Abstract
Background
Magnetic Resonance(MR) guided percutaneous procedures(MRgVABB) have been developed and largely employed to reduce the need of surgical biopsies for suspicious lesions which can be detected only by MR(MR-only lesion). The present study aims to investigate correlation between imaging, histological features of MRgVABB and surgical specimens of MR-only lesions.
Methods
We retrospectively enrolled 56 patients with a total of 61 lesions. Each finding was defined as Mass-Enhancement(ME) or Non-ME(NME) and classified according to BI-RADS. MRgVABB and surgical data were collected. Concordance between MR, MRgVABB and open biopsy was calculated. Underestimation Rate(UR) of MRgVABB with surgery was obtained.
Results
B2 and B5b lesions were statistically associated with NME and ME, respectively. No statistical association was found to B3 nor to B5a with radiological features. UR was 10 %; underestimated lesions were strongly associated with the presence of a ME on MR imaging. Moreover, B3 lesions are associated with higher UR.
Conclusion
Radiological features should influence patient management aiming to construct a correct diagnostic and therapeutic plan. When MR is prescribed for breast cancer staging for ME-MR-only lesions, we suggest surgical open biopsy instead of MRgVABB when upfront surgery is the treatment of choice.
Keywords: MRgVABB, Magnetic resonance, Imaging, Percutaneous biopsy, Breast cancer, Underestimation rate
1. Introduction
Magnetic resonance(MR) imaging has a paramount role in the management of breast cancer patients thanks to its great diagnostic sensitivity, mainly in cases of dense breast tissue [1,2]. In particular, MR improves detection of malignant lesions not identified by mammography(MMG) or Ultrasonography(US) [3,4]. With MR, in view of its higher sensitivity, it is possible to detect up to 20 % more malignant lesions compared with conventional imaging [[5], [6], [7]]. False-positive results represent a disadvantage of MR. Therefore, enhanced lesions with suspicious features should undergo to percutaneous biopsy with US-guided or MMG-guided procedures, to ensure accurate patient and disease management. In recent years, new MR-guided percutaneous procedures have been developed and employed worldwide to prevent unnecessary surgical biopsies, which carry associated morbidity risks, when the lesion cannot be reproduced on US or MMG [8,9]. According to the guidelines of American Cancer Society(ACR) and European Society of Breast Imaging(EUSOBI) MR-guided biopsy should be performed for suspicious lesions which can be detected only by MR(MR-only lesions) [10,11]. Although anesthesia and surgical techniques are aimed to reduce the surgery’s impact on patients(e.g. ERAS protocols) [[12], [13], [14], [15], [16]], several publications demonstrate how MR-guided vacuum-assisted breast biopsy(MRgVABB) is a safe and accurate technique for evaluation of MR-only breast lesions [6,8,10,11,[17], [18], [19], [20], [21]]. Nevertheless, there's a lack of evidence proving imaging predictive features of MR-only lesions according to existing literature [18,21,22].
The present study aims to investigate the correlation between imaging and histological features of all MR-guided breast biopsies performed at our institution. Another objective was to assess the diagnosis frequency of high-risk lesions through MRgVABB, and its underestimation rate(UR). Moreover, when surgery was performed, histology of surgical resection was compared with MRgVABB sample characteristics and with MR image in order to identify any factors that may predict an upgrading of lesions. Both types of evidence could be used to optimize diagnostic-therapeutic management for breast cancer loco-regional staging patients who are already candidates for surgery.
2. Materials and methods
This research was designed as retrospective monocentric study. Institutional Review Board of Policlinico Tor Vergata Foundation waived the need for a formal approval considering the retrospective design of the study. The study included 111 new MR findings in 103 patients who went through breast imaging evaluation in Tor Vergata University Hospital from July 2016 to November 2018. Main eligibility criteria were the presence of new lesion detected by MR and subsequent clinical management in our facilities. Exclusion Criteria were pregnancy and absence of complete Breast Study available in Tor Vergata Hospital PACS system. Abnormal MR findings were first investigated with “second-look” US.
As reported in literature, up to 57 % of lesions originally visible only on MR can be US correlated [23]. MR-guided procedures were restricted to MR-only lesions, according to published guidelines. 45 of these cases were excluded from study as breast MR findings were also demonstrated with US and were referred to US-guided VABB [9].
Although 66 lesions were indicated for MRgVABB, 5 patients did not perform the biopsy. In particular, two patients exhibited vanishing target phenomenon: MR-only lesion was no longer present at the time of VABB procedure [24], one other patient was excluded from procedure due to the MR-only lesion localization, and another due to inadequate thickness of breast under compression. In one case MRgVABB was interrupted on account of early bleeding, despite the lesions had already been targeted.
According to the primary aim of the study, we analyzed only patients who underwent MRgVABB and 61 breast findings from 56 patients were analyzed (mean age 53 years, range: 22–78).
Breast MR indications were classified according to the European Society of Breast Cancer Specialists(EUSOMA) working group recommendations, into five categories: Staging of biopsy-proven breast cancer(23;59,01 %), Evaluation of breast cancer recurrence(9;14,75 %), Assessment of unknown primary breast cancer(1;1,64 %), Screening of women at high risk of breast cancer(7;11,48 %), Characterization of equivocal findings with conventional imaging(21;34,43 %).
Among the 23 patients who underwent MR for preoperative breast cancer staging, ipsilateral MRgVABB was performed in 10 cases(43,48 %) and contralaterally to the index cancer in 13 cases(56,52 %).
All MRgVABBs were performed using a 1.5 TMR System(Philips Intera Achieva, Best, the Netherlands) equipped with a dedicated surface coil(Open Breast Array Coil, Invivo).
The biopsy system and the coil allowed only lateral approach to the breast. Biopsies were performed through a freehand approach by a radiologist with 10 years of experience in performing breast interventions. The patient was positioned prone. Compression plates immobilized breast allowing a lateral-only access to the breast through a grid localization system. A vitamin E capsule was taped in a cell grid near the expected lesion site as a fiducial marker.
MR exam included a T1-weighted(T1W) axial sequence(1.5-mm in-plane resolution) and sagittal sequence(2-mm in-plane resolution) that were performed before and after i.v. injection of 0.15 mmol/kg of gadobenate dimeglumine(Gd-BOPTA-Multihance; Bracco Imaging, Milan, Italy) and a 20-mL saline infusion. A subtraction of the unenhanced images was then performed. After reviewing the axial and sagittal images on the console, a cursor was placed over the lesion, and the distance between the cursor and the fiducial marker was calculated along three axes(horizontal, vertical, and depth coordinates, respectively). The lesion coordinates and guidance for needle positioning were then manually obtained based on the spatial relationship between the lesion, the fiducial marker, and grid lines. Local anesthesia(lidocaine 1%) was then administered following disinfection. A small incision was made in the skin and the introducer was inserted into the breast. If the correct correlation between the introducer and the lesion was confirmed by the imaging, the introducer was removed and replaced by the VABB device through which biopsies were collected. MRgVABB were performed with 8 or 11-gauge VABB device(Mammotome®, Roma, Italy). The median number of VABB specimens was 16,13(range 12–24). The median time required to perform MR-guided biopsies was 35 min(range 21–63 min). Following the sampling, the operator released an MR-compatible titanium clip(Mammotome®, Roma, Italy).
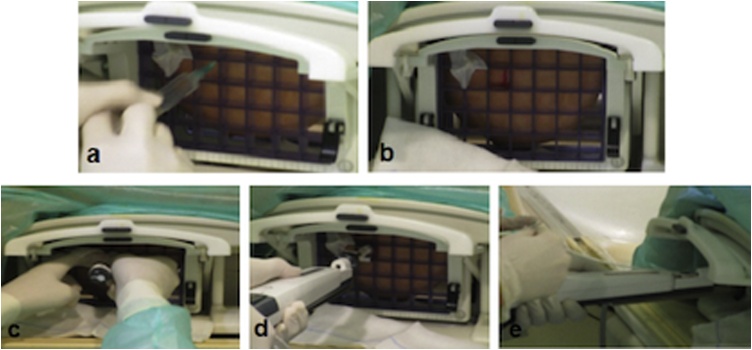
The technical sampling steps after lesion targeting are displayed in Fig. 1. Thereafter, biopsy site was compressed manually for at least 10 min beyond complete hemostasis. Sterile adhesives were then placed on the puncture site, antibiotics and cryotherapy was administered. After 2 weeks, cranio-caudal and medio-lateral MMG projections were acquired (Fig. 2).
Fig. 1.
Figure shows technical samplings steps in chronological order: local anaesthesia administration(a), incision of the skin(b), introducer insertion(c), VABB device insertion(d), clip insertion after specimens’ collection(e).
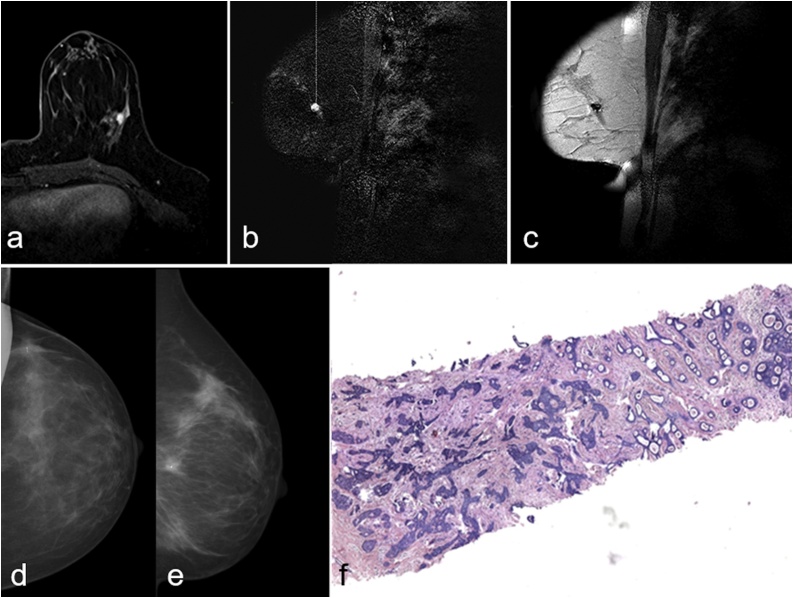
Fig. 2.
Figure shows a case of 54 years old woman with suspicious mass enhancement in the upper outer quadrant of the left breast classified as BI-RADS 4(a) undergone MRgVABB(b, c). Cranio-caudal(d) and medio-lateral(e) mammography projections acquired after 2 weeks show procedure effects and the presence of the metal clip. The histological examination resulted in invasive ductal carcinoma B5b(f).
Two radiologists with more than 10 and 5 years of experience in breast MR imaging, retrospectively reviewed all pre-biopsy MR images with knowledge of all preoperative data and the complete imaging, lacking information of the pathological or follow-up data. All the MR images were reviewed on a Picture Archiving and Communication System(PACS) workstation(Carestream, Genova, Italy). Axial T2 and T1 weighted images before injection were reviewed as well as an axial 3D T1 weighted DCE MR imaging(with a maximal slice thickness of 3 mm), with reformatted images in sagittal or coronal planes, as necessary. According to the ACR Breast Imaging and Reporting Data System(BI-RADS) MR lexicon [25], each MR lesion was categorized as mass enhancement(ME) or non-mass enhancement(NME). A final BI-RADS assessment category from 3 to 5 was assigned. All BI-RADS RM 4 and 5 need histological examination due to high risk of malignancy; BI-RADS RM 3 score patients were referred for MRgVABB due to clinical context and patients anxiety.
Histopathological results were classified into five diagnostic categories(B1-B5), according to the fourth edition of the European guidelines for quality assurance in breast cancer screening and diagnosis [26]. We compared MR features of the target lesion and histological outcomes of MRgVABB.
According to our internal guidelines, MR follow-up (6 months) is routinely recommended for all benign findings (B2), lesions classified as B3, B4 or B5 were assigned to subsequent surgical excision.
MRgVABB histopathological findings were compared to the final surgical findings in order to determine the UR. UR was calculated as the ratio between the number of upgraded lesions on surgical specimen and the number of total diagnosis with/by MRgVABB. Particularly, UR for atypical ductal hyperplasia(ADH) was defined when ADH lesions were diagnosed by MRgVABB and when ductal carcinoma in situ(DCIS) or invasive carcinoma were diagnosed by surgery, divided by the number of ADH diagnosed with MRgVABB. Moreover, underestimation for DCIS was determined when it was diagnosed by MRgVABB while invasive carcinoma was diagnosed by surgery, divided by the number of DCIS diagnosed with MRgVABB.
All data were recorded onto an EXCEL database(Microsoft, Redmond, Washington, United States). Continuous variables were reported as medians and ranges. Dummy variables were reported as numbers and percentages: Chi-squared and Fisher’s exact tests for statistical significance were performed. Variables with a P value <0.05 were considered statistically significant. SPSS statistical package version 23.0 was used(SPSS Inc., Chicago, IL).
3. Results
MRgVABB have been technically successful for all selected lesions. The only complication consisted of small hemorrhages requiring prolonged manual compression and took place in 9 patients (15 %). During surgeries, presence of the prior biopsy site was confirmed by pathologic analysis of surgical specimen in all cases.
According to retrospective analysis, MR-only lesions were classified as BI-RADS MR 4 lesions among 44 of the 61 cases and BI-RADS MR 5 lesions among 8 of the 61 cases. In 9 out of 61 cases MR-only lesions were classified as BI-RADS MR 3 lesions. In BI-RADS MR 3 lesions retrospective analysis of clinical notes demonstrated same BI-RADS RM 3 score, patients were referred for MRgVABB due to clinical context in 5 cases and according to patient’s choice in 4 cases.
As shown in Table 1, among the 61 analyzed findings, MRgVABB histological results were B2 in 35 cases(57,3 %), 10 of which were MEs(28,6 %) and 25 NMEs(71,4 %); B3 in 13 cases(21,3 %) of which 8 ME(61,5 %) and 5 NME(38,5 %); B5a in 8 cases(13,1 %) of which 2 ME(25 %) and 6 NME(75 %), B5b in 5 cases(8,2 %) of which 4 ME(80 %) and 1 NME(20 %).
Table 1.
Correlation between Magnetic Resonance features and pathologic classification of breast lesions diagnosed by Magnetic Resonance guided Vacuum Assisted Biopsy(n = 61). ME: Mass Enhancement; NME: Non-Mass Enhancement.
| B2 |
B3 |
B5a |
B5b |
|||||
|---|---|---|---|---|---|---|---|---|
| N° | (%) | N° | (%) | N° | (%) | N° | (%) | |
| ME | 10/24 | 41,6 % | 8/24 | 33,3 % | 2/24 | 8,3 % | 4/24 | 16,6 % |
| NME | 25/37 | 67,5 % | 5/37 | 13,5 % | 6/37 | 16,2 % | 1/37 | 2,7 % |
| Total | 35/61 | 57,3 % | 13/61 | 21,3 % | 8/61 | 13,1 % | 5/61 | 8,2 % |
ME were 24/61, of which 10/24(41,6 %) B2, 8/24(33,3 %) B3, 2/24(8,3 %) B5a and 4/24(16,6 %) B5b. NME were 37/61, of which 25/37(67,5 %) B2, 5/37(13,5 %) B3, 6/37(16,2 %) B5a and 1/37(2,7 %) B5b, as summarized in Table 2.
Table 2.
Pathologic classification and histological types of breast lesions diagnosed by Magnetic Resonance guided Vacuum Assisted Biopsy and corresponding Magnetic Resonance features(n = 61).
| Pathologic classification | N° of lesions(%) | Magnetic Resonance features |
|
|---|---|---|---|
| N° ME(%) | N° NME(%) | ||
| Benign lesions(B2) | 35(57.4) | 10(28.6) | 25(71.4) |
| Ductal epithelial hyperplasia | 26(42.6) | 7(26.9) | 19(73.1) |
| Sclerosing adenosis | 9(14.7) | 3(33.3) | 6(66.7) |
| High risk lesions(B3) | 13(21.3) | 8(61.5) | 5(38.5) |
| Papillary lesion | 1(1.6) | 1(100) | 0(0) |
| Atypical ductal hyperplasia | 6(9.8) | 2(33.3) | 4(66.7) |
| Lobular intraepithelial neoplasia | 2(3.3) | 2(100) | 0(0) |
| Flat epithelial atypia | 4(6.5) | 3(75.0) | 1(25.0) |
| Malignant lesions(B5) | 13(21.3) | 6(46.1) | 7(53.8) |
| Ductal Carcinoma in situ | 8(13.1) | 2(25.0) | 6(75.0) |
| Invasive carcinoma | 5(8.2) | 4(80.0) | 1(20.0) |
Among the 35 lesions classified as B2, 100 % were stable or showed decreased contrast enhancement at 6-months MR follow-ups confirming benign outcomes in all cases. These patients were addressed to periodic conventional breast imaging.
Among the 23 patients who underwent MR for preoperative breast cancer staging, 14 underwent conservative surgery (Fig. 3) and 9 underwent mastectomy (Fig. 4).
Fig. 3.
Figure shows a case of a 43 years old woman with invasive ductal carcinoma in the upper outer quadrant of the right breast classified as BI-RADS 6(a). During staging Magnetic Resonance another suspicious mass enhancement in the inner upper quadrant of the right breast was found, classified as BI-RADS 4(b) and underwent MRgVABB(c, d). Cranio-caudal(e) and medio-lateral(f) mammography projections acquired after 2 weeks show procedure effects and the presence of the metal clip. The histological examination resulted in ductal epithelial hyperplasia B2(g).
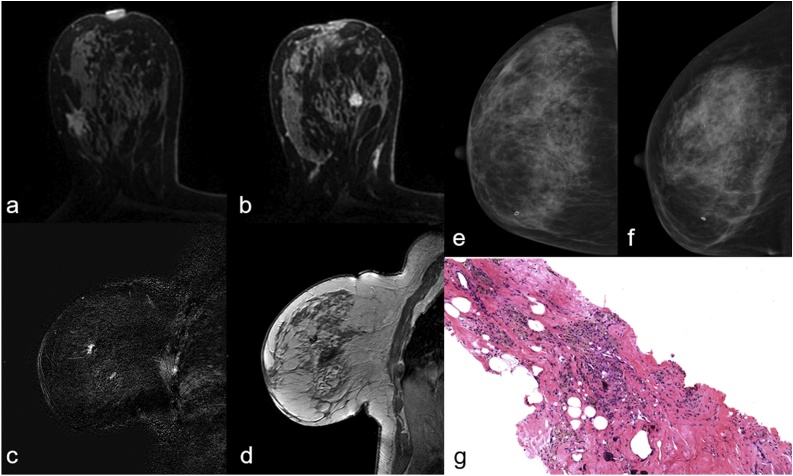
Fig. 4.
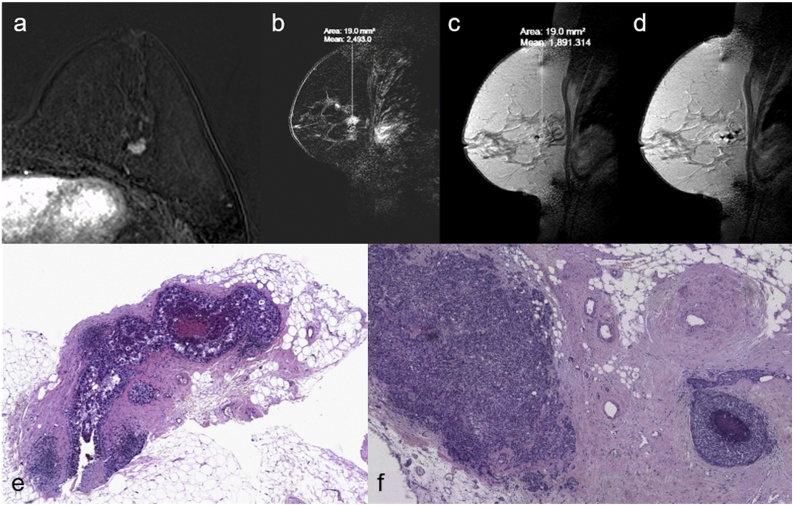
Figure shows a case of 47 years old woman with invasive lobular carcinoma classified as BI-RADS 6 in the right breast and a suspicious mass enhancement classified as BI-RADS 4 in left breast, evident on axial Maximum Intensity Projection(a). MRgVABB was performed on the new detected lesion with a histological diagnosis of invasive ductal carcinoma B5b(b).
Whereas B3 and B5a lesions were equally divided into ME and NME, a significant statistical difference was found between the distributions of B2 lesions as NME(p = 0.043) and B5b as ME(p = 0.046). Statistical evaluation underlined statistically significant association between NME and B2 lesions(p = 0.046; OR .342). Statistically non-significant associations were reported between B3 and B5a lesions to ME or NME, regardless of BI-RADS 3, 4 and 5 score.
Definitive surgical histological examination has confirmed in 55 out of 61 cases(90 %) MRgVABB histological outcomes with an underestimation in 6 cases(UR = 10 %). Underestimated cases belonged to ME in 5 out of 6(83,3 %) and in 1 case(16,6 %) to NME. Moreover, in 5 out of 6 underestimated cases ADH lesions were diagnosed by MRgVABB and DCIS was diagnosed by surgery, in 1 case DCIS was diagnosed by MRgVABB and invasive carcinoma by surgery (Fig. 5). On the other hand underestimation rate and BIRADS score at MR have no significant association.
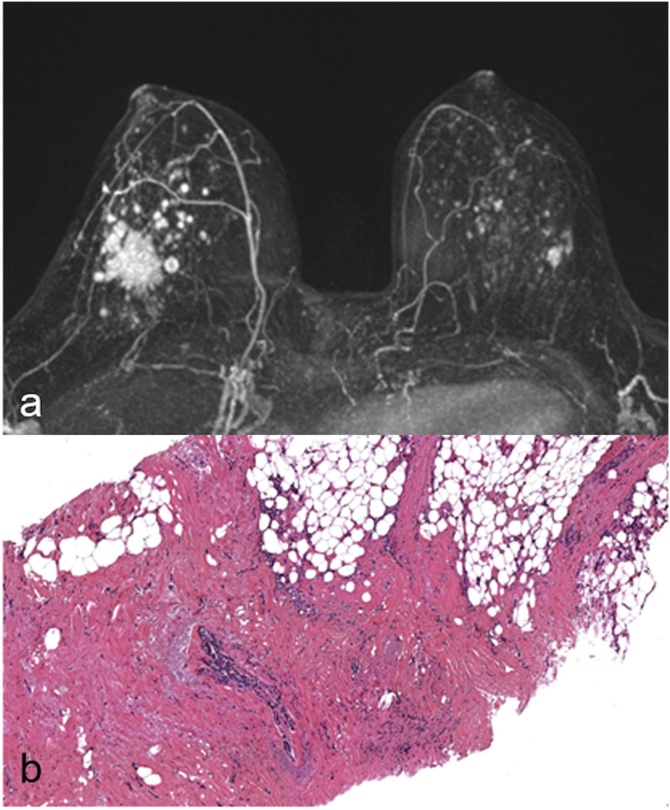
Fig. 5.
Figure shows a case of 47 years old woman with suspicious mass enhancement in the left breast classified as BI-RADS 4(a) undergone MRgVABB(b–d) with a histological diagnosis of ductal carcinoma in situ B5a(e). This biopsy underestimated the lesion, in fact final surgical resection resulted invasive ductal carcinoma B5b(f).
The UR was statistically higher for B3 lesions. Indeed, 5 out of 13 cases (38 %) of B3 lesions were underestimated. The UR of surgery was statistically higher for B3 lesions (38 %), specifically, the underestimated lesions were strongly associated with the presence of an ME on MR imaging (p = 0.003).
4. Discussion
MRgVABB, introduced in 1999 [27], is a safe and accurate technique for histological clarification of suspicious or equivocal lesions visible only by MR [[28], [29], [30]]. In cases of suspicious MR findings, clarification of the diagnoses is often required in view of the well-known disadvantage of breast MR - false-positive results. If such a lesion is not observable by conventional imaging, stereotactic and US-guided biopsy is precluded and MRgVABB must be performed.
The majority of MRgVABB procedures that have been reported in existing studies were performed with automated guided techniques designated software programs and medical devices [17,31]. A freehand VABB approach has been described in few other studies in the literature [18,32,33]. Freehand technique is more accessible and less expensive than automated techniques as it does not require any specifically designed software, hardware and medical devices.
The reported technical success rates of MR-guided biopsies with automated guided techniques are within the interval of 96–100 %, and the cancer yields are in the interval of 28–38 % [17,31]. Technical success of manual approaches has been reported to be approximately 95 %, with cancer yields of 14–20 % [18,32,33].
In our experience, with a careful selection of cases, technical success has been achieved in 100 % of cases, excluding one case in which we had to terminate the procedure due to bleeding.
As examined by Schrading [34], we used an MR-guided vacuum-assisted biopsy protocol that indicates collection of a larger amount of tissue, aiming for partial or complete ablation of the target-lesion. The rationale is avoiding biopsy failures(false-negative results due to under sampling), accomplished by collecting larger amounts of tissue.
The demonstration of target sampling is certainly more difficult following MR biopsy than following US or MMG guidance. MRgVABB is not as precise as other modes of guidance; the biopsy marker must be systematically placed following the procedure in order to determine the biopsy’s exact location. Additionally, MR lesion enhances only in vivo and its presence cannot be confirmed on biopsy samples. Moreover, even when the biopsy marker is correctly positioned, our results confirm that radio-pathological correlation is particularly difficult to be determined following MRgVABB. With aim of compensating over this difficulty, some researchers suggest a short-term follow-up MR after MR guided benign results as a mean to decrease the number of missed carcinomas. For MR biopsies and in view of 1% rate of missed carcinomas, the usual practice after a benign MR biopsy result is to perform MR short-term follow-up 6 months after the procedure [35,36].
However, some challenges do exist. Vacuum-assisted biopsy may be technically demanding in patients with very small or very large breasts and in those with specifically located target lesions such as the immediate retro-areolar, superficial subcutaneous, deep pre-pectoral, far medial parts of the breast, or in case of breast that is too thin under compression (at least 3 cm) [37,38].
Furthermore, a considerable proportion of lesions diagnosed by percutaneous breast biopsies are classified as high-risk lesions [39]. The high-risk histology includes atypical ductal hyperplasia(ADH), lobular neoplasia(LN)[regrouping of the former atypical lobular hyperplasia(ALH) and lobular carcinoma in situ(LCIS)], flat epithelial atypia(FEA), papillary lesions, radial scar/complex sclerosing lesion, phyllodes tumor and mucocele-like lesions [[40], [41], [42], [43], [44]]. When these lesions are diagnosed by image-guided biopsy, the presence of an underlying malignancy may be underestimated, yielding a challenge for clinical management [[45], [46], [47]]. The management of high-risk lesions diagnosed through stereotactic or US-guided VABB has been very well researched and surgical excision is usually recommended [[48], [49], [50], [51], [52], [53]]. There is limited data regarding UR of MRgVABB and management of high-risk lesions diagnosed by MRgVABB [54]. In our study, we reported lower UR than that reported in literature: 10 % of our total cases, compared to 25 % obtained by Verheyden et al. [55] and 23 % by Dratwa et al. [20]. In our series, 38 % of cases were classified as B3 lesions, compared to 50 % observed by Crystal et al. [54] and 29 % by Gristina et al. [18]. These discrepancies are partly justified by the fact that, according to Schrading [34], we have collected larger amounts of tissue for each procedure, allowing successful sampling, thus improving the diagnostic yield. In literature, lower needle gauge used for vacuum-assisted biopsy was significantly associated with a larger amount of tissues and a lower UR [34,55,56].
While a large number of existing studies in broader literature have described MRgVABB, only a few studies specifically analyze radiologic-pathologic concordance with scarce data regarding histological characteristics of suspicious MR lesions not visible with MMG or US [21,22,57]
Our results appear consistent with prior researches where B3 lesions were often underestimated [55]. Our study showed that underestimated lesions were significantly associated with the presence of a mass enhancement in MR [18]. In some studies, B3 lesions that were upgraded to B5 at final pathology represented true-positive results as well [21]. Therefore, according to our experience we believe that these patients should be evaluated by a multidisciplinary team in order to consider open biopsy after MR-guided metallic landmark positioning in case of mass enhancement features in MR, in particular in breast cancer loco-regional staging.
Our study has potential limitations, mainly due to the retrospective design of the study, single center institution and the small cohort of patients enrolled. Heterogeneity gave rise to an additional limitation: in 40 % of cases, patient study was performed in external facilities with different imaging system(US, MMG or MR). In our clinical practice we routinely perform “second-look” US to detect real MR-only lesions [58]. Moreover, we decide to retrospectively evaluate all breast imaging for this study(MMG, US and MR) in our PAC system to further reduce bias, and to exclude lacking cases. Any patient without a complete breast imaging in our PACS System available was excluded. A further limitation is represented by the fact that MRgVABB were performed mainly by one Breast Interventional Expert Radiologist(85 % of the cases) with more than 10 years of interventional breast clinical practice experience. This value highlights Breast Radiologists’ role in improving the success rate and clinical outcome, underlining the usefulness of this technique carried out by experienced hands.
5. Conclusion
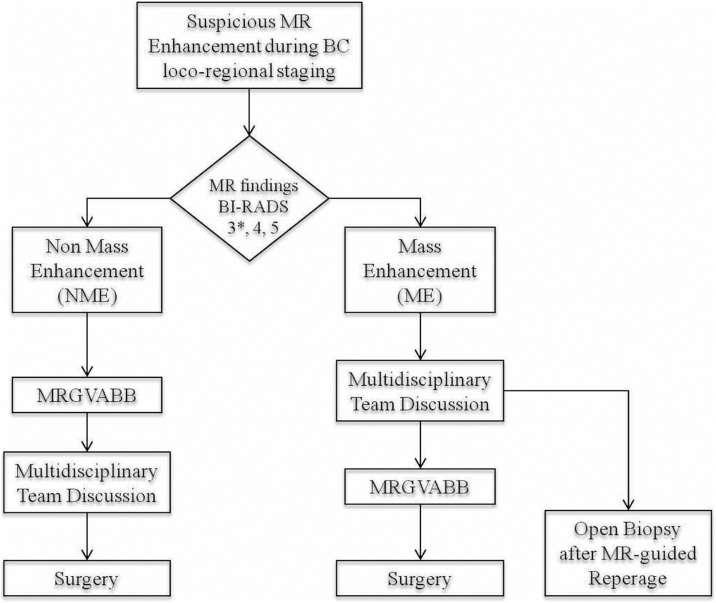
Based on the results obtained from our work, we can presume that NME MR-only lesions are most frequently associated with benignancy. In contrast, ME MR-Only lesions should be directly addressed to surgical evaluation due to higher malignancy rate and greater likelihood of histological upgrade at the definitive pathological diagnosis, with significant impact on the therapeutic outcomes. This presumption comprises a great importance regarding breast cancer loco-regional staging, when patients are already candidates for surgery. Due to above-mentioned reasons, radiological features(ME or NME) should influence management of these patients and in order to proceed on a correct diagnostic and therapeutic plan, we propose flow-chart shown in Fig. 6.
Fig. 6.
Figure shows flow-chart used to optimize the diagnostic and therapeutic plan for patients in breast cancer loco-regional staging with MR-only detectable lesions depending on features of enhancement (MRgVABB = Magnetic Resonance-guided Vacuum-Assisted Breast Biopsy BIRADS = Breast Imaging-Reporting and Data System). *BI-RADS RM 3 score patients were referred for MRgVABB due to clinical context and patients anxiety.
In conclusion, based on present findings, we suggest optimizing the diagnostic and treatment processes of patients in breast cancer staging which are highly dependent on features of MR suspicious enhancement. Particularly, considering the higher benignancy rate, in cases of NME we recommend execution of MRgVABB prior to the multidisciplinary team(MDT) evaluation. Contrarily, in cases of ME in MR-only lesions, when MR was prescribed for breast cancer staging, we propose case discussion during MDT meeting to consider surgical biopsy. In our opinion, when upfront surgery is the treatment of choice, surgical open biopsy should be the selected method instead of MRgVABB.
Authorship contributions
Category 1
Conception and design of study: R. Meucci, C.A. Pistolese, T. Perretta, O.C. Buonomo.
Acquisition of data: D. Ferrari, F. Lamacchia, C.P. Ryan, C. Di Stefano.
Analysis and/or interpretation of data: R. Meucci, G. Manenti, G. Vanni, M. Pellicciaro.
Category 2
Drafting the manuscript: A. Castrignanò, R. Meucci, M. Materazzo, C. Di Stefano.
Revising the manuscript critically for important intellectual content: A. Castrignanò, R. Meucci, I. Portarena.
Category 3
Approval of the version of the manuscript to be published (the names of all authors must be listed): Meucci Rosaria, Pistolese Chiara Adriana, Perretta Tommaso, Vanni Gianluca, Portarena Ilaria, Manenti Guglielmo, Ryan Colleen Patricia, Castrignanò Antonella, Di Stefano Carla, Ferrari Donatella, Lamacchia Feliciana, Pellicciaro Marco, Materazzo Marco, Buonomo Oreste Claudio.
Declaration of Competing Interest
The authors declare no conflicts of interest regarding this study.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Peters N.H.G.M., Borel Rinkes I.H.M., Zuithoff N.P.A., Mali W.P.T.M., Moons K.G.M., Peeters P.H.M. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246:116–124. doi: 10.1148/radiol.2461061298. [DOI] [PubMed] [Google Scholar]
- 2.Jackson V.P., Hendrick R.E., Feig S.A., Kopans D.B. Imaging of the radiographically dense breast. Radiology. 1993;188:297–301. doi: 10.1148/radiology.188.2.8327668. [DOI] [PubMed] [Google Scholar]
- 3.Abe H., Schmidt R.A., Shah R.N., Shimauchi A., Kulkarni K., Sennett C.A. MR-directed (“second-look”) ultrasound examination for breast lesions detected initially on MRI: MR and sonographic findings. Am. J. Roentgenol. 2010;194:370–377. doi: 10.2214/AJR.09.2707. [DOI] [PubMed] [Google Scholar]
- 4.Meissnitzer M., Dershaw D.D., Lee C.H., Morris E.A. Targeted ultrasound of the breast in women with abnormal MRI findings for whom biopsy has been recommended. Am. J. Roentgenol. 2009;193:1025–1029. doi: 10.2214/AJR.09.2480. [DOI] [PubMed] [Google Scholar]
- 5.Houssami N., Ciatto S., Macaskill P., Lord S.J., Warren R.M., Dixon J.M. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J. Clin. Oncol. 2008;26:3248–3258. doi: 10.1200/JCO.2007.15.2108. [DOI] [PubMed] [Google Scholar]
- 6.Schell A.M., Rosenkranz K., Lewis P.J. Role of breast MRI in the preoperative evaluation of patients with newly diagnosed breast cancer. Am. J. Roentgenol. 2009;192:1438–1444. doi: 10.2214/AJR.08.1551. [DOI] [PubMed] [Google Scholar]
- 7.Fischer U., Kopka L., Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology. 1999;213:881–888. doi: 10.1148/radiology.213.3.r99dc01881. [DOI] [PubMed] [Google Scholar]
- 8.Kuhl C.K., Jost P., Morakkabati N., Zivanovic O., Schild H.H., Gieseke J. Contrast-enhanced MR imaging of the breast at 3.0 and 1.5 T in the same patients: initial experience. Radiology. 2006;239:666–676. doi: 10.1148/radiol.2392050509. [DOI] [PubMed] [Google Scholar]
- 9.Sardanelli F., Boetes C., Borisch B., Decker T., Federico M., Gilbert F.J. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur. J. Cancer. 2010;46:1296–1316. doi: 10.1016/j.ejca.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Saslow D., Boetes C., Burke W., Harms S., Leach M.O., Lehman C.D. American cancer society guidelines for breast screening with MRI as an adjunct to mammography. Obstet. Gynecol. Surv. 2007;62:458–460. doi: 10.1097/01.ogx.0000269073.50925.38. [DOI] [PubMed] [Google Scholar]
- 11.Mann R.M., Kuhl C.K., Kinkel K., Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur. Radiol. 2008;18:1307–1318. doi: 10.1007/s00330-008-0863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanni G., Materazzo M., Perretta T., Meucci R., Anemona L., Buonomo C. Impact of awake breast cancer surgery on postoperative lymphocyte responses. Vivo (Brooklyn) 2019;33:1879–1884. doi: 10.21873/invivo.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanni G., Tacconi F., Sellitri F., Ambrogi V., Mineo T.C., Pompeo E. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann. Thorac. Surg. 2010;90:973–978. doi: 10.1016/j.athoracsur.2010.04.070. [DOI] [PubMed] [Google Scholar]
- 14.Calì Cassi L., Vanni G., Petrella G., Orsaria P., Pistolese C., Lo Russo G. Comparative study of oncoplastic versus non-oncoplastic breast conserving surgery in a group of 211 breast cancer patients. Eur. Rev. Med. Pharmacol. Sci. 2016;20:2950–2954. [PubMed] [Google Scholar]
- 15.Calì Cassi L., Biffoli F., Francesconi D., Petrella G., Buonomo O. Anesthesia and analgesia in breast surgery: the benefits of peripheral nerve block. Eur. Rev. Med. Pharmacol. Sci. 2017;21:1341–1345. [PubMed] [Google Scholar]
- 16.O. Buonomo, A.V. Granai, A. Felici, R. Piccirillo, N. De Liguori Carino, F. Guadagni, et al. Day-surgical management of ductal carcinoma in situ(DCIS) of the breast using wide local excision with sentinel node biopsy. Tumori n.d.; 88: S48-9. [DOI] [PubMed]
- 17.Meeuwis C., Mann R.M., Mus R.D.M., Winkel A., Boetes C., Barentsz J.O. MRI-guided breast biopsy at 3T using a dedicated large core biopsy set: feasibility and initial results. Eur. J. Radiol. 2011;79:257–261. doi: 10.1016/j.ejrad.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Gristina L., Rescinito G., Garlaschi A., Tosto S., Cevasco L., Calabrese M. Freehand 3T MR-guided vacuum-assisted breast biopsy(VAB): a five-year experience. Acta Radiol. 2018;59:540–545. doi: 10.1177/0284185117730102. [DOI] [PubMed] [Google Scholar]
- 19.Tozaki M., Yamashiro N., Sakamoto M., Sakamoto N., Mizuuchi N., Fukuma E. Magnetic resonance-guided vacuum-assisted breast biopsy: results in 100 Japanese women. Jpn. J. Radiol. 2010;28:527–533. doi: 10.1007/s11604-010-0464-7. [DOI] [PubMed] [Google Scholar]
- 20.Elsamaloty H., Elzawawi M.S., Mohammad S., Herial N. Increasing accuracy of detection of breast cancer with 3-T MRI. Am. J. Roentgenol. 2009;192:1142–1148. doi: 10.2214/AJR.08.1226. [DOI] [PubMed] [Google Scholar]
- 21.Ferré R., Ianculescu V., Ciolovan L., Mathieu M.C., Uzan C., Canale S. Diagnostic performance of MR-guided vacuum-assisted breast biopsy: 8 years of experience. Breast J. 2016;22:83–89. doi: 10.1111/tbj.12519. [DOI] [PubMed] [Google Scholar]
- 22.Dratwa C., Jalaguier-Coudray A., Thomassin-Piana J., Gonin J., Chopier J., Antoine M. Breast MR biopsy: pathological and radiological correlation. Eur. Radiol. 2016;26:2510–2519. doi: 10.1007/s00330-015-4071-y. [DOI] [PubMed] [Google Scholar]
- 23.Siegmann-Luz K.C., Bahrs S.D., Preibsch H., Hattermann V., Claussen C.D. Abklärung ausschließlich MRT-detektierbarer Mammaläsionen. RoFo Fortschritte Auf Dem Gebiet Der Rontgenstrahlen Und Der Bildgeb Verfahren. 2014;186:30–36. doi: 10.1055/s-0033-1335972. [DOI] [Google Scholar]
- 24.Schrading S., Simon B., Braun M., Wardelmann E., Schild H.H., Kuhl C.K. MRI-guided breast biopsy: influence of choice of vacuum biopsy system on the mode of biopsy of MRI-only suspicious breast lesions. Am. J. Roentgenol. 2010;194:1650–1657. doi: 10.2214/AJR.09.2550. [DOI] [PubMed] [Google Scholar]
- 25.Morris E.A., Comstock C.E., Lee C.H., Lehman C.D., Ikeda D.M., Newstead G.M. Am. Coll. Radiol.; Reston, VA: 2013. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System; pp. 56–71. [Google Scholar]
- 26.Perry N., Broeders M., de Wolf C., Tornberg S., Holland R., von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition—summary document. Ann. Oncol. 2007;19:614–622. doi: 10.1093/annonc/mdm481. [DOI] [PubMed] [Google Scholar]
- 27.Heywang-Köbrunner S.H., Heinig A., Schaumlöffel U., Viehweg P., Buchmann J., Lampe D. MR-guided percutaneous excisional and incisional biopsy of breast lesions. Eur. Radiol. 1999;9:1656–1665. doi: 10.1007/s003300050905. [DOI] [PubMed] [Google Scholar]
- 28.Perlet C., Heywang-Kobrunner S.H., Heinig A., Sittek H., Casselman J., Anderson I. Magnetic resonance-guided, vacuum-assisted breast biopsy: results from a European multicenter study of 538 lesions. Cancer. 2006;106:982–990. doi: 10.1002/cncr.21720. [DOI] [PubMed] [Google Scholar]
- 29.Orel S.G., Rosen M., Mies C., Schnall M.D. MR imaging-guided 9-gauge vacuum-assisted core-neddle breast biopsy: initial experience. Radiology. 2006;238:54–61. doi: 10.1148/radiol.2381050050. [DOI] [PubMed] [Google Scholar]
- 30.Malhaire C., El Khoury C., Thibault F., Athanasiou A., Petrow P., Ollivier L. Vacuum-assisted biopsies under MR guidance: results of 72 procedures. Eur. Radiol. 2010;20:1554–1562. doi: 10.1007/s00330-009-1707-9. [DOI] [PubMed] [Google Scholar]
- 31.Meeuwis C., Veltman J., Van Hall H.N., Mus R.D.M., Boetes C., Barentsz J.O. MR-guided breast biopsy at 3T: diagnostic yield of large core needle biopsy compared with vacuum-assisted biopsy. Eur. Radiol. 2012;22:341–349. doi: 10.1007/s00330-011-2272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung H.N., Han B.K., Ko E.Y., Shin J.H. Initial experience with magnetic resonance-guided vacuum-assisted biopsy in Korean women with breast cancer. J. Breast Cancer. 2014;17:270–278. doi: 10.4048/jbc.2014.17.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dogan B.E., Le-Petross C.H., Stafford J.R., Atkinson N., Whitman G.J. MRI-guided vacuum-assisted breast biopsy performed at 3 T with a 9-gauge needle: preliminary experience. Am. J. Roentgenol. 2012:199. doi: 10.2214/AJR.11.8055. [DOI] [PubMed] [Google Scholar]
- 34.Schrading S., Strobel K., Keulers A., Dirrichs T., Kuhl C.K. Safety and efficacy of magnetic resonance-guided vacuum-assisted large-volume breast biopsy(MR-Guided VALB) Invest. Radiol. 2017;52:186–193. doi: 10.1097/RLI.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 35.Sung Js, Lee Ch, Morris Ea, Comstock Ce, Dershaw Dd. Patient follow-up after concordant histologically benign imaging-guided biopsy of MRI-detected lesions. Am. J. Roentgenol. 2012;198:1464–1469. doi: 10.2214/AJR.11.7455. [DOI] [PubMed] [Google Scholar]
- 36.Li J., Dershaw D.D., Lee C.H., Kaplan J., Morris E.A. MRI follow-up after concordant, histologically benign diagnosis of breast lesions sampled by MRI-guided biopsy. Am. J. Roentgenol. 2009;193:850–855. doi: 10.2214/AJR.08.2226. [DOI] [PubMed] [Google Scholar]
- 37.Perlet C., Heinig A., Prat X., Casselman J., Baath L., Sittek H. Multicenter study for the evaluation of a dedicated biopsy device for MR-guided vacuum biopsy of the breast. Eur. Radiol. 2002;12:1463–1470. doi: 10.1007/s00330-002-1376-4. [DOI] [PubMed] [Google Scholar]
- 38.Brennan S.B. Breast magnetic resonance imaging for the interventionalist: magnetic resonance imaging-guided vacuum-assisted breast biopsy. Tech. Vasc. Interv. Radiol. 2014;17:40–48. doi: 10.1053/j.tvir.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Houssami N., Ciatto S., Bilous M., Vezzosi V., Bianchi S. Borderline breast core needle histology: predictive values for malignancy in lesions of uncertain malignant potential(B3) Br. J. Cancer. 2007;96:1253–1257. doi: 10.1038/sj.bjc.6603714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liberman L. Centennial dissertation. Percutaneous imaging-guided core breast biopsy: state of the art at the millennium. Am. J. Roentgenol. 2000;174:1191–1199. doi: 10.2214/ajr.174.5.1741191. [DOI] [PubMed] [Google Scholar]
- 41.Sewell C.W. Pathology of high-risk breast lesions and ductal carcinoma in situ. Radiol. Clin. N. Am. 2004;42:821–830. doi: 10.1016/j.rcl.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Wallis M., Tarvidon A., Helbich T., Schreer I. Guidelines from the European Society of Breast Imaging for diagnostic interventional breast procedures. Eur. Radiol. 2007;17:581–588. doi: 10.1007/s00330-006-0408-x. [DOI] [PubMed] [Google Scholar]
- 43.Costa A., Zanini V. Precancerous lesions of the breast. Nat. Clin. Pract. Oncol. 2008;5:700–704. doi: 10.1038/ncponc1239. [DOI] [PubMed] [Google Scholar]
- 44.Chivukula M., Bhargava R., Tseng G., Dabbs D.J. Clinicopathologic implications of “flat epithelial atypia” in core needle biopsy specimens of the breast. Am. J. Clin. Pathol. 2009;131:802–808. doi: 10.1309/AJCPLDG6TT7VAHPH. [DOI] [PubMed] [Google Scholar]
- 45.Liberman L. Clinical management issues in percutaneous core breast biopsy. Radiol. Clin. N. Am. 2000;38:791–807. doi: 10.1016/s0033-8389(05)70201-3. [DOI] [PubMed] [Google Scholar]
- 46.Jacobs T.W., Connolly J.L., Schnitt S.J. Nonmalignant lesions in breast core needle biopsies: to excise or not to excise? Am. J. Surg. Pathol. 2002;26:1095–1110. doi: 10.1097/00000478-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Lawton T.J., Georgian-Smith D. Excision of high-risk breast lesions on needle biopsy: is there a standard of core? AJR Am. J. Roentgenol. 2009;192:W268. doi: 10.2214/AJR.08.2227. [DOI] [PubMed] [Google Scholar]
- 48.Jackman R.J., Birdwell R.L., Ikeda D.M. Atypical ductal hyperplasia: can some lesions be defined as probably benign after stereotactic 11-gauge vacuum-assisted biopsy, eliminating the recommendation for surgical excision? Radiology. 2002;224:548–554. doi: 10.1148/radiol.2242011528. [DOI] [PubMed] [Google Scholar]
- 49.Schueller G., Jaromi S., Ponhold L., Fuchsjaeger M., Memarsadeghi M., Rudas M. US-guided 14-gauge core-needle breast biopsy: results of a validation study in 1352 cases. Radiology. 2008;248:406–413. doi: 10.1148/radiol.2482071994. [DOI] [PubMed] [Google Scholar]
- 50.Youk J.H., Kim E.-K., Kim M.J. Atypical ductal hyperplasia diagnosed at sonographically guided 14-gauge core needle biopsy of breast mass. Am. J. Roentgenol. 2009;192:1135–1141. doi: 10.2214/AJR.08.1144. [DOI] [PubMed] [Google Scholar]
- 51.Jang M., Cho N., Woo K.M., Jeong S.P., Min H.S., In A.P. Underestimation of atypical ductal hyperplasia at sonographically guided core biopsy of the breast. Am. J. Roentgenol. 2008;191:1347–1351. doi: 10.2214/AJR.07.3643. [DOI] [PubMed] [Google Scholar]
- 52.Eby P.R., Ochsner J.E., DeMartini W.B., Allison K.H., Peacock S., Lehman C.D. Frequency and upgrade rates of atypical ductal hyperplasia diagnosed at stereotactic vacuum-assisted breast biopsy: 9- versus II-gauge. Am. J. Roentgenol. 2009;192:229–234. doi: 10.2214/AJR.08.1342. [DOI] [PubMed] [Google Scholar]
- 53.Ji H.Y., Kim E.K., Min J.K., Ki K.O. Sonographically guided 14-gauge core needle biopsy of breast masses: a review of 2,420 cases with long-term follow-up. Am. J. Roentgenol. 2008;190:202–207. doi: 10.2214/AJR.07.2419. [DOI] [PubMed] [Google Scholar]
- 54.Crystal P., Sadaf A., Bukhanov K., McCready D., O’Malley F., Helbich T.H. High-risk lesions diagnosed at MRI-guided vacuum-assisted breast biopsy: can underestimation be predicted? Eur. Radiol. 2011;21:582–589. doi: 10.1007/s00330-010-1949-6. [DOI] [PubMed] [Google Scholar]
- 55.Verheyden C., Pages-Bouic E., Balleyguier C., Cherel P., Lepori D., Laffargue G. Underestimation rate at MR imaging-guided vacuumassisted breast biopsy: a multi-institutional retrospective study of 1509 breast biopsies. Radiology. 2016;281:708–719. doi: 10.1148/radiol.2016151947. [DOI] [PubMed] [Google Scholar]
- 56.Orsaria P., Grasso A., Carino R., Caredda E., Sammarra M., Altomare C. Heterogeneous risk profiles among B3 breast lesions of uncertain malignant potential. Tumori J. 2019;106(2):115–125. doi: 10.1177/0300891619868301. 030089161986830. [DOI] [PubMed] [Google Scholar]
- 57.Lourenco A.P., Khalil H., Sanford M., Donegan L. High-risk lesions at MRI-guided breast biopsy: frequency and rate of underestimation. Am. J. Roentgenol. 2014;203:682–686. doi: 10.2214/AJR.13.11905. [DOI] [PubMed] [Google Scholar]
- 58.Pistolese C.A., Lamacchia F., Tosti D., Anemona L., Ricci F., Censi M. Reducing the number of unnecessary percutaneous biopsies: the role of second opinion by expert breast center radiologists. Anticancer Res. 2020;40:939–950. doi: 10.21873/anticanres.14027. [DOI] [PubMed] [Google Scholar]