Highlights
-
•
EGIST in rectovaginal septum with unusual presentation (excessive vaginal bleeding).
-
•
EGIST was misdiagnosed by MRI as vaginal leiomyoma.
-
•
The first report of untreated EGIST associated with acute arterial occlusion.
Keywords: Abdominoperineal resection, Arterial occlusion, Bleeding, EGIST, Rectovaginal septum
Abstract
Extragastrointestinal stromal tumors (EGISTs) arise from atypical sites, such as the omentum, mesentery, retroperitoneal space, urinary bladder, or rectovaginal septum, and account for fewer than 10% of gastrointestinal stromal tumors (GISTs). Most EGISTs are asymptomatic at the time of diagnosis, due to the fact that they rarely cause symptoms until they grow to greater than 10 cm in diameter. Common presenting symptoms are a feeling of vaginal fullness and increased urinary frequency. Cases described in previous reports have been treated with surgery with or without targeted therapy. Here we report an unusual case of an EGIST at the rectovaginal septum presenting with excessive vaginal bleeding and acute arterial occlusion. This rectovaginal mass was successfully removed using the abdominoperineal approach and did not require targeted therapy.
1. Introduction
Gastrointestinal stromal tumors (GISTs) are common mesenchymal tumors that normally originate from the gastrointestinal (GI) tract, especially the stomach and intestine. Extragastrointestinal stromal tumors (EGISTs) which originate from outside the GI tract account for fewer than 10% of GISTs. It is even rarer for these tumors to occur in the vagina and rectovaginal septum, with only 22 cases having been reported (Cheng et al., 2019). Extragastrointestinal stromal tumors in the female reproductive tract have exhibited a wide variation of clinical presentations depending on size and location of the tumor, such as a sensation of being dragged down, constipation, dyspareunia, and vaginal bleeding (Hanayneh et al., 2018). Due to its rarity, this disease is often misdiagnosed as other conditions that originate from the uterine cervix such as cervical leiomyoma. Surgical removal is usually performed using a vaginal approach. We report a case of an EGIST in the rectovaginal septum originally diagnosed as cervical leiomyoma and successfully removed by abdominoperineal resection.
2. Case presentation
A 43-year-old woman, gravida 4, para 4, presented to our gynecological outpatient department in October 2016 after having had a protruded painless vaginal mass 5 cm in diameter without abnormal vaginal bleeding or abnormal urination for nine years. Core needle biopsy was performed and immunohistochemical studies revealed a spindle cell tumor that was strongly positive for CD34 but negative for desmin and S-100. She was lost to follow-up until April 2019, when she presented with excessive vaginal bleeding and acute left leg pain. Her hematocrit was 14%. She received 4 units of packed red cells. Computed tomographic angiography (CTA) of the lower extremities showed no contrast opacity at the left femoral or left popliteal arteries. She was diagnosed with superficial femoral artery occlusion and underwent immediate surgical embolectomy. After surgery, she received oral warfarin at 15 mg daily in order to prevent recurrence. Pervaginal examination revealed that the posterior vagina had an irregular surface with blood oozing and a mass approximately 10 cm in diameter at the rectovaginal septum. The lower edge of the mass was located at 2 cm above the hymen, which had a soft to firm consistency, was not tender, and was fixed. The cervix, uterus, and adnexa could not be evaluated. Rectovaginal examination revealed an extraluminal nodular surface mass at the anterior wall of the rectum.
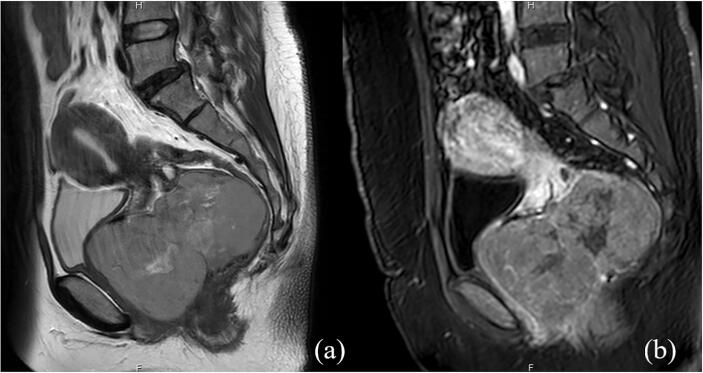
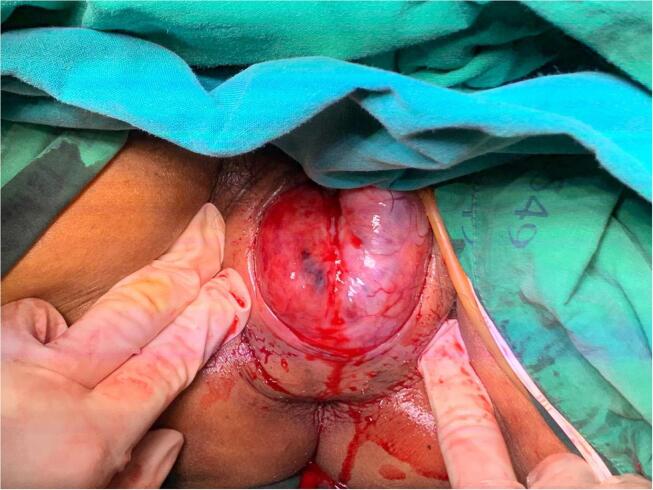
She underwent computed tomography (CT) of the whole abdomen, which showed a large enhancing mass in the vaginal pouch that extended to the cervix. Magnetic resonance imaging (MRI) of the lower abdomen was subsequently performed and revealed a vaginal mass measuring 11.2 × 7.6 × 7.8 cm with whirlpool-like heterogenous enhancement and an epicenter located within the vaginal canal. The mass was hyperintense on T2-weighted images, which indicated degeneration. This mass caused pressure and abutment to the urethra, posterior wall of the urinary bladder, and rectum without adjacent organ invasion (Fig. 1). The provisional diagnosis was cervical leiomyoma. Chest imaging was not performed because malignancy was not suspected. Since she had no desire for further pregnancy, we decided to perform a total abdominal hysterectomy (TAH) after obtaining informed consent. We discontinued warfarin administration for five days, bridging the warfarin with 1 mg/kg of enoxaparin every 12 h, and performed the procedure after enoxaparin administration had been discontinued for 12 h. Upon laparotomy, we noted the normal size of the uterus. However, after the uterus was removed, we found that there was no connection between the uterus and the vaginal mass. The mass was accessed through dissection of the area behind the vaginal cuff through the rectovaginal space and separated from the adjacent organs. The anterior wall of the rectum was then separated from the mass. Palpation revealed a well-defined, firm mass attached to the posterior vaginal wall (Fig. 2). The tumor was enucleated under the effort from intra-abdomen that gently pushing the mass down into vagina. Next, the posterior vaginal wall was opened at the midline from the posterior fourchette to the posterior vaginal vault. Electrocautery and blunt dissection were performed to completely separate the vaginal epithelium from the vaginal mass, followed by excision of the vaginal mass from the underlying rectovaginal septum without trauma to the rectal sphincter or rectal serosa. The vaginal wall was closed with interrupted 2–0 Vicryl. There were no complications related to the surgery. Enoxaparin administration was resumed 24 h after surgery at a dosage of 60 mg subcutaneously every 12 h until discharge.
Fig. 1.
Preoperative pelvic MRI (a) Sagittal T2-weighted MR images revealed an abnormally large vaginal mass with internal degeneration exhibiting homogeneous isosignal intensity with internal hypersignal intensity. (b) Contrast enhanced MR images showed whirl-pool like heterogeneous enhancement.
Fig. 2.
Intraoperative photo demonstrating an abnormally large mass located at the posterior vaginal wall.
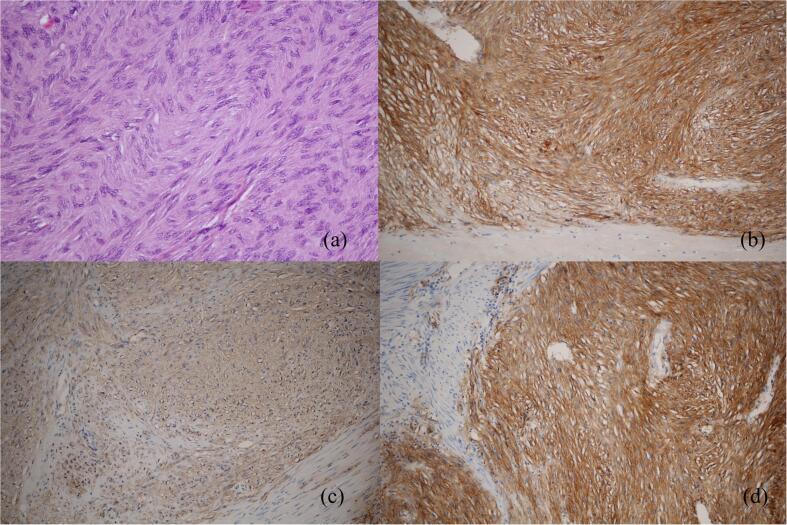
Gross examination revealed a 11.5 × 7 × 6 cm mass with rubbery tissue in the center on cut section. The pathological report revealed a well-circumscribed bland spindle cell lesion with moderate cellularity arranged in an intersecting fascicular pattern with prominent stromal hyalinization. The cells were spindle-shaped with a moderate amount of eosinophilic cytoplasm, spindle nuclei with blunt ends, dispersed chromatin, inconspicuous nucleoli, and mild pleomorphism. The mitotic count was 0–1/ 5 mm2. The tumor cells were strongly positive for CD117, CD34, DOG1, vimentin, and caldesmon, but negative for smooth muscle actin (SMA), desmin and S-100 (Fig. 3).
Fig. 3.
Histologic sections (a) Hematoxylin & Eosin stain showing spindle cell tumor. (b) Microscopic aspect (×100) showing the positive reaction to CD117. (c) Microscopic aspect (×100) showing the positive reaction to CD34. (d) Microscopic aspect (×100) showing the positive reaction to DOG1.
The patient did not receive any postoperative targeted therapy due to personal economic constraints. In November 2019, the patient discontinued anticoagulant drug treatment after CTA evaluation. In April 2020 (11 months post operation), the patient had no recurrent mass, abnormal vaginal bleeding, or left leg pain.
3. Discussion
Rectovaginal EGISTs are very rare and potentially malignant. They have been reported in women from 15 to 80 years of age, with a mean age of around 56 years (Cheng et al., 2019). Clinical presentations vary depending on tumor size and location. The most common presenting symptom is awareness of a mass in the vagina. Vaginal EGISTs can be asymptomatic or may have primary symptoms of bladder outlet obstruction or vaginal bleeding (Ceballos et al., 2004, Hanayneh et al., 2018, Weppler and Gaertner, 2005). Severe constipation and rectal mucosal involvement have also been reported to be associated with GISTs involving the rectovaginal septum (Nasu et al., 2004, Zhang et al., 2009). In our case, the severe bleeding may have been caused by ulceration and necrosis of the overlying vaginal epithelium.
There is a significantly greater amount of data available on venous thromboembolism (VTE) than on arterial thrombosis in cancer patients. This is because VTE is a common condition in patients with any active cancer, but the association between GISTs and venous thrombosis remains unclear. A previous review reported only five cases in which patients diagnosed with GISTs were found to have VTE (Galeano-Valle et al., 2020).
Thrombosis has been observed in the arteries of cancer patients, and the risk of cancer-related arterial thromboembolism (ATE) has been shown to vary over the course of disease (De Stefano, 2018). For instance, the rate of ATE is highest during the first twelve months after cancer confirmation and subsequently gradually declines (Aronson and Brenner, 2018, Navi et al., 2017). The most common cancers associated with arterial thromboembolic events (in descending order) are lung, colorectum, prostate, breast, non-Hodgkin lymphoma, pancreas, stomach, and uterus (Navi et al., 2017). The EGIST patient in this report is the first reported to subsequently develop acute arterial occlusion.
Accurate diagnosis of EGISTs remains a challenge due to their rarity and non-specific symptoms, which often lead to them being overlooked by physicians. Preoperative imaging may help determine whether an EGIST is benign or malignant prior to surgery, allowing for more effective treatment planning. Magnetic resonance imaging is the most accurate imaging technique for delineating the anatomy of the pelvic organs. Extragastrointestinal stromal tumors appear as intermediate signal intensity in both T1- and T2-weighted images (WI) with homogeneous enhancement, with central hyperintensity on T2WI reflecting cystic degeneration or necrosis (Vázquez et al., 2012). Although these findings are suggestive of EGISTs, diagnostic imaging is unable to distinguish this condition from sarcoma. Histopathology remains the gold standard for differentiation between the two conditions.
Similar to their GI tract counterparts, these tumors exhibit spindle cell morphology and expression of CD117 and CD34 (Akahoshi et al., 2018). However, definitive diagnosis of GISTs could not be made based solely on the presence of CD117, given that other spindle cell tumor also express strong positivity for CD117 (Novelli et al., 2010). Immunohistochemistry with a panel of antibodies including CD117, DOG1, CD34, SMA, S-100, and desmin is beneficial in the diagnosis of GISTs (Akahoshi et al., 2018, West et al., 2004). Leiomyomas and leiomyosarcomas are diagnosed when immunohistochemical analysis demonstrate the tumor to be positive for smooth muscle actin and desmin and negative for CD117 and s-100 (Akahoshi et al., 2018, Lam et al., 2006).
Surgery is the treatment of choice for localized GISTs (Akahoshi et al., 2018). In most of the reported, excision of the mass through the vaginal route was successful. However, when an EGIST is abnormally large (as our case) the abdomino-perineal approach may be more feasible. This technique has been reported to be safe in difficult cases of complete rectal resection in cancer patients (Abou-Zeid et al., 2015) and may offer an advantage in cases in which there is a large mass pressing against the rectum. Having two surgeons operate simultaneously allows for better access to the mass and safer dissection of the bowel from the mass. Laparoscopic surgery is also an option if performed under the supervision of an experienced surgeon. Since 10–30% of GISTs have malignant potential, recurrence is a concern. Routine follow-up gynecologic examination is warranted (Lam et al., 2006). Targeted therapy such as imatinib has been administered postoperatively in some previously reported cases (Hanayneh et al., 2018). A randomized controlled trial showed that adjuvant therapy was able to prolong recurrence-free survival, with no recurrence found at 5 years. However, evidence regarding the clinical benefits of adjuvant therapy in vaginal EGISTs is lacking. When considering whether or not to prescribe adjuvant therapy, benefits and side effects such as nausea/ vomiting, edema, diarrhea, skin rashes, and fever should be taken into account (Etherington and DeMatteo, 2019).
4. Conclusion
This unusual case, in which an EGIST presented as a vaginal mass and acute arterial occlusion, emphasizes the challenge of rare disease diagnosis. Some associated symptoms may give clues to whether or not the condition is malignant. Using the appropriate approach when excising the mass plays an important role in the success of the operation.
CRediT authorship contribution statement
Jen Sothornwit: Conceptualization, Writing - original draft. Teerayut Temtanakitpaisan: Supervision. Apiwat Aue-aungkul: Supervision, Writing - review & editing. Naratassapol Likitdee: Data curation. Pilaiwan Kleebkaow: Investigation, Validation.
Acknowledgments
Acknowledgements
We would like to thank Dylan Southard for editing the manuscript via the Khon Kaen University Publication Clinic (Thailand).
Disclosure
The authors declare they have no conflict of interest regarding the publication of this case report.
Patient Consent
Consent was obtained from the patient for publication of this case report and accompanying images.
References
- Abou-Zeid A.A., Ghamrini Y.E., Youssef T. The combined abdominal and perineal approach for dissection of the lower rectum. The development of new indications. Int. J. Surg. 2015;13:217–220. doi: 10.1016/j.ijsu.2014.11.046. [DOI] [PubMed] [Google Scholar]
- Akahoshi K., Oya M., Koga T., Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J. Gastroenterol. 2018;24:2806–2817. doi: 10.3748/wjg.v24.i26.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson D., Brenner B. Arterial thrombosis and cancer. Thromb. Res. 2018;164(Suppl 1):S23–S28. doi: 10.1016/j.thromres.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Ceballos K.M., Francis J.-A., Mazurka J.L. Gastrointestinal stromal tumor presenting as a recurrent vaginal mass. Arch. Pathol. Lab. Med. 2004;128:1442–1444. doi: 10.1043/1543-2165(2004)128<1442:GSTPAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Cheng M., Liu C.-H., Horng H.-C., Chen Y.-J., Lo P.-F., Lee W.-L., Wang P.-H. Gastrointestinal stromal tumor presenting as a rectovaginal septal mass: A case report and review of literature. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000015398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano V. Arterial thrombosis and cancer: the neglected side of the coin of Trousseau syndrome. Haematologica. 2018;103:1419–1421. doi: 10.3324/haematol.2018.197814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherington M.S., DeMatteo R.P. Tailored management of primary gastrointestinal stromal tumors. Cancer. 2019;125:2164–2171. doi: 10.1002/cncr.32067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeano-Valle F., Del-Toro-Cervera J., Demelo-Rodriguez P. Venous thromboembolism and gastrointestinal stromal tumour: A rare association. Mol. Clin. Oncol. 2020;12:57–59. doi: 10.3892/mco.2019.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanayneh W., Starr J., George T.J., Parekh H. Extragastrointestinal stromal tumors of the pelvic cavity and the vagina: Two case reports and review of the literature. Gynecol. Oncol. Rep. 2018;25:3–7. doi: 10.1016/j.gore.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M.M., Corless C.L., Goldblum J.R., Heinrich M.C., Downs-Kelly E., Rubin B.P. Extragastrointestinal stromal tumors presenting as vulvovaginal/rectovaginal septal masses: a diagnostic pitfall. Int. J. Gynecol. Pathol. 2006;25:288–292. doi: 10.1097/01.pgp.0000215291.22867.18. [DOI] [PubMed] [Google Scholar]
- Nasu K., Ueda T., Kai S., Anai H., Kimura Y., Yokoyama S., Miyakawa I. Gastrointestinal stromal tumor arising in the rectovaginal septum. Int. J. Gynecol. Cancer. 2004;14:373–377. doi: 10.1111/j.1048-891x.2004.014230.x. [DOI] [PubMed] [Google Scholar]
- Navi B.B., Reiner A.S., Kamel H., Iadecola C., Okin P.M., Elkind M.S.V., Panageas K.S., DeAngelis L.M. Risk of Arterial Thromboembolism in Patients With Cancer. J. Am. Coll. Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli M., Rossi S., Rodriguez-Justo M., Taniere P., Seddon B., Toffolatti L., Sartor C., Hogendoorn P.C.W., Sciot R., Van Glabbeke M., Verweij J., Blay J.Y., Hohenberger P., Flanagan A., Dei Tos A.P. DOG1 and CD117 are the antibodies of choice in the diagnosis of gastrointestinal stromal tumours. Histopathology. 2010;57:259–270. doi: 10.1111/j.1365-2559.2010.03624.x. [DOI] [PubMed] [Google Scholar]
- Vázquez J., Pérez-Peña M., González B., Sánchez A. Gastrointestinal stromal tumor arising in the rectovaginal septum. J. Low Genit. Tract. Dis. 2012;16:158–161. doi: 10.1097/LGT.0b013e31823b52af. [DOI] [PubMed] [Google Scholar]
- Weppler E.H., Gaertner E.M. Malignant extragastrointestinal stromal tumor presenting as a vaginal mass: report of an unusual case with literature review. Int. J. Gynecol. Cancer. 2005;15:1169–1172. doi: 10.1111/j.1525-1438.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- West R.B., Corless C.L., Chen X., Rubin B.P., Subramanian S., Montgomery K., Zhu S., Ball C.A., Nielsen T.O., Patel R., Goldblum J.R., Brown P.O., Heinrich M.C., van de Rijn M. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am. J. Pathol. 2004;165:107–113. doi: 10.1016/S0002-9440(10)63279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Peng Z., Xu L. Extragastrointestinal stromal tumor arising in the rectovaginal septum: report of an unusual case with literature review. Gynecol. Oncol. 2009;113:399–401. doi: 10.1016/j.ygyno.2009.02.019. [DOI] [PubMed] [Google Scholar]