Abstract
Objective
G6PC2 is predominantly expressed in pancreatic islet beta cells. G6PC2 hydrolyzes glucose-6-phosphate to glucose and inorganic phosphate, thereby creating a futile substrate cycle that opposes the action of glucokinase. This substrate cycle determines the sensitivity of glucose-stimulated insulin secretion to glucose and hence regulates fasting blood glucose (FBG) but not fasting plasma insulin (FPI) levels. Our objective was to explore the physiological benefit this cycle confers.
Methods
We investigated the response of wild type (WT) and G6pc2 knockout (KO) mice to changes in nutrition.
Results
Pancreatic G6pc2 expression was little changed by ketogenic diet feeding but was inhibited by 24 hr fasting and strongly induced by high fat feeding. When challenged with either a ketogenic diet or 24 hr fasting, blood glucose fell to 70 mg/dl or less in G6pc2 KO but not WT mice, suggesting that G6PC2 may have evolved, in part, to prevent hypoglycemia. Prolonged ketogenic diet feeding reduced the effect of G6pc2 deletion on FBG. The hyperglycemia associated with high fat feeding was partially blunted in G6pc2 KO mice, suggesting that under these conditions the presence of G6PC2 is detrimental. As expected, FPI changed but did not differ between WT and KO mice in response to fasting, ketogenic and high fat feeding.
Conclusions
Since elevated FBG levels are associated with increased risk for cardiovascular-associated mortality (CAM), these studies suggest that, while G6PC2 inhibitors would be useful for lowering FBG and the risk of CAM, partial inhibition will be important to avoid the risk of hypoglycemia.
Keywords: Glucose-6-phosphatase, Ketogenic diet, Glucose cycling, Hypoglycemia, G6PC2, Fasting, High fat diet, Islet, Glucose
Highlights
-
•
G6pc2 deletion lowers fasting blood glucose (FBG) in chow and high fat fed mice.
-
•
Elevated FBG increases the risk of cardiovascular-associated mortality (CAM).
-
•
G6pc2 deletion results in hypoglycemia in mice on a ketogenic diet.
-
•
G6pc2 deletion results in hypoglycemia in mice following prolonged fasting.
-
•
G6PC2 inhibitors may prevent CAM but increase risk of hypoglycemia.
1. Introduction
Glucose-6-phosphatase catalyzes the hydrolysis of glucose-6-phosphate (G6P) to glucose and inorganic phosphate [1]. It is located in the endoplasmic reticulum (ER) membrane and exists as a multi-component enzyme system in which a G6P transporter, encoded by the SLC37A4 gene, delivers substrate from the cytosol to the active site of a glucose-6-phosphatase catalytic subunit (G6PC) in the lumen of the ER, with transporters for inorganic phosphate and glucose returning the reaction products back to the cytosol [1]. The G6PC gene family is comprised of three members, namely G6PC1, G6PC2 and G6PC3 [1]. G6PC1 is predominantly expressed in liver and kidney where it catalyzes the terminal step in the gluconeogenic and glycogenolytic pathways, whereas G6PC3 is widely expressed, with especially high expression in kidney, testis, skeletal muscle and brain [1]. Based on Northern blotting, immunohistochemistry and RNA-Seq data, G6PC2, also known as IGRP, is predominantly expressed in pancreatic islets. In mice, G6pc2 is expressed at ∼20 fold higher levels in pancreatic islet beta cells than alpha cells whereas in humans G6PC2 is expressed at a ∼5 fold higher level in beta than alpha cells [[2], [3], [4]].
G6PC2 acts in conjunction with the beta cell glucose sensor, glucokinase, to create a futile substrate cycle that determines the rate of beta cell glycolytic flux [[5], [6], [7]]. In turn, this futile cycle determines the sensitivity of glucose-stimulated insulin secretion (GSIS) to glucose, and hence the influence of beta cells on fasting blood glucose (FBG) [5,6]. Key evidence in support of this model are the observations that, in isolated G6pc2 knockout (KO) islets, glucose-6-phosphatase activity [7] and glucose cycling [8], calculated as the percentage of the G6P generated from glucose that is converted back to glucose, are both reduced. This results in a leftward shift in the dose response curve for GSIS [7] such that under fasting conditions, insulin levels are the same in wild type (WT) and G6pc2 KO mice but FBG is reduced in KO mice [7,9]. This same shift in glucose sensitivity results in increased insulin secretion from isolated G6pc2 KO islets in response to sub-maximal glucose [7]. The same results are observed in both germline [7] and beta cell-specific [10] G6pc2 KO mice, consistent with the predominant expression of G6pc2 in beta cells and suggesting that trace G6pc2 expression in peripheral tissues is not biologically important. This model is also consistent with genome-wide association and molecular studies that have linked the rs560887 ‘A’ allele to reduced G6PC2 expression and reduced FBG [[11], [12], [13]].
A key question is the nature of the physiological benefit conferred by this glucokinase/G6PC2 substrate cycle. Experiments in mice in which glucocorticoids were injected or endogenous glucocorticoids were elevated by physical restraint induced G6pc2 expression [14,15]. The associated change in FBG in KO relative to WT mice suggests that G6PC2 may have evolved, in part, to confer a transient, beneficial elevation in FBG during periods of stress [14,15]. The experiments described here extend these studies by examining the role of G6PC2 in the response to different nutritional challenges and suggest that G6PC2 evolved to not only regulate FBG in response to stress, but also to prevent hypoglycemia associated with prolonged fasting and ketogenic diets.
2. Materials and methods
2.1. Animal care
The Vanderbilt University Medical Center Animal Care and Use Committee approved all protocols used. Mice were maintained on either a standard rodent chow diet (LabDiet 5001, PMI Nutrition International, which comprises 24% protein; 5% fat [linoleic acid, 1.16%; linolenic acid, 0.07%; total saturated fatty acids, 1.50%; total monounsaturated fatty acids, 1.58%]) and 49% carbohydrate (starch, 31.9%; glucose, 0.22%; fructose 0.3%; sucrose, 3.7%; lactose 2.0%) with calories from protein (29%), fat (13%) and carbohydrate (58%)) or a high fat diet (Mouse Diet F3282; BioServ, which comprises 21% protein; 36% fat [linoleic acid, 3.7%; linolenic acid, 0.4%; total saturated fatty acids, 14.1%; total monounsaturated fatty acids, 16.2%]) and 37% carbohydrate (monosaccharides, 0.1%; disaccharides, 14.6%; polysaccharides, 20%) with calories from protein (15%), fat (59%) and carbohydrate (26%) or a ketogenic diet (Mouse Diet F3666; BioServ, which comprises 8.6% protein; 75.1% fat [linoleic acid, 11.5%; linolenic acid, 0.7%; total saturated fatty acids, 30.3%; total monounsaturated fatty acids, 28.8%]) and 3.2% carbohydrate (monosaccharides, 0.7%; disaccharides, 2.5%; polysaccharides, 0%) with calories from protein (5%), fat (93%) and carbohydrate (2%) as indicated. Mice were placed on the diet at 8 weeks of age for 1–8 weeks for ketogenic diet studies, whereas for high fat diet studies, mice were placed on the diet at 33 weeks of age for 10–13 weeks. Food and water were provided ad libitum.
2.2. Generation of germline G6pc2 KO mice
The generation of germline G6pc2 KO mice on a pure C57BL/6J genetic background has been previously described [7,9].
2.3. In vivo phenotypic analyses
Intraperitoneal glucose tolerance tests (IPGTTs), insulin tolerance tests (ITTs), plasma cholesterol and blood glucose, were all measured in 6-hr fasted mice as previously described [7,16,17]. Plasma ketones were measured using the Ketone Body Assay Kit (Sigma). Plasma insulin and glucagon were assayed by the Vanderbilt Hormone Assay and Analytical Services Core using RIA or a highly specific and sensitive immunoassay (Mercodia; Kit #10-1271-01), respectively.
2.4. Analysis of gene expression
Pancreatic and liver RNA were isolated using the ToTALLY RNA kit (Ambion, Carlsbad, CA). The Turbo DNA-free DNAse Treatment Kit (Ambion, Carlsbad, CA) was then used to remove trace genomic DNA followed by cDNA generation using the iScript DNA Synthesis Kit (Bio-Rad, Hercules, CA). Gene expression was then quantitated by PCR using the dUTP-containing FastStart SYBR Green Master Mix in conjunction with Uracil-DNA-Glycosylase (Roche, Nutley, NJ). Fold induction of gene expression was calculated using the 2 (-ΔΔC(T)) method [18]. PCR primer sequences have been previously described [10]. The efficiency of primer amplification was tested in cDNA dilution analyses. These showed that correlation coefficients were ∼0.99 and PCR efficiency was ∼95%.
2.5. Mouse islet isolation and glucose cycling
Mouse islets were isolated by the Vanderbilt Islet Procurement and Analysis Core and glucose cycling was measured as previously described [8].
2.6. Statistical analyses
Mouse data were analyzed using a Student's t-test, two-sample assuming equal variance or a one-way ANOVA, assuming normal distribution and equal variance, as indicated. Post hoc analyses were performed using the Bonferroni correction for multiple comparisons. The level of significance was as indicated.
3. Results
3.1. G6PC2 confers protection against hypoglycemia upon ketogenic diet feeding
To understand why the G6PC2 gene would have been conserved across multiple species over the course of evolution, we considered the benefits that a glucokinase/G6PC2 futile substrate cycle might confer under different dietary conditions. We hypothesized that when carbohydrate-rich foods were scarce, G6PC2 might have been beneficial by limiting basal GSIS and therefore conferring protection against hypoglycemia. We investigated the response of WT and G6pc2 KO mice to ketogenic diet feeding to address that hypothesis.
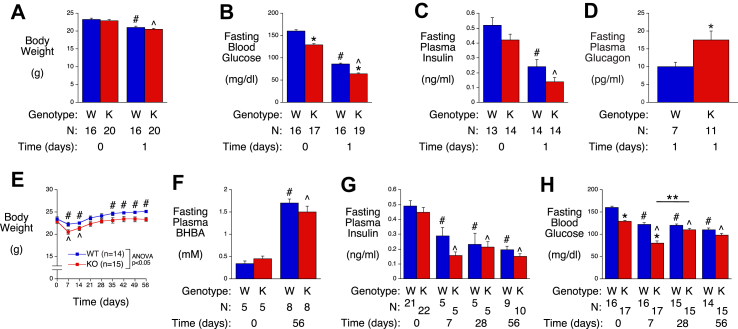
Significant weight loss was observed in WT and G6pc2 KO mice one day after switching mice from a chow diet to a ketogenic diet (Figure 1A) and this was associated with a marked decrease in both fasting blood glucose (FBG) (Figure 1B) and fasting plasma insulin (FPI) (Figure 1C), with KO mice becoming hypoglycemic (64−/+2 mg/dl glucose). Glucagon levels were significantly elevated in KO mice after 1 day on the ketogenic diet relative to WT mice, but this increase was insufficient to prevent hypoglycemia (Figure 1D). These data suggest that G6PC2 confers protection against hypoglycemia upon ketogenic diet feeding.
Figure 1.
Analysis of Metabolic Parameters in Male WT and G6pc2 KO Mice on a Ketogenic Diet. Body weight (Panel A), FBG (Panel B), FPI (Panel C) and plasma glucagon (Panel D) were compared in 6-hr fasted WT (W) and KO (K) mice before, and/or 1 day after, switching from a chow diet to ketogenic diet at 8 weeks of age. ∗p < 0.05 WT vs KO; #p < 0.05 WT at day 0 vs WT at day 1 of ketogenic diet feeding; ˆp < 0.05 KO at day 0 vs KO at day 1 of ketogenic diet feeding. Body weight (Panel E), plasma ketones (beta-hydroxybutyric acid; BHBA) (Panel F), FPI (Panel G) and FBG (Panel H) were compared in non-fasted (Panel E) or 6-hr fasted (Panels F–H) WT and KO mice that were switched from a chow diet to a ketogenic diet at 8 weeks of age. ∗p < 0.05 WT vs KO; #p < 0.05 WT at day 0 vs WT after ketogenic diet feeding; ˆp < 0.05 KO at day 0 vs KO after ketogenic diet feeding; ∗∗p < 0.05 KO at day 7 vs KO at day 28 of ketogenic diet feeding. Results show the mean data ± S.E.M. with the genotype and number of animals analyzed indicated. Statistical significance was analyzed using ANOVA (Panels A–E, G & H) or t-tests (Panel F).
We next investigated the response to prolonged ketogenic diet feeding. Figure 1E shows that the weight loss observed in both WT and G6pc2 KO mice upon switching from a chow diet to a ketogenic diet is transient. Both WT and G6pc2 KO mice returned to their initial starting weight after 21 days on the ketogenic diet (Figure 1E). After 35 days on the ketogenic diet, WT mice gained additional weight and exceeded their initial starting weight, whereas KO mice remained at their initial starting weight even after 56 days on the diet, resulting in a significant difference in body weight between WT and G6pc2 KO mice (Figure 1E). Ketone levels were significantly elevated in both WT and KO mice after 56 days on the ketogenic diet (Figure 1F).
FPI (Figure 1G) and FBG (Figure 1H) were significantly lower in both WT and G6pc2 KO mice after 7 days of ketogenic diet feeding and remained lower after 56 days. FPI did not differ between WT and G6pc2 KO mice at any time (Figure 1G). However, the fall in FBG observed in G6pc2 KO mice after 1 (Figure 1A) and 7 (Figure 1H) days of ketogenic diet feeding was partially reversed between 7 and 28 days of ketogenic diet feeding (Figure 1H). While a significant difference in FBG was observed in chow-fed WT and G6pc2 KO mice (Figure 1H; day 0) and after 1 (Figure 1A) and 7 (Figure 1H) days of ketogenic diet feeding, this difference between WT and KO mice was lost after 28 days on the ketogenic diet (Figure 1H).
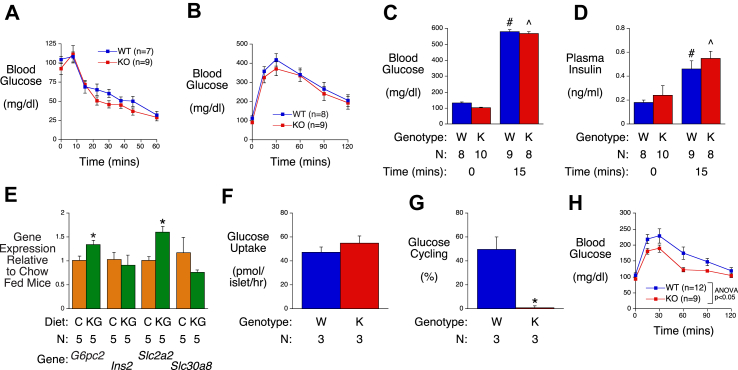
Additional studies were then performed to determine why prolonged ketogenic diet feeding appears to abolish the influence of G6PC2 on FBG. Similar to chow-fed mice [7], WT and G6pc2 KO mice on a ketogenic diet showed no differences in insulin sensitivity (Figure 2A) or glucose tolerance following intraperitoneal injection of 2 g/kg glucose (Figure 2B), and the rise in blood glucose following intraperitoneal glucose injection in WT and KO mice (Figure 2C) was not associated with a difference in the rise in plasma insulin (Figure 2D).
Figure 2.
Analysis of Metabolic Parameters, Glucose Cycling and Gene Expression in Male WT and G6pc2 KO Mice on a Ketogenic Diet. Insulin sensitivity (Panel A), intraperitoneal (IP) glucose tolerance (Panel B) and both blood glucose (Panel C) and plasma insulin (Panel D) before and after IP glucose injection (2 g/kg) were compared in 6-hr fasted 16–18 week old WT and KO mice after 8–10 weeks of ketogenic diet feeding. #p < 0.05 WT at t = 0 vs WT at t = 15; ˆp < 0.05 KO at t = 0 vs KO at t = 15. Comparison of pancreatic expression of genes known to be highly expressed in islets (Panel E) in 6-hr fasted 16 week old chow-fed (C) WT mice versus 6-hr fasted 16 week old WT mice that were switched to a ketogenic diet (KG) at 8 weeks of age. Data were corrected relative to Ppia (cyclophilin A) expression. ∗p < 0.05 chow vs ketogenic diet. Comparison of glucose uptake (Panel F) and glucose cycling (Panel G) rates at 11 mM glucose in islets isolated from 16 week old WT (W) and KO (K) mice that were switched to a ketogenic diet at 8 weeks of age. ∗p < 0.05 WT vs KO. Intraperitoneal glucose tolerance (Panel H) after IP glucose injection (0.75 g/kg) was analyzed in 6-hr fasted 16–18 week old WT and KO mice after 8–10 weeks of ketogenic diet feeding. Results show the mean data ± S.E.M. with the genotype and number of animal/islet preparations analyzed indicated. Statistical significance was analyzed using ANOVA (Panels A–D, H) or t-tests (Panels E-G).
To assess whether ketogenic diet feeding affected expression of G6pc2, we specifically analyzed G6pc2 gene expression in addition to other genes known to be highly expressed in islets but not acinar cells, in whole pancreas RNA preparations. This approach captures expression levels in the in vivo islet environment and avoids the confounding issue of gene expression changing during islet isolation. The switch from a chow to ketogenic diet was associated with a significant but modest induction of G6pc2 and Slc2a2 with no change in Ins2 or Slc30a8 gene expression (Figure 2E). The absence of a marked change in G6pc2 expression is consistent with the observation that the rate of glucose uptake (Figure 2F) and glucose cycling (Figure 2G) in islets isolated from mice on a ketogenic diet is similar to that in islets isolated from mice on a chow diet [8], with the deletion of G6pc2 abolishing glucose cycling as expected (Figure 2G). The small induction of G6pc2 expression would be predicted to protect against hypoglycemia but was surprising because glucose has a marked stimulatory effect on G6pc2 gene expression [19]. We therefore expected that the fall in FBG associated with ketogenic diet feeding would lead to a reduction in G6pc2 expression. Similarly, because Slc2a2 gene expression is also stimulated by glucose [20], we expected to observe a decrease rather than increase in expression (Figure 2E). These observations suggest that other unknown factors associated with ketogenic diet feeding modulate the expected effect of glucose on G6pc2 and Slc2a2 expression.
While these observations provide no explanation as to why prolonged ketogenic diet feeding abolishes the influence of G6PC2 on FBG, glucose tolerance tests using a lower intraperitoneal injection of 0.75 g/kg glucose showed that G6pc2 deletion was associated with a significant improvement in glucose tolerance (Figure 2H). This suggests a specific loss of influence of G6PC2 on FBG rather than a complete loss of function.
3.2. G6PC2 confers protection against hypoglycemia upon prolonged fasting
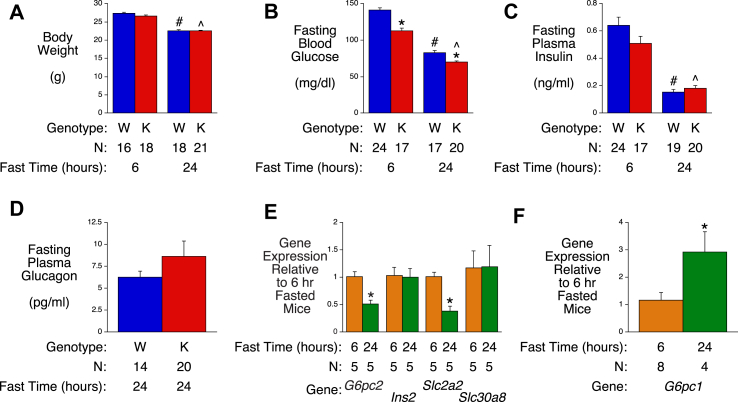
We next investigated the response of chow-fed WT and G6pc2 KO mice to prolonged fasting. A 24-hr fast resulted in significant reductions in body weight (Figure 3A), FBG (Figure 3B) and FPI (Figure 3C) relative to 6-hr fasted mice. FPI did not differ between WT and G6pc2 KO mice (Figure 3C) whereas the significant difference in FBG observed in 6-hr fasted WT and G6pc2 KO mice was still apparent after a 24-hr fast (Figure 3B), with KO mice becoming hypoglycemic (70−/+1 mg/dl glucose). A difference in glucagon levels between WT and KO mice was not observed despite the hypoglycemia in KO mice (Figure 3D). These data suggest that G6PC2 confers protection against hypoglycemia upon prolonged fasting.
Figure 3.
Analysis of Metabolic Parameters and Gene Expression in Male WT and G6pc2 KO Mice After Prolonged Fasting. Body weight (Panel A), FBG (Panel B), FPI (Panel C) and plasma glucagon (Panel D) were compared in 6-hr and/or 24-hr fasted 16 week old male WT (W) and KO (K) mice. ∗p < 0.05 WT vs KO; #p < 0.05 6-hr fasted WT vs 24-hr fasted WT; ˆ p < 0.05 6-hr fasted KO vs 24-hr fasted KO. Comparison of pancreatic expression of genes known to be highly expressed in islets (Panel E) or liver (Panel G) in 6-hr versus 24-hr fasted 16 week old male WT mice. Data were corrected relative to Ppia (cyclophilin A) expression. ∗p < 0.05 6-hr fasted WT vs 24-hr fasted WT. Results show the mean data ± S.E.M. with the genotype and number of animals analyzed indicated. Statistical significance was analyzed using ANOVA (Panels A-C) or t-tests (Panels D-F).
Prolonged fasting was associated with a significant ∼50% fall in pancreatic G6pc2 and Slc2a2 gene expression with no change in Ins2 or Slc30a8 gene expression (Figure 3E). Unlike the complex effects of ketogenic diet feeding on pancreatic G6pc2 and Slc2a2 gene expression (Figure 2), the fall observed with fasting is consistent with a reduction in the stimulatory effect of glucose on those genes (Figure 2D) [19,20]. Our previous studies on heterozygous G6pc2 mice have shown an intermediate effect on FBG relative to KO mice [7,9], therefore, we would speculate that this decrease in G6pc2 expression would lower FBG relative to WT, but not sufficiently to result in hypoglycemia and instead would serve to promote enhanced glucose clearance during re-feeding (Figure 2H). Furthermore, a partial reduction in G6pc2 expression during fasting would allow blood glucose to fall even more so as to minimize glucose usage while maintaining some insulin to brake proteolysis while keeping free fatty acid availability high.
In contrast to G6pc2, which is not expressed in the liver [10] and is not regulated by insulin (data not shown), hepatic G6pc1 expression increases significantly with fasting (Figure 3F). Because insulin represses [1] whereas glucose stimulates [21] G6pc1 expression, this suggests that the effect of reduced repression of G6pc1 expression by insulin associated with the reduction in FPI (Figure 3C) is dominant over the effect of reduced stimulation by glucose associated with the reduction in FBG (Figure 3B).
3.3. G6PC2 limits hyperglycemia upon high fat diet feeding
While prolonged fasting and ketogenic diets may have been common over the course of evolution, high fat diets are more prevalent in the modern world. We previously showed that 8 week old C57BL/6J G6pc2 KO male mice were not protected against diet induced obesity (DIO) when switched from a chow diet to a high fat diet and that the magnitude of the difference in FBG between WT and KO mice was not affected by 12 weeks of high fat feeding [7]. However, recent studies have shown that aging affects the response of islets to high fat feeding [22], so we reasoned that the outcome may be different in older mice.
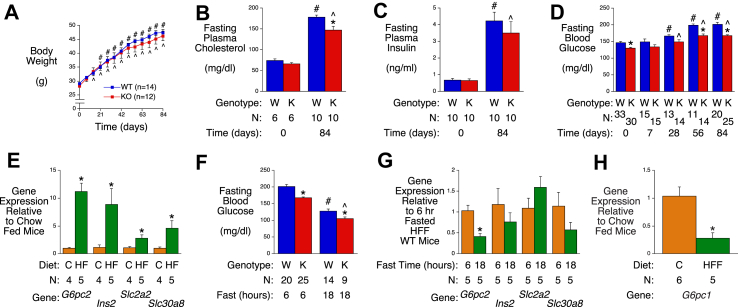
Switching 33 week old mice from a chow diet to a high fat diet resulted in significant weight gain in both WT and G6pc2 KO mice (Figure 4A) and, as in young mice [7], no difference in body weight emerged between WT and G6pc2 KO mice. Plasma cholesterol (Figure 4B), FPI (Figure 4C) and FBG (Figure 4D) were all significantly elevated following high fat diet feeding. The elevation in plasma cholesterol was greater in WT than KO mice after 84 days on the diet (Figure 4B), as previously reported [23]. The elevation in FBG in WT and KO mice was apparent after 28 days of high fat diet feeding and the significant difference in FBG between WT and G6pc2 KO mice remained after 84 days (Figure 4D). As concluded from studies in young mice [7], these data again suggest that inhibition of G6PC2 would limit but not prevent the hyperglycemia associated with high fat diet feeding.
Figure 4.
Analysis of Metabolic Parameters and Gene Expression in Male WT and G6pc2 KO Mice on a High Fat Diet. Body weight (Panel A), plasma cholesterol (Panel B), FPI (Panel C) and FBG (Panel D) were compared in non-fasted (Panel A) or 6-hr fasted (Panels B-D) WT and KO mice that were switched from a chow diet to a high fat (HF) diet at 33 weeks of age. ∗p < 0.05 WT vs KO; #p < 0.05 WT at day 0 vs WT after high fat diet feeding; ˆp < 0.05 KO at day 0 vs KO after high fat diet feeding. Comparison of pancreatic expression of genes known to be highly expressed in islets (Panel E) in 6-hr fasted 33 week old chow fed WT mice versus 6-hr fasted 45 week old WT mice that were switched to a high fat diet at 33 weeks of age. Data were corrected relative to Ppia (cyclophilin A) expression. ∗p < 0.05 chow vs high fat fed (HF). FBG (Panel F) in 45 week old male WT and KO mice that were placed on a high fat diet at 33 weeks of age after a 6-hr or 18-hr fast. ∗p < 0.05 WT vs KO; #p < 0.05 6-hr fasted WT vs 18-hr fasted WT; ˆp < 0.05 6-hr fasted KO vs 18-hr fasted KO. Comparison of pancreatic expression of genes known to be highly expressed in islets in 45 week old 6-hr fasted male WT mice that were placed on a high fat diet at 33 weeks of age versus 24 week old 18-hr fasted male WT mice that had been placed on a high fat diet at 12 weeks of age (Panel G). ∗p < 0.05 6-hr fasted WT vs 18-hr fasted WT. Comparison of hepatic G6pc1 expression in 6-hr fasted 33 week old chow-fed WT mice versus 6-hr fasted 45 week old WT mice that were switched to a high fat diet at 33 weeks of age (Panel H). Data were corrected relative to Ppia (cyclophilin A) expression. ∗p < 0.05 chow vs high fat fed (HFF). Results show the mean data ± S.E.M. with the genotype and number of animals indicated. Statistical significance was analyzed using ANOVA (Panels A-D, F) or t-tests (Panels E, G & H).
The switch from a chow diet to a high fat diet was associated with marked changes in pancreatic G6pc2 as well as Ins2, Slc2a2 and Slc30a8 gene expression (Figure 4E) that are highly likely to be due in part to the well-characterized effects of high fat diet feeding on pancreatic islet mass [24]. After 18-hr fasting, the FBG levels in previously high fat fed mice (Figure 4F) were similar to those in 6-hr fasted chow fed mice (Figure 1B). Prolonged fasting was associated with a significant ∼50% fall in pancreatic G6pc2 gene expression with no change in Ins2, Slc2a2 or Slc30a8 gene expression (Figure 4G). This fall in G6pc2 expression in previously high fat fed mice after prolonged fasting mimics that in mice that were previously fed a chow diet (Figure 3E) and is consistent with a reduction in the stimulatory effect of glucose [19]. It is unclear why a similar decrease in Slc2a2 expression is not observed in response to reduced FBG.
In contrast to the marked induction of G6pc2 expression by high fat feeding (Figure 4E), hepatic G6pc1 expression was significantly decreased (Figure 4H). Because insulin represses [1] whereas glucose stimulates [21] G6pc1 expression, this suggests that the effect of increased repression by insulin associated with the elevated FPI (Figure 4C) is dominant over the effect of increased stimulation by glucose associated with the elevated FBG (Figure 4D).
4. Discussion
The experiments described here suggest that G6PC2 may have evolved to not only confer a beneficial elevation in FBG in response to stress [14,15] but also to prevent hypoglycemia in response to ketogenic diet feeding (Figure 1) or prolonged fasting (Figure 3). In contrast, in response to high fat diet feeding, as is common in the modern world, the presence of G6PC2 amplifies the hyperglycemia (Figure 4).
Elevated FBG and HbA(1C) levels in humans have been associated with an increased risk for the development of cardiovascular-associated mortality (CAM) [[25], [26], [27]]. This correlation with CAM is observed in both women and men [27] and even at glucose concentrations in the non-diabetic range, although the risk of CAM increases still further in individuals with the high FBG levels characteristic of diabetes [[25], [26], [27], [28]]. Even small differences in FBG have a profound effect over time. For example, a reduction in FBG from 99 to 90 mg/dl in Asians is associated with a 25% reduction in the risk of CAM [27] and in Europeans an increase in FBG levels from less than 90 mg/dl to between 99 and 108 mg/dl is associated with a 30% increased risk of CAM [25]. Similarly, studies in pregnant women have shown continuous associations of maternal glucose levels below those diagnostic of diabetes with increased birth weight and increased cord-blood serum C-peptide levels [29]. Because deletion of G6pc2 is associated with a ∼20 mg/dl reduction in FBG [7,9,15] the magnitude of this effect, relative to the human data described above, suggests that inhibition of G6PC2 should already be considered as a novel therapeutic strategy for lowering FBG and hence preventing CAM. However, the potential risk of hypoglycemia under specific conditions (Figure 1, Figure 3) suggests that achieving partial inhibition may be the optimal approach.
We have previously observed increased insulin secretion in isolated islets from G6pc2 KO mice and in perfused KO pancreas in response to a challenge with sub-maximal glucose [7]. These observations are consistent with a model in which G6PC2 regulates glucose cycling [8], such that deletion of G6pc2 shifts the dose response curve for GSIS to the left [7]. We also see improvements in intraperitoneal glucose tolerance in both G6pc2 KO mice on a ketogenic diet when injected with submaximal 0.75 g/kg glucose (Figure 2H) and KO mice on a chow diet when injected with 0.4 g/kg glucose [7]. Based on these observations one would expect to see increased insulin secretion in KO mice in response to injection with submaximal glucose (0.4–0.75 g/kg) that would then explain the improvement in glucose tolerance. Surprisingly, we have seen no evidence for such an increase in chow-fed mice [7] and have not attempted this experiment in mice on a prolonged ketogenic diet because even the induction in plasma insulin following a 2 g/kg glucose injection is small and variable (Figure 2D). We hypothesize that the molecular mechanism for the improved glucose tolerance in the absence of enhanced insulin secretion might relate to an action of G6PC2 on insulin pulsatility, with G6pc2 deletion enhancing pulsatility and therefore improving glucose tolerance without a requirement for elevated insulin secretion. This concept was suggested by Watanabe and colleagues [30] based on human genetic data showing that reduced G6PC2 expression was associated with reduced FBG but also surprisingly reduced, rather than elevated, insulin levels in an oral glucose tolerance test. Future studies will directly address this hypothesis in G6pc2 KO mice.
We hypothesized that G6pc2 would be induced by fasting and ketogenic diet feeding to protect against hypoglycemia, however, G6pc2 expression was surprisingly reduced in response to fasting (Figure 3) and little changed in response to ketogenic diet feeding (Figure 2). In contrast, G6pc2 expression was markedly induced in response to high fat diet feeding (Figure 4). Because glucose stimulates G6pc2 expression in isolated islets (Figure 2), this could explain why G6pc2 expression is elevated in response to the hyperglycemia associated with high fat diet feeding (Figure 4) and reduced in response to the hypoglycemia associated with fasting (Figure 3). However, other factors must regulate G6pc2 expression because expression did not fall in response to the hypoglycemia associated with ketogenic diet feeding (Figure 2). Future experiments will explore the nature of these other regulatory factors and also whether the regulation by glucose occurs at a transcriptional or post-transcriptional level. The transcription factors ChREBP and CREB have been shown to be important for mediating the stimulatory effects on glucose on G6PC1, but whether these factors are also important for the regulation of G6PC2 expression is unknown. Future studies that include an examination of G6PC2 protein levels will also seek to understand why the marked induction of G6pc2 expression in mice on a high fat diet (Figure 4E) has little effect on the difference in FBG between WT and KO mice (Figure 4D).
Author contributions
K.J.B. performed most of the mouse phenotyping studies, gene expression analyses and some of the manuscript writing.
M.R. performed the islet glucose cycling studies.
J.K.O. performed some of the gene expression analyses.
O.P.M. contributed to the design of experiments and manuscript editing.
J.D.Y contributed to the design of the islet glucose cycling studies.
R.M.O. performed some of the mouse studies and manuscript writing.
Acknowledgments
We thank Susan Hajizadeh for performing insulin and glucagon assays. This research was supported by the following grants: R.O’B., DK92589; O.P.M., DK043748 and DK078188; J.D.Y DK106348. The isolation of mouse islets by the Vanderbilt Islet Procurement and Analysis Core and the measurement of plasma insulin and glucagon by the Vanderbilt Hormone Assay & Analytical Services Core were supported by NIH grant P60 DK20593 to the Vanderbilt Diabetes Research Training Center. K.J.B. was supported by the Vanderbilt Molecular Endocrinology Training Program grant 5T32 DK07563.
R.O’B. is the guarantor of this work, had full access to all data, and takes full responsibility for the integrity of the data and the accuracy of data analysis.
Conflict of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Hutton J.C., O'Brien R.M. The glucose-6-phosphatase catalytic subunit gene family. Journal of Biological Chemistry. 2009;284:29241–29245. doi: 10.1074/jbc.R109.025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arden S.D., Zahn T., Steegers S., Webb S., Bergman B., O'Brien R.M. Molecular cloning of a pancreatic islet-specific glucose-6-phosphatase catalytic subunit-related protein. Diabetes. 1999;48(3):531–542. doi: 10.2337/diabetes.48.3.531. [DOI] [PubMed] [Google Scholar]
- 3.Martin C.C., Bischof L.J., Bergman B., Hornbuckle L.A., Hilliker C., Frigeri C. Cloning and characterization of the human and rat islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) genes. Journal of Biological Chemistry. 2001;276(27):25197–25207. doi: 10.1074/jbc.M101549200. [DOI] [PubMed] [Google Scholar]
- 4.Xin Y., Kim J., Okamoto H., Ni M., Wei Y., Adler C. RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metabolism. 2016;24(4):608–615. doi: 10.1016/j.cmet.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Iynedjian P.B. Molecular physiology of mammalian glucokinase. Cellular and Molecular Life Sciences. 2009;66(1):27–42. doi: 10.1007/s00018-008-8322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matschinsky F.M., Wilson D.F. The central role of glucokinase in glucose homeostasis: a perspective 50 Years after demonstrating the presence of the enzyme in islets of Langerhans. Frontiers in Physiology. 2019;10:148. doi: 10.3389/fphys.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pound L.D., Oeser J.K., O'Brien T.P., Wang Y., Faulman C.J., Dadi P.K. G6PC2: a negative regulator of basal glucose-stimulated insulin secretion. Diabetes. 2013;62:1547–1556. doi: 10.2337/db12-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wall M.L., Pound L.D., Trenary I., O'Brien R.M., Young J.D. Novel stable isotope analyses demonstrate significant rates of glucose cycling in mouse pancreatic islets. Diabetes. 2015;64(6):2129–2137. doi: 10.2337/db14-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Martin C.C., Oeser J.K., Sarkar S., McGuinness O.P., Hutton J.C. Deletion of the gene encoding the islet-specific glucose-6-phosphatase catalytic subunit-related protein autoantigen results in a mild metabolic phenotype. Diabetologia. 2007;50:774–778. doi: 10.1007/s00125-006-0564-1. [DOI] [PubMed] [Google Scholar]
- 10.Bosma K.J., Rahim M., Singh K., Goleva S.B., Wall M.L., Xia J. Pancreatic islet beta cell-specific deletion of G6pc2 reduces fasting blood glucose. Journal of Molecular Endocrinology. 2020 doi: 10.1530/JME-20-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouatia-Naji N., Rocheleau G., Van Lommel L., Lemaire K., Schuit F., Cavalcanti-Proenca C. A polymorphism within the G6PC2 gene is associated with fasting plasma glucose levels. Science. 2008;320(5879):1085–1088. doi: 10.1126/science.1156849. [DOI] [PubMed] [Google Scholar]
- 12.Chen W.M., Erdos M.R., Jackson A.U., Saxena R., Sanna S., Silver K.D. Variations in the G6PC2/ABCB11 genomic region are associated with fasting glucose levels. Journal of Clinical Investigation. 2008;118:2620–2628. doi: 10.1172/JCI34566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baerenwald D.A., Bonnefond A., Bouatia-Naji N., Flemming B.P., Umunakwe O.C., Oeser J.K. Multiple functional polymorphisms in the G6PC2 gene contribute to the association with higher fasting plasma glucose levels. Diabetologia. 2013;56(6):1306–1316. doi: 10.1007/s00125-013-2875-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boortz K.A., Syring K.E., Lee R.A., Dai C., Oeser J.K., McGuinness O.P. G6PC2 modulates the effects of dexamethasone on fasting blood glucose and glucose tolerance. Endocrinology. 2016;157:4133–4145. doi: 10.1210/en.2016-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boortz K.A., Syring K.E., Dai C., Pound L.D., Oeser J.K., Jacobson D.A. G6PC2 modulates fasting blood glucose in male mice in response to stress. Endocrinology. 2016;157:3002–3008. doi: 10.1210/en.2016-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Oeser J.K., Yang C., Sarkar S., Hackl S.I., Hasty A.H. Deletion of the gene encoding the ubiquitously expressed glucose-6-phosphatase catalytic subunit-related protein (UGRP)/glucose-6-phosphatase catalytic subunit-beta results in lowered plasma cholesterol and elevated glucagon. Journal of Biological Chemistry. 2006;281(52):39982–39989. doi: 10.1074/jbc.M605858200. [DOI] [PubMed] [Google Scholar]
- 17.Pound L.D., Sarkar S.A., Ustione A., Dadi P.K., Shadoan M.K., Lee C.E. The physiological effects of deleting the mouse slc30a8 gene encoding zinc transporter-8 are influenced by gender and genetic background. PloS One. 2012;7(7) doi: 10.1371/journal.pone.0040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Petrolonis A.J., Yang Q., Tummino P.J., Fish S.M., Prack A.E., Jain S. Enzymatic characterization of the pancreatic islet-specific glucose-6-phosphatase-related protein (IGRP) Journal of Biological Chemistry. 2004;279:13976–13983. doi: 10.1074/jbc.M307756200. [DOI] [PubMed] [Google Scholar]
- 20.Bae J.S., Kim T.H., Kim M.Y., Park J.M., Ahn Y.H. Transcriptional regulation of glucose sensors in pancreatic beta-cells and liver: an update. Sensors. 2010;10(5):5031–5053. doi: 10.3390/s100505031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedersen K.B., Zhang P., Doumen C., Charbonnet M., Lu D., Newgard C.B. The promoter for the gene encoding the catalytic subunit of rat glucose-6-phosphatase contains two distinct glucose-responsive regions. American Journal of Physiology. Endocrinology and Metabolism. 2007;292(3):E788–E801. doi: 10.1152/ajpendo.00510.2006. [DOI] [PubMed] [Google Scholar]
- 22.De Leon E.R., Brinkman J.A., Fenske R.J., Gregg T., Schmidt B.A., Sherman D.S. Age-dependent protection of insulin secretion in diet induced obese mice. Scientific Reports. 2018;8(1):17814. doi: 10.1038/s41598-018-36289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boortz K.A., Syring K.E., Pound L.D., Mo H., Bastarache L., Oeser J.K. Effects of G6pc2 deletion on body weight and cholesterol in mice. Journal of Molecular Endocrinology. 2017;58(3):127–139. doi: 10.1530/JME-16-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ackermann A.M., Gannon M. Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. Journal of Molecular Endocrinology. 2007;38(1–2):193–206. doi: 10.1677/JME-06-0053. [DOI] [PubMed] [Google Scholar]
- 25.Coutinho M., Gerstein H.C., Wang Y., Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22(2):233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 26.DECODE Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care. 2003;26(3):688–696. doi: 10.2337/diacare.26.3.688. [DOI] [PubMed] [Google Scholar]
- 27.Lawes C.M., Parag V., Bennett D.A., Suh I., Lam T.H., Whitlock G. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care. 2004;27(12):2836–2842. doi: 10.2337/diacare.27.12.2836. [DOI] [PubMed] [Google Scholar]
- 28.Khaw K.T., Wareham N., Luben R., Bingham S., Oakes S., Welch A. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of european prospective investigation of cancer and nutrition (EPIC-Norfolk) BMJ. 2001;322(7277):15–18. doi: 10.1136/bmj.322.7277.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Group H.S.C.R., Metzger B.E., Lowe L.P., Dyer A.R., Trimble E.R., Chaovarindr U. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 30.Li X., Shu Y.H., Xiang A.H., Trigo E., Kuusisto J., Hartiala J. Additive effects of genetic variation in Gck and G6pc2 on insulin secretion and fasting glucose. Diabetes. 2009;58:2946–2953. doi: 10.2337/db09-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]