Introduction
Hair loss is a major problem for patients, and treatment of hair loss is a great challenge for doctors. In the 1970s, a new technique using artificial hair was introduced. Because of unsuitable materials and techniques, frequent severe adverse events were seen. As a result, the US Food and Drug Administration banned implantation with industrial fibers in 1983. In 1993, biocompatible fibers – biocompatible artificial hair (Biofibre®, Medicap®, Carpi, Italy) were developed, and clinical trials were performed with encouraging results. Therefore, the Council concerning Medical Devices in the European Union recognized the artificial hair implant technique as a medical act in 1996.1,2
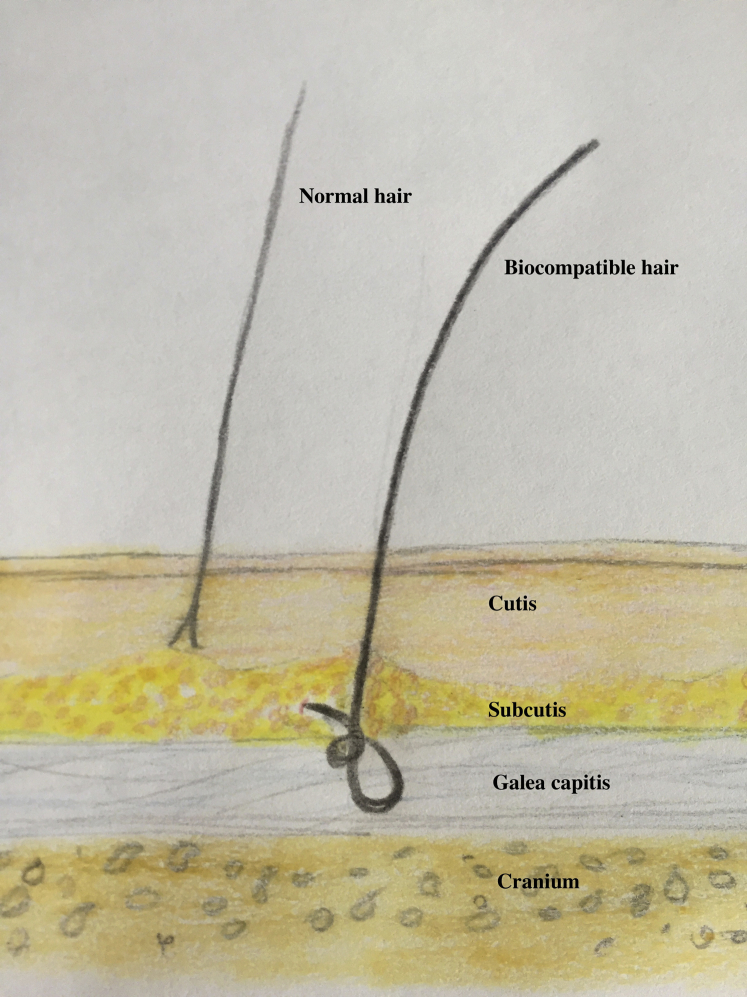
Biocompatible fiber is a polyamide fiber, which is 0.08 to 0.09 mm thick and 160 to 460 mm long.1 The implant technique is a surgery technique, performed by either a manual implanter or an automatic machine, under local anesthesia. A small hooking needle places the root of the fiber under the scalp at galea level (Fig 1) where the root can be held by fibrous tissue.2 The root is a reversible knot that ensures correct anchorage of the implant and allows total fiber extraction. With the appropriate traction, each fiber can be pulled out entirely with no residue in the scalp2 if needed in case of nontreatable side effects or unsatisfied result.
Fig 1.
Schematic picture of a biocompatible fiber. Anchorage of the fiber with the reversible knot under the scalp at galea level.
It is important that patients are properly selected; biocompatible fibers are not indicated in patients with risk of infection, autoimmune diseases, or chronic scalp disease including nonstabilized alopecia areata.
All patients should initially receive the implant with only a small amount of fibers to secure tolerance. If tolerated, larger sessions can be added. An average implant of 600 to 1000 fibers per session is often appropriate, performed at intervals of 1 month.2,3 An average amount of 2300 fibers is implanted per patient in total, varying depending on the size of the treated area.4 Spontaneous fiber loss is often less than10% per year. Follow-up and proper aftercare are essential to prevent possible complications such as infections and inflammation.2
Case report
Here we describe two patients with complications to artificial hair implants.
Case 1
A 27-year-old woman, with a history of alopecia since childhood and without desire for further investigation, started implantation with biocompatible fibers in a private clinic abroad in the summer of 2018. No tolerance test was done initially. In total, she had 9000 fibers implanted, distributed over 3 sessions. No aftercare controls were performed in the clinic. Her scalp became tender and erythematous after the third session of implantation in February 2019. The patient was seen in our department in April 2019, and examination found multiple, tender, erythematous nodules around the hairs. Pus was observed draining from many scalp areas (Fig 2, A and B). Constant pain and itching of the scalp were present in this period, and, consequently, she experienced sleeping problems and had sick leave from work. Culture found no growth of pathogenic bacteria or fungus. A scalp biopsy specimen showed a nonnecrotizing granulomatous foreign-body reaction (Fig 3). The scalp abscesses were reduced by injection of a single triamcinolone acetonide, 10 mg/mL, and the diffuse inflammation of the scalp was treated with topical mometasone. The treatment reduced pain and, together with an increased spontaneous fiber loss, resulted in the patient declining the opportunity of extraction.
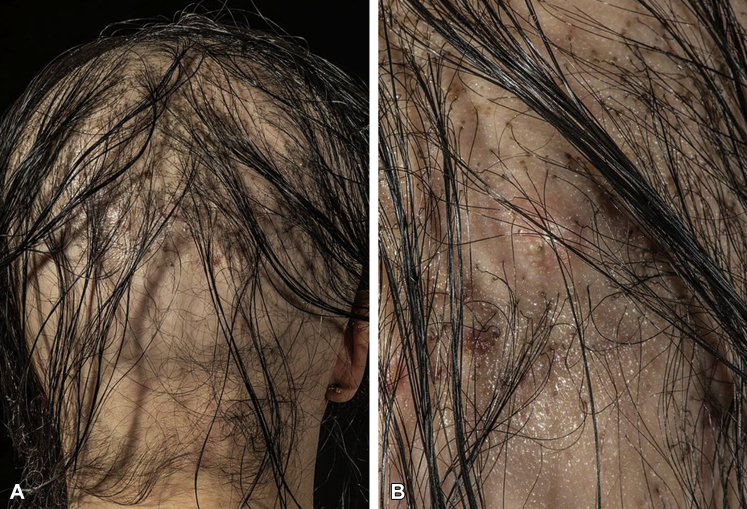
Fig 2.
Artificial hair implants and side effects. A 27-year-old woman, known to have congenital alopecia, had constant pain and itching of the scalp after implantation of 9000 biocompatible fibers. The examination found multiple, tender, erythematous nodules with pus. A, Overview of the back of the scalp. B, Close-up of the same area.
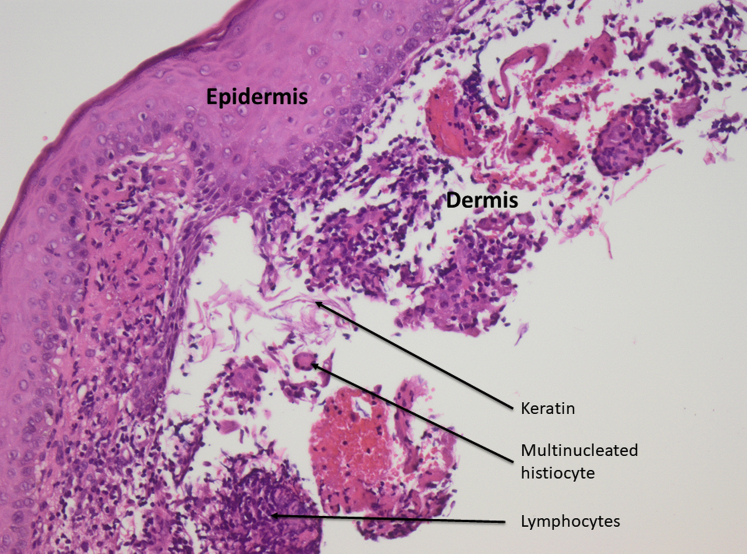
Fig 3.
Nonnecrotizing granulomatous foreign-body reaction. Superficial biopsy shows epidermis and a section of papillary dermis. In the dermis there is detached keratin infiltrated by lymphocytes and multinucleated histiocytes representing foreign body reaction. No follicles are represented in the biopsy. (Hematoxylin-eosin stain; original magnification: ×20.)
Case 2
A 54-year-old woman, known to have androgenic alopecia and hypothyroidism, had biocompatible fibers implanted into frontal bald areas in her scalp at a private clinic abroad in 2017. In total, 1000 fibers were implanted during one session, and no tolerance tests were done before this session. No follow-up to ensure correct aftercare was done at the performing clinic. Shortly after the procedure, she had tender, erythematous nodules with pus draining from the area. She was treated with systemic and topical antibiotics and corticosteroid without any effect. Eventually, the patient was referred to us, foreign-body reaction was suspected, and we extracted all the fibers leading to resolution of symptoms.
Discussion
These 2 cases show the potential side effect seen when using biocompatible fibers. Studies have shown approximately 95% to 98% satisfaction rate among patients.1,2,4,5 However, 6% to 13% reported allergic, inflammatory, or infectious complications in which approximately 2% were not solved and required fiber extraction.1, 2, 3, 4 Infections are mainly caused by Staphylococci aureus and epidermidis, Streptococcus pyogenes, and corynebacterium.1,5 Inflammatory complications are described as mild reddening and itching,6 histologically shown as peri-implant inflammation and folliculitis7 and in some cases foreign body reactions.8 During the initial tolerance test, some patients had a hypersensitivity reaction.1 Even severe adverse reactions have been reported, including osteomyelitis and infective endocarditis.9
The unsatisfactory cases described above were caused by unsuitable selection of patients, massive implant sessions, lack of patient's aftercare, and postoperative infection or badly treated inflammation.1,4
Alopecia, especially in women, has a major impact on quality of life, and there is a desperate need for treatment. Expensive artificial hair implants are often offered in private clinics, far away from the patient's residence. Therefore, the standards as described in the literature; initial tolerance test, recommended number of fibers implanted, and correct follow-up and aftercare, are not always followed. The complication rate is therefore probably higher than described above. It is crucial that the selection process, the number of fibers implanted, and aftercare are all done properly to avoid complications.
This case-report describes potential side effects and stresses the importance of compliance with the precautions. It also illuminates a procedure for treating alopecia that is not familiar to all dermatologists.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Said A.A.R., Albzour B.M., Santiago M. Automatic artifical hair implant: safety and efficacy in androgenetic alopecia: a prospective study with highly biocompatible fiber. Open Access Maced J Med Sci. 2017;6(1):38–42. doi: 10.3889/oamjms.2018.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roccia M., França K., Castillo D. Artificial hair: by the dawn to automatic biofibre hair implant. Open Access Maced J Med Sci. 2017;6(1):156–162. doi: 10.3889/oamjms.2018.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serdev N., D Erme A.M., Hercogova J. Polyamide hair implant (biofibre®): evaluation of efficacy and safety in a group of 133 patients. J Biol Regul Homeost Agents. 2015;29(1 Suppl):103–109. [PubMed] [Google Scholar]
- 4.Satolli F., Rovesti M., Bogdan Moran A.B. Biofibre artificial hair implant: retrospective study on 1,518 patients with alopecia and present role in hair surgery. Dermatol Ther. 2019;32(4):e12985. doi: 10.1111/dth.12985. [DOI] [PubMed] [Google Scholar]
- 5.Lotti T., Tirant M., Rateb Said A. Clinical updating Study at 3 years on 278 patients treated by modern artificial hair implant technique (Automatic Biofibre®) Dermatol Ther. 2020;33(1):e13194. doi: 10.1111/dth.13194. [DOI] [PubMed] [Google Scholar]
- 6.Santiago M., Pérez-Rangel R., D Úgo A. Artificial hair fiber restoration in the treatment of scalp scars. Dermatol Surg. 2007;33:35–44. doi: 10.1111/j.1524-4725.2007.33005.x. [DOI] [PubMed] [Google Scholar]
- 7.Cranwell W.C., Sinclair R. Familial frontal fibrosing alopecia treated with dutasteride, minoxidil and artificial hair transplantation. Australas J Dermatol. 2017;58:e94–e96. doi: 10.1111/ajd.12499. [DOI] [PubMed] [Google Scholar]
- 8.Kelly R.I., Marsden R.A. Complications of artificial hair implantation. J R Soc Med. 1994;87:291–292. doi: 10.1177/014107689408700518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai S., Okabayashi A., Tohda R. Case of infective endocarditis caused by implanted artificial hair pyoderma. J Dermatol. 2019;46(1):e35–e36. doi: 10.1111/1346-8138.14499. [DOI] [PubMed] [Google Scholar]