Abstract
This data in brief article explains the anti-cancer activity and characterization of oxadiazoles [1]. The main objective of this article was to provide general synthetic procedures, spectral discussion and physical data on the new oxadiazole tethered indazole (OTDs). This article discusses the 1H NMR, 13C NMR, mass spectroscopy, HPLC and melting point of the synthetic compounds. MTT assay was used to determine the anti-proliferative activity in hepatocellular cell lines.
Keywords: Indazole, Anti-cancer, MTT assay, Characterization
Specifications table
| Subject | Chemistry |
| Specific subject area | Organic Chemistry |
| Type of data | Figure, Spectra |
| How data were acquired | 1H NMR and 13C NMR spectra were recorded on the Agilent NMR instrument in DMSO-d6/CDCl3 solvent. Mass spectra have been recorded on an Agilent LC-MS.HPLC was recorded Waters (USA) connected to W2998 Photo-Diode Arrary Detector. Cytotoxicity was determined by MTT Assay. |
| Data format | Raw Analyzed |
| Parameters for data collection | All reagents and solvents were commercially available in the reagent grade. These were used without further purification. All the final compounds were purified by column chromatography on silica gel (60–120 mesh). |
| Description of data collection | All the final isolated compounds were characterized by NMR spectroscopy, LC-MS and melting points. |
| Data source location | Institution: University of Mysore, Manasagangotri, Mysore-570006 City/Town/Region: Mysore Country: India |
| Data accessibility | Data available with article |
| Related research article | Dukanya, M. K. Shanmugam, S. Rangappa, P. K. Metri, S. Mohan, Basappa, K. S Rangappa,Exploring the newer oxadiazoles as real inhibitors of human SIRT2 in hepatocellular cancer cells. Bioorg. Med. Chem. Lett. 30,2020,127330. doi:10.1016/j.bmcl.2020.127330. |
Value of the data
-
•
Indazole tethered oxadiazole (OTD) derivatives have significant anti-cancer activity against hepatocellular carcinoma (HCC) cells.
-
•
The given procedure for the synthesis of Indazole tethered oxadiazoles could be useful for organic chemists to develop novel drugs.
-
•
The given spectroscopic data on the Indazole tethered oxadiazoles could be useful for organic chemists for synthesis of OTDs.
1. Data description
A series of Indazole tethered oxadiazoles (OTDs) (3a-k) were synthesized by refluxing N-methyindazole-acid hydrazide with different aromatic carboxylic acids (2a-k) in phosphorous oxychloride [1]. The synthetic route and structure of final compounds were given in Fig. 1. All the final compounds (3a-k) were characterized by 1H NMR, 13C NMR, LCMS and HPLC for active compounds 3c, 3d, 3f, 3i. All the spectra data are provided in this article (Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, Fig. 11, Fig. 12, Fig. 13, Fig. 14, Fig. 15, Fig. 16, Fig. 17, Fig. 18, Fig. 19, Fig. 20, Fig. 21, Fig. 22, Fig. 23, Fig. 24, Fig. 25, Fig. 26, Fig. 27, Fig. 28, Fig. 29, Fig. 30, Fig. 31, Fig. 32, Fig. 33, Fig. 34, Fig. 35, Fig. 36, Fig. 37, Fig. 38). The anti-cancer activities of the newly synthesized OTDs are given in Fig. 39. The Crystalographic data for compounds 3a, 3 h & 3k have been deposited with the Cambridge Crystalographic Data centre., CCDC No. 1992373, 1992374 and 1992375. Copies of the data can be obtained free of charge upon request to the CCDC, 12 Union Road, Cambridge CB21EZ, UK (fax: 044 (0) 1223 336033 or e-mail: deposit@ccdc.cam.ac.uk).
Fig. 1.
Synthetic scheme and structure of Oxadiazoles 3a-3k.
Fig. 2.
1H NMR spectrum of 3a.
Fig. 3.
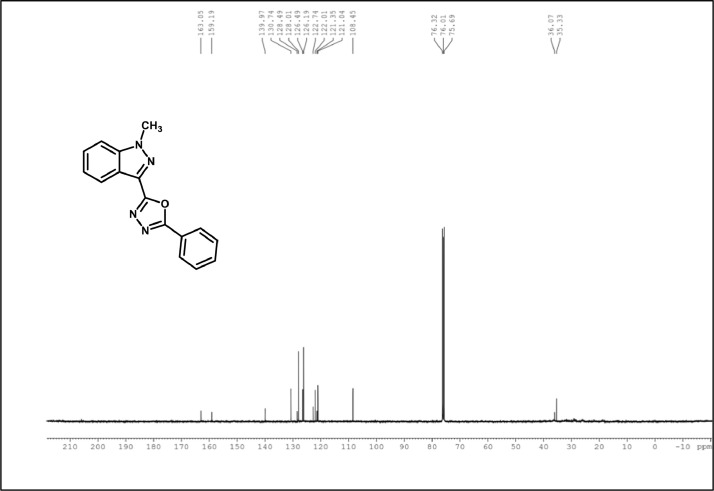
13C NMR spectrum of 3a.
Fig. 4.
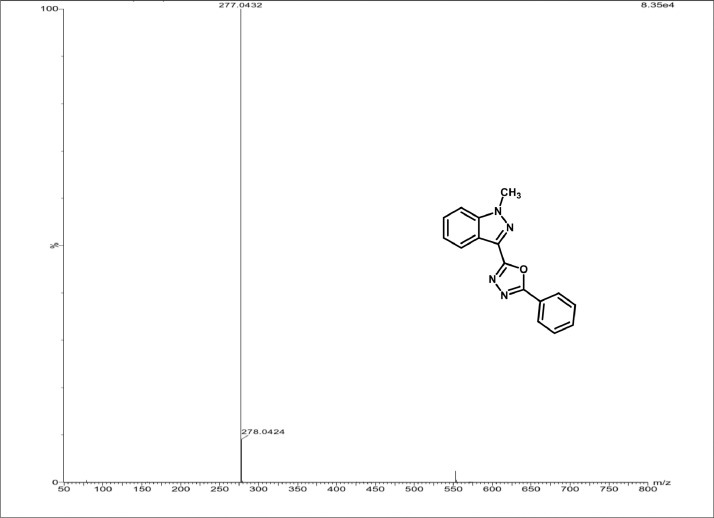
Mass spectrum of 3a.
Fig. 5.
1H NMR spectrum of 3b.
Fig. 6.
13C NMR spectrum of 3b.
Fig. 7.
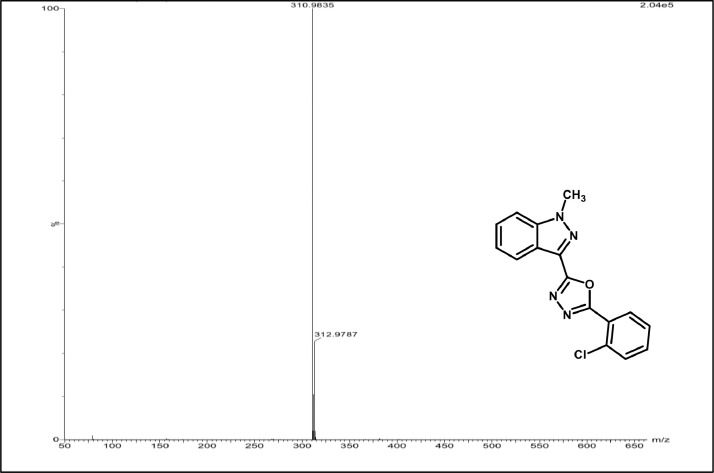
Mass spectrum of 3b.
Fig. 8.
1H NMR spectrum of 3c.
Fig. 9.
13C NMR spectrum of 3c.
Fig. 10.
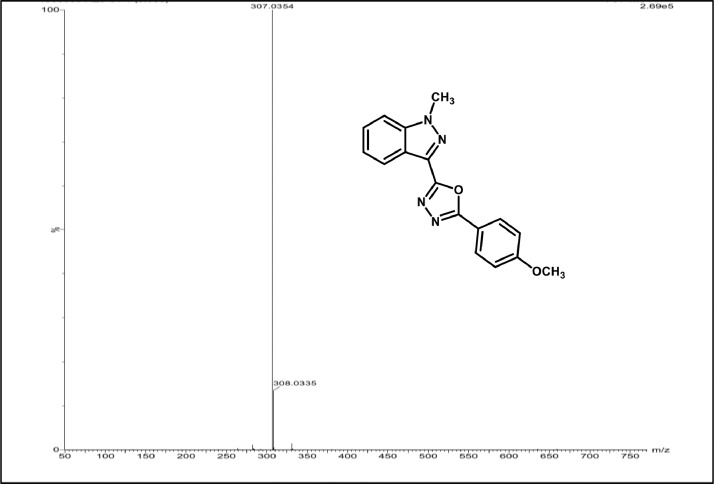
Mass spectrum of 3c.
Fig. 11.
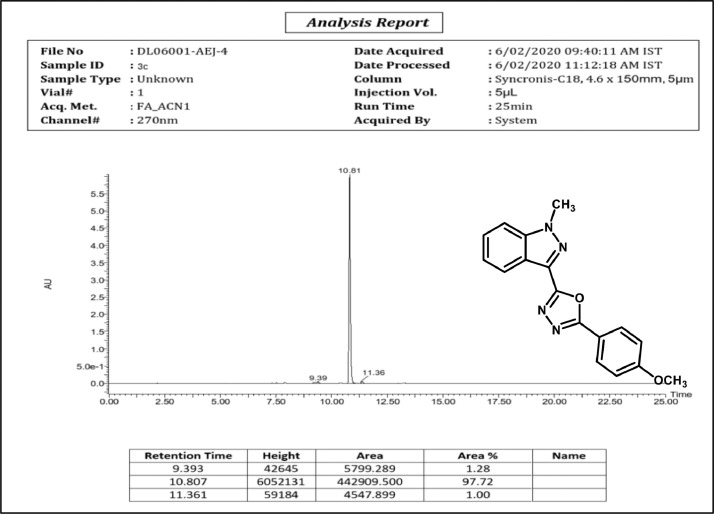
HPLC spectrum of 3c.
Fig. 12.
1H NMR spectrum of 3d.
Fig. 13.
13C NMR spectrum of 3d
Fig. 14.
Mass spectrum of 3d
Fig. 15.
HPLC spectrum of 3d
Fig. 16.
1HNMR spectrum of 3e.
Fig. 17.
13C NMR spectrum of 3e.
Fig. 18.
Mass spectrum of 3e.
Fig. 19.
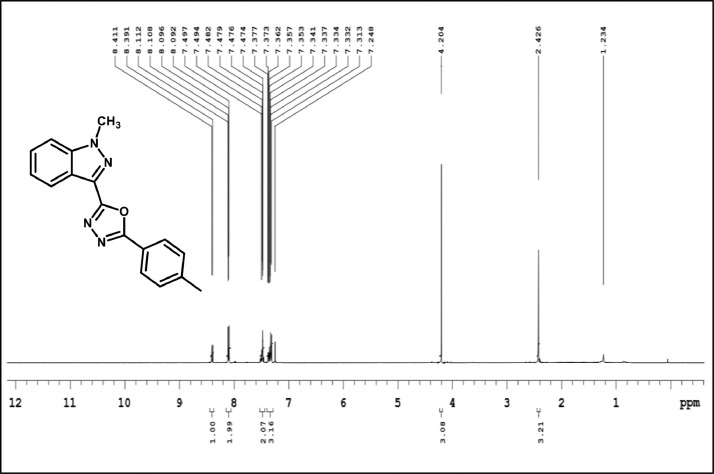
1H NMR spectrum of 3f.
Fig. 20.
13C NMR spectrum of 3f.
Fig. 21.
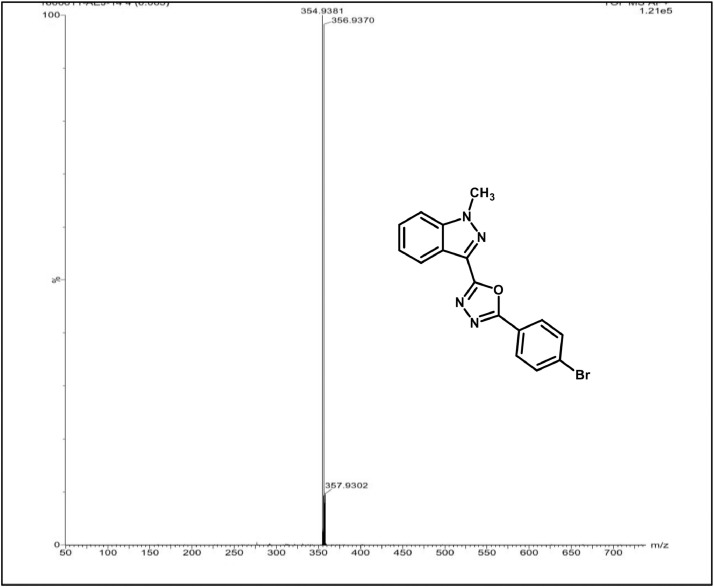
Mass spectrum of 3f.
Fig. 22.
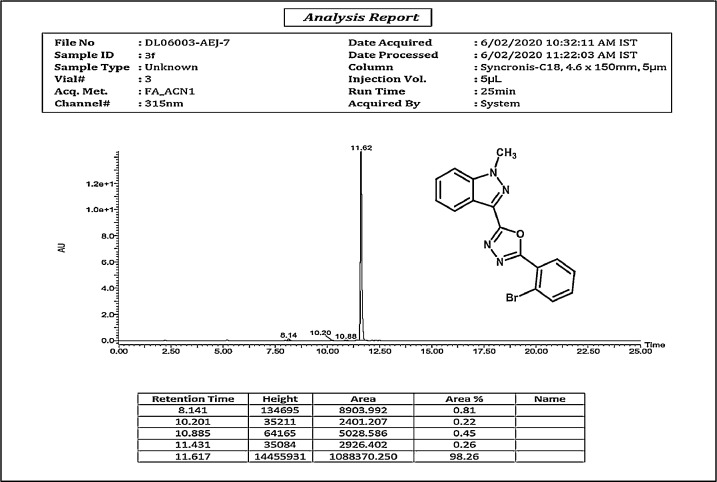
HPLC spectrum of 3f.
Fig. 23.
1HNMR spectrum of 3g.
Fig. 24.
13C NMR spectrum of 3g.
Fig. 25.
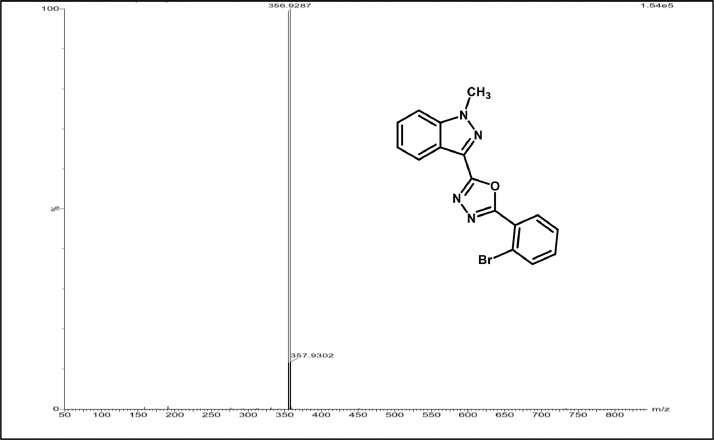
Mass spectrum of 3g.
Fig. 26.
1HNMR spectrum of 3h.
Fig. 27.
13C NMR spectrum of 3h.
Fig. 28.
Mass spectrum of 3h.
Fig. 29.
1HNMR spectrum of 3i.
Fig. 30.
13C NMR spectrum of 3i.
Fig. 31.
Mass spectrum of 3i.
Fig. 32.
HPLC spectrum of 3i.
Fig. 33.
1H NMR spectrum 3j.
Fig. 34.
13C NMR Spectrum of 3j.
Fig. 35.
Mass spectrum of 3j.
Fig. 36.
1H NMR spectrum of 3k.
Fig. 37.
13CNMR spectrum of 3k.
Fig. 38.
Mass spectrum of 3k.
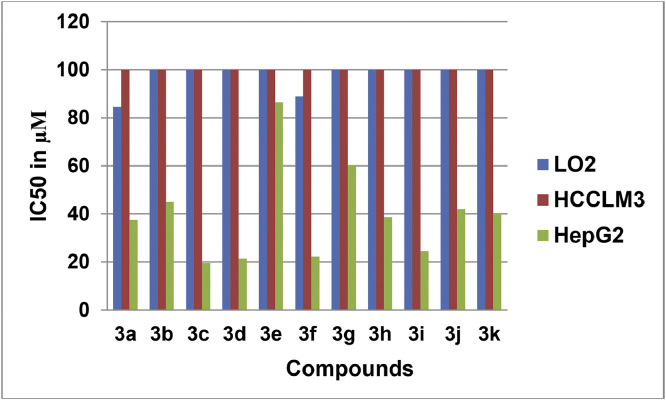
Fig. 39.
Anti-proliferative activity of Oxadiazoles as determined by MTT Assay.
2. Experimental design, materials and methods
2.1. General information
The reagents used for the reaction are commercially available reagent class and were used without further purification. The chemicals were purchased from Sigma-Aldrich, TCI or SD-Fine. The reactions were monitored by thin layer chromatography using pre-coated Merck silica gel 60 F254 plates and analytical TLCs were conducted using ethyl acetate and hexanes as eluent. Then the spots were observed under UV light. The final compounds were purified by silica gel column chomatography using ethyl acetate and hexane as eluent and organic solvents were concentrated under reduced pressure on the rotary evaporator.
The purified samples were dissolved in either CDCl3 or DMSO-d6 to acquire the NMR spectra using Agilent NMR. 1H NMR and 13C NMR were recorded at 400 and 100 MHz respectively. The 1H NMR Data are expressed in chemical shift (δ, ppm) and multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, dt = doublet of triplet, dd = doublet of doublets, m = multiplet), coupling constant (J, Hz) and integration. The LCMS measurments were performed on Agilent LC-MS using 2 mg/mL of the solutions in HPLC grade acetonitrile or methanol. The HPLC was recorded using Waters (USA) connected to W2998 Photo-Diode Array Detector, Column: Syncronis-C18, 4.6 × 150 mm, 5 μm, Mobile Phase-A: 0.1% Formic acid in Water, Mobile Phase-B: Acetonitrile.
The OTDs were screened for their anti-proliferative potency against human hepatocellular carcinoma cell lines (HepG2, HCCLM3) and normal Liver human cell line (LO2) using MTT assay [2].
2.2. General procedure
To a stirred solution of 1-methyl-1H-indazole-3-carboxylic acid (1 gm, 0.0056 mol) in anhydrous ethanol (15 mL). The catalytic amount of conc. Sulfuric acid was added and refluxed for 6 h. The excess of ethanol was concentrated off on a rota-evaporator. The reaction mixture was cooled and the contents of the flask were transferred to a separating funnel containing about 250 mL of distilled water. The synthesized ester was extracted three times with 50 mL Ethyl acetate each. The organic layers were mixed and washed with brine and dried over Sodium sulfate. Ethyl acetate was then distilled off under reduced pressure to get the ester 1 in 89% yield.
To a stirred solution of ethyl 1-methyl-1H-indazole-3-carboxylate 1 (0.5 gm, 0.0024 mol) taken in a round bottom flask containing 250 mL of ethanol, 99% hydrazine hydrate (0.15 mL, 0.0029 mol) was added and refluxed for 6 h. The excess of ethanol and hydrazine hydrate were distilled off under reduced pressure. Ice-cold water was poured into the above reaction mixture and the precipitate so obtained was filtered off and recrystallized from a suitable solvent and the product N-methy indazole-acid hydrazide [3] was obtained in 92% yield.
2.3. Procedure for the synthesis of 3a-k
A mixture of 1-methyl-1H-indazole-3-carbohydrazide (1eq), different aromatic acids (2a-k) (1eq) and phosphorus oxychloride (POCl3) (2 mL) were taken in round bottom flask and refluxed for 8 h. The contents of the reaction mixture were cooled and poured onto the crushed ice. The solid obtained was filtered, treated with potassium carbonate, washed with water and purified by column chromatography using ethyl acetate and hexane as eluent to afford oxadiazoles.
2. 3 characterization data
2-(1-methyl-1H-indazol-3-yl)-5-phenyl-1,3,4-oxadiazole, 3a:
White Solid; mp178–182˚C; 81% yield;1H NMR (CDCl3, 400 MHz) δ: 8.35(d, J=8.4 Hz, 1H), 8.18–8.16 (m, 2H), 7.44–7.49 (m,5 H), 7.31–7.33 (m, 1H), 4.16 (s, 3H); 13C NMR: 163.05, 159.19, 139.97, 130.74, 128.49, 128.01, 126.49, 126.19, 122.74, 122.01, 121.35, 121.04, 108.45, 35.33; LCMS (ESI): m/z Calcd for C16H12N4O [M + H] +: 277.1044; Found: 277.0432.
2-(2-chlorophenyl)-5-(1-methyl-1H-indazol-3-yl)-1,3,4-oxadiazole, 3b:White solid; mp 160–164˚C; 78% yield;1H NMR (CDCl3, 400 MHz) δ: 8.08–8.6(m,1H),8.05–7.45 (m,1H), 7.45–7.43 (m,1H),7.42–7.37(m,3H), 7.36–7.31(m,2H) 4.15 (s,3H);13CNMR:161.32,159.56, 139.97,132.34,131.36,130.38,130.21,128.22,126.45,126.03,122.08,122.37,120.90,108.49,35.36; LCMS (ESI): m/z Calcd for C16H11ClN4O [M + H] +: 311.0654; Found:310.9835.
2-(4-methoxyphenyl)-5-(1-methyl-1H-indazol-3-yl)-1,3,4-oxadiazole,3c:Off white solid; mp 196–200˚C; 83% yield;1H NMR (CDCl3, 400 MHz) δ: 8.39 (d, J=8 Hz,1H), 8.15 (d, J=8. 8 Hz,2H), 7.49–7.45(m,2H),7.37–7.34(m,1H),7.01(d, J=8.8 Hz, 2H),4.20(s,3H),3.87 (s, 3H); 13CNMR:164.00,162.35,159.73,140.94, 128.97, 128.544, 127.44,122.91, 122.28,122.08, 116.24, 114.43,109.42,55.46,36.29;LCMS (ESI): m/z Calcd for C17H14N4O2[M + H] +: 307.1150; Found:307.0354.
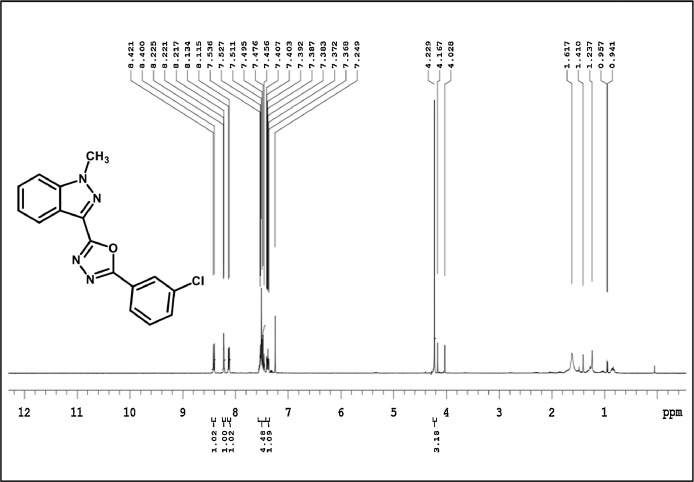
2-(3-chlorophenyl)-5-(1-methyl-1H-indazol-3-yl)-1,3,4-oxadiazole,3d: White solid; mp180–184˚C; 86% yield;1H NMR (CDCl3,400 MHz)δ: 8.41(d, J=8.4 Hz,1H), 8.23–8.22(m,1H),8.12 (d J=8 Hz, 1H),7.54–7.46(m,4H), 7.417.37 (m,1H), 4.22(s,3H); 13CNMR: 162.90, 160.47, 141.01, 135.21,131.75,130.36,129.31,127.55,127.09,125.42,125.26,123.13,122.40,122.02,109.47,36.33;LCMS (ESI): m/z Calcd for C16H11ClN4O[M + H] +: 311.0654; Found:310.9835.
4-(5-(1-methyl-1H-indazol-3-yl)-1,3,4-oxadiazol-2-yl)phenol,3e:off white solid; mp170–174˚C; 88% yield;1H NMR (DMSO-D6, 400 MHz) δ: 10.24 (s,1H),8.22 (s, 1H), 8.12(d, J=7.2 Hz,1H),7.9(d, J=8 Hz,1H),7.56 (d, J=7.6 Hz,1H),7.29–7.26 (m, 2H), 6.95(d, J=8.4 Hz,2H), 3.87 (s,3H);13C NMR:162.51, 160.89, 137.45, 132.16, 128.70, 124.89, 123.27, 121.86, 120.86, 116.61,114.97, 111.27, 99.03, 33.50;LCMS (ESI): m/z Calcd for C16H12N4O2[M + H] +: 293.0993; Found:293.0221.
2-(2-bromophenyl)-5-(1-methyl-1H-indazol-3-yl)-1,3,4-oxadiazole,3f:white solid; mp160–165˚C; 88% yield;1HNMR(CDCl3,400 MHz) δ:8.41(d, J=8.4 Hz,1H), 8.06–8.04(m,1H),7.68–64(m,1H),7.51–7.45(m,3H),7.41–7.35(m,2H),4.21(s,3H);13C NMR:162.85,160.61,140.9, 134.49,132.41,131.80,129.26,127.52,127.43,125.28,123.07,122.38,121.93(2),109.47,36.32;LCMS (ESI): m/z Calcd for C16H11BrN4O[M + H] +: 356.0095; Found:356.9287.
2-(1-methyl-1H-indazol-3-yl)-5-(m-tolyl)-1,3,4-oxadiazole,3 g:off white solid; mp150–152˚C; 85% yield;1H NMR (CDCl3, 400 MHz) δ: 8.41(d, J=8.4 Hz,1H), 8.04–8.01(m,2H), 7.50–7.34(m,5H), 4.21 (s,3H), 2.44 (s,3H); 13C NMR: 164.21, 160.10, 140.98, 138.89, 132.89, 132.54, 129.57, 128.90, 127.66, 127.47, 124.36, 123.61, 122.61, 122.97, 122.35, 122.08, 109.42, 36.30,29.67;LCMS (ESI): m/z Calcd for C17H14N4O[M + H] +: 291.1201; Found: 291.0395.
2-(1-methyl-1H-indazol-3-yl)-5-(p-tolyl)-1,3,4-oxadiazole,3h:off white solid; mp182–186˚C; 83% yield; 1H NMR (CDCl3, 400 MHz) δ: 8.4(d, J=8 Hz,1H), 8.11–8.09 (m,2H), 7.5–7.47(m,2H), 7.38–7.31(m,3H), 4.20(s,3H), 2.43(s,3H); 13C NMR: 164.20, 159.94, 142.30, 140.95,129.71,129.58,127.45,127.14,122.95,122.31,122.07,120.95,109.43,36.30,21.67;LCMS (ESI): m/z Calcd for C17H14N4O[M + H] +: 291.120; Found: 291.0395.
2-(3-bromophenyl)-5-(1-methyl-1H-indazol-3-yl)-1,3,4-oxadiazole,3i: Pale yellow solid; mp172–176˚C; 77% yield; 1H NMR (CDCl3, 400 MHz) δ: 8.42–8.37(m,2H),8.17 (d, J=8 Hz, 1H), 7.68 (d, J=8 Hz, 1H),7.52–7.50 (m,2H), 7.437.37 (m,2H), 4.23 (s,3H); 13C NMR: 162.75,160.48,140.99,134.70,130.59,129.92,129.26,127.58,125.72,125.57,123.15,123.08,122.37,122.02,109.51,36.38;LCMS (ESI): m/z Calcd for C16H11BrN4O[M + H] +: 355.0116; Found: 354.9381.
2-(4-chlorophenyl)-5-(1-methyl-1H-indazol-3-yl)-1,3,4-oxadiazole,3j:off white solid; mp180–186˚C; 87% yield;1H NMR (CDCl3, 400 MHz) δ: 8.38 (d, J= 8 Hz,1 H), 8 . 1 4 (d, J=8.4 Hz, 2H), 7.50–7.46 (m.4H), 7.38–7.34 (m,1H), 4.02(s,3H); 13C NMR: 163.21,160.30, 140.98, 137.98, 129.38, 128.41, 127.50, 123.06, 122.35, 122.25, 121.99,109.46,36.30;LCMS (ESI): m/z Calcd for C16H11ClN4O[M + H] +: 311.0621; Found: 310.9912.
2-(4-bromophenyl)-5-(1-methyl-1H-indazol-3-yl)-1,3,4-oxadiazole,3k:off white solid; mp184–190˚C; 85% yield;1H NMR (CDCl3, 400 MHz) δ: 8.39(d, J=8.4 Hz, 1H), 8.09–8.06 (m,2H), 7.68–7.65 (m,2H), 7.501–7.49 (m,2H),7.39–7.35(m,1H),4.21(s,3H);13C NMR:163.31, 160.32, 140.97, 132.36, 129.28, 128.56, 127.54, 126.44, 123.11, 122.65, 122.36, 121.99,109.49,36.36;LCMS (ESI): m/z Calcd for C16H11BrN4O[M + H] +: 356.0095; Found: 356.9370.
Declaration of Competing Interest
The authors declare that they are no known competing financial interests reported in this article.
Acknowledgments
This research was supported by the Council of Scientific and Industrial Research (No. 02(0291)17/EMR-II), Department of Biotechnology (BT/PR24978/NER/95/938/2017) and Vision Group on Science and Technology, Government of Karnataka, India to Basappa.
References
- 1.Dukanya, Shanmugam M.K., Rangappa S., Metri P.K., Mohan S., Basappa, Rangappa K.S. Exploring the newer oxadiazoles as real inhibitors of human SIRT2 in hepatocellular cancer cells. Bioorg. Med. Chem. Lett. 2020;30:127330. doi: 10.1016/j.bmcl.2020.127330. [DOI] [PubMed] [Google Scholar]
- 2.F.McAuliffe P., F.Meric-Bernstam G.B.Mills, Gonzalez-Angulo A.M. Deciphering the role of PI3K/Akt/mTOR pathway in breast cancer biology and pathogenesis. Clin. Breast Cancer. 2010;10:S59–S65. doi: 10.3816/CBC.2010.s.013. [DOI] [PubMed] [Google Scholar]
- 3.S.Zakir M.Patel, Farooqui M. Synthesis of new α aminophosphonate system bearingIndazole moiety and their biological activity. Eur. J. Med. Chem. 2012;50:39–43. doi: 10.1016/j.ejmech.2012.01.024. [DOI] [PubMed] [Google Scholar]