Abstract
Turbidimetry is used to characterize fibrin clot properties. In purified systems, maximum absorbance (MA) directly relates to fibrin fiber cross-sectional area. However, in plasma samples there are discrepancies in the relationships between MA and fibrinogen concentration, fiber diameter, other clot properties, and cardiovascular disease outcomes, which complicate data interpretation. This study aims to advance understanding of MA of plasma clots through testing how well it relates to fundamental dependence on fibrinogen concentration and fiber diameter as predicted by light scattering theory, other clot properties and lifestyle, and biochemical variables. Plasma samples from 30 apparently healthy individuals with a fibrinogen concentration from 2.4 to 6.4 g/L were included. We performed turbidimetry, permeability, scanning electron microscopy, and rheometry on in vitro formed plasma clots. MA correlated more strongly with fibrinogen concentration (r = 0.65; p < 0.001) than with fiber diameter (r = 0.47; p = 0.01), which combined explained only 46% of the MA variance. Of additional variables measured, only low-density lipoprotein cholesterol correlated with MA (r = 0.46; p = 0.01) and clot lysis (r = 0.62; p < 0.0001) but not with fiber diameter or fibrinogen concentration. MA correlated with clot lysis time (r = 0.59; p = 0.001), storage modulus (r = 0.61; p = 0.001), and loss modulus (r = 0.59; p = 0.001), and negatively with clot permeability (r =−0.60; p = 0.001) also after adjustment for fibrinogen concentration and fiber diameter. Increased MA is indicative of a prothrombotic clot phenotype irrespective of fibrinogen concentration. MA is more indicative of overall clot density than of fiber diameter. Other plasma components can alter internal fiber density without altering fiber diameter and should be considered when interpreting MA of plasma samples.
Keywords: turbidimetry, fibrinogen, fibrin structure, fibrin viscoelastic properties, blood plasma samples
Introduction
Turbidimetry is a high-throughput, highly sensitive technique, providing information about fibrin clot formation and structure, that can be used in large-scale clinical and epidemiological studies with good reproducibility.1 A turbidity curve is recorded by plotting light absorbance against time during in vitro clot formation.2 Three main variables are typically obtained from this curve, namely lag time (time required for protofibrils to grow to sufficient length to allow lateral aggregation), slope (rate of lateral aggregation), and maximum absorbance, which depends on fibrinogen concentration and clot structural parameters, such as fiber diameter and internal fiber density, defined as the internal mass density of the fiber measured in g/cm3.3–5 More recently, this method has been modified to also include clot lysis time (CLT) by the addition of tissue plasminogen activator (tPA), resulting in the breakdown of the formed clot.6 The interpretation of maximum absorbance in terms of clot structural properties started with the early observation by Ferry and Morrison7 that fibrin gels with varying maximum absorbance contain fibers with varying fiber diameters. In 1978, Carr and Hermans3 derived an equation that relates absorbance to fibrinogen concentration and specific structural properties of a clot. In this model, the fibrin gel is treated as a dilute solution of randomly oriented cylindrical, long, thin rods that attenuate transmitted light by scattering (absorption and other light-attenuating processes are ignored). According to this model, the turbidity, τ, which equals ln(10)·absorbance under common experimental conditions, see Supplementary Material (available in the online version), is given by
| (1) |
where λ is the wavelength of the incident and transmitted light, , and . NA is Avogadro’s number; ns is the refractive index of the solvent (1.33 for water); is the specific refractive index increment for fibrin (= 0.17594 cm3/g)—these values are typically constant for a given experiment; c is the concentration of fibrinogen in g/mL; μ is the molecular mass-to-length ratio of the fiber, measured in Daltons/cm; D is the diameter of the fiber. It is important to note that μ depends on the diameter of the fiber and on the internal fiber density, ρf, since it measures molecular mass per fiber length. For a homogenous, cylindrical fiber, , where ρf is the internal fiber density, measured in g/cm3. A thicker fiber will have a larger μ than a thinner fiber (at constant internal fiber density), and a denser fiber will have a larger μ than a less dense fiber (at constant diameter). Furthermore, it should be noted that the fibrinogen concentration, c, which appears in the prefactor, α, also has an effect on fiber diameter; higher fibrinogen concentrations have been shown to result in larger fiber diameters.5,8,9 Thus, according to this model (Eq. 1), the absorbance of a clot explicitly depends on the fibrinogen concentration, c, the fiber molecular mass per length, μ, and the fiber diameter, D, with the added complication that c, D, and μ are interrelated since c affects D, and D affects μ. Eq. (1), or modified versions of this equation, have also been used to determine fiber diameter, D, and average fiber mass-to-length ratio, μ, from turbidity versus wavelength measurements of fully formed clots.4,10–13
The model resulting in Eq. (1) was initially developed for purified fibrinogen solutions; however, turbidimetry is also used in plasma samples, typically when comparing fibrin clot properties of healthy control individuals with cardiovascular disease (CVD) patients since altered fibrin clot structure is considered a risk factor for arterial and venous thrombotic events.14 The correlation between τ and c, and between τ and D, as predicted by this equation, have, however, not been validated in plasma samples. Moreover, there are some apparent discrepancies in plasma studies when correlating maximum absorbance with c, D, other clot properties, and CVD outcomes. For example, in two case–control studies using plasma samples, maximum absorbance was increased in thrombotic patients.15,16 However, Undas et al16 reported these fibers to be thicker, whereas Mills et al15 reported thinner fibers when measured directly with scanning electron microscopy (SEM). Differences in maximum absorbance were also reported between healthy control and CVD patients despite having similar fibrinogen concentrations.17–22
Regarding other clot properties, not included in Eq. (1), some studies show increased maximum absorbance to be associated with higher clot permeability,17,19,23 whereas in others, it was associated with denser clots with decreased permeability.15,16,18,20–22 In terms of disease outcome itself, both decreased19,24 and increased15,20–22 maximum absorbance have been reported in CVD patients, as compared with healthy controls.
These inconsistencies indicate a need to better understand turbidity measurements in plasma samples. This study therefore aims to advance understanding of maximum absorbance in plasma samples through a series of association studies. Specifically, we tested how well maximum absorbance relates to (i) the predicted fundamental dependence on fibrinogen concentration and fiber diameter (Eq. 1), (ii) other clot properties (clot formation, permeability, lysis, and mechanical properties), and (iii) lifestyle and biochemical variables of the study participants. As expected (Eq. 1), maximum absorbance correlated with both, fiber diameter and fibrinogen concentration, with the latter association being the strongest. However, fibrinogen concentration and fiber diameter did not fully explain the variance in maximum absorbance, since, when keeping the fibrinogen concentration constant, a twofold difference in maximum absorbance was still found. This suggests that in plasma, there are additional parameters that affect maximum absorbance, most likely by altering the internal fiber density, which is very challenging to measure directly with a method independent from turbidity. Of additional lifestyle and biochemical variables measured, we found that low-density lipoprotein cholesterol (LDL-C) strongly and positively correlated with maximum absorbance and clot lysis but not with fiber diameter or fibrinogen concentration. This finding suggests that LDL-C increases the internal fiber density, which, in turn, increases the maximum absorbance. Finally, we also found positive correlations between maximum absorbance and CLT, storage modulus, and loss modulus, and a negative correlation with clot permeability.
Methods
Study Population and Design
Data for this study were obtained from apparently healthy, black South Africans, older than 30 years (not using chronic medication for noncommunicable diseases) enrolled in the South African arm of the International Prospective Urban and Rural Epidemiology study.25 Baseline data were collected in 2005 (n = 2,010) and follow-up data in 2010 (n = 1,288) and 2015 (n = 926). For this study, a subsample of 30 participants was systematically selected across the total fibrinogen concentration range (1.5–7.5 g/L) of the participants who partook in the 2015 data collection. All participants gave voluntary written informed consent and data collection complied with the Declaration of Helsinki of 1975 (as revised in 2013). The study was approved by the Health Research Ethics Committee of the North-West University, South Africa (NWU-00016–10-A1).
Questionnaire Data
Information regarding self-reported smoking, alcohol consumption, and contraceptive use was recorded using standardized, validated questionnaires.
Blood Sample Collection and Preparation
Fasting blood samples were collected between 07:00 a.m. and 11:00 a.m. from the antecubital vein using sterile winged infusion sets and syringes. For total and γ’ fibrinogen concentration, clot properties, and plasminogen activator inhibitor-1 (PAI-1) activity (PAI-1act), samples were collected in 3.2% sodium citrate tubes. For serum total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and LDL-C, triglycerides (TG), C-reactive protein (CRP), albumin, and creatinine, blood samples were collected in tubes without anticoagulants, and for plasma glucose, sodium fluoride tubes were used. Samples were centrifuged within 30 minutes of collection at 2,000 × g for 15 minutes and stored at −80°C until analyses.
Biochemical Analysis
Serum high-sensitivity CRP, TG, TC, HDL-C, albumin, creatinine, and plasma glucose were determined using the Cobas Integra 400 analyzer (Roche Diagnostics, Indianapolis, Indiana, United States). LDL-C was calculated according to the formula described by Sathiyakumar et al.26 PAI-1act was determined by an indirect enzymatic method (Spectrolyze PAI-1, Trinity Biotech, Bray, Ireland). Fibrinogen was analyzed with a modified Clauss method using an automated coagulation laboratory (Instrumentation Laboratory, Milan, Italy). Fibrinogen γ’ was determined with an enzyme-linked immunosorbent assay27 and reported as percentage of total fibrinogen.
Fibrin Clot Properties
Turbidity
Plasma fibrinolytic potential was determined using a turbidity assay.28 tPA (80 ng/mL tPA; Actilyse, Boehringer Ingelheim, Ingelheim, Germany) was added to plasma containing 17 mmol/L CaCl2, tissue factor (TF) (1,750 × diluted TF; Dade Innovin, Siemens Healthcare Diagnostics Inc., Marburg, Germany) and 10 mmol/L phospholipid vesicles (Rossix, Mölndal, Sweden). The tPA and TF concentrations were selected to obtain CLTs between 60 and 100 minutes. To determine clotting and lysis times from the resultant curves, sigmoidal curve fitting was used (Origin software version 8.5 [Origin lab, 2010]). Lag time (minutes), slope (×10−3 au/s), maximum absorbance (Δau), and CLT (minutes) were calculated as previously described.6
Permeability
Clot permeability (Ks), an indication of the intrinsic pore size of the network, was measured as described previously.29 Essentially, plasma clots were prepared in triplicate in 3-cm sections of 1 mL plastic serological pipettes. Plasma was clotted with the addition of 1 U/mL human α-thrombin (Merck, Darmstadt, Germany) and 20 mM CaCl2. Buffer was permeated through at a pressure height of Δh = 4 cm and Ks calculated from is the flow rate (flow through volume/time), η is the viscosity (ηwater = 1.0 cP at 20°C), L is the clot length, A is the cross-sectional area of the clot, and ΔP is pressure drop. ΔP = ρwater·g·Δh, where the density of water, ρwater = 1 g/cm3, the acceleration due to gravity, g = 980 cm/second2, and Δh = 4 cm is the height of the buffer above the clot.
SEM and Fiber Diameter Measurement
After completion of permeation, each clot container was rinsed with cacodylate buffer; thereafter, clots were fixed overnight in 2% glutaraldehyde (Merck) and recovered from the clot containers. Samples were then prepared for SEM imaging by dehydration with ethanol in successive dose increases and chemically dried with hexamethyldisilazane (Merck). Dried clots were mounted onto stubs and sputter coated with gold-palladium before being viewed and photographed with a FEI Corporation Quanta 200ESEM (Hillsboro, Oregon, United States). ImageJ (v 1.48, National Institutes of Health, Bethesda, Maryland, United States) was used to measure the fiber diameter of 100 systematically selected fibers in each of five micrographs per individual.
Rheometry
Plasma clots were prepared with 1 U/mL human α-thrombin and 20 mM CaCl2 and viscoelastic properties determined during plasma clot formation, by performing oscillatory shear measurements at 37°C on an ARES-G2 Rheometer (TA Instruments, New Castle, Delaware, United States). A time sweep test was performed using a 40-mm stainless steel cone, under an oscillation procedure of 3% strain, at an angular frequency of 5 rad/s (10 half sampling cycles) with a sampling interval of 3 points per second. The geometry (truncation) gap and loading gap were set at 0.045 and 15.0 mm, respectively. After lowering the stainless steel cone, immersion oil (100–120 mPa·s) (Merck) was placed around the plates to prevent the clot from drying out. The test was performed for 40 minutes, measuring the storage modulus (G’) and loss modulus (G”), that is, elastic and viscous properties, respectively, for each plasma sample at 3-second intervals. A summary of the methods used in this study and the data obtained from these methods, are presented in ►Fig. 1. The coefficient of variation for all methods was < 10%.
Fig. 1.
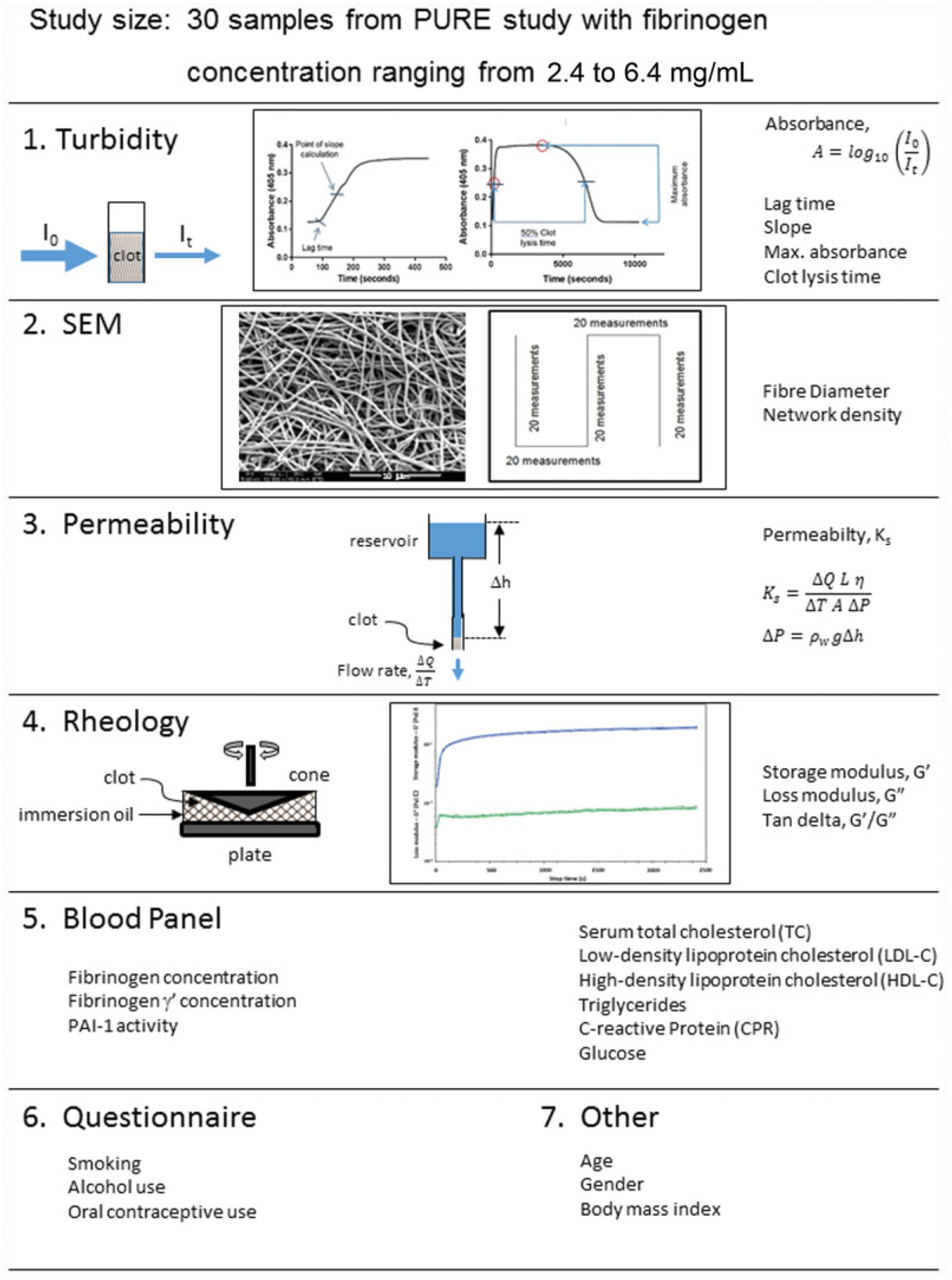
Summary of the methods used in this article. The turbidity curves of this figure are included with permission from Pieters et al.6
Statistical Analysis
Statistical analyses were performed using the Statistica software version 13. Significance was set at p ≤ 0.05. Normality was determined using histograms and the Shapiro–Wilks test. Normally distributed data (fibrinogen concentration, turbidimetry data, Ks, and fiber diameter) are reported as mean standard deviation. Nonnormally distributed data (% γ’ fibrinogen and rheometry data) were log transformed to improve normality for further statistical analyses but are reported as median (25th–75th percentiles). Multiple regression analysis was used to determine the contribution of fibrinogen concentration and fiber diameter to maximum absorbance variance. Pearson’s correlations and partial correlations (when adjusting for fibrinogen concentration or fiber diameter) were used to determine the correlation between maximum absorbance, fibrinogen concentration, and other fibrin clot properties. Scatterplots were inspected for outliers to ensure the correlations were not driven by single outlier data points. To determine whether there are other factors that are associated with maximum absorbance in plasma, Mann–Whitney U tests (because of the small sample size of the subdivisions) were used to compare maximum absorbance and fiber diameter between categorical variables (alcohol consumption, smoking, and oral contraceptive use) with analysis of covariance when adjusting for differences in fibrinogen concentration. Pearson’s correlations and partial correlations (adjusting for fibrinogen concentration) with visual inspection of scatterplots were used to determine associations with continuous variables (age, body mass index [BMI], glucose, CRP, blood lipids, albumin, and creatinine).
Results
The descriptive characteristics of the participants are presented in ►Table 1. Twenty-three of the 30 participants were women, 5 of whom used oral contraceptives. Eleven participants smoked and eight consumed alcohol. The mean age of the study group was 58.6 (53.5–66.7) years and the mean BMI was 26.2 (21.8–33.8) kg/m2. The mean fibrinogen concentration was 4.16 ± 0.85 g/L.
Table 1.
Descriptive characteristics of the participants
| Variable | Participants (n = 30) |
|---|---|
| Sex (male / female) n (%) | 7 (23.3) / 23 (76.7) |
| Age (years) | 58.6 (53.5–66.7) |
| Tobacco users, n (%) | 11 (36.6) |
| Alcohol users, n (%) | 8 (26.7) |
| Oral contraceptive users, n (%) | 5 (21.7) |
| BMI (kg/m2) | 26.2 (21.8–33.8) |
| Glucose (mmol/L) | 5.63 [1.97] |
| PAI-1 (U/mL) | 1.91 (0–8.45) |
| CRP (mg/L) | 5.09 (2.64–10.5) |
| TC (mmol/L) | 4.59 [1.43] |
| HDL-C (mmol/L) | 1.36 [0.42] |
| LDL-C (mmol/L) | 2.87 [1.38] |
| TG (mmol/L) | 1.18 [0.56] |
| Albumin (g/L) | 39.3 [6.88] |
| Creatinine (μmol/L) | 59.8 [17.3] |
| Fibrinogen (g/L) | 4.16 [0.85] |
| Fibrinogen γ’ (%) | 9.18 (8.36–12.9) |
| Lag time (min) | 4.18 [0.66] |
| Slope (au/s) | 10.8 [5.00] |
| Maximum absorbance (Δau) | 0.71 [0.24] |
| Clot lysis time (min) | 64.0 [9.47] |
| Fiber diameter (nm) | 191 [18.5] |
| Permeability (cm2 × 10−9) | 9.50 [3.20] |
| Storage modulus (G’) (Pa) | 56.2 (35.1–102) |
| Loss modulus (G”) (Pa) | 2.55 (1.69–3.94) |
| Tan (delta) (G’/G”) | 0.05 (0.04–0.06) |
Abbreviations: au/s, absorbance unit per second; BMI, body mass index; CRP, C-reactive protein; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PAI-1, plasminogen activator inhibitor-1; TC, total cholesterol; TG, triglycerides.
Note: Δau indicates change in absorbance units. Normally distributed data are reported as mean [standard deviation] and nonnormally distributed data as median (25th–75th percentile).
Association between Maximum Absorbance, Fibrinogen Concentration, and Fiber Diameter
In a first series of experiments, we investigated the correlation between maximum absorbance and fundamental clot parameters as predicted from light scattering theory (Eq. 1). Maximum absorbance correlated with both fibrinogen concentration and fiber diameter; however, the correlation with fibrinogen concentration was stronger than that with fiber diameter (r = 0.65; p < 0.001 compared with r = 0.47; p = 0.01) (►Table 2). Fiber diameter on the other hand demonstrated similar correlations with maximum absorbance and fibrinogen concentration (r = 0.47; p = 0.01 and r = 0.45; p = 0.01). After adjusting for differences in fibrinogen concentration between samples, maximum absorbance no longer correlated with fiber diameter (r = 0.26; p = 0.18) (►Table 3) indicating that this association is largely dependent on the fibrinogen concentration. In a separate analysis, when adjusting for fiber diameter, the association between maximum absorbance and fibrinogen concentration was only moderately reduced (r = 0.56; p = 0.002 vs. r = 0.65; p < 0.001). This suggests that this relationship is only partly attributable to fiber size with the remaining relationship likely reflecting clot density (volume occupied by fibers per total clot volume). ►Fig. 2 depicts scanning electron images of clots prepared from plasma samples with varying fibrinogen concentrations. Based on visual comparison, clots prepared from plasma with higher fibrinogen concentrations had thicker fibers and had a higher fiber density than clots prepared from plasma samples with a lower fibrinogen concentration.
Table 2.
Correlation between fibrin clot properties and fibrinogen concentration
| Clot property | Max abs | Fiber diameter | Fibrinogen | Fibrinogen γ’ (%) | Lag time | Slope | CLT | Permeability | Storage modulus | Loss modulus | Tan (delta) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Max abs (Δau) | 0.47b | 0.65c | −0.12 | 0.39a | 0.69c | 0.63c | −0.67c | 0.67c | 0.68c | −0.19 | |
| Fiber diameter (nm) | 0.47b | 0.45b | −0.21 | 0.04 | 0.38a | 0.30 | −0.05 | 0.24 | 0.26 | −0.09 | |
| Fibrinogen (g/L) | 0.65c | 0.45b | −0.13 | 0.11 | 0.64c | 0.29 | −0.54b | 0.37a | 0.41a | 0.04 | |
| Fibrinogen γ’ (%) | −0.12 | −0.21 | −0.13 | −0.19 | 0.17 | 0.03 | −0.04 | −0.15 | −0.10 | 0.26 | |
| Lag time (min) | 0.39a | 0.04 | 0.11 | −0.19 | −0.02 | 0.006 | −0.12 | 0.30 | 0.27 | −0.12 | |
| Slope (au/s) | 0.69c | 0.38a | 0.64c | 0.17 | −0.02 | 0.56c | −0.63c | 0.27 | 0.31 | 0.05 | |
| CLT (min) | 0.63c | 0.30 | 0.29 | 0.03 | 0.006 | 0.56c | −0.36a | 0.49b | 0.51b | −0.14 | |
| Permeability (cm2) | −0.67c | −0.05 | −0.54b | −0.04 | −0.12 | −0.63c | −0.36a | 0.41a | −0.46b | −0.18 | |
| Storage modulus (Pa) | 0.67c | 0.24 | 0.37a | −0.15 | 0.30 | 0.27 | 0.49b | −0.41a | 0.98c | −0.49b | |
| Loss modulus (Pa) | 0.68c | 0.26 | 0.41a | −0.10 | 0.27 | 0.31 | 0.51b | −0.46b | 0.98c | −0.33 | |
| Tan (delta) (G’/G”) | −0.19 | −0.09 | 0.04 | 0.26 | −0.12 | 0.05 | −0.14 | −0.18 | −0.49b | −0.33 |
Abbreviations: CLT, clot lysis time; Max abs, maximum absorbance; Δau, change in absorbance units.
p < 0.05.
p < 0.01.
p < 0.001.
Table 3.
Unadjusted and partial correlations of maximum absorbance with other clot properties
| Clot property | Unadjusted | Adjusted - fibrinogen | Adjusted - fiber diameter | Adjusted - fibrinogen and fiber diameter |
| r | r | r | r | |
| Fiber diameter (nm) | 0.47b | 0.26 | – | – |
| Lag time (min) | 0.39a | 0.43a | 0.42a | 0.45a |
| Slope (au/s) | 0.69c | 0.47b | 0.64c | 0.47b |
| Clot lysis time (min) | 0.63c | 0.61c | 0.59b | 0.59b |
| Permeability (cm2) | −0.67c | −0.49b | −0.73c | −0.60b |
| Storage modulus (Pa) | 0.67c | 0.62c | 0.65c | 0.61b |
| Loss modulus (Pa) | 0.68c | 0.60c | 0.65c | 0.59b |
| Tan (delta) (G’/G”) | −0.19 | −0.29 | −0.18 | −0.28 |
| Fibrinogen γ’ (%) | −0.12 | −0.06 | −0.04 | −0.01 |
Abbreviations: au/s, absorbance unit per second; Max abs, maximum absorbance.
p < 0.05.
p < 0.01.
p < 0.001.
Fig. 2.
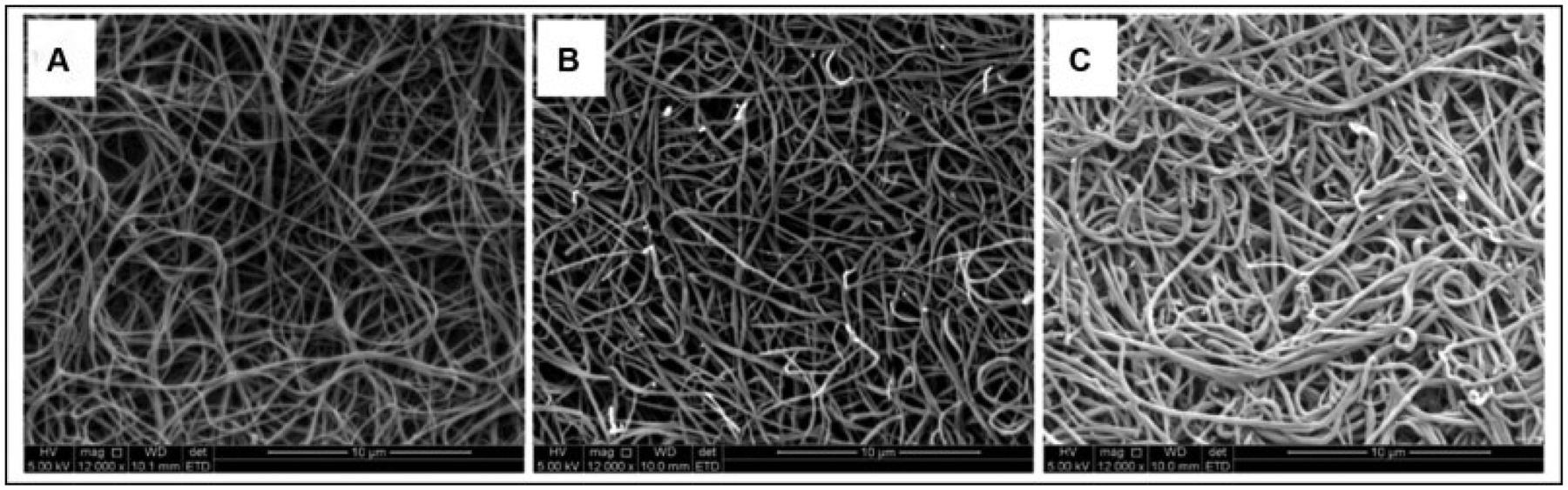
Scanning electron microscopy (SEM) images, at 12,000× magnification, of plasma clots with varying total fibrinogen concentration and increasing fiber diameters as follows: (A) 2.4 g/L, 162 nm; (B) 4.17 g/L, 196 nm; and (C) 6.34 g/L, 218 nm.
Despite the strong relationship between maximum absorbance and fibrinogen concentration, samples with similar fibrinogen concentrations, had maximum absorbance values that differed up to twofold (►Supplementary Table S1, available in the online version). In addition, fibrinogen concentration and fiber diameter combined, explained only 46% of the variance in maximum absorbance with fibrinogen concentration contributing 42% and fiber diameter 4%.
Association of Maximum Absorbance with Lifestyle and Other Measured Biochemical Variables
To identify other factors that may explain the remaining variance in maximum absorbance, maximum absorbance, fiber diameter, and fibrinogen concentration were correlated with other biochemical and lifestyle variables (►Supplementary Table S2, available in the online version). ►Table 4 presents the variables that correlated significantly with at least one of the three. Of these measured variables, LDL-C was the only variable that correlated significantly with maximum absorbance (r = 0.46; p = 0.01) but not with fiber diameter (r = 0.17; p = 0.36) or fibrinogen concentration (r = 0.18; p = 0.34). Referring to Eq. (1) (absorbance depends on variables c, D, and μ), this finding suggests that increased LDL-C is associated with an increased internal fiber density without increasing fiber diameter. LDL-C furthermore correlated significantly with CLT (r = 0.62 and r = 0.61 after adjusting for fibrinogen concentration; p < 0.0001 for both) suggesting a link between increased LDL-C, increased internal fiber density, and longer lysis time. LDL-C did not differ between smokers and nonsmokers, alcohol users and nonusers, or oral contraceptive users and nonusers. Maximum absorbance also correlated significantly with CRP (r = 0.47; p = 0.008) and fiber diameter with HDL-C (r = −0.38; p = 0.04), but significance disappeared after adjustment for fibrinogen concentration. There was no correlation with age, blood glucose, TC, TG, albumin, creatinine, or BMI (►Supplementary Material, available in the online version).
Table 4.
Other biochemical variables that correlated significantly with maximum absorbance, fiber diameter, and/or fibrinogen concentration
| Variable | Maximum absorbance | Fiber diameter | Fibrinogen concentration | |||
|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | |
| LDL-C - unadjusted - adjusted for fbg | 0.46 | 0.01 | 0.17 | 0.36 | 0.18 | 0.34 |
| 0.46 | 0.01 | 0.11 | 0.59 | - | ||
| HDL-C - unadjusted - adjusted for fbg | −0.32 | 0.08 | −0.38 | 0.04 | −0.41 | 0.02 |
| −0.08 | 0.69 | −0.24 | 0.22 | - | ||
| CRP - unadjusted - adjusted for fbg | 0.47 | 0.008 | 0.15 | 0.44 | 0.56 | 0.001 |
| 0.17 | 0.40 | −0.14 | 0.48 | - | ||
Abbreviations: CRP, C-reactive protein; fbg, fibrinogen; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
In addition, higher maximum absorbance values were observed for women using oral contraceptives compared with those who did not. However, after adjustment for the higher fibrinogen concentration in this group, the difference in maximum absorbance was no longer significant. Fiber diameter did not differ significantly between the two groups. There was no difference in either maximum absorbance or fiber diameter between smokers and nonsmokers (►Fig. 3). Although maximum absorbance tended to be higher in alcohol consumers, this difference disappeared after adjustment for fibrinogen concentration.
Fig. 3.
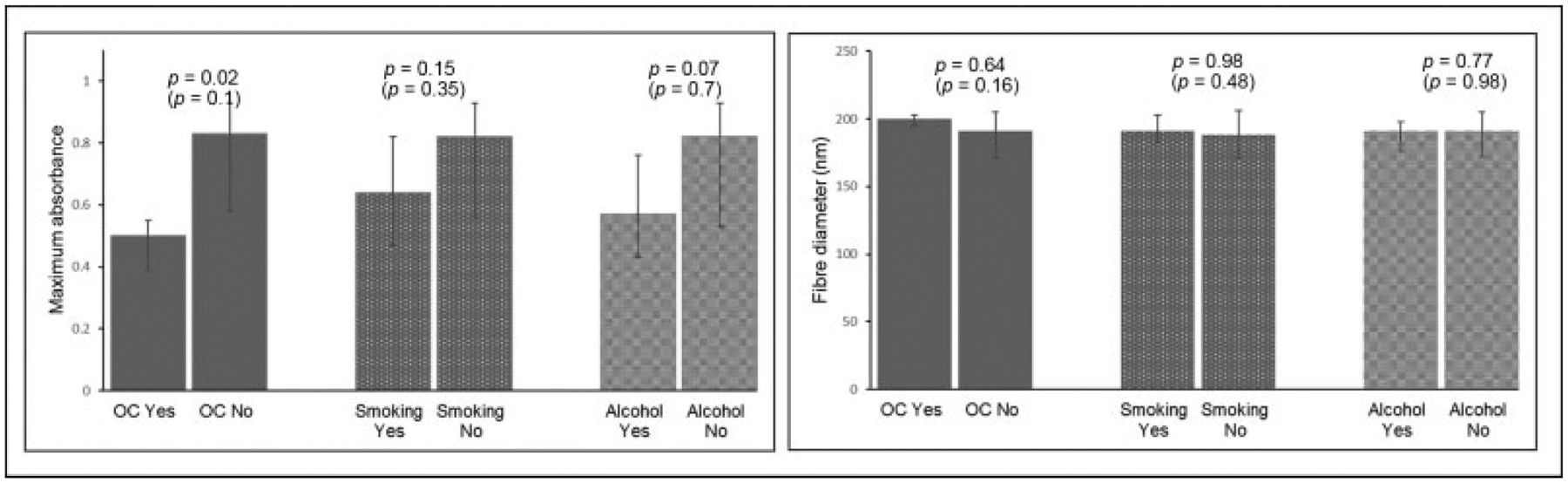
Maximum absorbance and fiber diameter in relation to oral contraceptive use, smoking, and alcohol consumption. Bars denote median and interquartile range. p-Values indicate significance between users and nonusers. p-Values in brackets were obtained after adjustment for fibrinogen concentration. OC, oral contraceptive users n=5; oral contraceptive nonusers n = 18; smokers n = 10; nonsmokers n = 19; alcohol consumers n = 8; alcohol nonusers n = 22.
Association between Maximum Absorbance and Other Clot Properties
In a third series of experiments, we determined the correlation between maximum absorbance and kinetic and bio-physical clot properties. Maximum absorbance had strong significant positive correlations with lag time (r = 0.39; p = 0.03), slope (r = 0.69; p < 0.001), CLT (r = 0.63; p < 0.001), stiffness (storage modulus, G′) (r = 0.67; p < 0.001), and plasticity (loss modulus, G″) (r = 0.68; p < 0.001), and a negative correlation with permeability (r = −0.67; p < 0.001) (►Table 2). These associations remained essentially unchanged after adjustment for fibrinogen concentration and fiber diameter, individually and combined (►Table 3). In contrast, aside from its significant correlation with maximum absorbance, fiber diameter correlated significantly with slope (r = 0.38; p = 0.04) only. After adjusting for fibrinogen concentration this association disappeared, indicating that this association is largely mediated through the fibrinogen concentration.
Discussion
In purified systems, maximum absorbance is considered to be directly related to the fibrin fiber cross-sectional area.3 This relationship was established through light scattering theory, which modeled fibrin fibers as diluted, randomly arranged, long, thin cylinders. It predicts that turbidity (maximum absorbance) depends on fibrinogen concentration, fiber diameter, and internal fiber density (as part of the molecular mass-to-length ratio, ), as can be seen from Eq. (1). Since maximum absorbance depends on all three, the direct association between maximum absorbance and fiber diameter is contingent on fibrinogen concentration and internal fibrin density being constant. The use of the turbidity assay has also been extended to plasma samples to characterize clot properties of mainly CVD patients, compared with healthy individuals. Superficially, plasma clots have a very similar appearance to fibrin clots prepared from purified fibrinogen. Thus, it may seem reasonable to assume that Eq. (1) also applies to plasma clots. However, this assumption needed to be tested as the plasma environment differs from purified systems in that the fibrinogen concentrations in the samples vary and plasma contains additional components, not present in purified fibrinogen systems. These components may modify internal fiber structure, which our data in fact suggest to be the case. In addition, in plasma studies, maximum absorbance is often interpreted to reflect fiber size, as is done in purified studies, without considering the influence of the varying fibrinogen concentrations or the additional plasma components. Validation studies in plasma samples are therefore required to advance our understanding of how to interpret maximum absorbance in plasma samples.
Traditionally, fibrin clot networks are defined as either fine or coarse networks.30,31 Clots composed of thinner fibers are typically denser and more rigid, with a stiffer network arrangement, reduced permeability (smaller pore sizes), and enhanced resistance to fibrinolysis (prothrombotic); whereas clots consisting of thicker fibers generally contain looser and less rigid networks that are more susceptible to fibrinolysis (antithrombotic).32–38 As mentioned in the introduction, in plasma (case–control) studies, these relationships are less clear, with inconsistencies existing regarding the association between other clot properties and maximum absorbance, which is often used as a proxy marker/indirect measurement of fiber diameter. Our data showed that clots with increased maximum absorbance, have an increased rate of lateral aggregation, have thicker fibers, increased clot stiffness, and decreased permeability and lysis. After adjustment for fibrinogen concentration, maximum absorbance no longer correlated with fiber diameter but all other associations remained. This suggests that increased maximum absorbance is indicative of a prothrombotic clot phenotype that is more dense, with increased stiffness and decreased lysis rate, irrespective of the fibrinogen concentration. These associations were further more not dependent on fiber diameter as fiber diameter did not associate significantly with any of the other clot properties nor did adjustment for fiber diameter alter the associations. In agreement with these associations, many studies in the literature found maximum absorbance to be increased in CVD patients regardless of whether fibrinogen concentration was increased or not.15,16,18,20–22,39,40
As predicted by Eq. (1), both fiber diameter and fibrinogen concentration associated positively with maximum absorbance. However, after adjusting for fibrinogen concentration, fiber diameter and maximum absorbance no longer correlated, indicating that this relationship is largely driven by the fibrinogen concentration. The relationship between maximum absorbance and fibrinogen concentration, however, remained after adjusting for fiber diameter. This indicates that fiber size only partly contributes to this relationship with the remainder likely reflecting the fiber density of the clot (i.e., volume occupied by fibers per total clot volume). Based on the SEM images, it is clear that clots with higher fibrinogen concentration formed both more and thicker fibers, increasing the total density of the clot. Maximum absorbance furthermore correlated more strongly with fibrinogen concentration than fiber diameter, further supporting the concept that it is a marker of overall clot density rather than simply reflecting fiber diameter.
Fibrinogen concentration and fiber diameter, however, explained only about half of the variance in maximum absorbance, and when keeping the fibrinogen concentration constant, a twofold difference in maximum absorbance still existed. As maximum absorbance is influenced not only by fibrinogen concentration and fiber diameter, but also by internal fiber density (Eq. 1), this suggests that the internal fiber density, ρf, which enters Eq. (1) through the mass-to-length ratio, μ, , is an additional important parameter that affects absorbance in plasma and which may be influenced by additional components present in plasma, but absent in the purified system. Several lifestyle and biochemical variables, for example, CRP and oral contraceptive use (with borderline significance for alcohol consumption), positively associated with maximum absorbance and not fiber diameter, but after adjustment for fibrinogen concentration, these associations disappeared indicating that the relationship was driven by the associated increased fibrinogen concentration. Only LDL-C was positively associated with maximum absorbance independent of fiber diameter and/or fibrinogen concentration. This suggests that LDL-C potentially increases internal fiber density by binding to fibrin fibers and in so doing increases maximum absorbance. It was furthermore also positively associated with CLT. Increased LDL-C has been reported in the literature to be associated with stiffer clots,41 slower lag time,42 lower clot permeability,43,44 and enhanced resistance to fibrinolysis.41,45 These associations, together with our data, suggest that this inhibited fibrinolysis may be the result of LDL-C-associated apolipoproteins binding to fibrin clots and thereby hindering permeation and action of lytic enzymes.41,46 In addition, surface-bound LDL has been found to bind to tPA, thus preventing the formation of plasmin.47 The oxidation of lipids may furthermore influence the effect of lipids on clot structure. It has been suggested that fibrin may be directly affected by highly oxidized LDL altering the ensuing clot network by enhancing hypercoagulation and fibrinolytic resistance in comparison to nonoxidized LDL.48 These results suggest a potential, novel role for LDL-C in thrombotic disease, which deserves further investigation. Intrafibrillar fibrin structure has recently been demonstrated to be modifiable and to significantly influence clot mechanical properties and resistance to lysis.49 Domingues et al50 demonstrated that thrombin concentration and γ’ fibrinogen altered protofibril content and protein density within fibers, altering the overall strength of the fibrin network. In support of this concept, in our study, maximum absorbance also correlated positively with fibrin clot stiffness even after adjusting for fiber diameter and fibrinogen concentration, although the exact mechanism behind this association remains to be determined.
A potential limitation of this study was that native plasma clots were used to obtain turbidimetric, permeability, and rheometry data, while fibrin clots were dehydrated for SEM analysis. The turbidity assay furthermore made use of TF as a clotting agent, while thrombin was used in the other methods. However, these assays were used with the intention of being complementary to one another, providing supporting data obtained from different methods. Furthermore, maximum absorbance demonstrated strong significant associations not only with the turbidimetry data, but also with the other assays, supporting internal consistency of the data. We could not investigate molecular mass-to-length ratio (μ), as an indirect marker of internal fiber density due to a lack of sample. Nor could we directly measure clot/protein density. We recommend the development and/or validation of such direct measures, to further assess the association between maximum absorbance and internal fiber density and overall clot protein density. Although we identified environmental and plasma components that contributed to altered clot properties, the study was not designed to identify an exhaustive list of fibrin clot structure determinants and follow-up experimentation is required to better characterize additional plasma components that may influence the internal fiber density of plasma clots. Being a cross-sectional study, we could furthermore not determine causality, which limits the conclusions that can be drawn from the data. It furthermore remains to be determined whether these results, obtained in apparently healthy participants, are also valid in patients with thrombosis.
Conclusion
In this study increased maximum absorbance was associated with a prothrombotic clot phenotype characterized by increased rate of lateral aggregation, increased clot stiffness, and decreased clot permeability and lysis irrespective of fiber diameter and fibrinogen concentration. Although maximum absorbance correlates positively with fiber diameter, in plasma samples it is more strongly associated with fibrinogen concentration and more indicative of overall clot density than of fiber diameter. Other components in plasma, such as LDL-C, can alter internal fiber density without altering fiber diameter, likely by binding to fibrin fibers and in so doing influence maximum absorbance. Our data show that while Eq. (1) is valid to use in plasma samples, the difference in fibrinogen concentration and potential influence of plasma constituents on internal fiber density should be considered when interpreting maximum absorbance.
Supplementary Material
What is known about this topic?
According to light scattering theory, the absorbance of a clot depends on the fibrinogen concentration, the internal fiber density (as part of the fiber molecular mass per length ratio), and the fiber diameter.
In purified fibrinogen systems, absorbance is indicative of fiber diameter at fixed fibrinogen concentrations but these associations remain to be validated in plasma samples.
There are discrepancies in published data from plasma samples when relating maximum absorbance to fibrinogen concentration, fiber diameter, other clot properties, and CVD outcomes.
What does this paper add?
In this study, increased maximum absorbance was indicative of a prothrombotic clot phenotype that was denser, with increased stiffness and decreased lysis, irrespective of fibrinogen concentration or fiber diameter.
Maximum absorbance correlated more strongly with fibrinogen concentration than fiber diameter, suggesting it to be a marker of overall clot density (volume occupied by fibers per total clot volume) rather than simply fiber diameter.
Fibrinogen concentration and fiber diameter explained only about half of the variance in maximum absorbance.
We demonstrated that other plasma components, such as LDL-C, can alter maximum absorbance by altering internal fiber density.
Acknowledgments
We thank all participants, fieldworkers, and supporting staff involved in the PURE data collection. We thank the PURE-SA research team and the office staff of the Africa Unit for Transdisciplinary Health Research of the Faculty of Health Sciences, NWU South Africa. In addition, we thank the PURE-International research team and the PURE-study office staff at the PHRI, Hamilton Health Sciences, and McMaster University, Ontario, Canada.
Funding
This work was supported by the National Research Foundation (grant numbers 120070 and 105700 to M.P.) and the Medical Research Society (Self-Initiated Research Grant to M.P.) of South Africa, by the National Heart, Lung, and Blood Institute of the National Institutes of Health (U.S.A.) under Award Number R15HL148842, and by Wake Forest University Pilot grant DM0741. Opinions expressed and conclusions arrived at are solely the responsibility of the authors and do not necessarily represent the official views of the funders.
Footnotes
Conflict of Interest
None declared.
References
- 1.Carter AM, Cymbalista CM, Spector TD, Grant PJ; EuroCLOT Investigators. Heritability of clot formation, morphology, and lysis: the EuroCLOT study. Arterioscler Thromb Vasc Biol 2007; 27(12):2783–2789 [DOI] [PubMed] [Google Scholar]
- 2.Sjøland JA, Sidelmann JJ, Brabrand M, et al. Fibrin clot structure in patients with end-stage renal disease. Thromb Haemost 2007;98(02):339–345 [PubMed] [Google Scholar]
- 3.Carr ME Jr, Hermans J. Size and density of fibrin fibers from turbidity. Macromolecules 1978;11(01):46–50 [DOI] [PubMed] [Google Scholar]
- 4.Yeromonahos C, Polack B, Caton F. Nanostructure of the fibrin clot. Biophys J 2010;99(07):2018–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisel JW, Nagaswami C. Computer modeling of fibrin polymerization kinetics correlated with electron microscope and turbidity observations: clot structure and assembly are kinetically controlled. Biophys J 1992;63(01):111–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pieters M, Philippou H, Undas A, de Lange Z, Rijken DC, Mutch NJ; Subcommittee on Factor XIII and Fibrinogen, and the Subcommittee on Fibrinolysis. An international study on the feasibility of a standardized combined plasma clot turbidity and lysis assay: communication from the SSC of the ISTH. J Thromb Haemost 2018;16(05):1007–1012 [DOI] [PubMed] [Google Scholar]
- 7.Ferry JD, Morrison PR. Preparation and properties of serum and plasma proteins; the conversion of human fibrinogen to fibrin under various conditions. J Am Chem Soc 1947;69(02):388–400 [DOI] [PubMed] [Google Scholar]
- 8.Blombäck B, Okada M. Fibrin gel structure and clotting time. Thromb Res 1982;25(1–2):51–70 [DOI] [PubMed] [Google Scholar]
- 9.Blombäck B, Carlsson K, Hessel B, Liljeborg A, Procyk R, Aslund N. Native fibrin gel networks observed by 3D microscopy, permeation and turbidity. Biochim Biophys Acta 1989;997(1–2):96–110 [DOI] [PubMed] [Google Scholar]
- 10.Kurniawan NA, Grimbergen J, Koopman J, Koenderink GH. Factor XIII stiffens fibrin clots by causing fiber compaction. J Thromb Haemost 2014;12(10):1687–1696 [DOI] [PubMed] [Google Scholar]
- 11.Piechocka IK, Jansen KA, Broedersz CP, Kurniawan NA, MacKintosh FC, Koenderink GH. Multi-scale strain-stiffening of semiflexible bundle networks. Soft Matter 2016;12(07):2145–2156 [DOI] [PubMed] [Google Scholar]
- 12.Leonidakis KA, Bhattacharya P, Patterson J, et al. Fibrin structural and diffusional analysis suggests that fibers are permeable to solute transport. Acta Biomater 2017;47:25–39 [DOI] [PubMed] [Google Scholar]
- 13.Ferri F, Calegari GR, Molteni M, Cardinali B, Magatti D, Rocco M. Size and density of fibers in fibrin and other filamentous networks from turbidimetry: beyond a revisited Carr–Hermans method, accounting for fractality and porosity. Macromolecules 2015;48(15):5423–5432 [Google Scholar]
- 14.Undas A, Ariëns RA. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol 2011;31(12):e88–e99 [DOI] [PubMed] [Google Scholar]
- 15.Mills JD, Ariëns RA, Mansfield MW, Grant PJ. Altered fibrin clot structure in the healthy relatives of patients with premature coronary artery disease. Circulation 2002;106(15):1938–1942 [DOI] [PubMed] [Google Scholar]
- 16.Undas A, Podolec P, Zawilska K, et al. Altered fibrin clot structure/function in patients with cryptogenic ischemic stroke. Stroke 2009;40(04):1499–1501 [DOI] [PubMed] [Google Scholar]
- 17.Pera J, Undas A, Topor-Madry R, Jagiella J, Klimkowicz-Mrowiec A, Slowik A. Fibrin clot properties in acute stroke: what differs cerebral hemorrhage from cerebral ischemia? Stroke 2012;43(05):1412–1414 [DOI] [PubMed] [Google Scholar]
- 18.Siudut J, Świat M, Undas A. Altered fibrin clot properties in patients with cerebral venous sinus thrombosis: association with the risk of recurrence. Stroke 2015;46(09):2665–2668 [DOI] [PubMed] [Google Scholar]
- 19.Undas A, Celinska-Löwenhoff M, Löwenhoff T, Szczeklik A. Statins, fenofibrate, and quinapril increase clot permeability and enhance fibrinolysis in patients with coronary artery disease. J Thromb Haemost 2006;4(05):1029–1036 [DOI] [PubMed] [Google Scholar]
- 20.Undas A, Zawilska K, Ciesla-Dul M, et al. Altered fibrin clot structure/function in patients with idiopathic venous thromboembolism and in their relatives. Blood 2009;114(19):4272–4278 [DOI] [PubMed] [Google Scholar]
- 21.Undas A, Zalewski J, Krochin M, et al. Altered plasma fibrin clot properties are associated with in-stent thrombosis. Arterioscler Thromb Vasc Biol 2010;30(02):276–282 [DOI] [PubMed] [Google Scholar]
- 22.Undas A, Nowakowski T, Cieśla-Dul M, Sadowski J. Abnormal plasma fibrin clot characteristics are associated with worse clinical outcome in patients with peripheral arterial disease and thromboangiitis obliterans. Atherosclerosis 2011;215(02): 481–486 [DOI] [PubMed] [Google Scholar]
- 23.Undas A, Stepien E, Tracz W, Szczeklik A. Lipoprotein(a) as a modifier of fibrin clot permeability and susceptibility to lysis. J Thromb Haemost 2006;4(05):973–975 [DOI] [PubMed] [Google Scholar]
- 24.Bouman AC, McPherson H, Cheung YW, et al. Clot structure and fibrinolytic potential in patients with post thrombotic syndrome. Thromb Res 2016;137:85–91 [DOI] [PubMed] [Google Scholar]
- 25.Teo K, Chow CK, Vaz M, Rangarajan S, Yusuf S; PURE Investigators-Writing Group. The Prospective Urban Rural Epidemiology (PURE) study: examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am Heart J 2009;158(01):1–7 [DOI] [PubMed] [Google Scholar]
- 26.Sathiyakumar V, Park J, Golozar A, et al. Fasting versus nonfasting and low-density lipoprotein cholesterol accuracy. Circulation 2018;137(01):10–19 [DOI] [PubMed] [Google Scholar]
- 27.Uitte de Willige S, de Visser MC, Houwing-Duistermaat JJ, Rosendaal FR, Vos HL, Bertina RM. Genetic variation in the fibrinogen gamma gene increases the risk for deep venous thrombosis by reducing plasma fibrinogen gamma’ levels. Blood 2005;106(13): 4176–4183 [DOI] [PubMed] [Google Scholar]
- 28.Lisman T, de Groot PG, Meijers JC, Rosendaal FR. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood 2005;105(03):1102–1105 [DOI] [PubMed] [Google Scholar]
- 29.Pieters M, Undas A, Marchi R, De Maat MP, Weisel J, Ariëns RA; Factor XIII And Fibrinogen Subcommittee Of The Scientific Standardisation Committee Of The International Society For Thrombosis And Haemostasis. An international study on the standardization of fibrin clot permeability measurement: methodological considerations and implications for healthy control values. J Thromb Haemost 2012;10(10):2179–2181 [DOI] [PubMed] [Google Scholar]
- 30.Mosesson MW, DiOrio JP, Siebenlist KR, Wall JS, Hainfeld JF. Evidence for a second type of fibril branch point in fibrin polymer networks, the trimolecular junction. Blood 1993;82(05): 1517–1521 [PubMed] [Google Scholar]
- 31.Siebenlist KR, Mosesson MW. Progressive cross-linking of fibrin gamma chains increases resistance to fibrinolysis. J Biol Chem 1994;269(45):28414–28419 [PubMed] [Google Scholar]
- 32.Blombäck B Fibrinogen and fibrin–proteins with complex roles in hemostasis and thrombosis. Thromb Res 1996;83(01):1–75 [DOI] [PubMed] [Google Scholar]
- 33.Weisel JW. Structure of fibrin: impact on clot stability. J Thromb Haemost 2007;5(Suppl 1):116–124 [DOI] [PubMed] [Google Scholar]
- 34.Campbell RA, Overmyer KA, Selzman CH, Sheridan BC, Wolberg AS. Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. Blood 2009;114(23): 4886–4896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carr ME Jr, Alving BM. Effect of fibrin structure on plasmin-mediated dissolution of plasma clots. Blood Coagul Fibrinolysis 1995;6(06):567–573 [DOI] [PubMed] [Google Scholar]
- 36.Collet JP, Woodhead JL, Soria J, et al. Fibrinogen Dusart: electron microscopy of molecules, fibers and clots, and viscoelastic properties of clots. Biophys J 1996;70(01):500–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fatah K, Silveira A, Tornvall P, Karpe F, Blombäck M, Hamsten A. Proneness to formation of tight and rigid fibrin gel structures in men with myocardial infarction at a young age. Thromb Haemost 1996;76(04):535–540 [PubMed] [Google Scholar]
- 38.Machlus KR, Cardenas JC, Church FC, Wolberg AS. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood 2011;117(18):4953–4963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Undas A, Slowik A, Wolkow P, Szczudlik A, Tracz W. Fibrin clot properties in acute ischemic stroke: relation to neurological deficit. Thromb Res 2010;125(04):357–361 [DOI] [PubMed] [Google Scholar]
- 40.Zolcinski M, Ciesla-Dul M, Undas A. Effects of atorvastatin on plasma fibrin clot properties in apparently healthy individuals and patients with previous venous thromboembolism. Thromb Haemost 2012;107(06):1180–1182 [DOI] [PubMed] [Google Scholar]
- 41.Skrzydlewski Z Coupling of fibrin with low density lipoproteins. Acta Med Acad Sci Hung 1976;33(02):171–177 [PubMed] [Google Scholar]
- 42.Kim JA, Kim JE, Song SH, Kim HK. Influence of blood lipids on global coagulation test results. Ann Lab Med 2015;35(01):15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhasin N, Ariëns RA, West RM, Parry DJ, Grant PJ, Scott DJ. Altered fibrin clot structure and function in the healthy first-degree relatives of subjects with intermittent claudication. J Vasc Surg 2008;48(06):1497–1503 [DOI] [PubMed] [Google Scholar]
- 44.Fatah K, Hamsten A, Blombäck B, Blombäck M. Fibrin gel network characteristics and coronary heart disease: relations to plasma fibrinogen concentration, acute phase protein, serum lipoproteins and coronary atherosclerosis. Thromb Haemost 1992;68(02):130–135 [PubMed] [Google Scholar]
- 45.Puccetti L, Pasqui AL, Pastorelli M, et al. Different mechanisms of fibrinolysis impairment among dyslipidemic subjects. Int J Clin Pharmacol Res 2001;21(3–4):147–155 [PubMed] [Google Scholar]
- 46.Kunz F, Pechlaner C, Erhart R, Zwierzina WD, Kemmler G. Increased lipid binding to thrombi in coronary artery disease. Findings in patients without premedication in native (not anti-coagulated) test systems. Arterioscler Thromb 1992;12(12): 1516–1521 [DOI] [PubMed] [Google Scholar]
- 47.Simon DI, Fless GM, Scanu AM, Loscalzo J. Tissue-type plasminogen activator binds to and is inhibited by surface-bound lipoprotein(a) and low-density lipoprotein. Biochemistry 1991;30(27): 6671–6677 [DOI] [PubMed] [Google Scholar]
- 48.Azizova OA, Roitman EV, Dement’eva II, Nikitina NA, Gagaeva EV, Lopukhin YM. Effects of low-density lipoproteins on blood coagulation and fibrinolytic activity. Bull Exp Biol Med 2000;129(06): 541–544 [DOI] [PubMed] [Google Scholar]
- 49.Ariëns RA. Novel mechanisms that regulate clot structure/function. Thromb Res 2016;141(Suppl 2):S25–S27 [DOI] [PubMed] [Google Scholar]
- 50.Domingues MM, Macrae FL, Duval C, et al. Thrombin and fibrinogen γ′ impact clot structure by marked effects on intrafibrillar structure and protofibril packing. Blood 2016;127(04):487–495 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


