Highlights
-
•
In this paper, we propose a Long Short-Term Memory (LSTM) based model trained on cumulative COVID-19 cases and deaths.
-
•
Our model can be adjusted based on the parameters in order to provide predictions as needed.
-
•
We provide results at both the country and county levels.
-
•
We compare mitigation measures in various counties in the United States based on the proposed LSTM model.
-
•
We can obtain insights based on the trends in the rate of infections and deaths.
-
•
Our proposed model can be of help for countries and counties deciding on mitigation and reopening strategies.
Keywords: COVID-19, Disease dynamics, Long short-term memory (LSTM), Mitigation measures, Social distancing
Abstract
The COrona VIrus Disease (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) has resulted in a challenging number of infections and deaths worldwide. In order to combat the pandemic, several countries worldwide enforced mitigation measures in the forms of lockdowns, social distancing, and disinfection measures. In an effort to understand the dynamics of this disease, we propose a Long Short-Term Memory (LSTM) based model. We train our model on more than four months of cumulative COVID-19 cases and deaths. Our model can be adjusted based on the parameters in order to provide predictions as needed. We provide results at both the country and county levels. We also perform a quantitative comparison of mitigation measures in various counties in the United States based on the rate of difference of a short and long window parameter of the proposed LSTM model. The analyses provided by our model can provide valuable insights based on the trends in the rate of infections and deaths. This can also be of help for countries and counties deciding on mitigation and reopening strategies. We believe that the results obtained from the proposed method will contribute to societal benefits for a current global concern.
1. Introduction
The outbreak of a novel coronavirus which spread from China in December 2019 led to severe health problems and fatalities worldwide, eventually turning into a global pandemic. The virus was named the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) and the disease caused due to it was named the COrona VIrus Disease (COVID-19). The most common symptoms are cough and fever, although symptoms range in severity including pneumonia-like symptoms and dyspnea [1]. It was confirmed that COVID-19 spread through infected respiratory droplets transmitted from an infected person to a healthy person [2]. Other symptoms in humans have been studied, including relations to the gastrointestinal system [3] and skin rashes [4]. More recent studies have focused on other possible zoonotic pathways, including other species that can possibly contract COVID-19 [5].
Till date, there is no definite medical treatment for COVID-19, although global efforts are in progress for development of a vaccine or specific drugs. Current methods for treatment have involved the use of antiviral medications commonly used for viral diseases [6], [7], [8].
Healthcare professionals were given special instructions and equipment in order to treat patients who tested positive for COVID-19. There was also a surge in demand for PPE (Personal Protective Equipment), especially for frontline healthcare workers as well as for ventilators in hospitals [9], [10].
Countries all over the world executed various mitigation measures for the COVID-19 pandemic, including social distancing, enforcing total lockdowns, and advising people to take precautions like wearing face masks and washing hands. These measures were implemented based on projection studies and statistical models that predicted the disease spread [11], [12], [13].
Usually individuals showing common COVID-19 symptoms were asked to self-isolate for two weeks and seek medical help if health conditions worsened [14], [15], [16]. COVID-19 was generally observed to affect specific groups of people in populations [17], [18], as well as people who have pre-existing medical conditions [19], [20].
It was noted that people may be capable of transmitting the virus despite being completely asymptomatic or in other words, testing positive for COVID-19 without any visible symptoms [21]. This may have expedited the spread of the disease [22] as certain individuals may have been unaware of their conditions and symptoms and transmitted the disease while socializing before they medically tested positive for COVID.
In the present work, we propose an LSTM based approach to understand and evaluate the effectiveness of mitigation measures at both the country and county levels. The rest of this paper is organized as follows: Section 2 discusses the related work, Section 3 discusses the proposed method, Section 4 discusses the results, and Section 5 concludes the paper and suggests future work.
2. Related work
The spread of COVID-19 led to global research efforts to understand the disease and its possible implications. We focus on discussing related work with regard to COVID-19 disease dynamics, especially studies involving simulations and computational learning algorithms.
Several studies have focused on projections of how the infections are likely to spread based on statistical analyses. Neher et al. [23] have proposed a seasonal transmissability model based on SIR [24], a class of mathematical epidemiological models to predict the trajectory of the virus re-infecting sub-populations in the world in future years. They have taken into account the infection, emigration, and population turnover rates respectively as part of their proposed model. A refined version of their model [25] considers visualizing the projected hospital resources necessary in order to support a pandemic like COVID-19.
Another similar hospital impact model CHIME (COVID-19 Hospital Impact Model for Epidemics) developed by Penn Medicine [26] suggests the projection of the hospitalized, ICU (Intensive Care Unit), and ventilated patients respectively for Penn hospitals. They have used a statistical model based on measures like social contact, hospitalization rate, and probability of detection.
With regard to COVID-19, there have been several machine learning based models proposed by various research teams globally in an effort to understand the different facets of COVID-19.
Hu et al. [27] proposed a machine learning model based on stacked auto-encoders to forecast cumulative COVID-19 cases in China till April 2020 based on past cumulative data. They also grouped the cities and provinces in clusters based on the features extracted from their proposed auto-encoder model. Similar LSTM approaches have also been explored in order to understand patient related data [28] as well as to study epidemic trends in China [29].
All COVID-19 forecast and projection models proposed so far have been more focused on graphical analyses and future curve prediction. However, in our proposed model we quantitatively evaluate the degree to which mitigation measures are working based on LSTM predictions.
Wang et al. [30] have proposed an “Ontology-based Side-effect Prediction Framework (OSPF)” for evaluating the “Traditional Chinese Medicine (TCM)” model using deep learning. They have trained an Artificial Neural Network (ANN) model with an architecture composed of three hidden layers on the TCM prescriptions used for the treatment of COVID-19 patients. They have also tested the validity of their OSPF model to evaluate the effectiveness and safety of use of TCM prescriptions for other flu-like diseases as a potential source of treatments for COVID-19. Their proposed model classifies a given TCM prescription as ‘Safe’ or ‘Unsafe’. They have identified seven TCM prescriptions that should take precedence over others while treating COVID-19 patients.
Wang et al. [31] have proposed BI-AT-GRU, a GRU (Gated Recurrent Unit) based model with bidirectionality and attention in order to classify six different respiratory patterns. They have specifically observed that abnormal breathing patterns in patients diagnosed with COVID-19 arise significantly due to Tachypnea, which is characterised as abnormally rapid and shallow breathing. They show that their proposed model is able to distinguish respiratory patterns of ‘Tachypnea’, ‘Central-Apnea’, ‘Bradypnea’, ‘Eupnea’, ‘Cheyne-Stokes’, and ‘Biots’ with high F-1 scores.
Song et al. [32] have proposed a deep learning model for studying CT (Computer Tomography) images of COVID-19 patients. They collected lung CT images from COVID-19 patients, bacterial pneumonia patients, and healthy people from two hospitals in China. Their proposed framework DeepPneumonia has an AUC (Area Under Curve) metric of 0.99 and sensitivity of 0.93 with regard to distinction of COVID-19 from other diseases.
Wang et al. [33] have proposed a CNN (Convolutional Neural Network) based model ‘Inception Migration Neuro Network’ to identify the radiographical features in volumetric chest CT images of patients obtained from two medical institutions in China. The aim of their proposed model is to identify whether COVID-19 is present or not in the CT images of the patients. They have evaluated AUC and F-1 metrics on their model and have claimed that their method is not invasive and is low cost.
Li et al. [34] have proposed a deep learning framework ‘COVID-19 detection neural network’ or ‘COVNet’ to distinguish CT scans of COVID-19 patients from those of ‘CAP (Community Acquired Pneumonia)’ and other non-pneumonia diseases. They have proposed a three-dimensional model involving ResNet50, max-pooling, and a fully connected layer to identify the presence of COVID-19 in the images.
Sethy and Behera [35] have proposed a similar machine learning model for X-Ray images based on ResNet50 and SVM (Support Vector Machines) in order to identify patients with COVID-19. They have evaluated the efficiency of their proposed model using False Positive Rate (FPR), F1 score, Kappa, and MCC (Matthews Correlation Coefficient) metrics.
In the present work, we explore the possibilities of using an LSTM-based approach to learn patterns in COVID-19 data. Statistical learning models require thorough understanding of data and coefficient tuning in order to fit models suitable for prediction. However, compared to statistical learning, machine learning approaches involve allowing the model to automatically learn complex patterns from the data based on the model constructed and tuned hyperparameters. This is particularly useful for handling epidemiological time-series data like that of COVID-19 where the cumulative curves of infections and deaths vary with respect to time and place.
3. Proposed method
Long Short-Term Memory Networks (LSTMs), originally proposed by Hochreiter and Schmidhuber [36], are a special kind of Recurrent Neural Networks (RNNs) that have the ability of capturing long-term dependencies. In this paper, we apply LSTMs to study the disease dynamics of COVID-19, specifically in forecasting cumulative cases and deaths. Most importantly, the capabilities of LSTMs allow us to gain a deeper understanding of where the model stands in terms of future predictions and will serve as an aid in taking important healthcare and mitigation measures.
3.1. Dataset
For the purpose of training, validation and testing, we utilize data from the Johns Hopkins University repository [37]. For experimental purposes, we consider data over the period from January 22, to June 30. The dataset provides time-series data of COVID-19 confirmed cases and deaths. We separately consider each of these scenarios, as well as look into more fine-grained data at the county-level in order to analyse the effectiveness of mitigation measures.
Separating time series data into train, validation, and test sets requires care since all the temporal relations have to be maintained. Since COVID-19 is an infectious viral disease, it spreads over time. This means that we can predict the infections/deaths at a particular point of time based on preceding values. It is also essential to monitor the generalization capabilities of the model and select hyperparameters for the model accordingly. In order to handle this, we partition the data based on time for training and validation as shown in Fig. 1 .
Fig. 1.
Dataset split for LSTM model.
The data is separately trained on three different train and validation splits and hyperparameters with minimum validation root mean squared error (RMSE). The hyperparameters we specifically focus on tuning are the lookback and lookahead. The split of train and test data for the tuned model is approximately maintained at an 80:20 ratio based on the initial lookback and lookahead of 1 and 1 respectively, although the model is capable of handling other splits based on the data. The smaller splits of train and validation sets are approximately maintained at a 60:20 ratio. Intuitively, an increase in the lookback and lookahead parameters reduces the number of training samples and testing samples respectively and leads to erroneous results. For experimentation purposes, we test lookback in the range of 1 and 7 and lookahead in the range of 5 and 7 for reasonable future predictions, while adhering to the index constraints on individual train, validation, and test data.
3.2. Model design
For the proposed LSTM model, Keras [38] was used as the framework with TensorFlow as backend.
In all our experiments, we train the LSTM for 10,000 epochs with 10 layers and a batch size of 10. All normalization is performed on the log scale and Adam [39] is used as the optimizer with a learning rate of 0.001. The window parameters we specifically focus on in this case are the lookback and lookahead parameters which are tuned based on the data of a particular location. In this case, the lookback parameter is indicative of the window size in terms of the number of consecutive days the LSTM needs to “look back” at in order to make a reasonable prediction. The lookahead parameter indicates the number of days in advance that the system needs to “look ahead” in order to make a prediction for a future date. Both the lookback and lookahead parameters are used by carrying out index slicing on the respective training, validation, and test data and can be formulated as data[:-(lookahead)] split into sets of lookback days based on time for the model input and data[():] for the model output.
In general, the model is able to provide future predictions of cumulative confirmed cases/deaths. However, from a research perspective, we plot the predictions obtained after training on the suitable hyperparameters by comparing them with the actual values of confirmed cases/deaths observed on those specific days for obtaining useful interpretations. The detailed analyses of the results and corresponding figures are discussed in Section 4.
4. Results and discussion
Our analyses based on our proposed LSTM model are divided into two main parts: the prediction models that show predictions of COVID-19 confirmed cases/deaths and evaluations on how well social distancing/mitigation measures are working in the present scenario. In all our plots, dates in January, February, March, April, May, and June are prefixed with ‘J’, ‘F’, ‘M’, ‘A’, ‘MA’, and ‘JU’ respectively followed by the day of the month. As aforementioned, all modeling is carried out based on the data provided for a specific location.
4.1. Prediction models
We discuss our results on two levels: country-level and county-level data on COVID-19 confirmed cases and deaths. In addition to plotting the predicted values and actual values, we also plot a baseline in all figures that is calculated as the moving average over the actual values in the lookback window. Overall, it was observed that it was harder to predict the number of comfirmed cases compared to the number of deaths. It was also observed in general that shorter values of lookback and lookahead resulted in lower values of both the train and test RMSE. However, in the present situation, it is of more interest to discuss the interpretation of what the LSTM predictions are indicative of instead of purely focusing on the values of the RMSE.
4.1.1. Predictions for the United States and other global locations
In this section, we discuss our results on five globally affected locations: (a) the United States of America, (b) Italy, (c) India, (d) Japan, and (e) Hubei, China.
Fig. 2 shows the plot of predictions for COVID-19 confirmed cases in the United States of America. Fig. 3 shows the plot of predictions for COVID-19 deaths in United States of America. The United States had different lockdown and phased reopening strategies for the various states [40]. Predictions on cases indicate the cumulative cases are still increasing. The actual COVID-19 cases are also rising. The predictions for deaths have a flattening trend. The trends indicate more mitigation measures may be needed.
Fig. 2.
Results on COVID-19 Cases in the United States of America.
Fig. 3.
Results on COVID-19 Deaths in the United States of America.
Fig. 4 shows the plot of predictions for COVID-19 confirmed cases in Italy. Fig. 5 shows the plot of predictions for COVID-19 deaths in Italy. Italy progressively enforced strict lockdown policies in various regions starting February 23, [41]. The LSTM predictions show similar flattening trend compared to the actual cumulative COVID-19 cases that implies the mitigation measures have been successful. The LSTM model is capable of predicting the flattened curve as achieved by Italy. The deaths in Italy show a similar trend as that of the cases. The small deviation in the predictions shows that mitigation measures may still need to be enforced.
Fig. 4.
Results on COVID-19 Cases in Italy.
Fig. 5.
Results on COVID-19 Deaths in Italy.
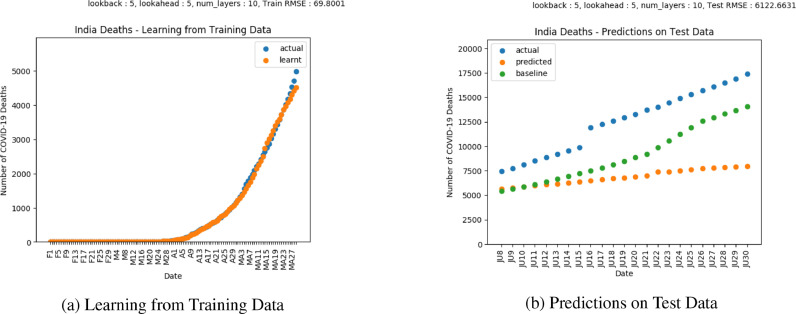
Fig. 6 shows the plot of predictions for COVID-19 confirmed cases in India. Fig. 7 shows the plot of predictions for COVID-19 deaths in India. India enforced nationwide lockdowns in several phases following the order issued on March 24, [42]. COVID-19 predictions for cases indicate cumulative cases are still increasing, which is also a trend indicated by the actual cases. Predictions on deaths indicate a flattening trend. The trends show that stronger mitigation measures need to be enforced to reduce infections.
Fig. 6.
Results on COVID-19 Cases in India.
Fig. 7.
Results on COVID-19 Deaths in India.
Fig. 8 shows the plot of predictions for COVID-19 confirmed cases in Japan. Fig. 9 shows the plot of predictions for COVID-19 deaths in Japan. Japan implemented a series of measures in March 2020 to control the spread of COVID-19 [43]. The slight surge in actual cases compared to the flattening of the LSTM predictions indicates that the mitigation measures are yet to fully work. The LSTM predictions show a similar trend as the confirmed cases, however, here the COVID-19 deaths in Japan show a flattening in the curve that is also indicated by the LSTM predictions.
Fig. 8.
Results on COVID-19 Cases in Japan.
Fig. 9.
Results on COVID-19 Deaths in Japan.
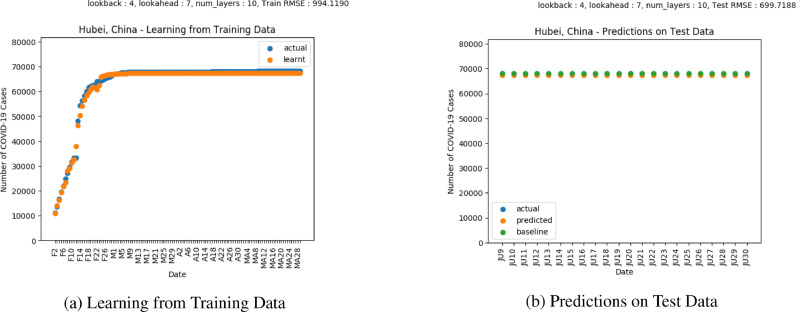
Fig. 10 shows the plot of predictions for COVID-19 confirmed cases in Hubei, China. Fig. 11 shows the plot of predictions for COVID-19 deaths in Hubei, China. Wuhan in Hubei, China was the location where the first cases of COVID-19 were observed [23]. China enforced mitigation measures in January [44]. Based on both the LSTM predictions and actual values, the COVID-19 cases seem to have flattened, suggesting that mitigation measures have worked. The trends in the LSTM predictions as well as the actual number of deaths indicate a flattening in the number of deaths. However, the difference suggests that some enforcement may still be needed before the mitigation measures are completely lifted.
Fig. 10.
Results on COVID-19 Cases in Hubei, China.
Fig. 11.
Results on COVID-19 Deaths in Hubei, China.
4.1.2. Predictions for counties in the United States
In this section, we show predictions of our proposed LSTM model on eight affected counties within the United States of America: (a) Los Angeles, California, (b) Dallas, Texas, (c) Hillsborough, Florida, (d) Maricopa, Arizona, (e) King, Washington, (f) Fulton, Georgia, (g) Cook, Illinois, and (h) New York City, New York. Our discussion is based on various lockdown and phased reopening measures in such states [45].
Figs. 12 and 13 show predictions for COVID-19 cases and deaths respectively in Los Angeles, California. The cases and deaths depict an increasing trend that is also indicated by the predictions. In this situation however, there is a small divergence between the predicted and actual curves, which shows that more mitigation measures may need to be introduced.
Fig. 12.
Results on COVID-19 Cases in Los Angeles, California.
Fig. 13.
Results on COVID-19 Deaths in Los Angeles, California.
Figs. 14 and 15 show the predictions for cases and deaths respectively in Dallas, Texas. Cases and deaths show a rising trend by the end of June, as shown by the actual and predicted plots. Stronger mitigation measures are also required here as the actual cases show a divergence over the LSTM predicted values.
Fig. 14.
Results on COVID-19 Cases in Dallas, Texas.
Fig. 15.
Results on COVID-19 Deaths in Dallas, Texas.
Figs. 16 and 17 show the predictions cases and deaths respectively in Hillsborough, Florida. The cases depict an increasing trend shown by the actual as well as predicted values. In the case of deaths, significant divergence is observed between the predicted and actual plots, which denotes that more mitigation measures need to be enforced.
Fig. 16.
Results on COVID-19 Cases in Hillsborough, Florida.
Fig. 17.
Results on COVID-19 Deaths in Hillsborough, Florida.
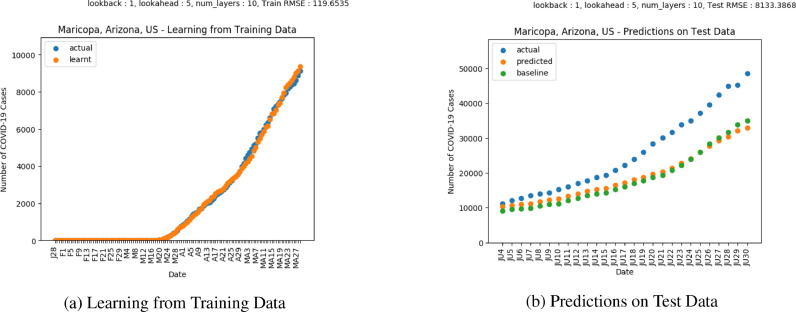
Figs. 18 and 19 show predictions for cases and deaths respectively in Maricopa, Arizona. The plots show a rise in cases in the month of June. The divergence between the actual and predicted values towards the end of June for both the predictions and deaths indicates that the mitigation measures have not worked.
Fig. 18.
Results on COVID-19 Cases in Maricopa, Arizona.
Fig. 19.
Results on COVID-19 Deaths in Maricopa, Arizona.
Figs. 20 and 21 show the predictions for cases and deaths respectively in King, Washington. The plots indicate that the mitigation measures were working appropriately until the end June when a slight divergence between the LSTM and actual curves appears for infections and deaths.
Fig. 20.
Results on COVID-19 Cases in King, Washington.
Fig. 21.
Results on COVID-19 Deaths in King, Washington.
Figs. 22 and 23 show the predictions for cases and deaths respectively in Fulton, Georgia. As indicated by the graph, the mitigation measures are working well in terms of deaths but not in the case of infections which is shown by the divergence between the actual and predicted curves during the last week of June.
Fig. 22.
Results on COVID-19 Cases in Fulton, Georgia.
Fig. 23.
Results on COVID-19 Deaths in Fulton, Georgia.
Figs. 24 and 25 show the predictions for cases and deaths respectively in Cook, Illinois. The predictions capture the flattening trend in terms of both cases and deaths. Though mitigation measures seem to be working for both cases and deaths, the increasing trends show that more mitigation measures are needed.
Fig. 24.
Results on COVID-19 Cases in Cook, Illinois.
Fig. 25.
Results on COVID-19 Deaths in Cook, Illinois.
Figs. 26 and 27 shows the predictions for cases and deaths respectively in New York City, New York. New York was one of the most heavily affected areas of the United States. Counties in New York enforced lockdowns in March similar to most other places of the United States [40]. The graphs suggest that mitigation measures are working based on the flattening of the infections. However, based on deaths, the LSTM predictions suggest that the lockdown measures have not fully worked yet as shown by the slight surge in deaths.
Fig. 26.
Results on COVID-19 Cases in New York City, New York.
Fig. 27.
Results on COVID-19 Deaths in New York City, New York.
4.2. How well are social distancing/mitigation measures working?
We now aim at understanding how well the current mitigation measures are working. Various countries placed nationwide lockdowns, while others implemented phased strategies based on affected individual locations. The goal is to understand how social distancing had made the situation better or worse. The analyses provided are based on our proposed LSTM model. In order to do this, we consider a shorter and longer window size. In this context, we fix the lookback parameter as 1 and use the line of best fit on the rate of change of the difference between the smaller lookahead parameter of 1 and a larger lookahead parameter of 5. The analyses can also be carried out on individual parameters, however, for experimental purposes we choose to compute the rate on the difference in order to obtain a smoother curve for the purpose of this work. Again, the model can be used for this type of analyses for the near future as well, which will be valuable for nations or specific locations to decide whether to tighten or loosen restrictions based on the trend of how well their mitigation policies will work in the near future.
Fig. 28 shows the various plots evaluating the trends of mitigation measures on rates of COVID-19 infections and deaths in eight affected counties in the United States: (a) Los Angeles, California, (b) Dallas, Texas, (c) Hillsborough, Florida, (d) Maricopa, Arizona, (e) King, Washington, (f) Fulton, Georgia, (g) Cook, Illinois, and (h) New York City, New York. The best_fit plots indicate the line of best fit in each figure while the LSTM_rate plots indicate the computed rate of difference of the two LSTM models as previously discussed. The line of best fit is of further interest as it provides valuable interpretations on how well the mitigation measures are working.
Fig. 28.
Evaluating effects of mitigation measures in various counties in the United States.
In order to quantify and evaluate how well mitigation and social distancing is working, we compare the slope, intercept and RMSE values (with respect to of the line of best fit for each of the counties as shown in Table 1 .
Table 1.
Comparing effects of mitigation measures in various counties in the United States.
| County | Mitigation measures for COVID-19 infections |
Mitigation measures for COVID-19 deaths |
||||
|---|---|---|---|---|---|---|
| Slope | Intercept | RMSE | Slope | Intercept | RMSE | |
| Los Angeles, California | 16.31 | −22.57 | 617.21 | −0.25 | 7.00 | 15.47 |
| Dallas, Texas | −6.07 | 10.01 | 100.17 | 0.08 | −0.38 | 3.73 |
| Hillsborough, Florida | 4.98 | 8.58 | 134.57 | −0.03 | 0.31 | 1.97 |
| Maricopa, Arizona | −10.73 | 165.94 | 409.28 | 0.08 | 2.44 | 5.97 |
| King, Washington | 1.52 | −1.80 | 24.73 | −0.00 | 0.27 | 2.19 |
| Fulton, Georgia | 1.94 | 1.65 | 44.21 | −0.02 | 0.25 | 4.36 |
| Cook, Illinois | 10.78 | −160.34 | 97.76 | −0.37 | 1.80 | 21.24 |
| New York City, New York | 1.14 | 35.57 | 65.40 | −0.24 | 6.99 | 5.89 |
The slope indicates the trend of the mitigation measures – a positive value of slope indicates that the current mitigation and social distancing are not working as expected while a negative value of slope indicates that the current mitigation and social distancing are working well. In other words, a positive slope indicates an increase in the rate of infections or deaths while a negative slope indicates a decrease in the rate of infections or deaths. The intercept gives an approximation of the increase/decrease in the rate of infections or deaths depending on a positive/negative value. The RMSE gives an estimate of the stabilization of the rate of infections/deaths.
Mitigation measures did not work in Los Angeles, California as rate of infections is increasing even though the rate of deaths shows a downward trend. Similarly, mitigation measures did not work in Hillsborough, Florida and Fulton, Georgia because of the rising infection rates even though the death rates show a trend of slowing down. Similar trends are also seen in New York City, New York and Cook, Illinois, which show a rise in infection rates. King, Washington, shows an increasing trend for infection rates even though the death rates appear to have stabilized. Although Dallas, Texas and Maricopa, Arizona show downward trends for infection rates, the rising trends for death rates show that mitigation measures are yet to work.
It is important to note that COVID-19 death rates are decreasing or nearly stabilized as shown by the eight counties in the United States. This happened due to the rapid increase in testing as well as improved treatment in hospitals, which reduced the mortality rate and increased the recovery rate.
Table 2 evaluates the success of mitigation measures based on COVID-19 infections and deaths respectively, where ✓ indicates mitigation measures being successful and ✗ indicates mitigation measures not being successful. Table 2 provides an overview of the success of mitigation measures based on the analyses in Table 1.
Table 2.
Evaluation of mitigation measures in various counties in the United States.
| County | Success of mitigation measures based on COVID-19 infections | Success of mitigation measures based on COVID-19 deaths |
|---|---|---|
| Los Angeles, California | ✗ | ✓ |
| Dallas, Texas | ✓ | ✗ |
| Hillsborough, Florida | ✗ | ✓ |
| Maricopa, Arizona | ✓ | ✗ |
| King, Washington | ✗ | ✓ |
| Fulton, Georgia | ✗ | ✓ |
| Cook, Illinois | ✗ | ✓ |
| New York City, New York | ✗ | ✓ |
Therefore, various effects of mitigation measures have been observed in different parts of the United States which may require additional measures like rapid testing, contact tracing, and phased reopening depending on the situation in various places before the entire nation resumes a normal lifestyle.
5. Conclusion and future work
The COVID-19 pandemic affected countries globally and led to an unprecedented number of infections and deaths. Countries took vital steps in mitigating the pandemic by enforcing lockdowns, social distancing, and a variety of other mitigation measures. In this paper, we propose an LSTM based learning model that can learn from the cumulative rise in COVID-19 confirmed cases and deaths and provide valuable insights on how well mitigation measures are working quantitatively in terms of the rate of infections and deaths. The predictions of the model can be helpful for countries deciding to make important decisions regarding the effects of currently implemented mitigation measures and aid in making plans for reopening various places. We provide analyses at both the country and county levels.
Future extensions of this work include implementing and understanding how this model can be transferred to studying the disease dynamics of other similar pandemics.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This project has been funded by the Jump ARCHES endowment through the Health Care Engineering Systems Center.
Appendix A. Code
Code for the proposed method is available here: https://github.com/sayantanibasu/covid19-models.
References
- 1.Vital Surveillances The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)—China, 2020. China CDC Weekly. 2020;2(8):113–122. [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Tech. Rep. World Health Organization; 2020. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations: scientific brief, 27 March 2020. [Google Scholar]
- 3.Gu J., Han B., Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020;158(6):1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 5.Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science. 2020;368(6494):1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov The. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 7.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Touret F., de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020;177:104762. doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranney M.L., Griffeth V., Jha A.K. Critical supply shortages - the need for ventilators and personal protective equipment during the COVID-19 pandemic. N Engl J Med. 2020;382(18) doi: 10.1056/NEJMp2006141. :e41(1)–e41(3) [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . Tech. Rep. World Health Organization; 2020. Rational use of personal protective equipment for coronavirus disease (COVID-19): interim guidance, 27 february 2020. [Google Scholar]
- 11.Alvarez F.E., Argente D., Lippi F. Tech. Rep. National Bureau of Economic Research; 2020. A simple planning problem for COVID-19 lockdown. [Google Scholar]
- 12.COVID-19 Projections (IHME). https://covid19.healthdata.org/united-states-of-america; 2020.
- 13.Das S, Ghosh P, Sen B, Mukhopadhyay I. Critical community size for COVID-19–a model based approach to provide a rationale behind the lockdown. arXiv preprint arXiv:200403126 2020.
- 14.Greenhalgh T., Koh G.C.H., Car J. COVID-19: a remote assessment in primary care. BMJ. 2020;368:1–5. doi: 10.1136/bmj.m1182. [DOI] [PubMed] [Google Scholar]
- 15.Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382(18):1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 16.Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395(10228):931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.COVIDCDC and Team, Response Severe outcomes among patients with coronavirus disease 2019 (COVID-19) United States, February 12–March 16, 2020. MMWR Morb Mortal Weekly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruz A.T., Zeichner S.L. COVID-19 in children: initial characterization of the pediatric disease. Pediatrics. 2020;145(6):1–3. doi: 10.1542/peds.2020-0834. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Lian N. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J Med Virol. 2020;2020:1–7. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndrome. 2020;14(3):247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai Y., Yao L., Wei T., Tian F., Jin D.-Y., Chen L. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai C.-C., Liu Y.H., Wang C.-Y., Wang Y.-H., Hsueh S.-C., Yen M.-Y. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARSCoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53(3):404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neher R.A., Dyrdak R., Druelle V., Hodcroft E.B., Albert J. Potential impact of seasonal forcing on a SARS-CoV-2 pandemic. Swiss Med Weekly. 2020;150(1112) doi: 10.4414/smw.2020.20224. [DOI] [PubMed] [Google Scholar]
- 24.Kermack W.O., McKendrick A.G.. Contributions to the mathematical theory of epidemics–I. 1927.1991. [DOI] [PubMed]
- 25.COVID-19 Scenarios. https://neherlab.org/covid19/; 2020.
- 26.COVID-19 Hospital Impact Model for Epidemics. https://penn-chime.phl.io; 2020.
- 27.Hu Z, Ge Q, Jin L, Xiong M. Artificial intelligence forecasting of COVID-19 in China. arXiv preprint arXiv:200207112 2020.
- 28.Bandyopadhyay SK, Dutta S. Machine learning approach for confirmation of COVID-19 cases: positive, negative, death and release. medRxiv 2020.
- 29.Yang Z., Zeng Z., Wang K., Wong S.-S., Liang W., Zanin M. Modified SEIR and AI prediction of the epidemics trend of COVID-19 in China under public health interventions. J Thorac Dis. 2020;12(3):165. doi: 10.21037/jtd.2020.02.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Li L, Yan J, Yao Y. Evaluating the traditional Chinese medicine (TCM) officially recommended in China for COVID-19 using ontology-based side-effect prediction framework (OSPF) and deep learning. Preprints 2020, arXiv:2020020230 2020a. [DOI] [PMC free article] [PubMed]
- 31.Wang Y, Hu M, Li Q, Zhang X-P, Zhai G, Yao N. Abnormal respiratory patterns classifier may contribute to large-scale screening of people infected with COVID-19 in an accurate and unobtrusive manner. arXiv preprint arXiv:200205534 2020b.
- 32.Song Y, Zheng S, Li L, Zhang X, Zhang X, Huang Z, et al. Deep learning enables accurate diagnosis of novel coronavirus (COVID-19) with CT images. medRxiv 2020. [DOI] [PMC free article] [PubMed]
- 33.Wang S, Kang B, Ma J, Zeng X, Xiao M, Guo J, et al. A deep learning algorithm using CT images to screen for Corona Virus Disease (COVID-19). medRxiv 2020c. [DOI] [PMC free article] [PubMed]
- 34.Li L., Qin L., Xu Z., Yin Y., Wang X., Kong B. Artificial intelligence distinguishes COVID-19 from community acquired pneumonia on chest CT. Radiology. 2020;296:E65–E71. doi: 10.1148/radiol.2020200905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sethy PK, Behera SK. Detection of coronavirus disease (COVID-19) based on deep features. Preprints 2020, arXiv:2020030300 2020.
- 36.Hochreiter S., Schmidhuber J. Long short-term memory. Neural Comput. 1997;9(8):1735–1780. doi: 10.1162/neco.1997.9.8.1735. [DOI] [PubMed] [Google Scholar]
- 37.Johns Hopkins COVID-19 Data Repository. https://github.com/CSSEGISandData/COVID-19; 2020.
- 38.Chollet F. Keras: The python deep learning library. ASCL. 2018 ascl–1806. [Google Scholar]
- 39.Kingma DP, Ba J. Adam: a method for stochastic optimization. arXiv preprint arXiv:14126980 2014.
- 40.Brzezinski A., Deiana G., Kecht V., Van Dijcke D. The COVID-19 pandemic: government vs. community action across the United States. Covid Econ. 2020;7:115–156. [Google Scholar]
- 41.Lazzerini M., Putoto G. COVID-19 in Italy: momentous decisions and many uncertainties. Lancet Global Health. 2020;8:e641–e642. doi: 10.1016/S2214-109X(20)30110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ray D., Subramanian S., Vandewalle L. Tech. Rep. Centre for Economic Policy Research; 2020. India’s lockdown. [Google Scholar]
- 43.Shaw R., Kim Y.-k., Hua J. Governance, technology and citizen behavior in pandemic: lessons from COVID-19 in East Asia. Progress Disaster Sci. 2020;6:100090. doi: 10.1016/j.pdisas.2020.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang S.X., Wang Y., Rauch A., Wei F. Unprecedented disruption of lives and work: health, distress and life satisfaction of working adults in China one month into the COVID-19 outbreak. Psychiatry Res. 2020;288:112958. doi: 10.1016/j.psychres.2020.112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.US Reopening of 50 States. https://www.nytimes.com/interactive/2020/us/states-reopen-map-coronavirus.html; 2020.