Introduction
Systemic therapy targeting CD20, such as rituximab, is a valuable first-line therapeutic option for moderate-to-severe pemphigus vulgaris (PV).1 However, for patients with PV who experience side effects (such as infusion reactions including anaphylaxis) or loss of treatment response to rituximab, this presents a challenging clinical situation. A prior report described a patient with severe PV, who experienced a serum sickness-like reaction with rituximab, achieving complete clinical clearance with durable response using off-label ofatumumab.2 Ofatumumab is a fully human, second-generation, high-affinity anti-CD20 monoclonal antibody approved for treatment of chronic lymphocytic leukemia, which targets a different CD20 epitope than rituximab.3 Here we report an additional patient with severe, refractory PV successfully treated with ofatumumab with a distinct clinical course.
Case report
A 34-year-old woman, with active mucocutaneous PV disease diagnosed at age 25, did not achieve remission with doxycycline, high-dose prednisone, mycophenolate mofetil (MMF), and methotrexate. Ten days after her first rituximab infusion, bilateral progressive lower extremity weakness developed, resulting in hospitalization. Although unclear if this reaction was related to rituximab, bilateral lower extremity weakness and Guillain-Barre syndrome have been reported in association with rituximab, although these adverse effects often occur several weeks after infusion.4 Given the severity of symptoms, rituximab was not re-trialed.
Over the next 18 months, the patient's disease remained intermittently controlled on prednisone, MMF, and intravenous immunoglobulin (IVIg). However, when the patient was discovered to be 8 weeks pregnant, given teratogenicity, MMF was discontinued, and IVIg monotherapy was initiated. Following delivery of a healthy child, the combination regimen of prednisone, 30 mg daily, MMF, 1500 mg twice daily, and IVIg monthly was restarted. Over the next 7 months while on prednisone, MMF, and IVIg, the patient continued to have severe oral mucosal involvement and innumerable flaccid bullae and erosions on her trunk and extremities (Fig 1, A to C), leading to recurrent hospitalizations, which prompted administration of several high-dose pulses of intravenous methylprednisolone (1 mg/kg for 3 days), an increase in her prednisone to 60 mg daily, and an increase in the frequency of IVIg infusions to every 3 weeks. However, 1 month later, the patient was hospitalized for lower extremity edema and tachycardia secondary to multiple acute pulmonary emboli. The patient decided to avoid IVIg given the risk of future thromboembolic events.
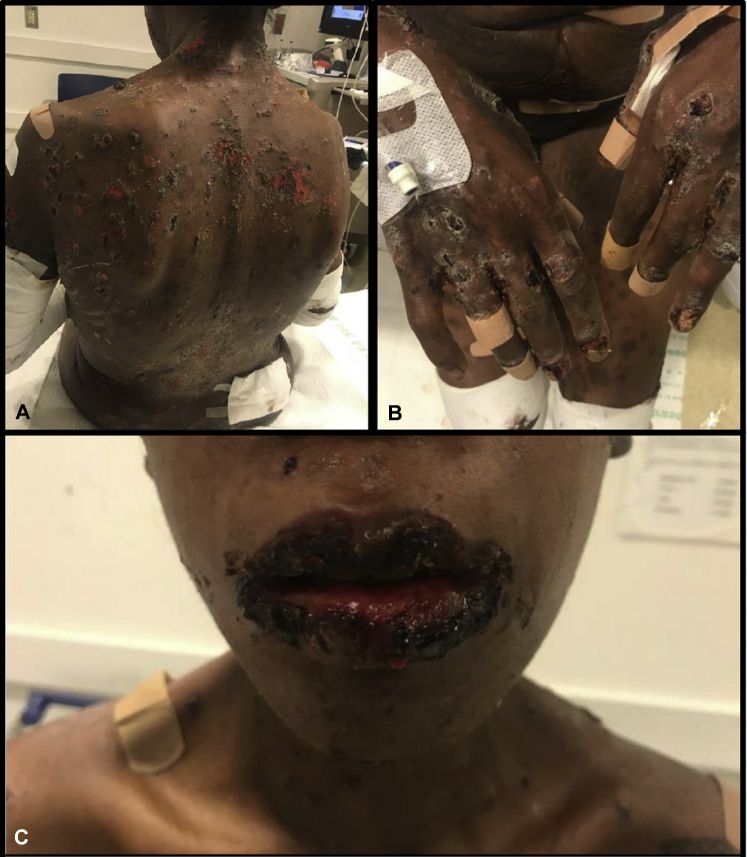
Fig 1.
A to C, Prior to ofatumumab initiation. The significant mucosal involvement, seen here as eroded plaques with heavy hemorrhagic crust on upper and lower lips, impaired the patient's ability to speak and open her mouth. In addition, the patient had widespread involvement of her trunk and extremities with numerous erosions and flaccid bullae.
Given the patient's recalcitrant disease and adverse events in the setting of prior systemic therapies, the care team elected to trial off-label treatment with ofatumumab, using the chronic lymphocytic leukemia dosing regimen, also used by Rapp et al2 (300 mg on day 1, 1000 mg on day 8, then 8 cycles of 1000 mg every 28 days). Similar to the case in Rapp et al2 (in which clinical response was noted after first infusion), our patient experienced clinical improvement after her second infusion, and following her fourth infusion, her prednisone was tapered below 20 mg for the first time since initial diagnosis. Unlike the case in Rapp et al,2 in which clinical clearance was achieved within 3 months of treatment, our patient only achieved complete clinical resolution 9 months after initiation of ofatumumab (1 month after completing 9 cycles of treatment) (Fig 2, A to C). The patient has tolerated ofatumumab well with one episode of transient neutropenia prior to her fourth infusion, which resolved in 3 days without intervention.
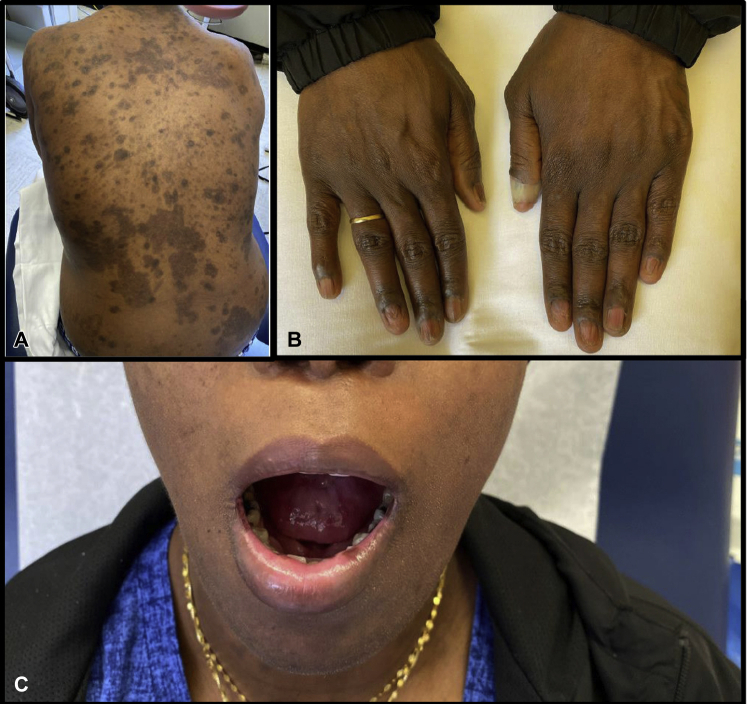
Fig 2.
A to C, After completion of ofatumumab treatment. One month after completing 9 cycles of ofatumumab, patient was seen in the outpatient clinic, where her skin examination found no erosions or flaccid bullae on lips, oropharynx, trunk, or extremities. Also notable is patient's nail plate regrowth and hyperpigmentation of previously involved skin.
Discussion
Ofatumumab may become an emerging therapy for PV. Although both rituximab and ofatumumab bind to the extracellular loop of CD20, ofatumumab's unique binding site more proximal to the cell membrane may induce a more robust complement-dependent cytotoxic response in vitro compared with rituximab.3,5 Given the fully human quality of ofatumumab, it may have less immunogenicity than rituximab, possibly explaining why it has been tolerated by patients who experienced adverse reactions to rituximab, including a patient who experienced anaphylaxis.6 Nonetheless, more investigation is needed to determine the comparative effectiveness of ofatumumab in PV. A phase III randomized controlled trial investigating the use of subcutaneous ofatumumab in 37 patients with PV was terminated without publication due to change of sponsor.7
Investigation is currently underway evaluating the role of other therapeutics targeting CD20 in PV treatment, like veltuzumab, which has high binding avidity to CD20 and can be administered subcutaneously, and obinutuzumab, which shows superior B-cell depletion compared with rituximab. Other therapeutics that affect B-cell differentiation by targeting BAFF and APRIL, such as belimumab and atacicept, respectively, may also be promising agents.8 Like rituximab and ofatumumab, these may be limited by cost, availability, and tolerability.
This case provides further evidence for ofatumumab as an effective alternative treatment for pemphigus vulgaris in patients who do not respond to or do not tolerate rituximab.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Joly P., Maho-Vaillant M., Prost-Squarcioni C. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389(10083):2031–2040. doi: 10.1016/S0140-6736(17)30070-3. [DOI] [PubMed] [Google Scholar]
- 2.Rapp M., Pentland A., Richardson C. Successful treatment of pemphigus vulgaris with ofatumumab. J Drugs Dermatol. 2018;17(12):1338. [PubMed] [Google Scholar]
- 3.Cang S., Mukhi N., Wang K., Liu D. Novel CD20 monoclonal antibodies for lymphoma therapy. J Hematol Oncol. 2012;5(1):64. doi: 10.1186/1756-8722-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaso R., Jaso R., Sierra M. Guillain–Barré syndrome after rituximab in a patient with idiopathic thombocytopenic purpura: a causal association? J Neurol. 2010;257(3):488–489. doi: 10.1007/s00415-009-5400-3. [DOI] [PubMed] [Google Scholar]
- 5.Teeling J.L., Mackus W.J., Wiegman L.J. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177(1):362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 6.McDonald V., Crowley M.P., Scully M. Ofatumumab for TTP in a patient with anaphylaxis associated with rituximab. N Engl J Med. 2018;378(1):92–93. doi: 10.1056/nejmc1714146. [DOI] [PubMed] [Google Scholar]
- 7.Efficacy and safety of ofatumumab in treatment of pemphigus vulgaris - full text view - ClinicalTrials.gov [Internet]. [cited Jan 20, 2020] https://clinicaltrials.gov/ct2/show/NCT01920477 Available at:
- 8.Didona D., Maglie R., Eming R., Hertl M. Pemphigus: current and future therapeutic strategies. Front Immunol. 2019;10:1418. doi: 10.3389/fimmu.2019.01418. [DOI] [PMC free article] [PubMed] [Google Scholar]