Abstract
Probiotics have been suggested as a potential intervention for improving outcomes, particularly ventilatory-associated pneumonia, in patients infected with coronavirus disease 2019 (COVID-19). However, with the rapid development of the COVID-19 pandemic, there is little direct evidence available in infected patients. The objective of this scoping review is to examine the availability and nature of literature describing the effect of probiotics in adults with conditions or infections similar to COVID-19 infection on related health outcomes. MEDLINE, Cumulative Index to Nursing & Allied Health Literature, and Cochrane Databases were searched for studies published from 1999 to May 1, 2020, examining the effect of probiotics in conditions applicable to individuals infected with COVID-19, including, but not limited to, other forms of coronavirus, critical illness, and mechanical ventilation. The databases search identified 1925 unique articles, 77 full-text articles were reviewed, and 48 studies were included in this scoping review, including 31 primary studies and 17 systematic reviews. Primary studies examined a range of interventions that varied by probiotic diversity and types, including 8 studies that focused on synbiotics, which include both pre- and probiotics. Several systematic reviews examined the effect of probiotics on ventilator-associated pneumonia and other infections. Although most systematic reviews concluded probiotics may improve these outcomes, most systematic review authors concluded that the evidence was low in quality and high in heterogeneity. In the absence of direct evidence with patients infected with COVID-19, studies in comparable populations are currently the best resource to guide probiotics interventions in conjunction with clinical expertise and multidisciplinary health care planning.
As the coronavirus disease 2019 (COVID-19) pandemic unfolds, dietitians are moving quickly to determine best methods for preventing and treating the effects of COVID-19 infection.1 Probiotics are living microorganisms that are consumed or applied for health benefits2 and have been suggested as a potential intervention to improve outcomes in patients infected with COVID-19. Probiotics may be delivered with in the form of a symbiotic, which also includes prebiotics to stimulate the growth or activity of probiotic microorganisms.2 Specific to COVID-19, probiotics have been suggested as a possible method of addressing the “cytokine storm” and inflammation caused by COVID-19, enhancing immune function, and decreasing infections common to patients in the intensive care unit (ICU), including ventilator-associated pneumonia.3, 4, 5, 6 In addition, literature has described the potential relationship between gut and lung microbiota and respiratory health.7, 8, 9, 10
Because of the rapid spread of COVID-19 across the globe, there has been little time for research on the efficacy of probiotics and other nutrition-related interventions on the prevention and treatment of signs and symptoms from COVID-19 infection specifically. Thus, to inform evidence-based practice, dietitians must rely on indirect evidence in addition to clinical expertise and critical thinking. For example, findings on the efficacy of probiotics in individuals with other forms of coronavirus, acute respiratory distress syndrome, critical illness, on ventilators, or with other viral infections may inform treatment decisions for adults infected with COVID-19. Evidence scoping reviews are a tool to determine if literature is available on a topic of interest,11 including systematic reviews12 and evidence-based practice guidelines.13 Identifying and mapping relevant studies can direct dietitians to the most current, applicable research with the highest-quality study designs to inform practice.
The objective of this scoping review was to answer the research question: In adults with conditions or infections similar to COVID-19 infection, what is the availability and nature of literature describing the effect of probiotics on health outcomes?
Methods
This scoping review was conducted based on the protocol by Arksey and O’Malley11 and later developed by Levac et al14 and the Joanna Briggs Institute.15 The protocol for this scoping review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist for scoping reviews16 and was registered at Open Science Framework (osf.io/2etbd).17
Eligibility Criteria
The research question was formulated using the Population-Concept-Context approach.15 A full description of the eligibility criteria can be found in Figure 1 . Studies were included if they included adults with conditions that were applicable to individuals with COVID-19 infection, including but not limited to adults with other forms of coronavirus, acute respiratory distress syndrome, critical illness, or on mechanical ventilation. Use of probiotics to prevent viral infections, such as rhinovirus or influenza, in healthy individuals were not included in this scoping review. The major concept explored was the intervention of probiotics. Interventions with synbiotics, which contain both pre- and probiotics, were included. Though the primary focus of this scoping review was to report studies targeting individuals in the ICU, the context was left open to also include free-living individuals with respiratory or viral infections similar to COVID-19. Study design was limited to primary intervention studies, systematic reviews, or evidence-based practice guidelines. Studies were limited to those published in the English language due to resource constraints and since 1999 to capture studies that may have been conducting during or following severe acute respiratory syndrome or Middle East respiratory syndrome outbreaks.
Figure 1.
Eligibility criteria for scoping review of studies examining the effect of probiotics on COVID-19-related outcomes.
| Category | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Study type | Articles published in peer-reviewed journals | Conference abstracts, gray literature such as organizational reports, government documents and white papers |
| Population | Adult humans who
|
Animal studies; cell or in vitro studies; children, healthy adults, athletes, pregnant women; individuals who do not have an infection or condition of interest; individuals with the following conditions: HIVe, AIDSf, HPVg, hepatitis, postsurgery, trauma or brain injury or burn, COPDh, acute pancreatitis |
| Intervention comparison outcomes | Probiotics, synbiotics | Herbal supplements |
| No limits | No limits | |
Outcomes including but not limited to:
|
Outcomes not related to COVID-19 or nutrition | |
| Setting | No limits | No limits |
| Sample size | No limits | No limits |
| Study designs | Intervention and observational primary studies and systematic review and meta-analyses | Narrative reviews, commentary, editorials, letters to the editor |
| Year range | January 1999 to May 1, 2020 | Articles published before 1999 or after the search on May 1, 2020 |
| Language | English | Non-English |
COVID-19 = coronavirus disease 2019.
SARS = severe acute respiratory syndrome.
MERS = Middle East respiratory syndrome.
ARDS = acute respiratory distress syndrome.
HIV = human immunodeficiency virus infection.
AIDS = acquired immune deficiency syndrome.
HPV = human papillomavirus.
COPD = chronic obstructive pulmonary disease.
Search Strategy
The literature was searched using MEDLINE (EBSCO), Cumulative Index to Nursing & Allied Health Literature (EBSCO), Cochrane Databases of Controlled Trials and Systematic Reviews for articles published in the English language from January 1999 until the search date of May 1, 2020. Databases were searched using terms for both population and for probiotics. Search terms for COVID-19 were adapted from the National Institute for Health and Care Excellence.18 The search plan for the MEDLINE database can be found in Figure 2 .
Figure 2.
Sample search strategy from MEDLINE database for scoping review examining the effect of probiotics on coronavirus disease 2019–related outcomes.
| No. | Query | Limiters and expanders | Last run via |
|---|---|---|---|
| S18 | S16 AND S17 | Limiters: date of publication: 19990101-20201231 Search modes: Boolean/phrase |
Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S17 | S1 OR S2 OR S3 | Search modes: Boolean/phrase | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S16 | S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 | Search modes: Boolean/phrase | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S15 | (MH ”Influenza, Human“) OR (MH ”Virus Diseases+") OR (MH ”Viremia+") | Search modes: Boolean/phrase | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S14 | (MH “Sepsis+") | Search modes: Boolean/phrase | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S13 | “acute respiratory distress syndrome” OR (MH “Respiratory Distress Syndrome, Adult") | Search modes: Boolean/phrase | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S12 | (MH “Respiratory Tract Infections+") | Search modes: Boolean/phrase | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S11 | (MH “Critical Illness") | Search modes: Boolean/phrase | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S10 | (MH “Respiration, Artificial+") | Search modes: Boolean/phrase | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S9 | (MH “Pneumonia, Ventilator-Associated”) OR (MH “Pneumonia+") | Search modes: Boolean/phrase | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S8 | (MH “Middle East Respiratory Syndrome Coronavirus") | Search modes: Boolean/phrase | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S7 | (MH “SARS Virus”) OR (MH “Severe Acute Respiratory Syndrome") | Search modes: Boolean/phrase | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S6 | coronavirus∗ OR coronovirus∗ OR coronavirinae∗ OR Coronavirus∗ OR Coronovirus∗ OR Wuhan∗ OR Hubei∗ OR Huanan OR “2019-nCoV” OR 2019nCoV OR nCoV2019 OR “nCoV-2019" OR “COVID-19" OR COVID19 OR “CORVID-19" OR CORVID19 OR “WN-CoV” OR WNCoV OR “HCoV-19" OR HCoV19 OR CoV OR “2019 novel∗" OR Ncov OR “n-cov” OR “SARS-CoV-2" OR “SARSCoV-2" OR “SARSCoV2” OR "SARS-CoV2” OR SARSCov19 OR “SARS-Cov19” OR “SARSCov-19" OR “SARS-Cov-19" OR Ncovor OR Ncorona∗ OR Ncorono∗ OR NcovWuhan∗ OR NcovHubei∗ OR NcovChina∗ OR NcovChinese∗ | Search modes: SmartText Searching | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S5 | ((corona∗ OR corono∗) N0 (virus∗ OR viral∗ OR virinae∗)) OR ((corona∗ OR corono∗) N0 (virus∗ OR viral∗ OR virinae∗)) | Search modes: SmartText Searching | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S4 | (MH “Coronavirus+") | Search modes: Boolean/phrase | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S3 | (MH “Bifidobacterium+") | Search modes: Boolean/phrase | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S2 | (MH “Lactobacillus+") | Search modes: Boolean/phrase | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
| S1 | (MM “Probiotics”) OR “probiotics" | Search modes: Boolean/phrase | Interface: EBSCOhost Research Databases Search Screen: Advanced Search Database: MEDLINE Complete |
Study Selection and Data Charting
Deduplicated studies were uploaded onto Rayyan, an online title and abstract screening program.19 Title and abstract screening was conducted in 2 phases. In the first phase, 1 reviewer (M.R.) excluded all studies that were conducted with animals or cells or did not examine the intervention of probiotics. All remaining eligible title and abstracts were screened independently by 2 reviewers using a priori eligibility criteria (Figure 1) (M.R. and F.W.C.) and discrepancies were settled by consensus or a third review (D.H.). All potentially included title and abstracts progressed to full-text review. For each potential study, a reviewer examined eligibility criteria and extracted data on the following: study design; disease condition of target population (eg, ICU, mechanically ventilated), intervention including the number and type of probiotic strains,20 whether the intervention was delivered in the context of a synbiotic, and mode of delivery; comparison treatment; and outcomes reported. Eligibility and data extraction were confirmed by a second reviewer, with questions and discrepancies determined by consensus or a third reviewer. As is customary for scoping reviews, eligibility criteria were clarified during the full-text review, and the authors determined that trauma, burn, and acute pancreatitis were conditions or infections not applicable to the COVID-19 population. The search and selection process was documented on a Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart.21 Results were synthesized narratively and were mapped using a heat map, pie chart, and bar graph.
Results
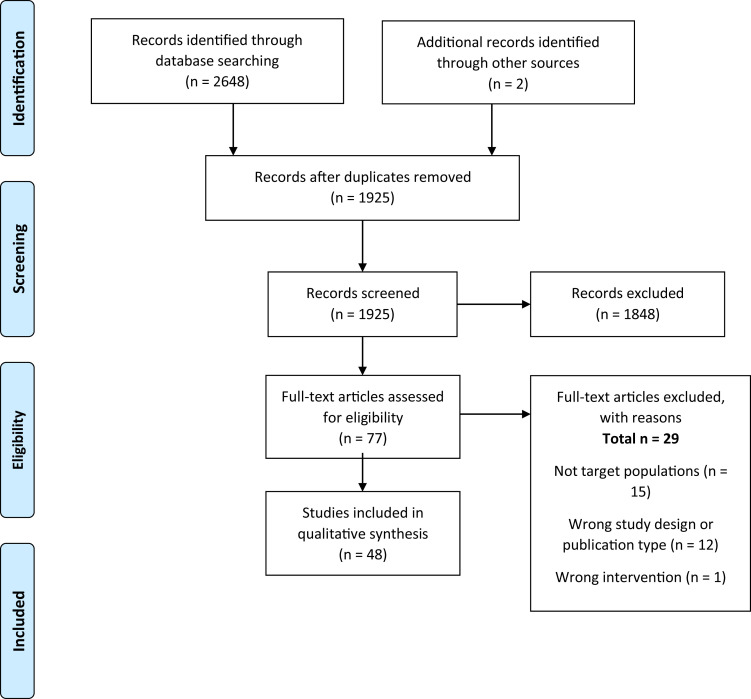
The databases and hand searches identified 1925 unique title or abstracts. Full texts of 77 studies were reviewed, and 48 studies were included in scoping review, including 17 systematic reviews,22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 26 randomized controlled trials,39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 and 5 nonrandomized controlled trials (including both nonrandomized controlled trials and observational studies)65, 66, 67, 68, 69 (Figure 3 ).
Figure 3.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for scoping review of literature examining the effects of probiotics on coronavirus disease 2019–related outcomes.
Overview of Included Articles
Of the 48 included articles, 23 articles23, 24, 25, 26 , 30 , 31 , 36 , 37 , 40 , 43, 44, 45, 46, 47 , 49 , 50 , 52 , 53 , 55 , 58 , 60 , 64 , 69 focused on participants who were critically ill but not mechanically ventilated, 20 articles22 , 27 , 28 , 32, 33, 34, 35 , 38 , 39 , 41 , 42 , 51 , 59 , 61, 62, 63 , 66, 67, 68, 69 targeted adults who were critically ill and mechanically ventilated, and 5 articles29 , 54 , 56 , 57 , 65 included individuals with various conditions, such as respiratory tract infections or influenza (Figure 4 ). All articles focused on the adult population, which may include older adults, but none of them focused exclusively on older populations.
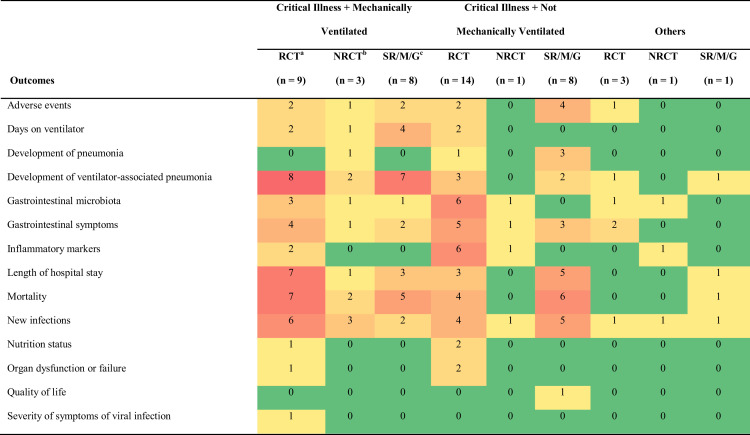
Figure 4.
Heat map describing interventions and outcomes according to study design in a scoping study investigating the effect of probiotics in conditions similar to coronavirus disease 2019 infection on health outcomes. Green cells indicate few included studies for the indicated population, outcome and study design, with yellow, orange, and red cells indicating progressively more available evidence. aRCT = randomized controlled trial. bNRCT = nonrandomized controlled study. cSR/M/G = systematic review/meta-analysis/guideline.
The most commonly reported outcomes were mortality, followed by development of ventilator-associated pneumonia, new infections, length of hospital, gastrointestinal symptoms, gastrointestinal microbiota, adverse events, inflammatory markers, days on ventilator, development of pneumonia, nutrition status, organ dysfunction or failure, quality of life, and severity of symptoms of viral symptoms. Availability and nature of included studies are demonstrated on a heat map (Figure 4), which illustrates the distribution of outcomes assessed in the included articles according to study design and patients’ condition. For example, of the 9 randomized controlled trials with critically ill and mechanically ventilated patients,28 , 39 , 41 , 42 , 49 , 51 , 59 , 61 , 62 development of ventilator-associated pneumonia was reported as an outcome in 8 of them.28 , 39 , 41 , 42 , 49 , 51 , 59 , 61
Primary Studies Included in Scoping Review
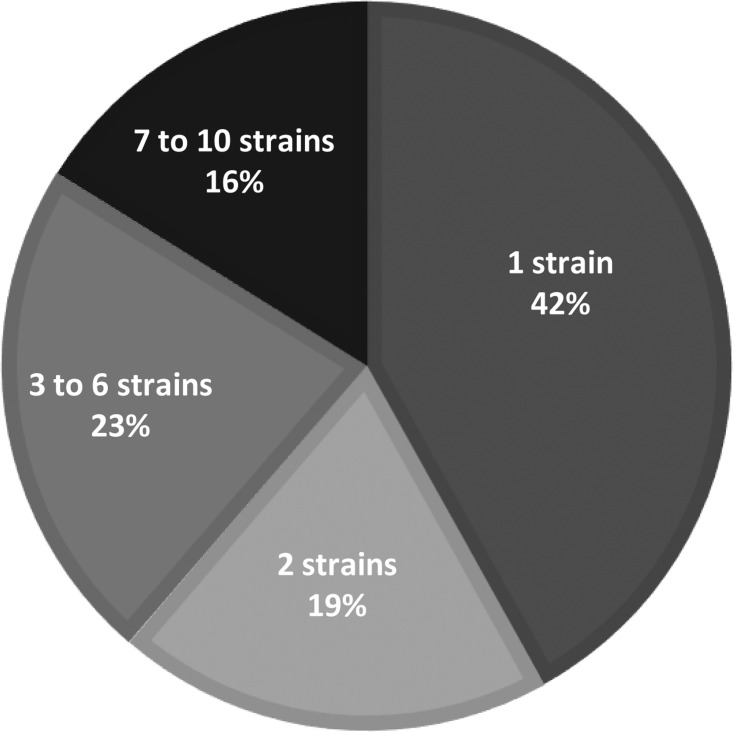
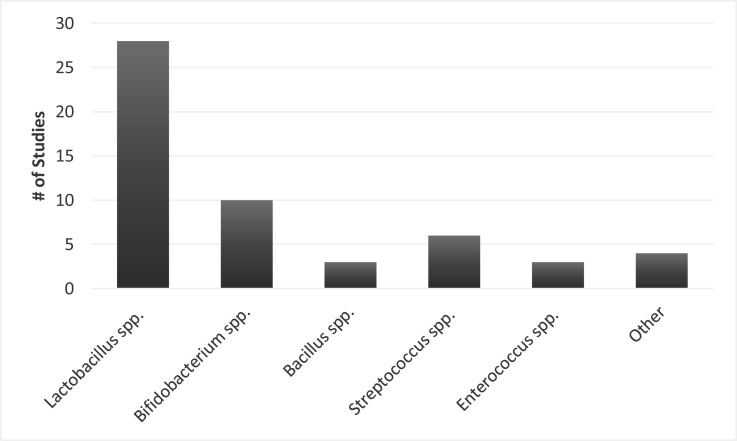
Of the 31 primary research studies included, sample sizes ranged from 15 to 259 participants and intervention durations ranged from 2 to 60 days. However, intervention durations were often variable even within a study depending on how long the participant was in the ICU or on mechanical ventilation. Eight of the included primary studies examined probiotics in the context of synbiotics (pre- and probiotics combined).59, 60, 61, 62, 63, 64 , 67 , 69 The number of probiotic strains varied between studies, with 42% of studies intervening with 1 probiotic strain and 16% intervening with 7 to 10 probiotic strains (Figure 5 ). The probiotic genus most frequently utilized in interventions was lactobacillus (90.3% of interventions), followed by bifidobacterium (32.2% of interventions) and streptococcus (19.4% of interventions) (Figure 6 ); several species of these genera was included across study interventions. Interventions were delivered enterally through a feeding tube due to the critical condition of nearly all participants in included studies, except in 2 studies each in which probiotics were ingested orally56 , 57 or applied topically.48 , 49 In 4 studies, authors indicated multiple routes of probiotics delivery. Patients were given probiotics orally vs through a feeding tube depending on patient condition in Kwon et al,50 McNaught et al,53 and Forestier et al,46 and probiotics were administered topically in the oropharynx combined with enterally in Morrow et al.54
Figure 5.
Proportion of primary research studies included in the scoping review according to the number of probiotics strains in the study interventions (n = 31).
Figure 6.
Frequency of probiotic genera in interventions of primary research studies included in the scoping review (n = 31).
Systematic Reviews and Meta-Analyses and Guidelines Included in Scoping Review
Seventeen systematic reviews and guidelines were included in this scoping review.22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 The authors’ conclusions and certainty of evidence for systematic reviews published from 2010 to 2020 are shown in Figure 7 . In these systematic reviews, authors’ conclusions are heterogeneous, though there were no systematic reviews describing high-quality evidence examining the effect of probiotics in the populations of interest. Most of the systematic reviews describe that probiotics decreased incidence of ventilator-associated pneumonia,26, 27, 28 , 34, 35, 36 although other systematic reviews that specifically focused on ventilator-associated pneumonia incidence concluded no beneficial effect from probiotics.22 , 29 , 32 Several authors describe that intervention heterogeneity22 , 25 , 26 , 29 , 32 , 34 , 36 or risk of bias24, 25, 26 , 29 , 34 , 36 were a concern. Although most systematic reviews did include an analysis of the risk of bias of included studies,22 , 24, 25, 26 , 28, 29, 30 , 33, 34, 35 , 37 , 38 few reported on the certainty of evidence for outcomes.30 , 34 The systematic review conducted by the Cochrane Collaboration in 2014 described low-quality evidence for the effect of probiotics on ventilator-associated pneumonia.34 There were fewer conclusions describing the effect of probiotics on other outcomes. Authors concluded that probiotics may decrease infections but had no effect on mortality. One systematic review focused specifically on the outcome of adverse events and found no increased risk for critically ill patients administered probiotics.30
Figure 7.
Authors’ conclusions in systematic reviews or guidelines published from 1999 to 2020 examining the effect of probiotics in individuals with conditions comparable to coronavirus disease 2019 infection.
| Systematic review or guideline | Target population/context | Authors conclusion | Grade for certainty of evidence |
|---|---|---|---|
| Fan et al 201935 | Prevention of VAPa | “Based on efficacy ranking, ‘B. longum + L. bulgaricus + S. thermophiles’ should be the first [symbiotic] choice for prevention of VAP, while Synbiotic 2000FORTE has the potential to reduce in-hospital mortality and ICU mortality.” | NRb; efficacy of interventions ranked in network meta-analysis |
| Manzanares et al 201636 | Critical illness | “Probiotics show promise in reducing infections, including VAP in critical illness. Currently, clinical heterogeneity and potential publication bias reduce strong clinical recommendations and indicate further high quality clinical trials are needed to conclusively prove these benefits.” | NR |
| Bo et al 201434 | Prevention of VAP | “Evidence suggests that use of probiotics is associated with a reduction in the incidence of VAP. However, the quality of the evidence is low . . . The available evidence is not clear regarding a decrease in ICU or hospital mortality with probiotic use . . . The results of this meta-analysis do not provide sufficient evidence to draw conclusions on the efficacy and safety of probiotics for the prevention of VAP in ICU patients.” | Incidence of VAP: low ICUc and hospital mortality: very low |
| Barraud et al 201333 | Critical illness | “The present meta-analysis suggests that the administration of probiotics did not significantly reduce ICU or hospital mortality rates but did reduce the incidence of ICU-acquired pneumonia and ICU length of stay.” | NR |
| Wang et al 201329 | Prevention of VAP | “Probiotic prophylaxis of [VAP] remained inconclusive and it failed to improve the prognosis of general mechanically ventilated patients. It was noteworthy that infections caused by P. aeruginosa was reduced by administration of probiotics. In further, it is recommended that advanced studies should exploit transformation in pathogenic microorganisms owing to administration of probiotics as well as the specific population.” | NR |
| Gu et al 201222 | Prevention of VAP | “The limited evidence suggests that probiotics show no beneficial effect in patients who are mechanically ventilated; thus, probiotics should not be recommended for routine clinical application. However, the results of this meta-analysis should be interpreted with caution because of the heterogeneity among study designs. Future studies should focus on the safety of probiotics.” | NR |
| Liu et al 201225 | Critical illness | “The use of probiotics was associated with a statistically significant reduction in the incidence of nosocomial pneumonia in critically ill patients. However, large, well-designed, randomized, multi-center trials are needed to confirm any effects of probiotics clinical endpoints such as mortality and length of ICU and hospital stay.” | NR |
| Petrof et al 201226 | Critical illness | “Probiotics appear to reduce infectious complications including [VAP] and may influence [ICU] mortality. However, clinical and statistical heterogeneity and imprecise estimates preclude strong clinical recommendations. Further research on probiotics in the critically ill is warranted.” | NR |
| Bailey et al 201132 | Prevention of VAP | “Clinical trials have failed to demonstrate a consistent beneficial effect of probiotics in mechanically ventilated patients; thus, they are not recommended for routine clinical use. However, heterogeneity among study designs may hinder this assessment and the designs should be unified in future research.” | NR |
| Hempel et al 201130 | Includes critical illness | “There is a lack of assessment and systematic reporting of adverse events in probiotic intervention studies, and interventions are poorly documented. The available evidence in RCTs [randomized controlled trials] does not indicate an increased risk; however, rare adverse events are difficult to assess, and despite the substantial number of publications, the current literature is not well equipped to answer questions on the safety of probiotic interventions with confidence.” | Insufficient, but critical illness not examined separately |
| Schultz et al 201127 | Prevention of VAP | “Prophylactic use of antibiotics in critically ill patients is effective in reducing the incidence of VAP. Probiotic strategies deserve consideration in future well-powered trials. Future studies are needed to determine if preventive . . . probiotic strategies are safe with regard to development of . . . probiotic infections. It should be determined whether the efficacy of probiotics improves when these agents are provided to the mouth and the intestines simultaneously.” | NR |
| Siempos et al 201028 | Prevention of VAP | “Administration of probiotics is associated with lower incidence of [VAP] than control. Given the increasing antimicrobial resistance, this promising strategy deserves consideration in future studies, which should have active surveillance for probiotic-induced diseases.” | NR |
| Jack et al 201023 | Critical illness | “Evidence to support probiotic use in the management of [enteral tube feeding] diarrhea in critically ill patients remains unclear. This paper argues that probiotics should not be administered to critically ill patients until further research has been conducted to examine the causal relationship between probiotics and mortality, irrespective of the patient’s disease state or projected prophylactic benefit of probiotic administration.” | NR |
| Koretz 200924 | Critical illness | “Probiotics did not appear to influence mortality or duration of hospitalization. However, the recipients of the probiotics had fewer infectious episodes . . . it is not clear that probiotics are beneficial (and they may even be harmful) in the critically ill patient group.” | NR |
| Isakow et al 200731 | Prevention of HAPd | “There is no current clinical evidence to support the use of probiotics to . . . reduce HAP rates.” | NR |
| Watkinson et al 200737 | Critical illness | “The use of pre- pro- or synbiotics in adult critically ill patients confers no statistically significant benefit [for nosocomial infections, length of ICU stay, hospital mortality and specifically pneumonia]. There is currently a lack of evidence to support the use of pre- pro- or synbiotics in patients admitted to adult ICUs, and a large well-designed trial is needed in this area.” | NR |
| Heyland et al 200338e | Critical illness, mechanically ventilated | “There are insufficient data to make a recommendation on the use of probiotics in critically ill patients.” | NR |
VAP = ventilator-associated pneumonia.
NR = not reported.
ICU = intensive care unit.
HAP = hospital-associated pneumonia.
Evidence-based practice guideline.
Discussion
This scoping review elucidated that there was considerable research, including recent systematic reviews, on the use of probiotics to treat ventilator-associated pneumonia in critically ill patients on mechanical ventilation, which may be applicable to patients infected with COVID-19. There were also systematic reviews available describing the effect of probiotics on length of hospital stay, mortality, new infections, and gastrointestinal symptoms in critically ill patients who were or were not mechanically ventilated. There were no systematic reviews or primary studies included that examined the effects of probiotics in patients infected with COVID-19 or other forms of the coronavirus, and there was little evidence regarding treating other viral infections such as influenza. There were important outcomes, including quality of life and severity of symptoms from a viral infection, that were not addressed in primary studies or systematic reviews.
Application to Practitioners in the Context of COVID-19 Pandemic
Evidence-based practice depends on practitioners staying abreast of the most recent evidence and interpreting and implementing it through the lens of clinical expertise and in consideration of each individual patient. The COVID-19 pandemic has developed so rapidly that practitioners are required to analyze indirect evidence in populations that may be comparable to determine which interventions will result in the most optimal outcomes.
This scoping review demonstrated that, at present, there are no systematic reviews or primary studies examining the effect of probiotics in patients with COVID-19 or other forms of coronavirus. Therefore, there is currently no direct evidence to demonstrate that probiotics may be effective in reducing COVID-19 symptoms for patients with mild or moderate infections who are managing care at home. There is evidence available in patients with critical illness, particularly those who are mechanically ventilated, and this body of research may be applicable to individuals infected with COVID-19 in critical care. Although there was 1 guideline describing probiotics use in mechanically ventilated critically ill adults,38 this guideline was from 2003 and described insufficient evidence to make a recommendation. Thus, for practitioners to find a starting point for guidance regarding probiotic interventions for patients with COVID-19, they may need to interpret findings from systematic reviews through the lens of clinical expertise, with consideration how the COVID-19 infection specifically may modify relationships observed in critically ill patients without COVID-19. In addition, practitioners will need to consider pragmatic considerations that are typically incorporated into guideline recommendations including feasibility and acceptability to other providers on the health care teams70 as well as factors specific to individuals infected with COVID-19. For example, a recent COVID-19 report on nutrition therapy by the Society of Critical Care Medicine and the American Society for Parenteral and Enteral Nutrition describe that supplemental nutrition given in discrete doses, such as probiotics, should be given once per day to cluster care.71
Any intervention can result in unintended consequences, and the risk-benefit ratio must be considered when determining whether to intervene with probiotics. The mechanisms of probiotics in regards to modulating the immune system to prevent and treat infections is not well understood,72 and thus, practitioners should proceed with caution when recommending probiotics to individuals infected with COVID-19.
Research Needs
The heterogeneity in findings described between systematic reviews may be indicative of the heterogeneous populations within critical care or due to the variation in types and doses of probiotics delivered in the interventions. Most of the included systematic reviews regarded probiotics as the intervention, but as demonstrated in the primary studies, probiotics can be delivered in a variety of genera, species, dosages, modes, and durations. In 14 studies, including 8 primary studies59, 60, 61, 62, 63, 64 , 67 , 69 and 6 systematic reviews,32, 33, 34, 35, 36, 37 authors included interventions with synbiotics, which include a prebiotic along with the probiotic to stimulate, activate, or improve survival of probiotic microorganisms.73 Although there were no clear differences in systematic review conclusions according to if the intervention was delivered in a synbiotic vs probiotic alone, this difference in included primary studies may have contributed to the heterogeneity demonstrated between the systematic reviews. Therefore, future systematic reviews should stratify narrative and quantitative results according to the types or diversity of strains in the interventions of primary studies to determine it using specific probiotics or a greater diversity of probiotic organisms is advantageous in improving outcomes. In addition, more research is needed on patient-centered outcomes such quality of life and severity of symptoms from viral infections.
The greater research need is to understand the efficacy and risks of utilizing probiotics in patients infected with COVID-19 specifically. Currently, research trials are underway to determine the effect of probiotics in treating COVID-19 infection.74, 75, 76 Dietitians who are working with individuals infected with COVID-19 and who are using probiotics in care are encouraged to document experiences using the Academy of Nutrition and Dietetics Health Informatics Infrastructure.77 This forum allows practitioners to contribute experiences to an evidence base for nutrition practice, with the goal of improving patient care.
Strengths and Limitations
This scoping review examined the effects of probiotics on a wide range of conditions that may be applicable to patients infected with COVID-19. However, due to the rapid development of the COVID-19 pandemic, there has been little time for published research regarding the effect of probiotics in patients infected with COVID-19. Therefore, though the evidence reported in this scoping review is a good starting place for finding applicable literature on probiotics that may apply to patients infected with COVID-19, the specific pathology and secondary complications of COVID-19 infection require that practitioners assess the potential benefits and risk for each individual patient before recommending probiotics.
Conclusion
Probiotics have been suggested as a potential method of modulating the immune system to improve outcomes, such as ventilator-associated pneumonia, in patients infected with COVID-19. There is currently no direct evidence examining the use of probiotics in improving outcomes in patients infected with COVID-19 or other similar viral infections. There have been several systematic reviews examining the effects of probiotics in individuals with critical illness with or without mechanical ventilation on patient-centered outcomes such as mortality and new infections, including ventilator-associated pneumonia. However, risk of bias in these studies and heterogeneity between studies preclude consistent conclusions between systematic reviews, and practitioners should consider these limitations when determining treatment priorities for critically ill patients with COVID-19.
Biographies
M. Rozga is nutrition researchers, Academy of Nutrition and Dietetics Evidence Analysis Center, Chicago, IL.
F. W. Cheng is nutrition researchers, Academy of Nutrition and Dietetics Evidence Analysis Center, Chicago, IL.
D. Handu is senior scientific director, Academy of Nutrition and Dietetics Evidence Analysis Center, Chicago, IL.
Footnotes
STATEMENT OF POTENTIAL CONFLICT OF INTEREST No potential conflict of interest was reported by the authors.
FUNDING/SUPPORT This work was supported by the Academy of Nutrition and Dietetics.
AUTHOR CONTRIBUTIONS All authors wrote sections of the first draft, thoroughly edited the manuscript, and approved the final draft.
References
- 1.Handu D., Moloney L., Rozga M., Cheng F. Malnutrition care during the COVID-19 pandemic: Considerations for registered dietitian nutritionists evidence analysis center. J Acad Nutr Diet. 2021;121(5):979–987. doi: 10.1016/j.jand.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Center for Complementary and Integrative Health Probiotics: What you need to know. US Department of Health and Human Services. https://www.nccih.nih.gov/health/probiotics-what-you-need-to-know Published 2020. Updated August 2019. Accessed June 2, 2020.
- 3.Mak J.W.Y., Chan F.K.L., Ng S.C. Probiotics and COVID-19: one size does not fit all. Lancet Gastroenterol Hepatol. 2020;5:644–645. doi: 10.1016/S2468-1253(20)30122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Q.Y., Chen Y.X., Fang J.Y. 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21(3):125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: A review. Diabetes Metab Syndr. 2020;14(4):367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romano L., Bilotta F., Dauri M. Short report—Medical nutrition therapy for critically ill patients with COVID-19. Eur Rev Med Pharmacol Sci. 2020;24(7):4035–4039. doi: 10.26355/eurrev_202004_20874. [DOI] [PubMed] [Google Scholar]
- 7.Chan C.K.Y., Tao J., Chan O.S., Li H.B., Pang H. Preventing respiratory tract infections by synbiotic interventions: A systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;11:979–988. doi: 10.1093/advances/nmaa003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wypych T.P., Wickramasinghe L.C., Marsland B.J. The influence of the microbiome on respiratory health. Nat Immunol. 2019;20(10):1279–1290. doi: 10.1038/s41590-019-0451-9. [DOI] [PubMed] [Google Scholar]
- 9.Dumas A., Bernard L., Poquet Y., Lugo-Villarino G., Neyrolles O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol. 2018;20(12) doi: 10.1111/cmi.12966. [DOI] [PubMed] [Google Scholar]
- 10.Anand S., Mande S.S. Diet, microbiota and gut-lung connection. Front Microbiol. 2018;9:2147. doi: 10.3389/fmicb.2018.02147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arksey H., O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 12.Handu D., Moloney L., Wolfram T., Ziegler P., Acosta A., Steiber A. Academy of Nutrition and Dietetics methodology for conducting systematic reviews for the evidence analysis library. J Acad Nutr Diet. 2016;116(2):311–318. doi: 10.1016/j.jand.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Papoutsakis C., Moloney L., Sinley R.C., Acosta A., Handu D., Steiber A.L. Academy of Nutrition and Dietetics methodology for developing evidence-based nutrition practice guidelines. J Acad Nutr Diet. 2017;117(5):794–804. doi: 10.1016/j.jand.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Levac D., Colquhoun H., O’Brien K.K. Scoping studies: Advancing the methodology. Implement Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters MDJ GC, McInerney P, Munn Z, Tricco AC, Khalil, H. Chapter 11: Scoping Reviews (2020 version). In: Aromataris E, Munn Z, (Eds.), Joanna Briggs Institute Reviewer's Manual, Adelaide, Australia: Joanna Briggs Institute. https://wiki.jbi.global/display/MANUAL; 10.46658/JBIRM-20-01. [DOI]
- 16.Tricco A.C., Lillie E., Zarin W. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 17.Rozga M. Effect of probiotics on COVID-19-related outcomes: A scoping review. Open Science Framework. osf.io/2etbd Published 2020. Accessed May 13, 2020.
- 18.National Institute for Health and Care Excellence Interim Process and Methods for Developing Rapid Guidelines on COVID-19. https://www.nice.org.uk/process/pmg35/resources/interim-process-and-methods-for-developing-rapid-guidelines-on-covid19-pdf-72286777565125 Published 2020. Accessed June 25, 2020.
- 19.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khalighi A. Probiotics: A comprehensive review of their classification, mode of action and role in human nutrition. In: IntechOpen; 2016. https://doi.org/10.5772/63646.
- 21.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Gu W.-J., Wei C.-Y., Yin R.-X. Lack of efficacy of probiotics in preventing ventilator-associated pneumonia probiotics for ventilator-associated pneumonia: A systematic review and meta-analysis of randomized controlled trials. Chest. 2012;142(4):859–868. doi: 10.1378/chest.12-0679. [DOI] [PubMed] [Google Scholar]
- 23.Jack L., Coyer F., Courtney M., Venkatesh B. Probiotics and diarrhoea management in enterally tube fed critically ill patients—what is the evidence? Intensive Crit Care Nurs. 2010;26(6):314–326. doi: 10.1016/j.iccn.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Koretz R.L. Probiotics, critical illness, and methodologic bias. Nutr Clin Pract. 2009;24(1):45–49. doi: 10.1177/0884533608329296. [DOI] [PubMed] [Google Scholar]
- 25.Liu K-x, Zhu Y-g, Zhang J. Probiotics’ effects on the incidence of nosocomial pneumonia in critically ill patients: A systematic review and meta-analysis. Crit Care. 2012;16(3):R109. doi: 10.1186/cc11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrof E.O., Dhaliwal R., Manzanares W., Johnstone J., Cook D., Heyland D.K. Probiotics in the critically ill: A systematic review of the randomized trial evidence. Crit Care Med. 2012;40(12):3290–3302. doi: 10.1097/CCM.0b013e318260cc33. [DOI] [PubMed] [Google Scholar]
- 27.Schultz M.J., Haas L.E. Antibiotics or probiotics as preventive measures against ventilator-associated pneumonia: A literature review. Crit Care. 2011;15(1):R18. doi: 10.1186/cc9963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siempos, Ntaidou T.K., Falagas M.E. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: A meta-analysis of randomized controlled trials. Crit Care Med. 2010;38(3):954–962. doi: 10.1097/CCM.0b013e3181c8fe4b. [DOI] [PubMed] [Google Scholar]
- 29.Wang J., Liu K-x, Ariani F., Tao L.-L., Zhang J., Qu J.-M. Probiotics for preventing ventilator-associated pneumonia: A systematic review and meta-analysis of high-quality randomized controlled trials. PloS One. 2013;8(12) doi: 10.1371/journal.pone.0083934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hempel S., Newberry S., Ruelaz A. Safety of probiotics used to reduce risk and prevent or treat disease. Evid Rep Technology Assess. 2011;(200):1–645. [PMC free article] [PubMed] [Google Scholar]
- 31.Isakow W., Morrow L.E., Kollef M.H. Probiotics for preventing and treating nosocomial infections: Review of current evidence and recommendations. Chest. 2007;132(1):286–294. doi: 10.1378/chest.06-2156. [DOI] [PubMed] [Google Scholar]
- 32.Bailey J.L., Yeung S.Y. Probiotics for disease prevention: a focus on ventilator-associated pneumonia. Ann Pharmacother. 2011;45(11):1425–1432. doi: 10.1345/aph.1Q241. [DOI] [PubMed] [Google Scholar]
- 33.Barraud D., Bollaert P.-E., Gibot S. Impact of the administration of probiotics on mortality in critically ill adult patients: A meta-analysis of randomized controlled trials. Chest. 2013;143(3):646–655. doi: 10.1378/chest.12-1745. [DOI] [PubMed] [Google Scholar]
- 34.Bo L., Li J., Tao T. Probiotics for preventing ventilator-associated pneumonia. Cochrane Database Syst Rev. 2014;(10):CD009066. doi: 10.1002/14651858.CD009066.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan Q.L., Yu X.M., Liu Q.X., Yang W., Chang Q., Zhang Y.P. Synbiotics for prevention of ventilator-associated pneumonia: A probiotics strain-specific network meta-analysis. J Int Med Res. 2019;47(11):5349–5374. doi: 10.1177/0300060519876753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manzanares W., Lemieux M., Langlois P.L., Wischmeyer P.E. Probiotic and synbiotic therapy in critical illness: A systematic review and meta-analysis. Crit Care. 2016;19:262. doi: 10.1186/s13054-016-1434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watkinson P.J., Barber V.S., Dark P., Young J.D. The use of pre- pro- and synbiotics in adult intensive care unit patients: Systematic review. Clin Nutr. 2007;26(2):182–192. doi: 10.1016/j.clnu.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Heyland D.K., Dhaliwal R., Drover J.W. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenteral Enteral Nutr. 2003;27(5):355–383. doi: 10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- 39.Rongrungruang Randomized controlled study of probiotics containing Lactobacillus casei (Shirota strain) for prevention of ventilator-associated pneumonia. J Med Assoc Thai. 2015;98(3):253–259. [PubMed] [Google Scholar]
- 40.Alberda C., Gramlich L., Meddings J. Effects of probiotic therapy in critically ill patients: A randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2007;85(3):816–823. doi: 10.1093/ajcn/85.3.816. [DOI] [PubMed] [Google Scholar]
- 41.Barraud D., Blard C., Hein F. Probiotics in the critically ill patient: A double blind, randomized, placebo-controlled trial. Intensive Care Med. 2010;36(9):1540–1547. doi: 10.1007/s00134-010-1927-0. [DOI] [PubMed] [Google Scholar]
- 42.Cook D.J., Johnstone J., Marshall J.C. Probiotics: Prevention of Severe Pneumonia and Endotracheal Colonization Trial-PROSPECT: A pilot trial. Trials. 2016;17:377. doi: 10.1186/s13063-016-1495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Castro Soares G.G., Marinho C.H., Pitol R. Sporulated Bacillus as alternative treatment for diarrhea of hospitalized adult patients under enteral nutrition: A pilot randomized controlled study. Clin Nutr ESPEN. 2017;22:13–18. doi: 10.1016/j.clnesp.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Ebrahimi-Mameghani M., Sanaie S., Mahmoodpoor A., Hamishehkar H. Effect of a probiotic preparation (VSL#3) in critically ill patients: A randomized, double-blind, placebo-controlled trial (pilot study) Pak J Med Sci. 2013;29(2):490–494. doi: 10.12669/pjms.292.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrie S., Daley M. Lactobacillus GG as treatment for diarrhea during enteral feeding in critical illness: Randomized controlled trial. JPEN J Parenter Enteral Nutr. 2011;35(1):43–49. doi: 10.1177/0148607110370705. [DOI] [PubMed] [Google Scholar]
- 46.Forestier C., Guelon D., Cluytens V. Oral probiotic and prevention of Pseudomonas aeruginosa infections: A randomized, double-blind, placebo-controlled pilot study in intensive care unit patients. Crit Care. 2008;12(3) doi: 10.1186/cc6907. R69-R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frohmader T.J., Chaboyer W.P., Robertson I.K., Gowardman J. Decrease in frequency of liquid stool in enterally fed critically ill patients given the multispecies probiotic VSL#3: A pilot trial. Am J Crit Care. 2010;19(3):e1–e11. doi: 10.4037/ajcc2010976. [DOI] [PubMed] [Google Scholar]
- 48.Klarin B., Molin G., Jeppsson B., Larsson A. Use of the probiotic Lactobacillus plantarum 299 to reduce pathogenic bacteria in the oropharynx of intubated patients: A randomised controlled open pilot study. Crit Care. 2008;12(6):R136. doi: 10.1186/cc7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klarin B., Adolfsson A., Torstensson A., Larsson A. Can probiotics be an alternative to chlorhexidine for oral care in the mechanically ventilated patient? A multicentre, prospective, randomised controlled open trial. Crit Care. 2018;22(1):272. doi: 10.1186/s13054-018-2209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon J.H., Bommarito K.M., Reske K.A. Randomized controlled trial to determine the impact of probiotic administration on colonization with multidrug-resistant organisms in critically ill patients. Infec Control Hosp Epidemiol. 2015;36(12):1451–1454. doi: 10.1017/ice.2015.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahmoodpoor A., Hamishehkar H., Asghari R., Abri R., Shadvar K., Sanaie S. Effect of a probiotic preparation on ventilator-associated pneumonia in critically ill patients admitted to the intensive care unit: A prospective double-blind randomized controlled trial. Nutr Clin Pract. 2019;34(1):156–162. doi: 10.1002/ncp.10191. [DOI] [PubMed] [Google Scholar]
- 52.Malik A.A., Rajandram R., Tah P.C., Hakumat-Rai V.-R., Chin K.-F. Microbial cell preparation in enteral feeding in critically ill patients: A randomized, double-blind, placebo-controlled clinical trial. J Crit Care. 2016;32:182–188. doi: 10.1016/j.jcrc.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 53.McNaught C.E., Woodcock N.P., Anderson A.D.G., MacFie J. A prospective randomised trial of probiotics in critically ill patients. Clin Nutr. 2005;24(2):211–219. doi: 10.1016/j.clnu.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Morrow L.E., Kollef M.H., Casale T.B., Morrow L.E., Kollef M.H., Casale T.B. Probiotic prophylaxis of ventilator-associated pneumonia: A blinded, randomized, controlled trial. Am J Respir Crit Care Med. 2010;182(8):1058–1064. doi: 10.1164/rccm.200912-1853OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanaie S., Ebrahimi-Mameghani M., Hamishehkar H., Mojtahedzadeh M., Mahmoodpoor A. Effect of a multispecies probiotic on inflammatory markers in critically ill patients: A randomized, double-blind, placebo-controlled trial. J Res Med Sci. 2014;19(9):827–833. [PMC free article] [PubMed] [Google Scholar]
- 56.Song H.J., Kim J.-Y., Jung S.-A. Effect of probiotic Lactobacillus (Lacidofil® cap) for the prevention of antibiotic-associated diarrhea: A prospective, randomized, double-blind, multicenter study. J Korean Med Sci. 2010;25(12):1784–1791. doi: 10.3346/jkms.2010.25.12.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stadlbauer V., Horvath A., Komarova I. Dysbiosis in early sepsis can be modulated by a multispecies probiotic: A randomised controlled pilot trial. Benef Microbes. 2019;10(3):265–278. doi: 10.3920/BM2018.0067. [DOI] [PubMed] [Google Scholar]
- 58.Zeng J., Wang C.-T., Zhang F.-S. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: A randomized controlled multicenter trial. Intensive Care Med. 2016;42(6):1018–1028. doi: 10.1007/s00134-016-4303-x. [DOI] [PubMed] [Google Scholar]
- 59.Giamarellos-Bourboulis E.J., Bengmark S., Kanellakopoulou K., Kotzampassi K. Pro- and synbiotics to control inflammation and infection in patients with multiple injuries. J Trauma. 2009;67(4):815–821. doi: 10.1097/TA.0b013e31819d979e. [DOI] [PubMed] [Google Scholar]
- 60.Jain P.K., McNaught C.E., Anderson A.D.G., MacFie J., Mitchell C.J. Influence of synbiotic containing Lactobacillus acidophilus La5, Bifidobacterium lactis Bb 12, Streptococcus thermophilus, Lactobacillus bulgaricus and oligofructose on gut barrier function and sepsis in critically ill patients: A randomised controlled trial. Clin Nutr. 2004;23(4):467–475. doi: 10.1016/j.clnu.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Knight D.J.W., Gardiner D., Banks A. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: A randomised, double-blind, placebo-controlled trial. Intensive Care Med. 2009;35(5):854–861. doi: 10.1007/s00134-008-1368-1. [DOI] [PubMed] [Google Scholar]
- 62.Kotzampassi K., Giamarellos-Bourboulis E.J., Voudouris A., Kazamias P., Eleftheriadis E. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically ill trauma patients: Early results of a randomized controlled trial. World J Surg. 2006;30(10):1848–1855. doi: 10.1007/s00268-005-0653-1. [DOI] [PubMed] [Google Scholar]
- 63.Shimizu K., Yamada T., Ogura H. Synbiotics modulate gut microbiota and reduce enteritis and ventilator-associated pneumonia in patients with sepsis: A randomized controlled trial. Crit Care. 2018;22(1):239. doi: 10.1186/s13054-018-2167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spindler-Vesel A., Bengmark S., Vovk I., Cerovic O., Kompan L. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: A randomized study in trauma patients. JPEN J Parenter Enteral Nutr. 2007;31(2):119–126. doi: 10.1177/0148607107031002119. [DOI] [PubMed] [Google Scholar]
- 65.Hu X., Zhang H., Lu H. The effect of probiotic treatment on patients infected with the H7N9 influenza virus. PloS One. 2016;11(3) doi: 10.1371/journal.pone.0151976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kenna J., Mahmoud L., Zullo A.R. Effect of probiotics on the incidence of healthcare-associated infections in mechanically ventilated neurocritical care patients. Nutr Clin Pract. 2016;31(1):116–120. doi: 10.1177/0884533615620349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shimizu K., Ogura H., Kabata D. Association of prophylactic synbiotics with reduction in diarrhea and pneumonia in mechanically ventilated critically ill patients: A propensity score analysis. J Infect Chemother. 2018;24(10):795–801. doi: 10.1016/j.jiac.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 68.Oudhuis G.J., Bergmans D.C., Dormans T. Probiotics versus antibiotic decontamination of the digestive tract: Infection and mortality. Intensive Care Med. 2011;37(1):110–117. doi: 10.1007/s00134-010-2002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klarin B., Wullt M., Palmquist I., Molin G., Larsson A., Jeppsson B. Lactobacillus plantarum 299v reduces colonisation of Clostridium difficile in critically ill patients treated with antibiotics. Acta Anaesthesiol Scand. 2008;52(8):1096–1102. doi: 10.1111/j.1399-6576.2008.01748.x. [DOI] [PubMed] [Google Scholar]
- 70.Alonso-Coello P., Schünemann H.J., Moberg J. GRADE Evidence to Decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016;353:i2016. doi: 10.1136/bmj.i2016. [DOI] [PubMed] [Google Scholar]
- 71.Martindale R.P.J., Teylor B., Warren M., McClave S.A. Society of Critical Care Medicine, American Society of Parenteral and Enteral Nutrition; 2020. Nutrition Therapy in the Patient with COVID-10 Disease Requiring ICU Care.https://www.sccm.org/getattachment/Disaster/Nutrition-Therapy-COVID-19-SCCM-ASPEN.pdf?lang=en-US [Google Scholar]
- 72.Suez J., Zmora N., Segal E., Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25(5):716–729. doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 73.Markowiak P., Slizewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9(9) doi: 10.3390/nu9091021. 1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.ClinicalTrials.gov. Study to evaluate the effect of a probiotic in COVID-19 NCT04390477. U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04390477. Published 2020. Updated May 15, 2020. Accessed May 15, 2020.
- 75.International Scientific Association for Probiotics and Prebiotics How some probiotic scientists are working to address COVID-19. https://isappscience.org/how-some-probiotic-and-prebiotic-scientists-are-working-to-address-covid-19/ Published 2020. Updated May 4, 2020. Accessed May 15, 2020.
- 76.World Health Organization Clinical Trials A prospective, multicenter, open-label, randomized, parallel-controlled trial for probiotics to evaluate efficacy and safety in patients infected with 2019 novel coronavirus pneumonia (COVID-19) ChiCTR2000029974. https://apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000029974 Published 2020. Updated 2-18-2020. Accessed May 15, 2020.
- 77.Academy of Nutrition and Dietetics ANDHII Academy of Nutrition and Dietetics Health Informatics Infrastructure. https://www.andhii.org/info/ Published 2020. Accessed May 15, 2020.