Introduction
Coronavirus disease of 2019 (COVID-19) has currently reached pandemic levels and neurological manifestations, including stroke as the initial presentation, have been increasingly recognized.1, 2, 3 Majority of the reported ischemic strokes are large vessel occlusion or embolic appearing strokes and are frequently described in critically ill patients with severe COVID-19 disease2, but small ischemic strokes have also been reported.4 Recently, stroke as the presenting symptom in younger patients (< 50 years of age) with mild COVID-19 disease has gained increased attention.5
We report the case of a young female with history of Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) virus with acute ischemic stroke as the sole manifestation. At the time of submission, there were no other prior publication reporting the rare combination of acute ischemic stroke in a CADASIL patient with SARS-CoV-2 virus.
Case report
A 37 years old African American female with prior medical history of controlled hypertension on a beta-blocker, migraines without aura, history of cigarette smoking, and genetically proven CADASIL mutation (heterozygous missense mutation; c.3062A>G; p.Tyr1021Cys), that was on aspirin 81 mg daily as outpatient, who presented to our Emergency Room with the chief complaint of left leg weakness, dysarthria and ataxia. Symptoms were discovered by the patient in the morning upon wakening and resolved by the time she presented to ER. Review of symptoms was negative for gastrointestinal or flu-like symptoms, including cough, fevers, sore throat, malaise, shortness of breath, and myalgias. In addition, the patient denied exposure to individuals with flu-like symptoms or who were known to be positive for COVID-19. The general exam and the neurologic exam were unrevealing except for chronic left facial numbness. Vital signs on presentation were temperature of 98.1 degrees Celsius, heart rate of 69 beats per min, blood pressure of 140/99 mmHg and oxygen saturation of 100% on room air. Head computed tomography was negative for acute findings. MRI brain without contrast revealed an acute ischemic stroke in the right pons (Fig. 1 ) along with extensive chronic white matter signal abnormalities characteristic of CADASIL (Fig. 2 ) which were unchanged compared to a previous study.6 MR angiogram of the head and neck showed patent cerebral vasculature with no evidence of large vessel occlusion (Fig. 3 ). Comprehensive metabolic profile on admission was unremarkable. Hemoglobin A1C was 5.5% and low-density lipoprotein cholesterol levels were 88 mg/dL. As part of the hospital admission protocol established at the beginning of the COVID-19 pandemic, the patient was screened for SARS-CoV-2 by using RT-PCR and nasopharyngeal swab which was positive. Inflammatory markers were not checked given patient being asymptomatic. Electrocardiogram revealed normal sinus rhythm. The patient had an echocardiogram in 2018 which was unremarkable and workup for procoagulability which was negative. Due to the low likelihood of cardioembolism and low pre-test probability, performing an echocardiogram was deferred to be done as outpatient when she cleared her COVID-19 infection. A chest X-Ray showed no evidence of consolidation or infiltration (Fig. 4 ). A lumbar puncture was not performed as the patient remained clinically asymptomatic throughout hospital stay with no evidence of CNS viral involvement. For the same reason, no treatment was initiated for COVID-19 infection. The patient was discharged on her home medication, aspirin 81 mg daily. In addition, atorvastatin, which the patient had self-discontinued, was resumed.
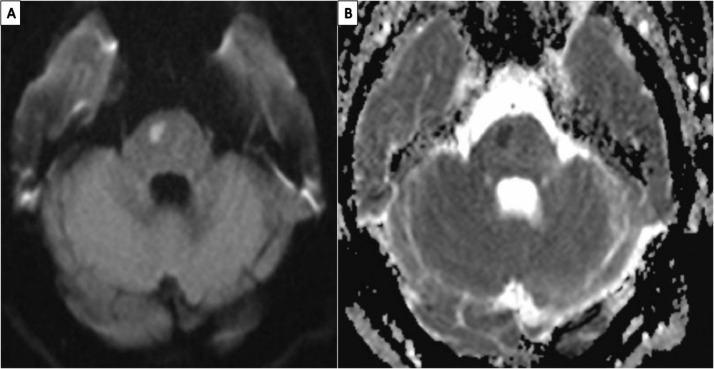
Fig. 1.
MRI brain, axial view: A) diffusion weighed imaging showing an acute ischemic stroke in the R pons; B) Apparent diffusion coefficient sequence showing a matched reduction in the same location in the R pons, indicative of acute ischemic stroke
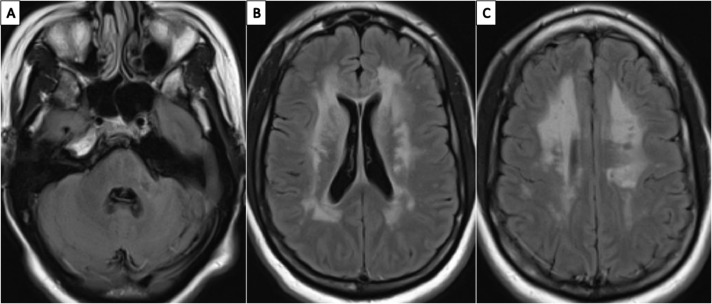
Fig. 2.
A–C: MRI brain non-contrast, axial views of the Fluid-Attenuated Inversion Recovery sequences showing chronic lacunar infarct in the L cerebellar peduncle and symmetric, bilateral periventricular and deep white matter hyperintensities, characteristic of CADASIL imaging findings
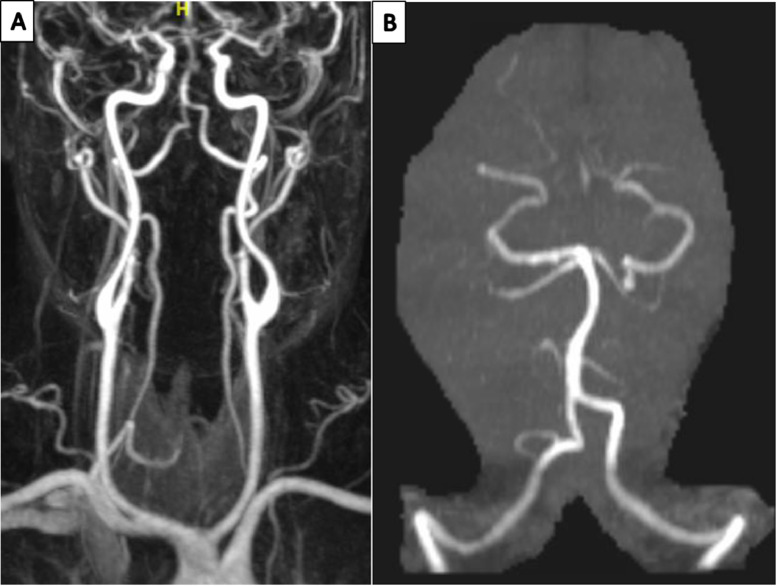
Fig. 3.
A) 3-D reconstitution of the non-enhanced MRA head and neck showing no evidence of acute stenosis or occlusion; B) 3-D reconstitution of the MRA head, showing intact posterior circulation without evidence of stenosis or occlusion
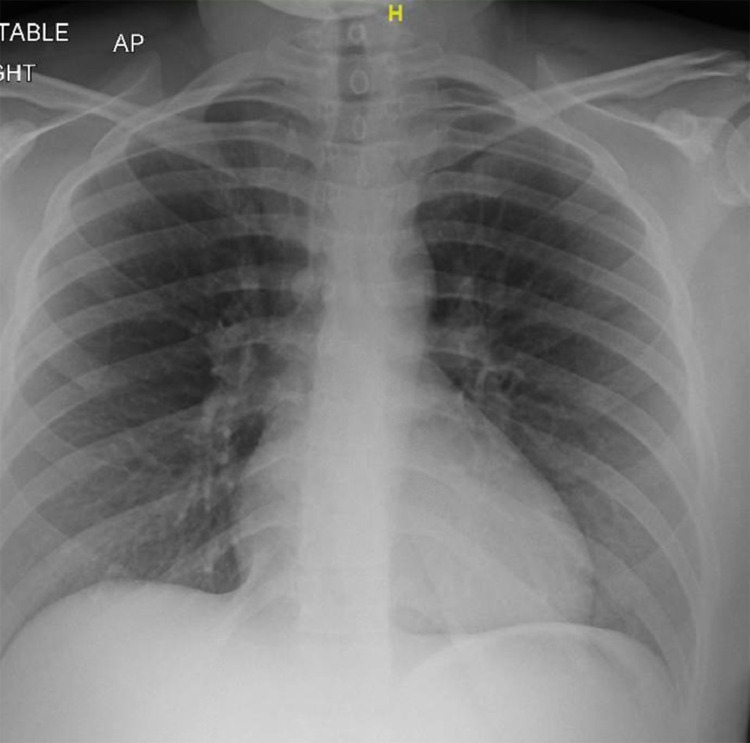
Fig. 4.
Chest X-ray, Anterior-posterior view, showing no evidence of an acute cardio-pulmonary process
Discussion
At the time of the submission of this case report, there were no other reported cases of acute ischemic stroke in a CADASIL patient with concomitant SARS-CoV-2 infection. CADASIL is a known cause of recurrent subcortical ischemic strokes due to small vessel arteriopathy caused by mutations in the NOTCH3 gene.7 Smoking and hypertension, two cardiovascular risk factors present in our patient, are associated with one to three-fold increase in the risk of stroke in CADASIL8 In our patient, both vascular risk factors were well controlled and vessel imaging revealed a patent vertebro-basilar arterial system (Fig. 3) raising the question if SARS-CoV-2 is associated with the ischemic insult. The association between infection and stroke is complex and not completely understood. It has been shown that herpesviruses, such as varicella zoster virus, can invade the vessel wall causing cerebral vasculopathy. In comparison, cytomegalovirus, Chlamydia pneumonie and other pathogens have been associated with atherogenesis and atheromatous plaque instability. Furthermore, recent bacterial or viral infections are known to transiently exacerbate the deleterious effect of pre-existing vascular risk factors.9, 10, 11 It has been proposed that infections create an inflammatory milieu that predisposes to stroke by activating prothrombotic pathways, affecting plaque stability, and inducing endothelial dysfunction, intimal thickening, and arterial wall remodeling. In the particular case of SARS-CoV-2, stroke has been associated with the activation of the renin-angiotensin system, procoagulability and the massive release of inflammatory cytokines (cytokine storm).12, 13, 14, 15 SARS-CoV-2 invades cells through a spike surface glycoprotein on the surface of the viral envelope that binds the angiotensin-converting enzyme (ACE)2 found on the surface of epithelial cells in the host.16 ACE2 participates in the conversion of angiotensin-2 (AT2) to angiotensin-1-7. AT2 binds the angiotensin-receptor type 1 and causes vasoconstriction, neuro-inflammation, oxidative stress and apoptosis. Angiotensin-1-7, in comparison, counterbalances the effects of AT2 and is associated with vasodilation and has anti-apoptotic, anti-inflammatory, anti-thrombotic, and antioxidant properties. It has been proposed that, by competing with AT2 for binding ACE2, SARS-Cov-2 induces activation of the angiotensin-receptor type 1 and causes stroke through its vasoconstrictor effect.17
Stroke in CADASIL has a predilection for subcortical structures. Brainstem involvement is more rare.18 Interestingly, autopsy and animal studies have shown that coronaviruses may have a particular tropism to the brainstem, supporting the potential implication of SARS-Cov-2 in the development of stroke in our case. At the same time, studies done in preclinical models have shown that when coronaviruses invade medulla, there is an increase in mortality rates in the animals, presumably from dysfunction of the cardiorespiratory center in the brainstem.19 , 20 Our patient had vascular risk factors for stroke, including smoking, hypertension, prior history of stroke and CADASIL. Her hypertension was well controlled and the CADASIL associated imaging changes have remained stable throughout the years. However, she continued to smoke. Thus, while it is possible that SARS-Cov-2 infection may have contributed to the etiology of the acute pontine stroke of our patient, we cannot conclusively prove causation.
It should be noted that most of our knowledge in relation to SARS-Cov-2-associated strokes is based on data obtained in single-center case series. Most of these studies include patients who, unlike our case, have severe respiratory distress syndrome, multi-organ failure and laboratory studies indicative of hypercoagulability and/or severe inflammatory state.5 Multicenter efforts are currently underway to better characterize the full spectrum of clinical manifestations, etiologies risk factors, and outcomes of stroke associated SARS-CoV-2. 21
Declaration of Competing Interest
None.
References
- 1.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S. COVID-19 presenting as stroke. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.04.077. S0889-1591(20)30685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020:382(20):e60. [DOI] [PMC free article] [PubMed]
- 6.Yousry TA, Seelos K, Mayer M, Bruning R, Uttner I, Dichgans M. Characteristic MR lesion pattern and correlation of T1 and T2 lesion volume with neurologic and neuropsychological findings in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) AJNR Am J Neuroradiol. 1999;20(1):91–100. [PubMed] [Google Scholar]
- 7.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383(6602):707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 8.Adib-Samii P, Brice G, Martin RJ, Markus HS. Clinical spectrum of CADASIL and the effect of cardiovascular risk factors on phenotype: study in 200 consecutively recruited individuals. Stroke. 2010;41(4):630–634. doi: 10.1161/STROKEAHA.109.568402. [DOI] [PubMed] [Google Scholar]
- 9.Miller EC, Elkind MS. Infection and stroke: an update on recent progress. Curr Neurol Neurosci Rep. 2016;16(1):2. doi: 10.1007/s11910-015-0602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. 2003;34(10):2518–2532. doi: 10.1161/01.STR.0000089015.51603.CC. [DOI] [PubMed] [Google Scholar]
- 11.Grau A, Buggle F, Becher H, Zimmermann E, Spiel M, Fent T. Recent bacterial and viral infection is a risk factor for cerebrovascular ischemia: clinical and biochemical studies. Neurology. 1998;50:196–203. doi: 10.1212/wnl.50.1.196. [DOI] [PubMed] [Google Scholar]
- 12.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirano T, Murakami M. COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immun. 2020;52(5):731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Divani AA, Andalib S, Di Napoli M, Lattanzi S, Hussain MS, Biller J. Coronavirus disease 2019 and stroke: clinical manifestations and pathophysiological insights. J Stroke Cerebrovasc Dis. 2020 doi: 10.1016/j.jstrokecerebrovasdis.2020.104941. 104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chabriat H, Mrissa R, Levy C, Vahedi K, Taillia H, Iba-Zizen MT. Brain stem MRI signal abnormalities in CADASIL. Stroke. 1999;30(2):457–459. doi: 10.1161/01.str.30.2.457. [DOI] [PubMed] [Google Scholar]
- 19.Li Y-C, Bai W-Z, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li K, Wohlford-Lenane C, Perlman S, Zhao J, Jewell AK, Reznikov LR. Middle east respiratory syndrome coronavirus causes multiple organ damage and Lethal disease in Mice Transgenic for human Dipeptidyl Peptidase 4. J Infect Dis. 2016;213(5):712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azarpazhooh MR, Morovatdar N, Avan A, Phan TG, Divani AA, Yassi N, et al. COVID-19 pandemic and burden of non-communicable diseases: an ecological study on data of 185 countries: COVID-19 and non-communicable diseases. J Stroke Cerebrovasc Dis 2020;29(9):105089. [DOI] [PMC free article] [PubMed]