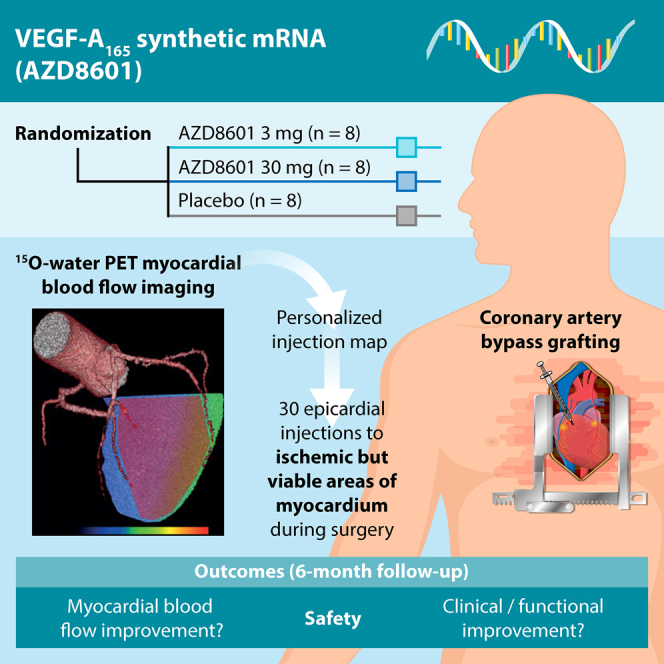
Abstract
Therapeutic angiogenesis may improve outcomes in patients with coronary artery disease undergoing surgical revascularization. Angiogenic factors may promote blood vessel growth and regenerate regions of ischemic but viable myocardium. Previous clinical trials of vascular endothelial growth factor A (VEGF-A) gene therapy with DNA or viral vectors demonstrated safety but not efficacy. AZD8601 is VEGF-A165 mRNA formulated in biocompatible citrate-buffered saline and optimized for high-efficiency VEGF-A expression with minimal innate immune response. EPICCURE is an ongoing randomized, double-blind, placebo-controlled study of the safety of AZD8601 in patients with moderately decreased left ventricular function (ejection fraction 30%–50%) undergoing elective coronary artery bypass surgery. AZD8601 3 mg, 30 mg, or placebo is administered as 30 epicardial injections in a 10-min extension of cardioplegia. Injections are targeted to ischemic but viable myocardial regions in each patient using quantitative 15O-water positron emission tomography (PET) imaging (stress myocardial blood flow < 2.3 mL/g/min; resting myocardial blood flow > 0.6 mL/g/min). Improvement in regional and global myocardial blood flow quantified with 15O-water PET is an exploratory efficacy outcome, together with echocardiographic, clinical, functional, and biomarker measures. EPICCURE combines high-efficiency delivery with quantitative targeting and follow-up for robust assessment of the safety and exploratory efficacy of VEGF-A mRNA angiogenesis (ClinicalTrials.gov: NCT03370887).
Keywords: ▪▪▪
In the EPICCURE study, patients undergoing coronary artery bypass grafting receive epicardial injections of synthetic mRNA encoding vascular endothelial growth factor A or placebo. Imaging of myocardial blood flow with 15O-water PET is used to target injections to ischemic but viable regions in each participant and to monitor potential improvement.
Graphical Abstract
Main Text
Ischemic cardiovascular diseases remain the leading cause of morbidity and mortality worldwide,1 despite the success of revascularization therapy and medical treatments (e.g., statins and anti-hypertensive and anti-platelet drugs). Patients with myocardial infarction may suffer from impaired cardiac function owing to the loss of billions of cardiomyocytes, which normally do not regenerate following ischemic injury.2 Myocardial regions surrounding the core of a myocardial infarction are poorly perfused at rest or during stress. Although this myocardial tissue has reduced contractility, it remains viable and may be recoverable with revascularization therapy.3
Combining therapeutic angiogenesis with revascularization could improve outcomes in patients with myocardial infarction by promoting the growth of new blood vessels and regeneration of injured myocardium.4 Modalities for targeted delivery of angiogenic factors include transplantation of ex vivo expanded cells, gene therapy with naked DNA plasmids or viral vectors, and administration of recombinant proteins.5 Vascular endothelial growth factor (VEGF) family members are key regulators of angiogenesis and remain among the most promising candidates for therapeutic angiogenesis.6 VEGF-A has been tested in patients with myocardial infarction using gene therapy with plasmid DNA or adenoviral vectors, or administration of recombinant protein, but with inconclusive evidence of efficacy.7,8 Clinical trials have, however, demonstrated the safety of epicardial injections in patients undergoing coronary artery bypass grafting.9, 10, 11, 12, 13, 14, 15, 16 Lack of efficacy may result from poor gene transfer efficiency, poor expression levels, low bioactivity of the expressed protein, suboptimal pharmacokinetics with constitutive promoters, and inaccurate targeting of ischemic but viable myocardium.17
Enhanced targeting and delivery of VEGF therapy to regions of viable but non-functional myocardium could transform prospects for improved cardiac function and clinical benefits with therapeutic angiogensis.17,18 Recent advances in cardiac 15O-water positron emission tomography (PET) allow quantitative assessment of myocardial blood flow and viability, which enables identification of ischemic but viable myocardium for targeted treatment with angiogenic therapy.19, 20, 21 Synthetic chemically modified mRNA offers enhanced VEGF-A expression levels and kinetics compared with previous gene therapy modalities.22 Modified mRNA mediates highly efficient transient protein expression in vivo without eliciting an innate immune response and with no need for lipid-based carriers.23, 24, 25, 26 A study in a mouse model of myocardial infarction showed that injection of VEGF-A mRNA into cardiac muscle mediated transient expression of VEGF-A and was superior to gene therapy with plasmid DNA in reducing infarct size, enhancing myocardial perfusion, and improving survival.27
AZD8601 is a clinical-grade VEGF-A165 mRNA formulated in biocompatible citrate-buffered saline and optimized for efficient VEGF-A protein production with minimal induction of innate immune responses.28, 29, 30 AZD8601 injection elicits sustained transient expression of functional VEGF-A protein in the skin, heart, and skeletal muscle in animal models,28, 29, 30 with no apparent acute toxicity or inflammation in rats and cynomolgus monkeys.29 Injection of AZD8601 also enhanced blood flow and increased blood vessel density in the skin and heart in animal models.28,29 Furthermore, epicardial injection of AZD8601 improved cardiac function in pigs following experimental myocardial infarction.29
AZD8601 was injected intradermally in men with type 2 diabetes in a randomized, double-blind, placebo-controlled, first-in-human phase 1 study.31 Intradermal VEGF-A mRNA was well tolerated and led to local functional VEGF-A protein expression 4−24 h after administration as well as transient local enhancement of basal skin blood flow at 4 h and 7 days after administration.31 Here, we describe the design of EPICCURE, an ongoing safety study of epicardial injections of AZD8601 in patients with moderately impaired systolic function who are undergoing elective coronary artery bypass grafting. Before surgery, quantitative 15O-water PET is used to identify and map each patient’s areas of ischemic but viable myocardium for targeted epicardial injections of AZD8601. During follow-up, quantitative 15O-water PET is used to measure potential improvements in myocardial perfusion.
Study Design
Overview
EPICCURE is a randomized, placebo-controlled, double-blind, multicenter, 6-month, phase 2a clinical trial of the safety, tolerability, and exploratory efficacy of epicardial injections of AZD8601 in patients with stable coronary artery disease and moderately decreased left ventricular ejection fraction who are undergoing coronary artery bypass grafting surgery.
Ethics and Conduct
The study started in February 2018 and is recruiting patients at two sites in Finland. In addition, one site in Germany, two sites in the Netherlands, and one other site in Finland (pending) have ethical and regulatory approval. EPICCURE is registered as ClinicalTrials.gov: NCT03370887 and conforms to the principles of the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice, the AstraZeneca policy on Bioethics and Human Biological Samples, and all applicable regulatory requirements. Local ethics committees reviewed and approved the study protocol, and participants gave their written informed consent before study enrollment. A 10-min extension of cardioplegia was considered ethically acceptable to allow epicardial injections after completion of the peripheral anastomoses.
Objectives
The primary objective of EPICCURE is to assess the safety and tolerability of AZD8601. Exploratory objectives include assessing efficacy based on 15O-water PET quantification of myocardial blood flow as well as echocardiography, clinical symptoms, biomarkers, and functional tests (Box 1).
Box 1. Objectives.
Primary
-
•
Safety and tolerability of AZD8601
Exploratory
-
•Effect of AZD8601 on
-
○regional and global stress myocardial blood flow measured with 15O-water PET
-
○regional and global myocardial blood flow reserve measured with 15O-water PET
-
○left ventricular end-diastolic volume, left ventricular end-systolic volume, and left ventricular ejection fraction measured by echocardiography
-
○regional myocardial wall motion measured by echocardiography and strain analysis
-
○cardiac function under adenosine stress measured by echocardiography, including CFVR in the left anterior descending artery
-
○clinical symptoms: NYHA functional class, SAQ, and KCCQ
-
○
-
•
Change in troponin T and NT-proBNP levels from baseline
-
•
VEGF-A protein concentration in plasma
-
•Sample collection for
-
○biomarkers
-
○AZD8601 plasma concentrations
-
○anti-drug immunogenicity
-
○
-
•
Optional digital 6MWT and MCWS in some centers
6MWT, 6-minute walk test; CFVR, coronary flow velocity reserve; KCCQ, Kansas City Cardiomyopathy Questionnaire; MCWS, maximum continuously walked steps; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PET, positron emission tomography; SAQ, Seattle Angina Questionnaire; VEGF-A, vascular endothelial growth factor A.
Participants
Eligible patients are men and women aged 18 years or older who are scheduled for elective coronary artery bypass grafting surgery within 15–90 days and whose left ventricular ejection fraction is between 30% and 50%. Patients taking angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, statins, or beta-blockers must be on a stable dose for at least 2 weeks before screening. Men must be surgically sterile or using barrier contraception to prevent pregnancy in partners, and women must be unable to have children (post-menopausal or surgically sterile). Key exclusion criteria are listed in Box 2.
Box 2. Key exclusion criteria.
-
•
BMI > 35 kg/m2 or poor image window for echocardiography
-
•
Indication for emergency coronary artery bypass grafting
-
•
Severe comorbidities or history of disease or disorder that would put the patient at risk, affect participation, or influence study results
-
•
eGFR ≤ 30 mL/min
-
•
History of ventricular arrhythmia (Lown grade ≥ 3) without implantable cardiac defibrillator
-
•
Contraindication for CFVR or sMBF measurement procedure
-
•
Concomitant use of medications associated with Torsades de Pointes
-
•
History of QT prolongation associated with medication that required discontinuation of that medication
-
•
Congenital long QT syndrome
-
•
Symptomatic arrythmia or arrythmia that requires treatment
-
•
Atrial fibrillation (including paroxysmal atrial fibrillation)
-
•
Hepatitis B or C virus or HIV seropositivity
-
•
History of drug or alcohol abuse
BMI, body mass index; CFVR, coronary flow velocity reserve; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; QT, electrocardiographic interval from the onset of the QRS complex to the end of the T wave; sMBF, stress myocardial blood flow.
Randomization and Blinding
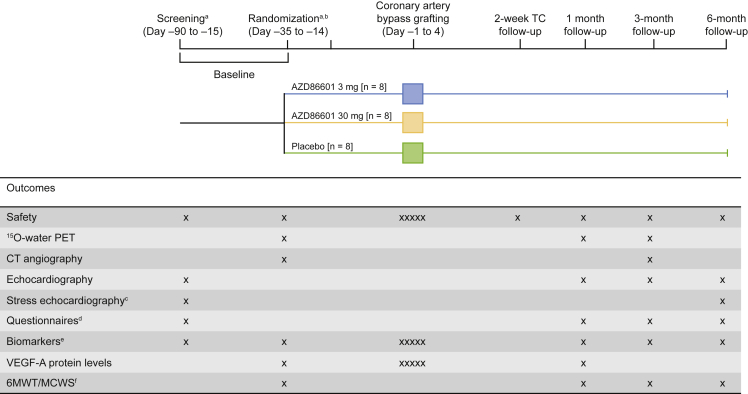
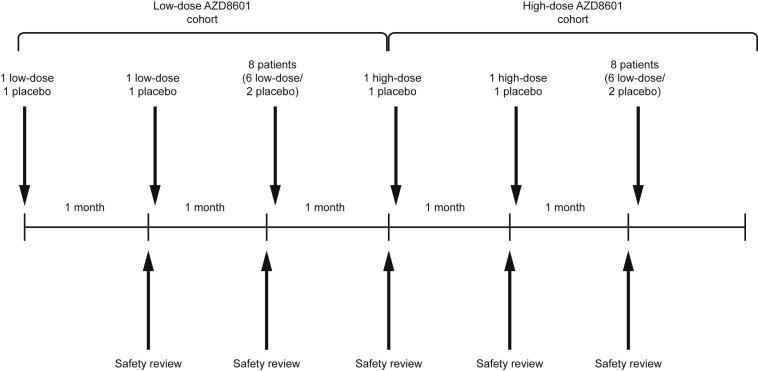
Enrolled participants (N = 24) are randomized at least 14 days before coronary artery bypass grafting to receive AZD8601 3 mg, AZD8601 30 mg, or placebo in a ratio of 1:1:1 (n = 8 per group) (Figure 1). Participants are divided into two sequential ascending-dose cohorts (n = 12 per cohort) and randomized 2:1 to AZD8601 (n = 8) or placebo (n = 4), with AZD8601 doses of 3 mg in the first cohort and 30 mg in the second cohort (Figure 2). Within each cohort, two sentinel participants are randomized 1:1 to AZD8601 or placebo, followed by another two sentinel participants randomized 1:1 to AZD8601 or placebo, followed by the remaining eight participants randomized to 3:1 to AZD8601 or placebo. Safety data from up to 1 month after administration are reviewed by the safety review committee before the treatment of participants in the next sentinel sub-group within each cohort and before the treatment of participants in the high-dose cohort (Figure 2).
Figure 1.
Study Flow Chart
aScreening and randomization visits can be combined provided that 15O-water PET and CFVR assessments are carried out on separate days.
bThe 14-day time-window before surgery on day 0 can be reduced if the pre-operative conference, randomization, and delivery of AZD8601/placebo can be accommodated within a shorter period.
cIncludes CFVR in the left anterior descending artery (for sites able to assess CFVR).
dKansas City Cardiomyopathy Questionnaire, Seattle Angina Questionnaire, and NYHA classification.
eIncludes high-sensitivity troponin T and NT-proBNP.
fOptional digital assessments via mobile phone app.
X, assessment times; XXXXX, daily assessments during stay in hospital; 6MWT, 6-min walking test; CFVR, coronary flow velocity reserve; CT, computed tomography; MCWS, maximum continuously walked steps; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PET, positron emission tomography; TC, teleconference; VEGF-A, vascular endothelial growth factor.
Figure 2.
Sentinel and Sequential Ascending-Dose Cohorts
Sequential low-dose and high-dose cohorts each include 12 participants, with 8 randomized to AZD8601 and 4 randomized to placebo. Each dose cohort includes 3 sequential sentinel sub-cohorts: the first and second sub-cohort each include 2 participants, randomized to AZD8601 (n = 1) or placebo (n = 1), and the third sentinel cohort includes 8 participants, randomized to AZD8601 (n = 6) or placebo (n = 2). Safety data at 1 month is reviewed before initiation of the next cohort or sub-cohort.
Participants and the study team will remain blinded to treatment assignments throughout the study. Investigators will remain blinded unless they need to know a patient’s assigned treatment in a medical emergency. The safety review committee is unblinded. The placebo solution for injection matches the appearance of AZD8601, so the identity of the treatment cannot be discerned. Randomization is carried out by the contract research organization (Parexel) using an algorithm provided by the sponsor (AstraZeneca).
Procedures
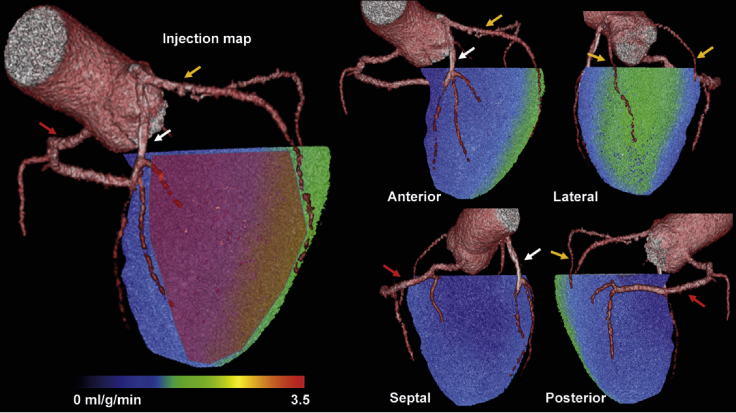
At baseline, participants have a 15O-water PET scan to quantify pre-operative myocardial blood flow at rest and during adenosine stress. This is paired with the baseline contrast-enhanced coronary computed tomography (CT) angiogram to generate a perfusion map that combines coronary anatomy and myocardial blood flow. The perfusion map is used to design an individualized injection map that guides placement of each patient’s injections during surgery (Figure 3). Ischemic regions are identified as those with stress myocardial blood flow below 2.3 mL/g/min (±0.3 mL/g/min) or below 80% (±10%) of the segment with highest stress myocardial blood flow on 15O-water PET.32,33 Target regions of ischemic but viable myocardium are identified as ischemic regions with resting myocardial blood flow above 0.6 mL/g/min on 15O-water PET. On the individualized injection map, 30 epicardial injection sites with approximately 1 cm spacing are identified within the target regions. The individualized injection map is designed in a pre-operative conference involving the responsible surgeon, PET physician and cardiologist from the study site, the responsible person from the PET core laboratory, and at least one other cardiothoracic surgeon from another hospital.
Figure 3.
Example Individualized Injection Map for Targeting Ischemic but Viable Myocardium
The individualized injection map is based on a hybrid image showing 3D-rendered coronary anatomy (coronary CT angiography) and left ventricular myocardial blood flow during adenosine stress (15O-water PET). Green or blue shading indicates stress myocardial blood flow < 2.3 mL/g/min. The red overlay on the left panel indicates the target region of the left coronary artery territory for epicardial injections of AZD8601 (stress myocardial blood flow < 2.3 mL/g/min and resting myocardial blood flow > 0.6 mL/g/min). Treatment is focused on the largest ischemic area, with any remaining injections used for partial treatment of the second-largest area. White arrows, left anterior descending coronary artery; yellow arrows, left circumflex coronary artery branches; red arrows, right coronary artery.
During surgery, participants receive 30 epicardial injections of 200 μL volume each, guided by their individualized injection map to target ischemic but viable myocardial regions. Injections are given under cardioplegia immediately after bypass grafting and before reperfusion. Participants receive their randomly assigned treatment of AZD8601 3 mg (0.1 mg per injection site; 0.5 mg/mL solution), AZD8601 30 mg (1 mg per injection site; 5 mg/mL solution), or matching placebo. The injection procedure is documented by filming or photography, and the surgeon records the actual injection sites on the individualized injection map (Figure 3).
Injections are performed using a 30G (approximately 0.3 mm diameter) × 13 mm hypodermic needle coupled to a 1 mL syringe. Comments are recorded on the presence or absence of potential or suspected perforation, potential sustained bleeding at the injection site, and other adverse events.
Patients remain in the hospital for at least 4 days after surgery and then attend follow-up visits at 1, 3, and 6 months after surgery to assess safety and efficacy (Figure 1). Adverse events are also assessed by telephone interviews 2 weeks after surgery.
Safety Outcomes
Safety outcomes include monitoring of adverse events and serious adverse events, physical examinations, electrocardiography, monitoring of vital signs (blood pressure, pulse, and oxygen saturation), laboratory assessments, and echocardiographic assessment of hemopericardium, tamponade, and left ventricular ejection fraction. Adverse events are recorded from coronary artery bypass grafting until the end of follow-up, and serious adverse events are recorded from informed consent until the end of follow-up. All adverse events are followed up by the investigator until resolved or for as long as medically indicated. Investigators assess whether adverse events are causally related to the investigational medical product and also whether serious adverse events are causally related to other medications, to study procedures, and to the drug injection procedure.
Exploratory Outcomes
Cardiac Imaging
Regional and global stress myocardial blood flow and myocardial flow reserve (stress-to-rest ratio in myocardial blood flow) will be assessed using 15O-water PET at baseline and at 1 month and 3 months after surgery (Figure 1). Regional wall motion, global longitudinal strain, and cardiac volumes will be assessed using comprehensive echocardiography at baseline and at 1, 3, and 6 months after surgery. Participants also have follow-up CT angiograms 3 months after surgery. Echocardiography at baseline and 6 months after surgery will assess left ventricular function and coronary flow velocity reserve at rest and during adenosine-induced hyperemia (if possible at the study site). Coronary flow velocity reserve is the stress-to-rest ratio in mean diastolic Doppler flow velocity in the left anterior descending artery.34,35
Clinical and Functional Outcomes
Patients will complete the Kansas City Cardiomyopathy Questionnaire36 and the Seattle Angina Questionnaire,37 and investigators will perform New York Heart Association classifications38 at baseline and at 1, 3, and 6 months after surgery (Figure 1). Participants optionally engage in a digital 6-min walk test and maximum continuous walked steps assessments, via a mobile phone app, at baseline and at 1, 3, and 6 months after surgery (Figure 1).
Pharmacokinetics and Pharmacodynamics
Plasma samples will be collected to assess VEGF-A protein levels at baseline, during the hospital stay (day –1 and 3, 6, 24, 34, 48, and 72 h after first epicardial injections), and at 1 month after surgery (Figure 1). Plasma samples to assess levels of AZD8601 will also be taken during the hospital stay (day –1 and 1, 3, and 48 h after first injection).
Biomarkers
Samples will be collected at baseline, during the hospital stay (day –1 and 3, 6, 24, 34, 48, and 72 h after the first epicardial injection), and at 1, 3, and 6 months after surgery for analysis of cardiovascular biomarkers. Biomarkers of particular interest are high-sensitivity troponin T and N-terminal pro-B-type natriuretic peptide.
Statistical Methods
The study sample size is not based on formal statistical considerations. Twenty-four patients will be randomized for an estimated 21 evaluable patients (seven per group). Safety will be assessed in all randomized patients who have received at least one injection of AZD8601 or placebo. Exploratory efficacy will be assessed in all randomized patients who have received at least one injection of AZD8601 or placebo and for whom data are available at baseline and at least one follow-up visit.
Changes from baseline in exploratory outcome measures will be tested using two-sided analysis of covariance (with treatment as a fixed effect and baseline value as covariate) or repeated-measures model (with treatment, visit, and interaction between treatment and visit as fixed effects and baseline as covariate). Analyses will not be adjusted for multiplicity and all p values will be nominal.
Current Status
As of November 2019, we have enrolled and randomized five participants. We expect to enroll the last patient and complete the last follow-up visit in 2021.
Discussion
The EPICCURE study of AZD8601 is the first clinical trial of mRNA in the human heart. By combining high-efficiency transient VEGF-A expression from mRNA with quantitative ischemia-guided treatment and follow-up, EPICCURE provides a new and unique opportunity to obtain compelling proof-of-concept for therapeutic angiogenesis in patients with cardiovascular disease. To our knowledge, EPICCURE is also the first multicenter therapeutic study to use 15O-water PET not only for accurate targeting of VEGF-A mRNA injections to potentially salvageable regions of ischemic but viable myocardium, but also for quantitative assessment of potential improvement in myocardial blood flow during follow-up.
Functional VEGF-A protein expression after injection of AZD8601 was demonstrated in a first-in-human phase 1 trial, consistent with findings in animal models.28, 29, 30, 31 Sustained increases in local blood flow were detected in both humans and animals following intradermal AZD8601 injections, together with transient local production of VEGF-A protein.28, 29, 30, 31 AZD8601 promoted new blood vessel formation in animal models and improved cardiac function in a pig model of myocardial infarction.28, 29, 30 Furthermore, AZD8601 did not induce innate immune responses in animals and was well tolerated in human volunteers, with minimal local reactions.29, 30, 31
Previous studies of VEGF-A gene therapy in patients with ischemic heart disease provided a strong evidence base for safety, but did not convincingly demonstrate efficacy.6,17 A possible efficacy signal was observed, however, in the Kuopio angiogenesis trial.39 Intracoronary infusion of VEGF adenovirus vector was associated with significant improvement in regional myocardial perfusion in patients receiving percutaneous coronary interventions, but no effects were seen on the primary efficacy outcomes of luminal stenosis or minimal diameter.39 Previous studies generally did not identify patients most likely to benefit from therapeutic angiogenesis, did not target gene delivery to ischemic but viable myocardial regions, did not demonstrate adequate VEGF-A expression levels and kinetics from plasmid or viral vectors, and did not use quantitative outcome measures.17 All of these issues are addressed by the design of EPICCURE.
A key strength of EPICCURE is the targeting of AZD8601 or placebo injections to mapped regions of ischemic but viable myocardium using quantitative 15O-water PET imaging. Target regions have a sub-normal stress myocardial blood flow (<2.3 mL/g/min) but residual resting myocardial blood flow (>0.6 mL/g/min), which means they may regain contractile function when reperfused.32,33 Additional strengths of the study include the randomized, double-blind, placebo-controlled study design and the use of quantitative and objective exploratory efficacy outcomes as well as subjective clinical and functional assessments. The sequential design with sentinel cohorts minimizes the risk to participants. A key limitation of the study is that patients must undergo coronary artery bypass grafting to participate. Injections cannot be administered to parts of the ventricular septum or inferior wall during surgery, although most of the left ventricle is accessible. Adverse events may be difficult to attribute to study drug administration or surgery (e.g., arrythmia, atrial fibrillation, pericardial effusion, and excessive postoperative bleeding).
Inclusion of patients with moderately reduced left ventricular ejection fraction (30%–50%) limits the number of patients who are eligible to participate in EPICCURE. This inclusion criterion also selects those most likely to benefit from improved myocardial reperfusion following coronary artery bypass grafting. Augmentation of coronary artery bypass grafting with angiogenic therapies may allow more patients to benefit from revascularization compared with surgery alone, particularly those with reduced left ventricular ejection fraction. Future studies will investigate endocardial administration of AZD8601 in patients receiving percutaneous coronary interventions, once the technical feasibility of injecting AZD8601 with a cardiac catheter has been confirmed.
The use of placebo injections is a strength of the study but may subject participants to additional risk during surgery. Intramyocardial placebo injections during coronary artery bypass grafting have been employed in 11 published studies, with no safety concerns.40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 Furthermore, intramyocardial injection of investigational medicinal products during coronary artery bypass grafting had a similar safety profile to surgery alone in seven published studies.9, 10, 11, 12, 13, 14, 15 The choice of a short narrow-gauge needle for intramyocardial injections in the present study minimizes the risks of perforation and bleeding at the injection site.
In conclusion, EPICCURE combines novel VEGF-A delivery using mRNA with novel ischemia-guided administration using quantitative 15O-water PET imaging. The study is designed not only to assess the safety of AZD8601, but also to detect potentially beneficial angiogenic effects of VEGF-A mRNA therapy on myocardial perfusion and cardiac function in patients with obstructive coronary artery disease who need bypass surgery.
Author Contributions
Conceptualization, V.A., A.S., J.K., P.J., M.H., S.S., M.L.-F., A.J., and L.-M.G.; Methodology, M.L.-F., M.K., S.S., and J.K.; Formal analysis and data curation, M.K.; Investigation, V.A., A.S., J.K., P.J., M.H., S.S., and A.J.; Reviewing, editing, and approving the manuscript, V.A., A.S., J.K., P.J., M.H., S.S., M.L.-F., M.K., A.J., and L.-M.G.
Conflicts of Interest
V.A., P.J., and S.S. declare no conflicts of interest. M.H. has received speaker’s fees from Amgen. A.J. has received speaker’s fees and consultancy fees from AstraZeneca and research grants from AstraZeneca unrelated to the present study. A.S. has received consultancy fees from AstraZeneca. J.K. has received speaker’s fees from GE Healthcare, Merck, and Lundbeck Inc. and consultancy fees from AstraZeneca and GE Healthcare. M.K., M.L.-F., and L.-M.G. are employees of AstraZeneca and may own stock or stock options.
Acknowledgments
We thank the patients and study site staff who are taking part in the study. This study is funded by AstraZeneca. AstraZeneca develops and markets treatments for cardiovascular disease. AZD8601 is an investigational medical product with no approved indication.
Under the direction of the authors, Drs. Matt Cottingham and Sarah Sabir of Oxford PharmaGenesis provided medical writing support funded by AstraZeneca. AstraZeneca participates in study design, data collection, data analysis, data interpretation, and writing of the study protocol. AstraZeneca reviewed this publication, without influencing the opinions of the authors, to ensure medical and scientific accuracy and to protect intellectual property. The authors had access to all study protocols, all authors approved the manuscript for submission, and the corresponding author had the final responsibility for the decision to submit the manuscript for publication.
References
- 1.Moran A.E., Forouzanfar M.H., Roth G.A., Mensah G.A., Ezzati M., Flaxman A., Murray C.J., Naghavi M. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1493–1501. doi: 10.1161/CIRCULATIONAHA.113.004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foglia M.J., Poss K.D. Building and re-building the heart by cardiomyocyte proliferation. Development. 2016;143:729–740. doi: 10.1242/dev.132910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan M.J., Perera D. Identifying and managing hibernating myocardium: what’s new and what remains unknown? Curr. Heart Fail. Rep. 2018;15:214–223. doi: 10.1007/s11897-018-0396-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes G.C., Annex B.H. Angiogenic therapy for coronary artery and peripheral arterial disease. Expert Rev. Cardiovasc. Ther. 2005;3:521–535. doi: 10.1586/14779072.3.3.521. [DOI] [PubMed] [Google Scholar]
- 5.Johnson T., Zhao L., Manuel G., Taylor H., Liu D. Approaches to therapeutic angiogenesis for ischemic heart disease. J. Mol. Med. (Berl.) 2019;97:141–151. doi: 10.1007/s00109-018-1729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ylä-Herttuala S., Baker A.H. Cardiovascular gene therapy: past, present, and future. Mol. Ther. 2017;25:1095–1106. doi: 10.1016/j.ymthe.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giacca M., Zacchigna S. VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond. Gene Ther. 2012;19:622–629. doi: 10.1038/gt.2012.17. [DOI] [PubMed] [Google Scholar]
- 8.Gaffney M.M., Hynes S.O., Barry F., O’Brien T. Cardiovascular gene therapy: current status and therapeutic potential. Br. J. Pharmacol. 2007;152:175–188. doi: 10.1038/sj.bjp.0707315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmadi H., Baharvand H., Ashtiani S.K., Soleimani M., Sadeghian H., Ardekani J.M., Mehrjerdi N.Z., Kouhkan A., Namiri M., Madani-Civi M. Safety analysis and improved cardiac function following local autologous transplantation of CD133(+) enriched bone marrow cells after myocardial infarction. Curr. Neurovasc. Res. 2007;4:153–160. doi: 10.2174/156720207781387141. [DOI] [PubMed] [Google Scholar]
- 10.Ang K.L., Chin D., Leyva F., Foley P., Kubal C., Chalil S., Srinivasan L., Bernhardt L., Stevens S., Shenje L.T., Galiñanes M. Randomized, controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium during CABG versus CABG alone. Nat. Clin. Pract. Cardiovasc. Med. 2008;5:663–670. doi: 10.1038/ncpcardio1321. [DOI] [PubMed] [Google Scholar]
- 11.Katayama Y., Takaji K., Shao Z.Q., Matsukawa M., Kunitomo R., Hagiwara S., Moriyama S., Kawasuji M. The value of angiogenic therapy with intramyocardial administration of basic fibroblast growth factor to treat severe coronary artery disease. Ann. Thorac. Cardiovasc. Surg. 2010;16:174–180. [PubMed] [Google Scholar]
- 12.Mocini D., Staibano M., Mele L., Giannantoni P., Menichella G., Colivicchi F., Sordini P., Salera P., Tubaro M., Santini M. Autologous bone marrow mononuclear cell transplantation in patients undergoing coronary artery bypass grafting. Am. Heart J. 2006;151:192–197. doi: 10.1016/j.ahj.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Patel A.N., Geffner L., Vina R.F., Saslavsky J., Urschel H.C., Jr., Kormos R., Benetti F. Surgical treatment for congestive heart failure with autologous adult stem cell transplantation: a prospective randomized study. J. Thorac. Cardiovasc. Surg. 2005;130:1631–1638. doi: 10.1016/j.jtcvs.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 14.Stamm C., Kleine H.D., Choi Y.H., Dunkelmann S., Lauffs J.A., Lorenzen B., David A., Liebold A., Nienaber C., Zurakowski D. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J. Thorac. Cardiovasc. Surg. 2007;133:717–725. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 15.Trifunović Z., Obradović S., Balint B., Ilić R., Vukić Z., Šišić M., Kostić J., Rusović S., Dobrić M., Ostojić G. Functional recovery of patients with ischemic cardiomyopathy treated with coronary artery bypass surgery and concomitant intramyocardial bone marrow mononuclear cell implantation--a long-term follow-up study. Vojnosanit. Pregl. 2015;72:225–232. doi: 10.2298/vsp140109071t. [DOI] [PubMed] [Google Scholar]
- 16.Losordo D.W., Vale P.R., Symes J.F., Dunnington C.H., Esakof D.D., Maysky M., Ashare A.B., Lathi K., Isner J.M. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–2804. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 17.Ylä-Herttuala S., Bridges C., Katz M.G., Korpisalo P. Angiogenic gene therapy in cardiovascular diseases: dream or vision? Eur. Heart J. 2017;38:1365–1371. doi: 10.1093/eurheartj/ehw547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robich M.P., Chu L.M., Oyamada S., Sodha N.R., Sellke F.W. Myocardial therapeutic angiogenesis: a review of the state of development and future obstacles. Expert Rev. Cardiovasc. Ther. 2011;9:1469–1479. doi: 10.1586/erc.11.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grönman M., Tarkia M., Stark C., Vähäsilta T., Kiviniemi T., Lubberink M., Halonen P., Kuivanen A., Saunavaara V., Tolvanen T. Assessment of myocardial viability with [15O]water PET: A validation study in experimental myocardial infarction. J. Nucl. Cardiol. 2019 doi: 10.1007/s12350-019-01818-5. Published online July 17, 2019. 31317328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartikainen J., Hassinen I., Hedman A., Kivelä A., Saraste A., Knuuti J., Husso M., Mussalo H., Hedman M., Rissanen T.T. Adenoviral intramyocardial VEGF-DΔNΔC gene transfer increases myocardial perfusion reserve in refractory angina patients: a phase I/IIa study with 1-year follow-up. Eur. Heart J. 2017;38:2547–2555. doi: 10.1093/eurheartj/ehx352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murthy V.L., Bateman T.M., Beanlands R.S., Berman D.S., Borges-Neto S., Chareonthaitawee P., Cerqueira M.D., deKemp R.A., DePuey E.G., Dilsizian V., SNMMI Cardiovascular Council Board of Directors. ASNC Board of Directors Clinical Quantification of Myocardial Blood Flow Using PET: Joint Position Paper of the SNMMI Cardiovascular Council and the ASNC. J. Nucl. Med. 2018;59:273–293. doi: 10.2967/jnumed.117.201368. [DOI] [PubMed] [Google Scholar]
- 22.Chien K.R., Zangi L., Lui K.O. Synthetic chemically modified mRNA (modRNA): toward a new technology platform for cardiovascular biology and medicine. Cold Spring Harb. Perspect. Med. 2014;5:a014035. doi: 10.1101/cshperspect.a014035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karikó K., Muramatsu H., Keller J.M., Weissman D. Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol. Ther. 2012;20:948–953. doi: 10.1038/mt.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karikó K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kormann M.S., Hasenpusch G., Aneja M.K., Nica G., Flemmer A.W., Herber-Jonat S., Huppmann M., Mays L.E., Illenyi M., Schams A. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 2011;29:154–157. doi: 10.1038/nbt.1733. [DOI] [PubMed] [Google Scholar]
- 26.Warren L., Manos P.D., Ahfeldt T., Loh Y.H., Li H., Lau F., Ebina W., Mandal P.K., Smith Z.D., Meissner A. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zangi L., Lui K.O., von Gise A., Ma Q., Ebina W., Ptaszek L.M., Später D., Xu H., Tabebordbar M., Gorbatov R. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013;31:898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun N., Ning B., Hansson K.M., Bruce A.C., Seaman S.A., Zhang C., Rikard M., DeRosa C.A., Fraser C.L., Wågberg M. Modified VEGF-A mRNA induces sustained multifaceted microvascular response and accelerates diabetic wound healing. Sci. Rep. 2018;8:17509. doi: 10.1038/s41598-018-35570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlsson L., Clarke J.C., Yen C., Gregoire F., Albery T., Billger M. Biocompatible, purified VEGF-A mRNA improves cardiac function after intracardiac injection 1 week post-myocardial Infarction in swine. Mol. Ther. Methods Clin. Dev. 2018;9:330–346. doi: 10.1016/j.omtm.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pehrsson S., Hölttä M., Linhardt G., Danielson R.F., Carlsson L. Rapid production of human VEGF-A following intradermal injection of modified VEGF-A mRNA demonstrated by cutaneous microdialysis in the rabbit and pig in vivo. BioMed Res. Int. 2019;2019:3915851. doi: 10.1155/2019/3915851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gan L.M., Lagerström-Fermér M., Carlsson L.G., Arfvidsson C., Egnell A.C., Rudvik A., Kjaer M., Collén A., Thompson J.D., Joyal J. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat. Commun. 2019;10:871. doi: 10.1038/s41467-019-08852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kajander S.A., Joutsiniemi E., Saraste M., Pietilä M., Ukkonen H., Saraste A., Sipilä H.T., Teräs M., Mäki M., Airaksinen J. Clinical value of absolute quantification of myocardial perfusion with (15)O-water in coronary artery disease. Circ. Cardiovasc. Imaging. 2011;4:678–684. doi: 10.1161/CIRCIMAGING.110.960732. [DOI] [PubMed] [Google Scholar]
- 33.Danad I., Uusitalo V., Kero T., Saraste A., Raijmakers P.G., Lammertsma A.A., Heymans M.W., Kajander S.A., Pietilä M., James S. Quantitative assessment of myocardial perfusion in the detection of significant coronary artery disease: cutoff values and diagnostic accuracy of quantitative [(15)O]H2O PET imaging. J. Am. Coll. Cardiol. 2014;64:1464–1475. doi: 10.1016/j.jacc.2014.05.069. [DOI] [PubMed] [Google Scholar]
- 34.Gan L.M., Svedlund S., Wittfeldt A., Eklund C., Gao S., Matejka G., Jeppsson A., Albertsson P., Omerovic E., Lerman A. Incremental value of transthoracic doppler echocardiography-assessed coronary flow reserve in patients with suspected myocardial ischemia undergoing myocardial perfusion scintigraphy. J. Am. Heart Assoc. 2017;6:6. doi: 10.1161/JAHA.116.004875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blomster J.I., Svedlund S., U Westergren H., Gan L.M. Coronary flow reserve as a link between exercise capacity, cardiac systolic and diastolic function. Int. J. Cardiol. 2016;217:161–166. doi: 10.1016/j.ijcard.2016.04.179. [DOI] [PubMed] [Google Scholar]
- 36.Green C.P., Porter C.B., Bresnahan D.R., Spertus J.A. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J. Am. Coll. Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 37.Spertus J.A., Winder J.A., Dewhurst T.A., Deyo R.A., Prodzinski J., McDonell M., Fihn S.D. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J. Am. Coll. Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 38.The Criteria Committee of the New York Heart Association . Ninth Edition. Little, Brown & Co.; Boston: 1994. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. [Google Scholar]
- 39.Hedman M., Hartikainen J., Syvänne M., Stjernvall J., Hedman A., Kivelä A., Vanninen E., Mussalo H., Kauppila E., Simula S. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003;107:2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Q., Sun Y., Xia L., Chen A., Wang Z. Randomized study of mononuclear bone marrow cell transplantation in patients with coronary surgery. Ann. Thorac. Surg. 2008;86:1833–1840. doi: 10.1016/j.athoracsur.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 41.Schumacher B., Stegmann T., Pecher P. The stimulation of neoangiogenesis in the ischemic human heart by the growth factor FGF: first clinical results. J. Cardiovasc. Surg. (Torino) 1998;39:783–789. [PubMed] [Google Scholar]
- 42.Ruel M., Laham R.J., Parker J.A., Post M.J., Ware J.A., Simons M., Sellke F.W. Long-term effects of surgical angiogenic therapy with fibroblast growth factor 2 protein. J. Thorac. Cardiovasc. Surg. 2002;124:28–34. doi: 10.1067/mtc.2002.121974. [DOI] [PubMed] [Google Scholar]
- 43.Ruel M., Beanlands R.S., Lortie M., Chan V., Camack N., deKemp R.A., Suuronen E.J., Rubens F.D., DaSilva J.N., Sellke F.W. Concomitant treatment with oral L-arginine improves the efficacy of surgical angiogenesis in patients with severe diffuse coronary artery disease: the Endothelial Modulation in Angiogenic Therapy randomized controlled trial. J. Thorac. Cardiovasc. Surg. 2008;135:762–770, 770.e1. doi: 10.1016/j.jtcvs.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 44.Pätilä T., Lehtinen M., Vento A., Schildt J., Sinisalo J., Laine M., Hämmäinen P., Nihtinen A., Alitalo R., Nikkinen P. Autologous bone marrow mononuclear cell transplantation in ischemic heart failure: a prospective, controlled, randomized, double-blind study of cell transplantation combined with coronary bypass. J. Heart Lung Transplant. 2014;33:567–574. doi: 10.1016/j.healun.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Noiseux N., Mansour S., Weisel R., Stevens L.M., Der Sarkissian S., Tsang K., Crean A.M., Larose E., Li S.H., Wintersperger B. The IMPACT-CABG Trial: A Multicenter, Randomized Clinical Trial of CD133(+) Stem Cell Therapy During Coronary Artery Bypass Grafting for Ischemic Cardiomyopathy. J Thorac Cardiovasc Surg. 2016;152:1582–1588.e1582. doi: 10.1016/j.jtcvs.2016.07.067. [DOI] [PubMed] [Google Scholar]
- 46.Nasseri B.A., Ebell W., Dandel M., Kukucka M., Gebker R., Doltra A., Knosalla C., Choi Y.H., Hetzer R., Stamm C. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur. Heart J. 2014;35:1263–1274. doi: 10.1093/eurheartj/ehu007. [DOI] [PubMed] [Google Scholar]
- 47.Menasché P., Alfieri O., Janssens S., McKenna W., Reichenspurner H., Trinquart L., Vilquin J.T., Marolleau J.P., Seymour B., Larghero J. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 48.Kilian E.G., Sadoni S., Vicol C., Kelly R., van Hulst K., Schwaiger M., Kupatt C., Boekstegers P., Pillai R., Channon K. Myocardial transfection of hypoxia inducible factor-1α via an adenoviral vector during coronary artery bypass grafting. - A multicenter phase I and safety study - Circ. J. 2010;74:916–924. doi: 10.1253/circj.cj-09-0594. [DOI] [PubMed] [Google Scholar]
- 49.Hu S., Liu S., Zheng Z., Yuan X., Li L., Lu M., Shen R., Duan F., Zhang X., Li J. Isolated coronary artery bypass graft combined with bone marrow mononuclear cells delivered through a graft vessel for patients with previous myocardial infarction and chronic heart failure: a single-center, randomized, double-blind, placebo-controlled clinical trial. J. Am. Coll. Cardiol. 2011;57:2409–2415. doi: 10.1016/j.jacc.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 50.Hendrikx M., Hensen K., Clijsters C., Jongen H., Koninckx R., Bijnens E., Ingels M., Jacobs A., Geukens R., Dendale P. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial. Circulation. 2006;114(1, Suppl):I101–I107. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]