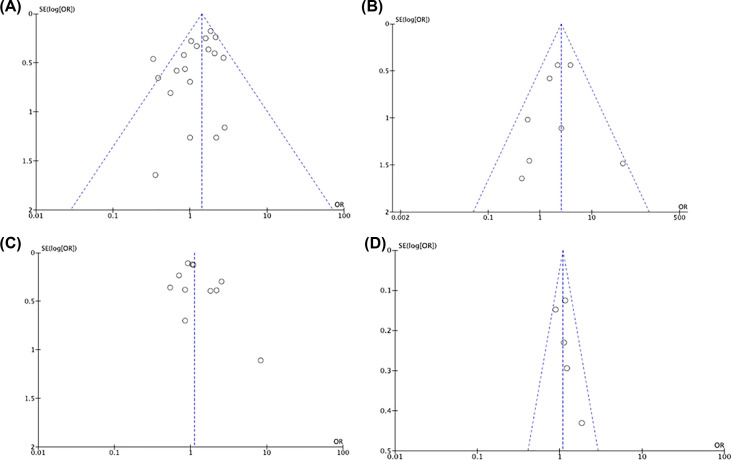
The shapes of the funnel plots appeared to be symmetrical in all genetic comparisons, indicating the lack of publication bias and the reliability of the meta-analysis in both overall and subgroups (Figure 3). We further performed Egger’s tests in the analyses that proposed significant ORs. The results demonstrated no significant publication bias (P>0.05, data not shown).
Figure 3. Representative funnel plots.
(A) TT vs. CT+CC for MTHFR C667T polymorphisms in PCOS. (B) CC vs. AC+AA for A1298C polymorphisms in PCOS. (C) TT vs. CT+CC for MTHFR C667T polymorphisms in ovarian cancer. (D) CC vs. AC+AA for A1298C polymorphisms in ovarian cancer.
Discussion
Polycystic ovary syndrome consists a heterogeneous endocrinological disorder, which is characterized by oligo- or anovulation, hyperandrogenism and polycystic ovaries [50]. These pathophysiological malfunctions are associated with clinical manifestations like oligomenorrhea, amenorrhea, infertility, hirsutism, obesity, acne, Type 2 diabetes mellitus, skin hyperpigmentation, etc [51,52]. The long term of treatments and the consequential infertility seriously affect women’s living qualities, even though many symptoms could be meliorated by oral contraceptive and metformin. Contradictory evidence exists regarding PCOS and risk of ovarian cancer. Some studies suggested that self-reported PCOS was related to higher risk of ovarian cancer when age was adjusted, while others concluded no significant association between the two diseases. Moreover, several studies reported that PCOS might decrease the risk of ovarian cancer because the use of oral contraceptives were common among such patients [53,54]. The etiology of PCOS and ovarian cancer involve multiple genetic and epigenetic alterations that cause changes in metabolic enzymes such as MTHFR. It has been reported that polymorphisms of the MTHFR gene might reduce MTHFR enzyme activity. As a result, the concentration of Hcy in plasma would be increased, which is commonly discovered in PCOS and ovarian cancer patients. Even recently, several trials have explored the efficacy of folate receptor antagonist in ovarian cancer [55]. This clinically reflects the significance of folate metabolic pathways in ovarian carcinogenesis. Therefore, it is assumed that the abnormality (including several polymorphisms) of MTHFR, which is a key component of the folate pathways, could contribute to increased PCOS and ovarian cancer risk.
In the present study, we performed an updated meta-analysis to explore MTHFR C667T and A1298C polymorphisms in the PCOS and ovarian cancer risk. Twenty-nine articles including 45 case–control studies were included. We found that MTHFR C677T polymorphisms were correlated with elevated PCOS risk, which were more obvious in Middle Eastern subgroups. Also, MTHFR A1298C polymorphisms were associated with overall PCOS risk, which were mainly reflected in Asians. For ovarian cancer, MTHFR C677T polymorphisms were only related with elevated ovarian cancer risk in Asians, while no significant association was found for A1298C polymorphisms. Several points could be noted based on current results. First, both MTHFR C677T and MTHFR A1298C polymorphisms were correlated with elevated PCOS risk. Although polymorphisms of A1298C seem to be less prevalent than C677T, the current findings suggest that any impairment on folate methylation could impact the development of PCOS, regardless of its frequency [16]. As such, new genetic abnormalities in folate metabolism pathways are of great potential in unrevealing the critical pathogenic factor of PCOS. Second, ethnicity difference seems to have an impact on the disease susceptibility. Despite confounding factors that could not be excluded from the study, such findings may indicate that different genetic components could play different extends of roles in the disease development, leading to different manifestations. For instance, it was reported that East Asian patients with PCOS were more likely to have diabetes compared with Caucasian patients [56]. Thus, continuous genome-based studies are needed to personalize the diagnosis and management of PCOS across different ethnicities. Third, the relationship between MTHFR and ovarian cancer is less conclusive compared with PCOS. While both polymorphisms are associated with increased PCOS risk in overall population, significant result was only observed in MTHFR C667T Asian subgroup for ovarian cancer. PCOS has been hypothesized to increase ovarian cancer risk through androgen exposure in pre-clinical settings and oral contraceptive use has been proved to reduce the risk of ovarian cancer, suggesting the potential metabolic relationship between PCOS and ovarian cancer [57,58]. However, it is hard to conclude a concrete relationship between PCOS and ovarian cancer under the mechanism of MTHFR gene polymorphisms based on the current results. Whereas such findings could not rule out the potential relationship between PCOS and ovarian cancer risk. In a population-based, case–control study of 476 subjects with histologically confirmed epithelial ovarian cancer, Schildkraut et al. found that ovarian cancer risk was found to increase 2.5-fold (95% CI: 1.1–5.9) among women with PCOS [59]. Moreover, the possibility that the pathogenesis of PCOS and ovarian cancer might be interacted on the pathways of hormone metabolism still exist. In fact, recent identification of several proteins overexpressed in both PCOS and ovarian cancer, including calreticulin, fibrinogen, superoxide dismutase, and vimentin gave us a clue on the two diseases and even promising subgroup identification of ovarian cancer based on PCOS development. As such, whether MTHFR polymorphisms played a role in this intriguing contribution remains to be further studied.
Despite our efforts to pool the results of currently published studies, some disadvantages of the present meta-analysis still existed. First, the number of enrolled studies was so far the largest among all relevant meta-analysis but was still limited. It is possible that the results of further investigations and unpublished articles might be different from the present conclusion, thus cautions should be paid to explain the results. Second, this meta-analysis was based on unadjusted estimations. Although matched parameters were carefully reviewed, it is known that some unreported risk factors like family history and genetic information (e.g. BRCA mutation, HRD status) were also important in the development of PCOS or ovarian cancer [60]. Meanwhile, no histological subtypes of ovarian cancer were provided in the source studies. Since it is reported that an elevation in ovarian cancer risk might be relevant to only certain histological subtypes [9,10], the absence or enrichment of certain types of ovarian cancer in the present study might not reveal the real-world disease landscape. All those confounding factors mentioned above might affect the validity of the results. In addition, MTHFR polymorphisms and elevated homocysteine levels may increase risks of several other diseases such as thromboembolism, endometrial cancer, hypertension, diabetes, etc. Such conditions themselves may interact with diet, concurrent medication and lifestyle, thus affecting the susceptibility of PCOS and ovarian cancer. Third, we confirmed the risk prone effects of MTHFR polymorphisms in Middle Eastern population for PCOS and particular MTHFR A1298C polymorphisms in Asian population for ovarian cancer. The ethnicities should be deliberately illustrated because of two reasons. One is that the ethnicity distribution in the overall group did not reflect the real-world percentages. The sample size changes due to future publications in Asian population for C667T in ovarian cancer risk might affect the final readout of overall group analysis. The other is that only Turkish and Iranian were included in the Middle Eastern subgroup and most of the stratified Asians were Chinese, while other populations such as Hispanics and Black were not within this discussion. Thus, studies enrolling diverse ethnicities were required.
Conclusion
To our knowledge, the present study was the most updated meta-analysis exploring the association between MTHFR C677T and A1298C polymorphisms and the PCOS and ovarian cancer. It was also the first study to explore the MTHFR polymorphisms in both diseases. Although it was hard to conclude a concrete association between PCOS and ovarian cancer under the mechanism of MTHFR gene polymorphisms, the present study suggested that MTHFR C677T and A1298C polymorphisms were correlated with elevated PCOS risk while MTHFR C677T polymorphisms only posed a higher risk for ovarian cancer in Asians. Further studies are needed to validate the conclusion.
Abbreviations
- AMH
Anti-Müllerian hormone
- BMI
body mass index
- DHEA-S
dehydroepiandrosterone sulfate
- FSH
follicle-stimulating hormone
- HDL-C
high density lipoprotein-cholesterol
- LDL-C
low density lipoprotein-cholesterol
- LH
luteinizing hormone
- MTHFR
methylenetetrahydrofolate reductase
- NOS
Newcastle–Ottawa scale
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by Key Research Projects of Science and Technology Department Foundation of Sichuan Province [grant number 2017SZ0141] and Applied Basic Research Programs of Science and Technology Department Foundation of Sichuan Province [grant number 2016JY0122].
Author Contribution
Y.X., C.B. and X.Z. contributed to study concept and design. C.B. and X.L. contributed to literature search and data extractions. X.W. and Y.X. contributed to data analysis and the study quality evaluation. K.X. and X.Z. contributed to the methodology. K.X. and X.Z. contributed to the supervision of all the study processes. All the authors contributed to writing and revising the manuscript. All authors approved the final version of the manuscript
References
- 1.Franks S. (1995) Polycystic ovary syndrome. N. Engl. J. Med. 333, 853–861 10.1056/NEJM199509283331307 [DOI] [PubMed] [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 81, 19–25 [DOI] [PubMed] [Google Scholar]
- 3.Chambers J.C. and Kooner J.S. (2001) Homocysteine: a novel risk factor for coronary heart disease in UK Indian Asians. Heart 86, 121–122 10.1136/heart.86.2.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang R.J. (2004) A practical approach to the diagnosis of polycystic ovary syndrome. Am. J. Obstet. Gynecol. 191, 713–717 10.1016/j.ajog.2004.04.045 [DOI] [PubMed] [Google Scholar]
- 5.Carmina E. (2006) Metabolic syndrome in polycystic ovary syndrome. Minerva Ginecol. 58, 109–114 [PubMed] [Google Scholar]
- 6.Zhang J., Bao Y., Zhou X. and Zheng L. (2019) Polycystic ovary syndrome and mitochondrial dysfunction. Reproduct. Biol. Endocrinol. 17, 67 10.1186/s12958-019-0509-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumesic D.A. and Lobo R.A. (2013) Cancer risk and PCOS. Steroids 78, 782–785 10.1016/j.steroids.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 8.Barry J.A., Azizia M.M. and Hardiman P.J. (2014) Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. Update 20, 748–758 10.1093/humupd/dmu012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen C.M., Green A.C., Nagle C.M., Jordan S.J., Whiteman D.C., Bain C.J. et al. (2008) Epithelial ovarian cancer: testing the ‘androgens hypothesis’. Endocr. Relat. Cancer 15, 1061–1068 10.1677/ERC-08-0075 [DOI] [PubMed] [Google Scholar]
- 10.Harris H., Titus L., Cramer D. and Terry K. (2016) Long and irregular menstrual cycles, polycystic ovary syndrome, and ovarian cancer risk in a population-based case–control study. Int. J. Cancer 140, 285–291 10.1002/ijc.30441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamanti-Kandarakis E. (2008) Polycystic ovarian syndrome: pathophysiology, molecular aspects and clinical implications. Exp. Rev. Mol. Med. 10, e3 10.1017/S1462399408000598 [DOI] [PubMed] [Google Scholar]
- 12.Goodarzi M.O., Dumesic D.A., Chazenbalk G. and Azziz R. (2011) Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 7, 219–231 10.1038/nrendo.2010.217 [DOI] [PubMed] [Google Scholar]
- 13.Mtiraoui N., Ezzidi I., Chaieb M., Marmouche H., Aouni Z., Chaieb A. et al. (2007) MTHFR C677T and A1298C gene polymorphisms and hyperhomocysteinemia as risk factors of diabetic nephropathy in type 2 diabetes patients. Diabetes Res. Clin. Pract. 75, 99–106 10.1016/j.diabres.2006.05.018 [DOI] [PubMed] [Google Scholar]
- 14.Muslumanoglu M.H., Tepeli E., Demir S., Uludag A., Uzun D., Atli E. et al. (2009) The analysis of the relationship between A1298C and C677T polymorphisms of the MTHFR gene with prostate cancer in Eskisehir population. Genetic Testing Mol. Biomarkers 13, 641–645 10.1089/gtmb.2009.0046 [DOI] [PubMed] [Google Scholar]
- 15.Hickey S.E., Curry C.J. and Toriello H.V. (2013) ACMG Practice Guideline: lack of evidence for MTHFR polymorphism testing. Genet. Med. 15, 153–156 10.1038/gim.2012.165 [DOI] [PubMed] [Google Scholar]
- 16.Yang B., Liu Y., Li Y., Fan S., Zhi X., Lu X. et al. (2013) Geographical distribution of MTHFR C677T, A1298C and MTRR A66G gene polymorphisms in China: findings from 15357 adults of Han nationality. PLoS ONE 8, e57917 10.1371/journal.pone.0057917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glueck C.J., Wang P., Fontaine R.N., Sieve-Smith L., Tracy T. and Moore S.K. (1999) Plasminogen activator inhibitor activity: an independent risk factor for the high miscarriage rate during pregnancy in women with polycystic ovary syndrome. Metabolism 48, 1589–1595 10.1016/S0026-0495(99)90250-0 [DOI] [PubMed] [Google Scholar]
- 18.Lee Y.H. and Song G.G. (2014) Plasminogen activator inhibitor-1 4G/5G and the MTHFR 677C/T polymorphisms and susceptibility to polycystic ovary syndrome: a meta-analysis. Eur. J. Obstetr. Gynecol. Reproduct. Biolol. 175, 8–14 10.1016/j.ejogrb.2013.12.030 [DOI] [PubMed] [Google Scholar]
- 19.Fu L.Y., Dai L.M., Li X.G., Zhang K. and Bai Y. (2014) Association of methylenetetrahydrofolate reductase gene C677T polymorphism with polycystic ovary syndrome risk: a systematic review and meta-analysis update. Eur. J. Obstet. Gynecol. Reproduct. Biolol. 172, 56–61 10.1016/j.ejogrb.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 20.Karimi-Zarchi M., Moghimi M., Abbasi H., Hadadan A., Salimi E., Morovati-Sharifabad M. et al. (2019) Association of MTHFR 677C>T Polymorphism with Susceptibility to Ovarian and Cervical Cancers: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 20, 2569–2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He L. and Shen Y. (2017) MTHFR C677T polymorphism and breast, ovarian cancer risk: a meta-analysis of 19,260 patients and 26,364 controls. OncoTargets Ther. 10, 227–238 10.2147/OTT.S121472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlus S.J., Sarkar S., Bansal S.K., Singh V., Singh K., Jha R.K. et al. (2016) Is MTHFR 677 C>T Polymorphism Clinically Important in Polycystic Ovarian Syndrome (PCOS)? A Case-Control Study, Meta-Analysis and Trial Sequential Analysis PLoS ONE. 11, e0151510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi Q., Zhang H., Yu M., Wang X., Wang Z., Xu L. et al. (2015) Association of methylenetetrahydrofolate reductase gene polymorphisms with polycystic ovary syndrome. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 32, 400–404 [DOI] [PubMed] [Google Scholar]
- 24.Wu J.B., Zhai J.F. and Yang J. (2016) Role of methylenetetrahydrofolate reductase C677T and A1298C polymorphisms in polycystic ovary syndrome risk. Genetic and Mol. Res. 15, 10.4238/gmr15048570 [DOI] [PubMed] [Google Scholar]
- 25.Choi S.W., Gu B.H., Ramakrishna S., Park J.M. and Baek K.H. (2009) Association between a single nucleotide polymorphism in MTHFR gene and polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reproduct. Biol. 145, 85–88 10.1016/j.ejogrb.2009.04.013 [DOI] [PubMed] [Google Scholar]
- 26.Idali F., Zareii S., Mohammad-Zadeh A., Reihany-Sabet F., Akbarzadeh-Pasha Z., Khorram-Khorshid H.R. et al. (2012) Plasminogen activator inhibitor 1 and methylenetetrahydrofolate reductase gene mutations in iranian women with polycystic ovary syndrome. Am. J. Reprod. Immunol. 68, 400–407 10.1111/aji.12002 [DOI] [PubMed] [Google Scholar]
- 27.Jain M., Pandey P., Tiwary N.K. and Jain S. (2012) MTHFR C677T polymorphism is associated with hyperlipidemia in women with polycystic ovary syndrome. J. Human Reproduct. Sci. 5, 52–56 10.4103/0974-1208.97802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karadeniz M., Erdogan M., Zengi A., Eroglu Z., Tamsel S., Olukman M. et al. (2010) Methylenetetrahydrofolate reductase C677T gene polymorphism in Turkish patients with polycystic ovary syndrome. Endocrine 38, 127–130 10.1007/s12020-010-9370-0 [DOI] [PubMed] [Google Scholar]
- 29.Orio F. Jr, Palomba S., Di Biase S., Colao A., Tauchmanova L., Savastano S. et al. (2003) Homocysteine levels and C677T polymorphism of methylenetetrahydrofolate reductase in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 88, 673–679 10.1210/jc.2002-021142 [DOI] [PubMed] [Google Scholar]
- 30.Ozegowska K., Bogacz A., Bartkowiak-Wieczorek J., Seremak-Mrozikiewicz A. and Pawelczyk L. (2016) Is there an association between the development of metabolic syndrome in PCOS patients and the C677T MTHFR gene polymorphism? Ginekol. Pol. 87, 246–253 10.17772/gp/61751 [DOI] [PubMed] [Google Scholar]
- 31.Palep-Singh M., Picton H.M., Yates Z.R., Barth J. and Balen A.H. (2007) Polycystic ovary syndrome and the single nucleotide polymorphisms of methylenetetrahydrofolate reductase: a pilot observational study. Human Fertility (Camb) 10, 33–41 10.1080/14647270600950157 [DOI] [PubMed] [Google Scholar]
- 32.Sills E.S., Genton M.G., Perloe M., Schattman G.L., Bralley J.A. and Tucker M.J. (2001) Plasma homocysteine, fasting insulin, and androgen patterns among women with polycystic ovaries and infertility. J. Obstetetrics Gynaecologic Res. 27, 163–168 10.1111/j.1447-0756.2001.tb01241.x [DOI] [PubMed] [Google Scholar]
- 33.Tsanadis G., Vartholomatos G., Korkontzelos I., Avgoustatos F., Kakosimos G., Sotiriadis A. et al. (2002) Polycystic ovarian syndrome and thrombophilia. Hum. Reprod. 1, 314–319 10.1093/humrep/17.2.314 [DOI] [PubMed] [Google Scholar]
- 34.Geng J.X., Zhang C.L. and Hu S.W. (2016) Role of methylenetetrahydrofolate reductase genetic polymorphisms in polycystic ovary syndrome risk. Int. J. Clin. Exp. Pathol. 9, 8532–8537 [Google Scholar]
- 35.Jiang Y., Yan L. and Li Y. (2015) Study on the correlation between methylenetetrahydrofolate reductase gene polymorphisms and polycystic ovary syndrome. Maternal & Child Health Care of China, vol. 30, pp. 3831–3833 [Google Scholar]
- 36.Jiao X., Chen W., Zhang J., Wang W., Song J., Chen D. et al. (2018) Variant Alleles of the ESR1, PPARG, HMGA2, and MTHFR genes are associated with polycystic ovary syndrome risk in a Chinese population: A case-control study. Front. Endocrinol. (Lausanne) 9, 504 10.3389/fendo.2018.00504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee K.W., Jeong Y.M. and Lee S.H. (2003) The study of 5,10- methylenetetrahydrofolate reductase variation (MTHFR C677T) in infertile females with polycystic ovarian syndrome. Korean J. Fert. Ster. 30, 217–222 [Google Scholar]
- 38.Naghavi A., Mozdarani H., Garshasbi M. and Yaghmael M. (2015) Prevalence of methylenetetrahydrofolate reductase C677T polymorphism in women with polycystic ovary syndrome in southeast of Iran. J. Med. Life 8, 229–232 [PMC free article] [PubMed] [Google Scholar]
- 39.Szafarowska M., Segiet A. and Jerzak M.M. (2016) Methylenetetrahydrofolate reductase A1298C and C677T polymorphisms and adverse pregnancy outcome in women with PCOS. Neuro Endocrinol. Lett. 37, 141–146 [PubMed] [Google Scholar]
- 40.Gao S., Liu N., Ma Y. and Ying L. (2012) Methylenetetrahydrofolate reductase gene polymorphisms as predictive and prognostic biomarkers in ovarian cancer risk. Asian Pac. J. Cancer Prev. 13, 569–573 10.7314/APJCP.2012.13.2.569 [DOI] [PubMed] [Google Scholar]
- 41.Jakubowska A., Gronwald J., Menkiszak J., Górski B., Huzarski T., Byrski T. et al. (2007) Methylenetetrahydrofolate reductase polymorphisms modify BRCA1-associated breast and ovarian cancer risks. Breast Cancer Res. Treat. 104, 299–308 10.1007/s10549-006-9417-3 [DOI] [PubMed] [Google Scholar]
- 42.Ozkilic A.C., Cetin A. and Bayoglu B. (2016) Association between MTHFR C677T polymorphism and folate, vitamin B12, homocysteine, and DNA fragmentation in patients with ovarian cancer. Turk Biyokimya Dergisi. 41, 459–465 [Google Scholar]
- 43.Pawlik P., Mostowska A., Lianeri M., Sajdak S., Kędzia H. and Jagodzinski P.P. (2012) Folate and choline metabolism gene variants in relation to ovarian cancer risk in the Polish population. Mol. Biol. Rep. 39, 5553–5560 10.1007/s11033-011-1359-0 [DOI] [PubMed] [Google Scholar]
- 44.Prasad V.V.T.S. and Wilkhoo H. (2011) Association of the functional polymorphism C677T in the methylenetetrahydrofolate reductase gene with colorectal, thyroid, breast, ovarian, and cervical cancers. Onkologie 34, 422–426 10.1159/000331131 [DOI] [PubMed] [Google Scholar]
- 45.Terry K.L., Tworoger S.S., Goode E.L., Gates M.A., Titus-Ernstoff L., Kelemen L.E. et al. (2010) MTHFR polymorphisms in relation to ovarian cancer risk. Gynecol. Oncol. 119, 319–324 10.1016/j.ygyno.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webb P.M., Ibiebele T.I., Hughes M.C., Beesley J., van der Pols J.C., Chen X. et al. (2011) Australian cancer study (Ovarian Cancer), Australian ovarian cancer study group. Folate and related micronutrients, folate-metabolising genes and risk of ovarian cancer. Eur. J. Clin. Nutr. 65, 1133–1140 10.1038/ejcn.2011.99 [DOI] [PubMed] [Google Scholar]
- 47.Wu Y., Zhang J. and Zuo W. (2007) Association between genetic polymorphisms of methylenetetrahydrofolate reductase C677T and susceptibility to ovarian cancer. Prog. Obstet. Gynecol. 16, 811–813 [Google Scholar]
- 48.Zhang L., Liu W., Hao Q., Bao L. and Wang K. (2012) Folate intake and methylenetetrahydrofolate reductase gene polymorphisms as predictive and prognostic biomarkers for ovarian cancer risk. Int. J. Mol. Sci. 13, 4009–4020 10.3390/ijms13044009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song C.X., Ping L. and Ting W. (2012) Folate. MTHFR C677T and A1298C polymorhpisms with the relationship with ovarian cancer risk among Chinese females. African J. Microbiologic Res. 6, 4761–4766 [Google Scholar]
- 50.Franks S., Gharani N., Waterworth D., Franks S., Gharani N. and Waterworth D. (1997) The genetic basis of polycystic ovary syndrome. Hum. Reprod. 12, 2641–2648 10.1093/humrep/12.12.2641 [DOI] [PubMed] [Google Scholar]
- 51.Wild R.A. (1995) Obesity, lipids, cardiovascular risk, and androgen excess. Am. J. Med. 98, 27s–32s 10.1016/S0002-9343(99)80056-4 [DOI] [PubMed] [Google Scholar]
- 52.Marcondes J.A., Hayashida S.A., Barcellos C.R., Rocha M.P., Maciel G.A. and Baracat E.C. (2007) Metabolic syndrome in women with polycystic ovary syndrome: prevalence, characteristics and predictors. Arquivos Brasileiros De Endocrinologia Metabologia 51, 972–979 10.1590/S0004-27302007000600012 [DOI] [PubMed] [Google Scholar]
- 53.Norman R.J., Dewailly D., Legro R.S. and Hickey T.E. (2007) Polycystic ovary syndrome. Lancet 370, 685–697 10.1016/S0140-6736(07)61345-2 [DOI] [PubMed] [Google Scholar]
- 54.Lim S.S., Davies M.J., Norman R.J. and Moran L.J. (2012) Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. Update 18, 618–637 10.1093/humupd/dms030 [DOI] [PubMed] [Google Scholar]
- 55.Cheung A., Bax H.J., Josephs D.H., Ilieva K.M., Pellizzari G., Opzoomer J. et al. (2016) Targeting folate receptor alpha for cancer treatment. Oncotarget 7, 52553–52574 10.18632/oncotarget.9651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim J.J. and Choi Y.M. (2019) Phenotype and genotype of polycystic ovary syndrome in Asia: Ethnic differences. J. Obstet. Gynaecologic Res. 45, 2330–2337 10.1111/jog.14132 [DOI] [PubMed] [Google Scholar]
- 57.Beral V., Doll R., Hermon C., Peto R., Reeves G. and Collaborative Group on Epidemiological Studies of Ovarian Cancer (2008) Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet 371, 303–314 [DOI] [PubMed] [Google Scholar]
- 58.Shen C.C., Yang A.C., Hung J.H., Hu L.Y. and Tsai S.J. (2015) A nationwide population-based retrospective cohort study of the risk of uterine, ovarian and breast cancer in women with polycystic ovary syndrome. Oncologist 20, 45–49 10.1634/theoncologist.2014-0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schildkraut J.M., Schwingl P.J., Bastos E., Evanoff A. and Hughes C. (1996) Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet. Gynecol. 88, 554–559 10.1016/0029-7844(96)00226-8 [DOI] [PubMed] [Google Scholar]
- 60.Joseph N., Reddy A.G., Joy D., Patel V., Santhosh P., Das S. et al. (2016) Study on the proportion and determinants of polycystic ovarian syndrome among health sciences students in South India. J. Nat. Sci. Biol. Med. 7, 166–172 10.4103/0976-9668.184704 [DOI] [PMC free article] [PubMed] [Google Scholar]