Abstract
Introduction: Parkinson’s disease (PD) is one of the most common neurodegenerative disorders. The neuroinflammation in the brain of PD patients is one of the critical processes in the immune pathogenesis of PD leading to the neural loss in the substantia nigra. Due to the anti-inflammatory effects of curcumin (CU) and low-level laser therapy (LLLT), we examined the protective effect of CU and LLLT on PC12 cells treated with 6-hydroxydopamine (6-OHDA) as a Parkinson model.
Methods: PC12 cells were pretreated using various concentrations of 6-OHDA for 24 hours to induce oxidative and cellular damages. PC12-6-OHDA cells were co-treated with CU and LLLT. The effects of CU and LLLT on Bax/Bcl2 and LC3/ATG10 expression were analyzed by real-time PCR and cell viability was assessed by MTT assay. Cell A Software was used to calculate the length of the Neurite and cell body areas.
Results: The results of this study show that the combination of CU dose-dependently and LLLT has a significant neuroprotective effect on cells and cellular death significantly decreases by increasing CU concentration. CU+LLLT decreases Bax/Bcl2 ratio which is an indicator of apoptosis and it also rescued a decrease in LC3 and ATG10 expression in comparison with 6-OHDA group.
Conclusion: This study shows that the combination of 5 μM CU and LLLT has the best neuroprotective effect on PC12 cells against 6-OHDA by decreasing the BAX/BCL2 ratio.
Keywords: LLLT, Curcumin, LC3, BAX/BCL2, ATG10, 6-OHDA
Introduction
Parkinson’s disease (PD) is a neurodegenerative movement disorder characterized by bradykinesia, muscular rigidity, and tremor. In the pathogenicity of PD, genetic and environmental factors play an important role.1 The precise molecular mechanisms underlying PD remain to be completely elucidated. The impaired redox signaling, mitochondrial dysfunction, excitotoxicity, chronic inflammation, early apoptosis, and synaptic loss in dopaminergic neurons are several factors that are associated with PD pathogenicity.2 Based on recent evidence, stress-mediated neuroinflammation in the brain is evident.3 Reactive oxygen species (ROS) could be generated through different mechanisms such as dopamine metabolism, NADPH oxidases, Fenton reaction, and aging, and they are associated with the pathogenicity of neurodegenerative disorders.4 Thus, the prevention of oxidative stress could be considered as a potential therapeutic strategy. After treating nerve-growth-factor-differentiated PC12 cells with neurotoxins 6-hydroxydopamine (6-OHDA), neurons take up 6-OHDA which causes damage due to mitochondrial dysfunction, resulting in over-production of ROS and DNA damage.5,6 Curcumin (CU) is a polyphenol compound and a major component of the Curcuma longa plant. Due to the inhibition of cyclooxygenase-2, lipoxygenase, and inducible nitric oxide synthase by CU, it has anti-inflammatory and antioxidant activities.7-9 CU can reduce the level of lipid peroxidation and regulate antioxidant enzymes.10-12 Photobiomodulation or low-level laser therapy (LLLT) improves electron transport in mitochondria and increases mitochondrial membrane potential and it also induces the production of low levels of ROS in the normal cell.13,14 In the present study, we provide evidence that CU and LLLT inhibit ROS production and apoptosis in PC12 cells treated with 6-OHDA.
Materials and Methods
Cell Culture
PC12 cells were cultured and seeded in a DMEM/F12/NGF medium which was supplemented by (penicillin –streptomycin)100 U/mL), 5% fetal bovine serum and 5% horse serum using poly-L-lysine coated T-75 culture flasks. A humidified incubator with 5% CO2 was used to incubate cells at 37°C. The cells between passages 2 and 8 were used for this experiment because of the easiness in their isolation. After passage 10, all cells got clumpy and the isolation was difficult. A cell scraper was used to dislodge cultured cells from the flask after 80% confluency. Then the poly-L-lysine coated 96-well microplate was used to grow these cells at a density of 1 × 105 cells/mL. After overnight incubation, the cells adhered to the substrate. Then all cells were treated with 100 μM concentrations of 6-OHDA for 24 hours. The cells in the negative control group were only cultured in DMEM/F12 Cell Culture Media and the cells in the positive control group were exposed only to 6-OHDA.
Application of CU
DMSO was used to prepare the CU stock solution (50 mM). CU was diluted before adding to cell culture because the final concentration of DMSO should be kept at 1% in the culture medium. One hour after seeding, CU was added to the cell culture medium in different concentrations of 1.0, 2.5, 5.0, 7.5, and 10.0 μM.
PC12-6-OHDA Treatment
The PC12 cells were cultured in DMEM/F12/NGF 5% FBS, PC, and SP (1%). Afterward, these cells were exposed to CU and LLLT (LLL Protocol: 0.0022 W/cm2, 635 nm, 4, 6, 8 mW, 1.5 J/cm2). The laser beam diameter was 1.56 cm, and the laser beam surface area was 1.96 cm2.13,14
Determination of Cell Viability
In order to investigate the cytotoxicity effect of CU treatment on 6-OHDA induced PC12, 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used. MTT reagent, a yellow tetrazole, is converted to insoluble purple formazan after reducing in the mitochondria of living cells. The resultant shift in color was measured by absorbance microplate readers at 570 nm. The cell viability mean percentage of each group was compared with the untreated control mean percentage and they were reported as mean ± standard error of the mean (SEM).
Examination of PC12 Cell Morphology
The 6-well plates were used for seeding the PC12 cell. Fifty cells were selected on a random basis and they were examined in each group. The Cell A program was used to analyze the findings. Afterward, images were taken randomly from each well (20 images per well) for morphological analysis purposes. The cell body area and neurite length were measured after the administration of LLLT and CU. The neurite length was considered the sum of the lengths of all primary branches plus associated twigs. The cell body area was considered as the area of a cell body without its branches. The minimum number of quantified cells per treatment was 50.
Real-Time PCR
To quantify the transcripts of genes including ATG10, Bax, Bcl2, and LC3, the total RNA of these cells from the dissected spine was extracted using the RNA Extraction kit in the first week (Invitrogen, USA). The Prime Script TM RT reagent Kit (Fermentas, Lithuania) was used to produce cDNAs. Syber Green PCR Master Mix (Applied Biosystems, USA) was used for performing reverse transcription using Step OnePlus™ Real-Time PCR System (Applied Biosystems, USA) based on the protocols provided by the company. Initial denaturation was carried out at 95˚C, for 30 seconds, annealing at 56˚C for 45 seconds, extension at 72˚C for the duration of 45 seconds and a final polymerization at 65˚C for 10 minutes. The forward and reverse primer sequences for bax, bcl2, and GAPDH genes were briefed in Table 1. For gene target expression analysis (2ΔΔCt), a comparative Ct was applied. GAPDH level was used for normalizing all samples as the loading control.
Table 1. Primer Sequences Used in the qRT-PCR .
| Gene | Primer Type | Sequence | Annealing Temperature | Product Size (Base Pairs) |
| Bax | F | CCCGAGAGGTCTTTTTCCGAG | 65 | 210bp |
| R | CCAGCCCATGATGGTTCTGAT | |||
| Bcl2 | F | TACAGGCTGGCTCAGGACTAT | 65 | 230bp |
| R | CGCAACATTTTGTAGCACTCTG | |||
| LC3 | F | TGTTAGGCTTGCTCTTTTGG | 58 | 218bp |
| R | GCAGAGGAAATGACCACAGAT | |||
| ATG10 | F | CCTGTTTGCTTGGGATAGTGG | 61 | 198bp |
| R | ACTTCCCCATCAATCTCCAC | |||
| GAPDH | F | CCACAACTC TTCCATTCTC | 59 | 200bp |
| R | CCAAGATTCACGGTAGATAC |
Data Analysis
One-way ANOVA and Tukey post hoc test were conducted for statistical analysis. The data were presented as mean ± SEM and the P value < 0.05 was considered as statistically significant.
Results
The Morphological Characteristics of PC12 Cells Post-Treatment With LLLT and CU
Based on our morphological findings, cell exposure to 6-OHDA for 24 hours decreased length and width of the neurite which was rescued by the treatment of cells with LLLT and/or CU. An increase in cell body area induced by 6-OHDA were rescued in the group that received CU, LLLT, and CU+LLLT (Figure. 1).
Figure 1.
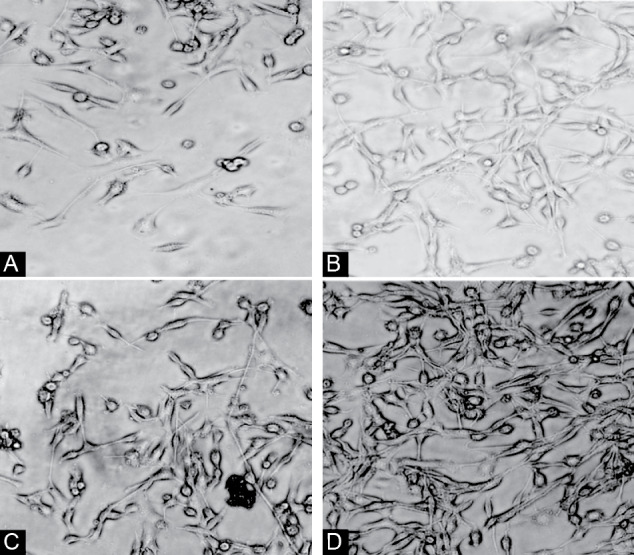
Morphology of PC12 cells after treating by 6OHDA (A), CU(B), LLLT(C), and LLLT+CU (D).
Dose-Response of CU Toxicity
In order to find the half-maximal inhibitory concentration of CU, different concentrations of CU were investigated using 10 000 cells/well. The cell viability decreased significantly in PC12 cells incubated with an increasing concentration of CU from 0 to 10 μM for 24 hours. It was found that there is an association between increasing CU concentration and increased cell death. By using 10 µM of CU, the mortality rate rose dramatically in comparison with the other groups. The survival rate decreased in all groups in comparison with the control group except in the presence of 5.0 micro molar of CU. (Figure 2).
Figure 2.
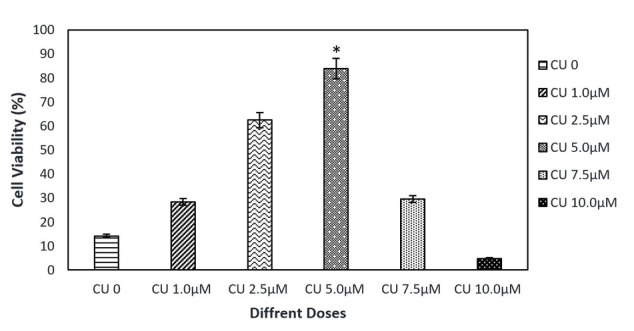
The Effect of Different Doses of CU on Cell Viability. The results indicated that 5 µM of CU was the optimal dose for cell viability.
6-OHDA-Induced Cell Death in Different Groups
After treating cells with 6-OHDA 100 µM for 24 hours, CU, LLLT, and CU+LLLT, the apoptosis rate was evaluated using MTT. The results showed that the presence of 6-OHDA increased cell death and mortality rate dramatically in comparison with the other groups. However, in all three groups, survival was reduced significantly in comparison with the 6-OHDA group (Figure 3).
Figure 3.
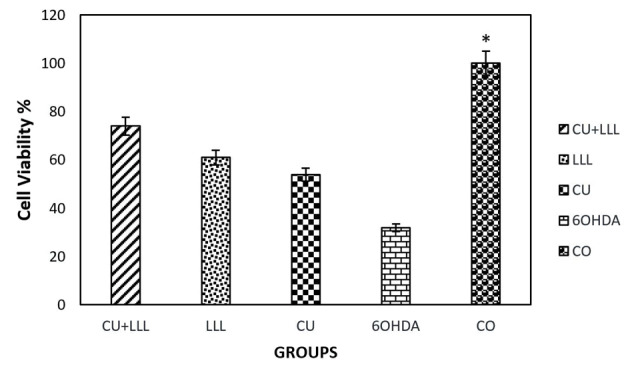
The Effect of 6-OHDA, CU, LLLT, CU+LLLT on Cell Viability. The results indicated that the mortality rates increased after treating by 6-OHDA and decreased after treating by CU, LLLT, and CU+ LLLT (*P < 0.5).
The Effect of LLLT+CU on the Neuritis Length and Cell Body Area of PC12 Cells After 6-OHDA Treatment
Based on our findings using the Cell A program, the neuritis length decreased significantly after treating cells with 6-OHDA which is responsible for inducing oxidative stress. The decrease in 6-OHDA group was rescued by CU, LLL, and LLLT+CU treated group. The level of rescue is higher LLLT+CU other two groups (Figures 4 and 5).
Figure 4.
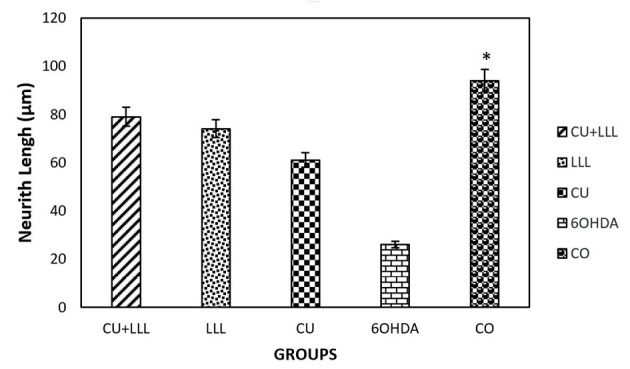
The effect of CU, LLLT, and CU+LLLT on the PC12 Neurith length was defined as the sum of lengths of all primary branches and their associated twigs. *P < 0.05 significantly different from the 6OHDA groups.
Figure 5.
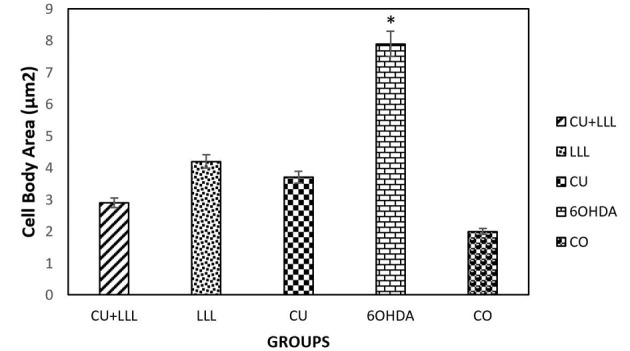
The Effect of CU+LLLT, LLLT, CU, and 6OHDA on the Morphology of PC12 cells. The cell body area was considered without its branches. The cell body area was significantly higher in the 6OHDA group than the other groups (*P < 0.05).
Effect of LLLT+CU on the BAX/BCL2 Ratio in 6-OHDA-Treated Cells
Based on the results of real-time PCR, the Bax/Bcl2 ratio increased in the 6-OHDA-treated cells in comparison with the control group which was rescued by LLL, CU, LLL+CU groups, but the level of rescue is highest in the LLLT+CU-treated group in comparison with the control group (Figure 6).
Figure 6.
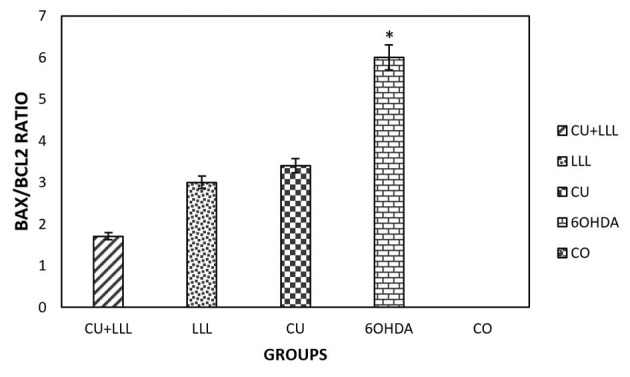
The Bax/Bcl2 Expression Ratio in Different Groups. The results indicated that an increase in the Bax/Bcl2 ratio in the 6-OHDA group was significantly rescued by CU, LLL, CU+LLL groups (*P < 0.05).
Effect of LLLT+CU on the LC3 and ATG10 in 6-OHDA-Treated Cells
The real-time PCR analysis showed that the expression of LC3 and ATG10 decreased in 6-OHDA-treated cells. ATG10 and LC3 are indicators of the accumulation of autophagosomes and the decrease in their expression level in 6-OHDA treated group were rescued significantly by LLLT+CU, LLL and CU treatment (Figure 7).
Figure 7.
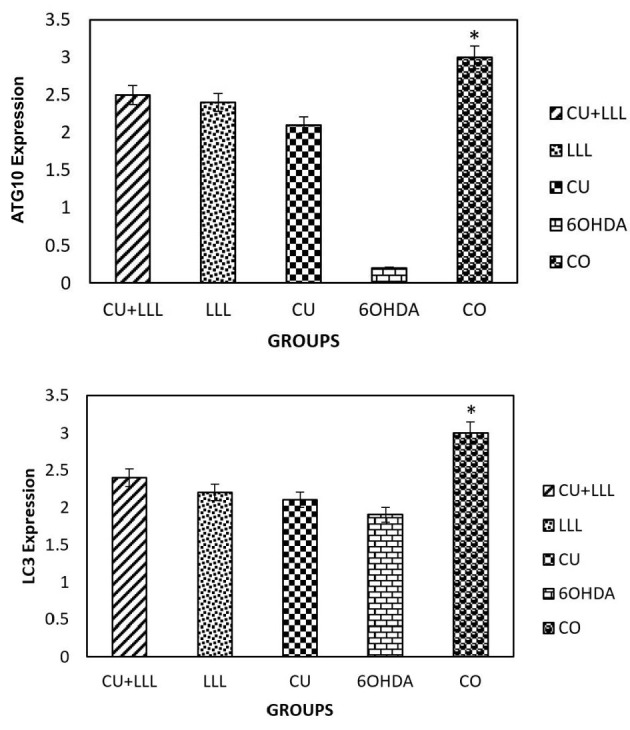
The Expression of LC3 and ATG10 in Different Groups. A decrease in theLC3 and ATG10 expression in the 6-OHDA group was significantly rescued by CU, LLL, CU+LLL groups (*P <0.05).
Discussion
In this study, we investigated the neuroprotective effects of CU and LLLT on the LC3/ATG10 expression and BAX/BCL2 expression ratio in PC12 Cells induced by 6-OHDA. First, the PC12 cells were treated with 6-OHDA and the viability rate of them was examined using the MTT technique. Due to the apoptosis of PC12 cells, the Bax/Bcl2 ratio and LC3/ATG10 expression were measured for evaluating apoptosis and autophagy in different groups by the real-time PCR technique. Since cell survival and viability increased in LLLT+CU-6-OHDA-treated cells in comparison with the control group. Thus, LLLT+CU significantly decreased the apoptosis rate which was associated with oxidative stress caused by 6-OHDA. Due to the neuroprotective effects of CU and LLLT, the viability of PC12 cells pretreated with 6-OHDA increased significantly. The results of other researchers confirmed our findings.15 PC12 cells were used to exhibit the morphology of the nerve-like cell, due to the high passage capability of PC12 cells and good proliferation and differentiation; they are suitable for the study of physiology and pathology of the nervous system as well as the neurodegenerative disease.16 PC12 cells have a similar phenotype to sympathetic ganglionic cells so they have been used in hypoxia studies.17 Many pieces of evidence have proven that the generation of excessive ROS may induce oxidative stress that may lead to neuroinflammation and neurodegeneration, particularly in Alzheimer’s and Parkinson’s disorders.18,19 In this study, the treatment of cells with increasing concentration of 6-OHDA may increase ROS exceeding the level of antioxidant production in the cells which may result in the oxidative stress of the cell, mitochondrial dysfunction, and neuronal death.20 Free radicals generated by 6-OHDA may cause DNA damage, lipid peroxidation, and cytoskeletal disorganization that ultimately trigger neuronal apoptosis.21 Flavonoid polyphenols, particularly CU, have been proposed as a potential candidate for protecting dopaminergic neurons against ROS.22 In this study, the neuroprotective effect of CU on 6-OHDA-Induced neurotoxicity in PC12 cells was investigated, which could be considered as a cellular model of PD. It was shown that the combination of LLLT and CU may have a protective role for PC12 cells against 6-OHDA. The pretreatment of PC12 cells with 5 μM CU showed an increase in cell viability. In fact, pretreating PC12 cells with CU may result in an increase in antioxidant enzymatic activity23 that functions in eliminating ROS before inducing cellular damage.24
Conclusion
The combination of LLLT and CU has a neuroprotective effect on PC12 cells against 6-OHDA-induced neurotoxicity. Due to an increase cell viability and decrease an increase in the Bax/Bcl2 ratio which shows cell susceptibility to apoptosis.
CU+LLLT decreases Bax/Bcl2 ratio and rescue a decrease the formation of autophagosome induced by 6-OHDA in the cellular model of Parkinson’s disease.
Ethical Considerations
This study was approved by the ethics committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1399.127).
Conflict of Interests
The authors declare no conflict of interest.
Acknowledgment
We are thankful for the funding provided by, Laser Application in Medical Sciences Research Center Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grant number#22116).
Please cite this article as follows: Tabatabaei Mirakabad FS, Khoramgah MS, Tahmasebinia F, Darabi S, Hojjat Allah Abbaszadeh HA, Khoshsirat S. The effect of low-level laser therapy and curcumin on the expression of LC3, ATG10 and BAX/BCL2 ratio in PC12 cells induced by 6-hydroxide dopamine. J Lasers Med Sci. 2020;11(3):299-304. doi:10.34172/jlms.2020.50.
References
- 1.Chao Y, Wong SC, Tan EK. Evidence of inflammatory system involvement in Parkinson’s disease. Biomed Res Int. 2014;2014:308654. doi: 10.1155/2014/308654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebrahimi-Fakhari D, Wahlster L, McLean PJ. Protein degradation pathways in Parkinson’s disease: curse or blessing. Acta Neuropathol. 2012;124(2):153–72. doi: 10.1007/s00401-012-1004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niknazar S, Nahavandi A, Peyvandi AA, Peyvandi H, Roozbahany NA, Abbaszadeh HA. Hippocampal NR3C1 DNA methylation can mediate part of preconception paternal stress effects in rat offspring. Behav Brain Res. 2017;324:71–6. doi: 10.1016/j.bbr.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis. 2013;3(4):461–91. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li B, Xiao L, Wang ZY, Zheng PS. Knockdown of STIM1 inhibits 6-hydroxydopamine-induced oxidative stress through attenuating calcium-dependent ER stress and mitochondrial dysfunction in undifferentiated PC12 cells. Free Radic Res. 2014;48(7):758–68. doi: 10.3109/10715762.2014.905687. [DOI] [PubMed] [Google Scholar]
- 6.Wang YH, Xuan ZH, Tian S, Du GH. Echinacoside protects against 6-Hydroxydopamine-induced mitochondrial dysfunction and inflammatory responses in PC12 cells via reducing ROS production. Evid Based Complement Alternat Med. 2015;2015:189239. doi: 10.1155/2015/189239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004;44(2):97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 8.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75(4):787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 10.Alexander JA, Castillo M, Hoffman JC Jr. Magnetic resonance findings in a patient with internuclear ophthalmoplegia Neuroradiological-clinical correlation. J Clin Neuroophthalmol. 1991;11(1):58–61. [PubMed] [Google Scholar]
- 11.Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr Metab (Lond) 2007;4:8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan WH, Wu HJ, Hsuuw YD. Curcumin inhibits ROS formation and apoptosis in methylglyoxal-treated human hepatoma G2 cells. Ann N Y Acad Sci. 2005;1042:372–378. doi: 10.1196/annals.1338.057. [DOI] [PubMed] [Google Scholar]
- 13.Mohsenifar Z, Fridoni M, Ghatrehsamani M, Abdollahifar MA, Abbaszadeh H, Mostafavinia A. et al. Evaluation of the effects of pulsed wave LLLTT on tibial diaphysis in two rat models of experimental osteoporosis, as examined by stereological and real-time PCR gene expression analyses. Lasers Med Sci. 2016;31(4):721–32. doi: 10.1007/s10103-016-1916-9. [DOI] [PubMed] [Google Scholar]
- 14.Fallahnezhad S, Piryaei A, Darbandi H, Amini A, Ghoreishi SK, Jalalifirouzkouhi R. et al. Effect of lowlevel laser therapy and oxytocin on osteoporotic bone marrow‐derived mesenchymal stem cells . J Cell Biochem. 2018;119(1):983–97. doi: 10.1002/jcb.26265.. [DOI] [PubMed] [Google Scholar]
- 15.Darabi SH, Tiraihi T, Noori-Zadeh A, Rajaei F, Darabi L, Abbaszadeh HA. Creatine and retinoic acid effects on the induction of autophagy and differentiation of adipose tissue-derived stem cells into GABAergic-like neurons. J Babol Univ Med Sci. 2017;19(8):41–9. [Google Scholar]
- 16.Awasthi S, Pandya U, Singhal SS, Lin JT, Thiviyanathan V, Seifert WE Jr. et al. Curcumin-glutathione interactions and the role of human glutathione S-transferase P1-1. Chem Biol Interact. 2000;128(1):19–38. doi: 10.1016/s0009-2797(00)00185-x. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Tang XQ, Zhi JL, Cui Y, Yu HM, Tang EH. et al. Curcumin protects PC12 cells against 1-methyl-4-phenylpyridinium ion-induced apoptosis by bcl-2-mitochondria-ROS-iNOS pathway. Apoptosis. 2006;11(6):943–53. doi: 10.1007/s10495-006-6715-5. [DOI] [PubMed] [Google Scholar]
- 18.Chang CH, Chen HX, Yü G, Peng CC, Peng RY. Curcumin-protected PC12 cells against glutamate-induced oxidative toxicity. Food Technol Biotechnol. 2014;52(4):468–78. doi: 10.17113/ftb.52.04.14.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim KM, Pae HO, Zhung M, Ha HY, Ha YA, Chai KY. et al. Involvement of anti-inflammatory heme oxygenase-1 in the inhibitory effect of curcumin on the expression of pro-inflammatory inducible nitric oxide synthase in RAW2647 macrophages. Biomed Pharmacother. 2008;62(9):630–6. doi: 10.1016/j.biopha.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Abramova NA, Cassarino DS, Khan SM, Painter TW, and Bennett JP Jr. Inhibition by R(+) or S(-) pramipexole of caspase activation and cell death induced by methylpyridinium ion or beta amyloid peptide in SH-SY5Y neuroblastoma. J Neurosci Res. 2002;67(4):494–500. doi: 10.1002/jnr.10127. [DOI] [PubMed] [Google Scholar]
- 22.Tang XQ, Feng JQ, Chen J, Chen PX, Zhi JL, Cui Y. et al. Protection of oxidative preconditioning against apoptosis induced by H2O2 in PC12 cells: mechanisms via MMP, ROS, and Bcl-2. Brain Res. 2005;1057(1-2):57–64. doi: 10.1016/j.brainres.2005.07.072. [DOI] [PubMed] [Google Scholar]
- 23.Ghorabi MT, Aliaghaei A, Sadeghi Y, Shaerzadeh F, Rad AA, Mohamadi R. et al. Evidence supporting the neuroprotective effect of adipose derived stem cells on PC12 cells against oxidative stress induced by H2O2. Cell Mol Biol (Noisy-le-grand) 2017;63(3):1–6. doi: 10.14715/cmb/2017.63.3.1. [DOI] [PubMed] [Google Scholar]
- 24.Clementi ME, Pani G, Sampaolese B, Tringali G. Punicalagin reduces H2O2-induced cytotoxicity and apoptosis in PC12 cells by modulating the levels of reactive oxygen species. Nutr Neurosci. 2018;21(6):447–454. doi: 10.1080/1028415X.2017.1306935. [DOI] [PubMed] [Google Scholar]


