Introduction
Initially described by Shulman in 1974, eosinophilic fasciitis (EF) is a rare fibrosing disorder involving the fascia.1 Patients with EF are often treated with systemic glucocorticoids as a first-line treatment.1 The pathogenesis is unclear; immunophenotyping studies have found involvement of immune-mediated mechanisms involving macrophages and CD8+ T lymphocytes.2 There are increasing reports of cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) in cancer therapy.3 Reported cutaneous autoimmune or autoinflammatory conditions induced by ICIs include scleroderma, dermatomyositis, and cutaneous lupus erythematosus.3 More recently, there are reports of EF in patients on anti–programmed death-1 or anti–programmed death ligand-1 therapy, usually requiring treatment interruption and systemic immunosuppression.4, 5, 6, 7, 8, 9, 10 We present a case of new-onset EF during nivolumab therapy managed without treatment interruption or systemic immunosuppression.
Case report
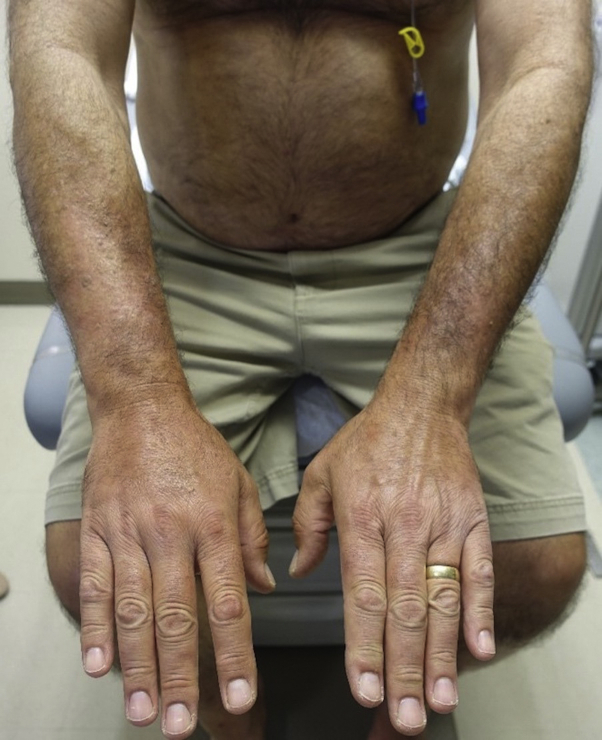
A 59-year-old white man with metastatic neuroendocrine carcinoma presented to the skin toxicity clinic at the Dana-Farber Cancer Institute with new-onset eruption on the bilateral forearms and left lower extremity after treatment with nivolumab for 2 years with partial response. Physical examination found woody, indurated plaques with overlying alopecia on the bilateral dorsal forearms and left anterior lower leg (Fig 1). Dense pitting edema was limited to the right dorsal hand. The skin overlying the left lower leg and bilateral forearms, right greater than left, was bound down and immobile. Foci on the flanks showed subtle dimpling without decreased range of motion (ROM). Flexion and extension of the wrists showed reduced ROM, right greater than left; there was slightly reduced ROM in left ankle plantar flexion and dorsiflexion. There were no nail fold capillary changes, Raynaud phenomenon, telangiectasia, or sclerodactyly. There was no history of recent excessive exercise.
Fig 1.
Clinical image of patient's arms. Area of woody, indurated edema with linear demarcation, overlying hair loss, and sclerosis. No sclerodactyly or edema of the fingers.
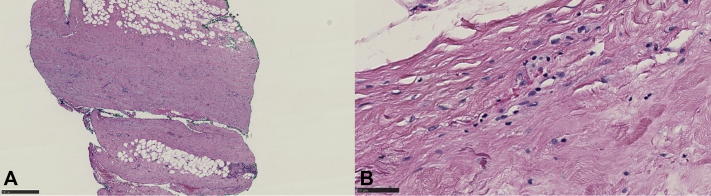
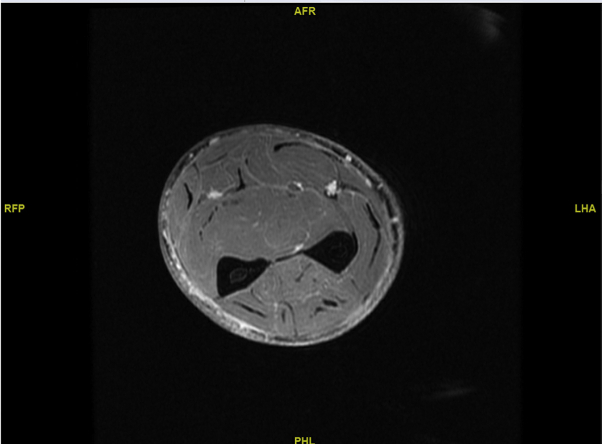
Skin biopsy from the right forearm showed diffuse dermal and subcutaneous sclerosis. Biopsy from the deep fascia of the right forearm showed prominent fascial sclerosis compatible with EF (Fig 2). Eosinophil count and aldolase levels were normal. A serum protein electrophoresis did not show a polyclonal hypergammaglobulinemia. Pulmonary function tests (PFTs) found no restrictive abnormalities. Magnetic resonance imaging (MRI) of the right forearm showed intrafascial and intrafascicular enhancement of the muscles with subdermal enhancement (Fig 3).
Fig 2.
Histopathology of right forearm deep fascia biopsy at ×4 (A) and ×40 (B) original magnification, respectively. Prominent fascial sclerosis with eosinophils, compatible with eosinophilic fasciitis.
Fig 3.
Magnetic resonance imaging, T1, of the right forearm shows mild intrafascial and intrafasicular enhancement.
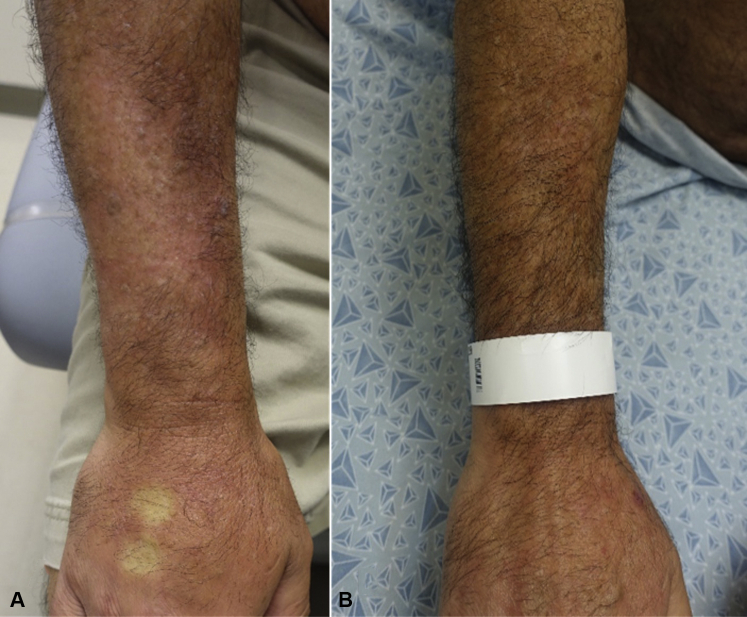
The patient was started on topical clobetasol 0.05% ointment under occlusion to affected areas twice daily with partial response. Natural ultraviolet light exposure was recommended for 10 to 20 minutes 4 times weekly in addition to physical therapy. Given his aggressive malignancy responsive to checkpoint blockade, lack of restrictive findings on PFTs, and close monitoring, nivolumab was continued. After 2 months and at subsequent follow-up visits, there were no new lesions and slow improvement of edema and overlying alopecia on his bilateral forearms and left lower extremity. He had gradual improvement in stiffness and ROM of the forearms with hair regrowth over the next 1.5 years with treatment (Fig 4, A and B).
Fig 4.
A, Clinical image of patient's right arm at presentation. Area of woody, indurated edema with linear demarcation on the proximal forearm, overlying hair loss, and sclerosis. B, Clinical image of patient's right arm after topical steroids under occlusion with ROM exercises, showing resolution of edema and hair regrowth after 1.5 years.
Discussion
Although immunotherapy-induced EF has been reported in the literature, we present an unusual case of EF associated with ICI therapy that did not require immunotherapy interruption or systemic immunosuppressive treatment. True incidence for EF from immunotherapy is unknown.1 Although it remains to be validated, the most recent criteria for EF diagnosis requires either both major criteria or 1 major and 2 minor criteria.2 Major criteria include (1) swelling, induration, and thickening of the skin and subcutaneous tissue, diffuse or localized and (2) fascial thickening with accumulation of lymphocytes and macrophages with or without eosinophilic infiltration on full-thickness biopsy. Minor criteria include eosinophilia greater than 0.5 × 109/L, hypergammaglobulinemia greater than 1.5 g/L, muscle weakness and/or elevated aldolase levels, groove sign and/or peau d'orange, and hyperintense fascia on magnetic resonance T2-weighted images.2 Although our patient did not have eosinophilia (seen in 63%-93% of patients2) or a polyclonal hypergammaglobulinemia (up to 50%2), he satisfied the 2 major criteria for the diagnosis of EF. He fulfilled 2 minor criteria including changes consistent with peau d'orange and intrafascial and intrafascicular enhancement on MRI. Our study supports the use of additional diagnostic tools for EF including MRI and full-thickness skin-to-fascia biopsy. We also performed workup for systemic involvement including PFTs.
In a recent literature review, the authors found 15 cases of fasciitis from immunotherapy, with 9 fulfilling the proposed diagnostic criteria for EF.5 To our knowledge, there are 10 reported cases of EF from ICI therapy in the literature.4, 5, 6, 7, 8, 9, 10 Among these 10 cases, the median age was 55 years (range, 43-77), 5 were females, median onset was 11 months (3-22) after initiation of ICI therapy, and 8 of 9 (89%) had peripheral eosinophilia. Immunotherapy included atezolizumab, nivolumab, pembrolizumab, or nivolumab and ipilimumab combination therapy. All had discontinuation of their respective ICIs due to new-onset EF, and 9 of 10 (90%) were started on systemic immunosuppressive therapy, including prednisone (regimens ranging from 40-80 mg daily), infliximab (3 mg/kg), mycophenolate mofetil (3 g/d), solumedrol (24 mg 6-day taper), or methotrexate (20 mg/wk).5
Given its rarity, the guidelines for stratification and treatment for patients with EF are lacking. This situation is further complicated for those on ICIs. For our patient, given the response of his malignancy to nivolumab, absence of systemic involvement, and our ability to closely monitor his toxicity, immunotherapy was not interrupted. Progression of EF was halted by 2 months' follow-up, with gradual improvement of skin findings over the next 1.5 years with topical corticosteroids under occlusion, diligent physical therapy, and natural ultraviolet light. Although no randomized trials have evaluated EF therapies, systemic glucocorticoids are typically the mainstay and first-line treatment.1 Other steroid-sparing therapies include methotrexate, mycophenolate, or hydroxychloroquine.1 The treatment of patients with EF on ICIs poses a unique challenge. There are increasing data to suggest that glucocorticoids may negatively affect the antitumor efficacy of ICIs, including overall survival.11 Moreover, the effect may be dose dependent as seen in patients with ICI-induced hypophysitis, with patients receiving higher doses (maximum average daily dose >7.5 mg during initial 2 months in one study) of glucocorticoids having reduced survival compared with those on lower doses (maximum average daily dose <7.5 mg).11 Further characterization of the dose-dependent effect of systemic glucocorticoids for other irAEs remains to be studied. We carefully considered our patient's aggressive malignancy and his response to immunotherapy as well as the severity of his EF. With localized involvement and our ability to monitor him very closely, we continued his ICI, deferred systemic immunosuppression treatment, and managed his symptoms with topical steroids, physical therapy and natural ultraviolet light. He had a positive response, and these interventions were well tolerated. The judicious use of systemic immunosuppressive therapy for patients on ICIs is increasingly important as the range of cutaneous irAEs increases. Our case supports the consideration of local rather than systemic immunosuppressive therapies with close monitoring in the treatment of immunotherapy-induced EF with localized involvement.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed
References
- 1.Mazori D.R., Femia A.N., Vleugels R.A. Eosinophilic fasciitis: an updated review on diagnosis and treatment. Curr Rheumatol Rep. 2017;19(12):74. doi: 10.1007/s11926-017-0700-6. [DOI] [PubMed] [Google Scholar]
- 2.Fett N., Arthur M. Eosinophilic fasciitis: current concepts. Clin Dermatol. 2018;36(4):487–497. doi: 10.1016/j.clindermatol.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19(3):345–361. doi: 10.1007/s40257-017-0336-3. [DOI] [PubMed] [Google Scholar]
- 4.Andres-Lencina J.J., Burillo-Martinez S., Aragon-Miguel R. Eosinophilic fasciitis and lichen sclerosus in a patient treated with nivolumab. Australas J Dermatol. 2018;59(4):e302–e304. doi: 10.1111/ajd.12836. [DOI] [PubMed] [Google Scholar]
- 5.Chan K.K., Magro C., Shoushtari A. Eosinophilic fasciitis following checkpoint inhibitor therapy: four cases and a review of literature. Oncologist. 2020;25:140. doi: 10.1634/theoncologist.2019-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Tallec E., Ricordel C., Triquet L. An original case of an association of eosinophilic fasciitis with cholangitis induced by nivolumab. J Thorac Oncol. 2019;14(1):e13–e15. doi: 10.1016/j.jtho.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Toussaint F., Hammon M., Erdmann M. Checkpoint inhibitor-induced eosinophilic fasciitis following high eosinophilia associated with complete response. Rheumatology (Oxford) 2019;58(10):1875–1877. doi: 10.1093/rheumatology/kez164. [DOI] [PubMed] [Google Scholar]
- 8.Khoja L., Maurice C., Chappell M. Eosinophilic fasciitis and acute encephalopathy toxicity from pembrolizumab treatment of a patient with metastatic melanoma. Cancer Immunol Res. 2016;4(3):175–178. doi: 10.1158/2326-6066.CIR-15-0186. [DOI] [PubMed] [Google Scholar]
- 9.Lidar M., Giat E., Garelick D. Rheumatic manifestations among cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev. 2018;17(3):284–289. doi: 10.1016/j.autrev.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Wissam Y., Belcaid L., Wittoek R. Eosinophilic fasciitis in a patient treated by atezolizumab for metastatic triple-negative breast cancer. J Immunother Precis Oncol. 2019;2(3):101–105. [Google Scholar]
- 11.Faje A.T., Lawrence D., Flaherty K. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124(18):3706–3714. doi: 10.1002/cncr.31629. [DOI] [PubMed] [Google Scholar]