Abstract
Introduction
Wolfram syndrome (WFS) is a neurodegenerative disorder characterized by childhood-onset diabetes mellitus, optic nerve atrophy, diabetes insipidus, hearing impairment and commonly bladder and bowel dysfunction. We hypothesize that there is an association between smaller pons, which contains the pontine micturition center, and abnormal lower urinary tract function.
Materials and Methods
Patients with genetically confirmed WFS attend an annual multidisciplinary research clinic. Patients undergo non-invasive urodynamic testing, brain MRI and complete validated patient-reported outcome measures. Bowel and bladder diaries are filled out prior to visits. Age and sex-corrected linear and logistic mixed-effects models are used to correlate pons volume, corrected for whole brain size, to urodynamic and patient-reported outcomes.
Results
36 patients attended 142 visits between 2010 and 2016. Mean age was 16.9 years (range 7–30) and 64% were female. Functional bladder capacity was decreased in 31%, normal in 54%, and increased in 14%. 44% and 54% had abnormal uroflowmetry and post-void residual, respectively, on at least one occasion. There was no increase over time in incidence of lower urinary tract dysfunction. Lower pons volume was associated with increased post-void residual (p = 0.048) and with higher Pediatric Incontinence Quality of Life score (p = 0.011), indicating lower quality of life and higher levels of dysfunction.
Conclusions
A significant number of children, adolescents and young adults with WFS have objective evidence of lower urinary tract dysfunction. Lower pons volume was associated with more abnormal urinary function and lower quality of life in WFS patients.
Keywords: Wolfram Syndrome, urinary function, non-invasive urodynamics, pontine micturition center
Introduction
Wolfram syndrome (WFS) is a rare, autosomal recessive disease characterized by early childhood onset diabetes, optic nerve atrophy, vision and hearing loss, diabetes insipidus, and commonly bowel and bladder dysfunction (BBD).1,2 WFS patients have reduced life expectancy due in some cases to brainstem atrophy-induced respiratory failure.3 Brainstem neurodegeneration may uniquely explain the high prevalence of BBD in WFS patients, unlike most BBD seen in otherwise young, healthy patients.4–6 The causative gene, WFS1, encodes a particular endoplasmic reticulum protein, the mutation of which leads to stress-mediated apoptosis.7–10 Pancreatic β-cells are susceptible, but WFS1 is also expressed throughout the brain, and neuronal cell death underlies neurodegeneration in WFS, leading to the constellation of symptoms found in these patients.9,11,12 Elucidation and treatment of this disorder may shed light on other neurodegenerative diseases known to affect urinary function and quality of life, including multiple sclerosis, Alzheimer disease, and Parkinson disease.
Historically, WFS patients were described as having marked dilation of the upper and lower urinary tracts—often labeled megacystis—and this was originally attributed to polyuria from diabetes insipidus.13,14 More recently, however, groups have recognized that WFS patients exhibit a broad spectrum of lower urinary tract phenotypes and voiding behaviors with poor correlation between diabetes insipidus and urinary tract dilation.13,15 As such, BBD likely represents a primary manifestation of the underlying disease process (progressive neurodegeneration), rather than a secondary one.14,16
To date, longitudinal assessments of urinary function in WFS have not been reported. Prior studies have shown there to be decreased volumes in the brain, particularly in the pons, in WFS patients. The goal of the current study is to determine the pattern of urological abnormalities in WFS longitudinally using non-invasive urodynamic instruments as they relate to brain imaging and patient-reported outcomes (PROs). We hypothesize there will be a significant association between pons volume, corrected for whole brain size, and various urodynamic and quality of life measures.
Methods
This study was approved by the Human Research Protection Office at Washington University in St. Louis. All participants provided written informed consent before testing. For children under age 18, parents/guardians gave written consent and children assented to testing.
Participants
WFS patients were a part of a longitudinal natural history study involving annual data collection at the Washington University WFS Research Clinic, primarily recruited from a registry website (http://wolframsyndrome.dom.wustl.edu).2 Patients were required to be age 30 or younger and have genetically confirmed WFS (mutations of the WFS1 gene). Participants filled out questionnaires and were evaluated by a multi-disciplinary team of investigators including a pediatric neurologist, audiologist, endocrinologist, ophthalmologist and urologist. In addition, magnetic resonance imaging (MRI) was conducted on those patients without contraindications. Some of these data have been previously reported.6,17 Data from urology exams and MRI are presented in this paper.
Urological Testing
Patients underwent non-invasive urodynamic testing including pre- and post-void pelvic ultrasound and uroflowmetry. Bladder volumes were estimated using the formula for a prolate ellipsoid (bladder length × width × height × 0.52 in mL), and a post-void residual (PVR) was calculated. Subjective symptoms such as urinary frequency, urgency, and urinary/fecal incontinence were recorded. Patients were contacted prior to clinic and provided instructions on completing a 48-hour urine frequency-volume chart and 7-day bowel and bladder diary. Participants were invited to fill out three validated PROs, including the Pediatric Incontinence Quality of Life (PinQ), Toronto Bowel and Bladder Dysfunction, and Compass-31 questionnaires.18–20
Urodynamic Definitions
Functional bladder capacity (FBC) provides a general estimate for actual bladder capacity and was recorded as the maximum value of one of the following: voided volume on uroflowmetry, calculated volume on pre-void pelvic ultrasound, or voided volume recorded on voiding diary. Estimated bladder capacity (EBC) was calculated using (age + 2) × 30 mL up to a maximum of 500 mL.21 FBC was denoted as either increased (≥ 133% EBC) or decreased (≤ 66% EBC).
Uroflowmetry was performed in all patients who could void spontaneously. Both quantitative and qualitative measures were recorded, including average flow, maximum flow, voided volume, curve shape (bell-shaped, tower, plateau, staccato, intermittent or other). A flow index was calculated for each patient using equations derived by Franco et al.22 Each test was categorized as either normal or disordered if voided volume was ≥ 50 mL. A disordered uroflow corresponded to flow indices < 0.7 for males and < 0.68 for females.
PVR was categorized as either normal or increased (≥ 10% EBC remaining in bladder). Patients were categorized as having polyuria on voiding diaries if 24-hour total voided volume was greater than 6 × EBC up to a maximum of 3,000 mL.
Magnetic Resonance Imaging
Scans were acquired on a Siemens 3T Tim Trio MRI scanner, including T1-weighted and T2-weighted images. To determine regional brain volumes, we used the semi-automatic segmentation program Freesurfer (v5.3) using previously published techniques.23 Regional brain volumes, including the pons, were corrected for whole brain size to allow for comparison between patients.6,17
Statistical Analysis
Study data were collected and managed using REDCap electronic data capture tools.24 Descriptive statistics were calculated. Age and sex-corrected linear or logistic mixed-effects models were used to correlate corrected pons volume to both continuous and dichotomous non-invasive urodynamic measures and PROs. Similar models were created to determine if these measures worsened over time. Mixed effects models account for multiple measures from the same patient. Two-sided p values < 0.05 were considered significant. All analyses were performed with R, version 3.4.3.
Results
36 unique patients were evaluated at 142 separate research visits between 2010 and 2016. In the most recent year, mean age was 16.9 years (range 7–30, Table 1). 61% of patients were diagnosed with diabetes insipidus at a median age of 11.2 years (range 5.5–22), and objective evidence of polyuria was noted in 29% of patients. Three perform clean-intermittent catheterization to assist with bladder emptying (each via continent catheterizable channels).
Table 1.
Demographics, comorbidities, non-invasive urodynamics, voiding diaries and results of patient-reported outcomes (PROs). PinQ = Pediatric Incontinence Quality of Life questionnaire, BBD = bowel and bladder dysfunction
| All patients |
||
|---|---|---|
| n | % | |
| Unique patients | 36 | 100% |
| Total visits | 142 | 100% |
| Demographics | ||
| Age (range) | 16.9 years (7.2–30.7) | |
| F | 23 | 64% |
| M | 13 | 36% |
| Comorbidities | ||
| Diabetes mellitus | 33/36 | 92% |
| Diabetes insipidus | 22/36 | 61% |
| Optic atrophy | 34/36 | 94% |
| Hearing loss | 29/36 | 81% |
| Clean-intermittent catheterization | 3/36 | 8% |
| Polyuria (based on voiding diaries) | 7/24 | 29% |
| Non-invasive urodynamics | ||
| Uroflowmetry | 84 tests in 32 patients | |
| Normal uroflowmetry | 62 | 74% |
| Disordered uroflowmetry | 22 | 26% |
| Unique patients with any disordered uroflow | 15 | 44% |
| Post-void residual | 96 tests in 35 patients | |
| Normal post-void residual | 65 | 68% |
| Increased post-void residual | 31 | 32% |
| Unique patients with any increased PVR | 19 | 54% |
| Functional bladder capacity | 70 measurements in 34 patients | |
| Decreased | 22 | 31% |
| Normal | 38 | 54% |
| Increased | 10 | 14% |
| Diaries | ||
| Voiding diary | 57 diaries in 23 patients | |
| Stool diary | 27 diaries in 17 patients | |
| Mean voids per day (range) | 5.7 voids (1–14) | |
| Mean stools per day (range) | 2.1 stools (0.3–5.0) | |
| Mean Bristol stool score (range) | 4 (1–7) | |
| Proportion hard (Bristol 1–2) | 48 | 23% |
| Proportion normal (Bristol 3–4) | 71 | 34% |
| Proportion loose (Bristol 5–7) | 90 | 43% |
| Questionnaires | ||
| PinQ (max score = 80) | 70 questionnaires in 31 patients | |
| Mean score (range) | 10.1 (0–67) | |
| Toronto BBD (max score = 30) | 71 questionnaires in 32 patients | |
| Mean score (range) | 11.7 (1–31) | |
| Compass-31 (max score = 31) | 41 questionnaires in 28 patients | |
| Mean score (range) | 16.4 (0–31) | |
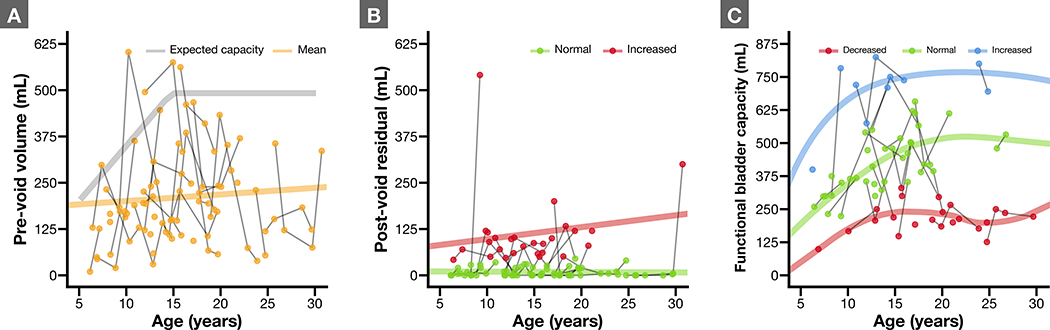
Pre- and post-void volumes are shown for each patient over time in Figures 1A and B. Not unexpectedly, patients arrived at visits with widely varying bladder volumes that do not necessarily indicate true functional capacity. Post-void, there was a significant fraction of patients with incomplete emptying and elevated PVR (32% of all tests or 54% of patients at any time point).
Figure 1.
Scatterplots of various important measures. Individual patients have multiple values shown over time, connected with a solid line. A) Plot of WFS patients age versus pre-void bladder volumes. Expected capacity (as a function of age) is shown in gray for comparison. Mean across the whole population is shown as solid line and increases slightly over time as expected. B) Post-void residual bladder volumes. C) Functional bladder capacity for all patients, categorized as either decreased (< 66% estimated bladder capacity), normal, or increased (> 133% estimated bladder capacity).
FBC was decreased in 41% (n = 14), normal in 65% (n = 22) or increased in 21% (n = 7) (Figure 1C). Totals are greater than 100% as patients with repeat testing fell into different categories during subsequent visits. Despite some crossover, FBC did not vary significantly across time for individual patients (p = 0.90). 23 patients returned 57 voiding diaries, indicating normal daytime voiding patterns only about half the time (47%).
21 patients underwent two or more MRIs, and 76% experienced deterioration in pons volume corrected for whole brain size between their first and last scan (p = 0.002 by paired Wilcoxon signed-rank test). Abnormal objective non-invasive urodynamic measures were associated with lower pons volumes, across the different testing modalities, although only significantly so for increased PVR (p = 0.048 by logistic mixed effects modeling). Table 2 shows that other objective and subjective measures did not covary significantly on linear or logistic mixed effects modeling.
Table 2.
List of linear mixed effects models (for continuous variables) and logistic mixed effects models (for dichotomous variables). All models were adjusted for age and sex. Bolded and red values indicate statistical significance (p < 0.05). PinQ = Pediatric Incontinence Quality of Life questionnaire, BBD = bowel and bladder dysfunction, QmaxFI = maximum flow index, QavgFI = mean flow index
|
Linear mixed-effects models |
||||
| Estimate (increase in 100 units of pons volume) | P value (adjusted for age and sex) | Observations | Unique patients | |
| Post-void bladder volume | −0.077 | 0.972 | 64 | 24 |
| Functional bladder capacity | 3.341 | 0.263 | 47 | 24 |
| Maximum urine flow index (QmaxFI) | 0.005 | 0.280 | 56 | 22 |
| Mean urine flow index (QavgFI) | −0.003 | 0.577 | 43 | 22 |
| Voids per day | −0.026 | 0.664 | 27 | 15 |
| Bowel movements per day | 0.021 | 0.186 | 16 | 10 |
| Bristol stool score | −0.002 | 0.868 | 16 | 10 |
| PinQ score | −0.442 | 0.011 | 40 | 20 |
| Toronto BBD score | −0.218 | 0.062 | 41 | 21 |
| Compass-31 score | −0.187 | 0.254 | 22 | 16 |
| Hemoglobin A1c | −0.024 | 0.219 | 96 | 25 |
|
Logistic mixed-effect models |
||||
| Odds ratio (increase in 100 units of total pons volume) | P value (adjusted for age and sex) | Observations | Unique patients | |
| Increased post-void residual | 0.958 | 0.048 | 64 | 24 |
| Disordered uroflowmetry | 0.985 | 0.614 | 56 | 22 |
| Subjective urinary frequency * | 0.902 | 0.219 | 21 | 11 |
| Subjective urinary urgency | 0.873 | 0.089 | 17 | 10 |
| Subjective urinary incontinence | 1.055 | 0.646 | 17 | 10 |
| Diagnosis of diabetes insipidus | 0.208 | 0.046 | 97 | 25 |
| Objective polyuria on voiding diary | 0.913 | 0.148 | 30 | 15 |
Models devolved into fixed effects models
Similar mixed effects models were created to determine if any measures changed over time for individual patients. Increased PVR, disordered uroflowmetry, polyuria, subjective symptoms of urgency and incontinence did not change significantly over time for individual patients. Maximum urine flow index (QmaxFI) decreased significantly over time (−0.033 [p = 0.007]). Compass-31 scores did increase (3.0 point increase per year, p = 0.049). Compass-31 is a measure of autonomic dysfunction, with higher scores indicating worsening function. Additionally, a small but significant increase in hemoglobin A1c was noted (0.1% increase per year, p = 0.034).
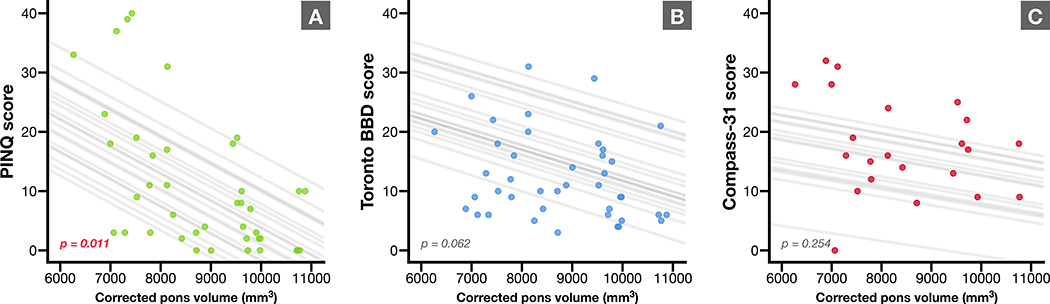
Figure 2 shows a strong inverse correlation on linear mixed effects modelling, adjusting for age and sex, between PROs and corrected pons volumes. Higher scores indicate lower quality of life and higher levels of dysfunction across the domains each test, and these higher scores were associated with smaller pons on MRI. The relationship between PinQ and corrected pons volume was statistically significant (p = 0.011).
Figure 2.
Scatterplots of pons volumes corrected for whole brain volume on annual MRI correlated to three validated patient-reported outcome measures, including A) Pediatric Incontinence Quality of Life (PinQ), B) Toronto Bowel and Bladder score, and C) Compass-31 questionnaire, which assesses autonomic function. Trendlines correspond to individual patients in each linear mixed-model, adjusted for age and sex (projected using mean values of non-visualized variables). P value for each model is shown.
As part of the urological workup, bowel function was assessed with stool diaries. Stool frequency and consistency using the Bristol Stool Scale were obtained for 17 patients (27 diaries).25 Bowel function proved to be highly variable, with 50% noting issues with constipation (Bristol 1–2) or encopresis. Contrasted with normal, ideal bowel function (soft, daily Bristol 3–4 bowel movement), we observed marked variation in WFS patients.
Discussion
This study offers several new, unique insights into BBD in WFS patients followed longitudinally over a period of seven years. We assessed patients with a combination of non-invasive urodynamics, bowel and bladder diaries, MRI scans of the brain and brainstem, and patient-reported outcomes (PROs), and we demonstrated that WFS patients exhibit a wide spectrum of voiding phenotypes and showed a significant association between lower pons volume (corrected for whole brain volume), increased PVR, and decreased quality of life, thus confirming our original hypothesis. Our findings significantly support the relationship between neuro-anatomical alterations with quality of life as it relates to bladder function. The PinQ is a validated pediatric questionnaire that measures the emotional impact of bladder function and dysfunction, has been tested across different cultural and language settings, and showed significant correlation between higher scores (worse function) and lower pons volumes.18
Past studies have shown that brain volumes are significantly lower in WFS patients compared to either diabetic or heathy age-matched controls, and this disproportionately affects the brainstem and pons, where Barrington’s nucleus (also known as the pontine micturition center) is located.4,6,17 As voiding and bowel function are inter-related and both rely on a complex network of volitional and spinobulbospinal reflexes to function properly, neurodegenerative disease processes like WFS produce varied elimination patterns. The data demonstrate this well, reinforcing the downstream effects of the WFS disease process: namely that underlying cell stress dysregulation leads to neurodegeneration and cell death, thus producing features commonly seen in WFS patients, including BBD. Interestingly, although pons volumes did trend downward over time in our patients, objective measures of urinary function did not appear to change within individual patients. This might be due to small number of patients, insufficient follow up, diffusion of downstream effects of the underlying primary disease process, or contributions from other factors to the observed elimination phenotype.
Tekgül et al provided the first report to disprove that all WFS patients shared a common urinary tract pathology. The authors described three distinct bladder types (large and atonic, normal, or small and non-compliant), emphasizing the wide spectrum of urologic pathology.16 Other groups described the use of formal urodynamics and pressure-flow studies, noting that detrusor-sphincter dyssynergia was present in some patients, perhaps explaining upper urinary tract dilation.26,27 Ribière and colleagues took this a step further with the largest description of urinary symptoms, collected by telephone interview, noting that 22 of 33 patients voided spontaneously but a large proportion (73%) exhibited ongoing urinary symptoms, many characterized as severe.28 While in the past, urinary tract dilation was felt to be common and occur secondary to diabetes insipidus, it is now recognized that not all patients are affected by diabetes insipidus and there has been some suggestion that altered innervation of the urinary tract might also be responsible for hydroureteronephrosis.14
Wragg et al recently published the largest experience to date with assessment of urological features of a group of WFS patients at a single point in time through the use of non-invasive urodynamics, suggesting that patients progress to megacystis with age.15 Our data challenge this assertion. We captured urological assessments longitudinally over time, and Figure 1C shows that only 3 of 34 patients progressed from either a low or normal FBC to elevated FBC (> 130% of expected capacity), while two patients converted from high FBC to low or normal. 21% of WFS in our report were classified as having high FBC, and these patients tended to continue with the same picture over time and few patients progressed to megacystis. Their finding might be attributed to the fact that bladder capacity normally increases with age and thus, older patients will have larger bladders on average. Assessment over time is key to providing insight into ongoing dysfunction, as a clearer picture tends to emerge for individual patients. WFS patients studied in this same way reinforce this observation.
Treatment of BBD is largely the same as with non-WFS patients with similar complaints.27,29,30 WFS patients exhibited a significant degree of bowel dysfunction, fluctuating between constipation and diarrhea in a one-week time frame, mirroring past reports.29 This degree of bowel dysfunction is difficult to treat. Despite many WFS patients having instituted behavior modification and appropriate medications, objective evidence of BBD was still very evident and will require ongoing attention by a multi-disciplinary team.
Strengths of this analysis rest on the use of longitudinal, objective, non-invasive urodynamic data correlated to MRI findings and PROs in patients genetically-confirmed to have WFS. Non-invasive urodynamic testing of this sort, however, is subject to variability with regards to time of day, patient hydration, recent voiding and stooling behaviors, anxiety, or other undetected perturbations of the urinary track (e.g., urinary tract infection). Interpretation of urodynamic testing is also subject to inter- and intraobserver variation, although using calculated flow index to help normalize uroflometry data eliminates a great deal of interpretation subjectivity.22 We did not ascertain presence of upper tract dilation or relation to degree of diabetes insipidus or polyuria. Past reports have noted poor correlation between diabetes insipidus and hydronephrosis.16 Family and patient motivation to participate in this registry may also contribute bias to these results, as patients must often travel long distances and undergo several days of testing by various services. Other limitations include lack of correction for use of medications known to alter these parameters. Voiding and bowel diaries were filled out voluntarily, and response bias may have skewed these results.
Conclusion
Neurodegenerative diseases that impact areas of the brain responsible for the coordination of voiding, such as WFS, appear to result in a wide spectrum of lower urinary tract dysfunction. Patients with smaller pons volumes have worse urinary function and lower quality of life. Hopefully, as we march toward future therapies for WFS, this will provide a template for relief in other neurodevelopmental and neurodegenerative disorders known to affect urinary function.
Acknowledgements
We thank the Washington University Wolfram Study Group Members: P. Austin, MD (Surgery), B. Beato, BA (Psychiatry), E. Bihun, MA (Psychiatry), G. Earhart, PhD (Physical Therapy), S. Eisenstein, PhD (Psychiatry), J. Hoekel, DO (Ophthalmology), R. Karzon, PhD (Saint Louis Children’s Hospital), A. Licis, MD (Neurology), H. Lugar, MA (Psychiatry), L. Manwaring, MS (Pediatrics), A.R. Paciorkowski, MD (Neurology, URMC), T. Pearson, MD (Neurology), Y. Pepino de Gruev, PhD (Medicine), A. Permutt, MD (Medicine, Deceased), K. Pickett, PhD (Physical Therapy, U Wisconsin), A. Reiersen, MD (Psychiatry), J. Rutlin, BS (Psychiatry), J. Shimony, MD, PhD (Radiology), L. Tychsen, MD (Ophthalmology), F. Urano MD, PhD (Medicine), A. Viehoever, MD (Neurology, UCSF), J. Wasson, MS (Medicine, Deceased), and N. H. White MD, CDE (Pediatrics).
Funding
This work was supported by the NICHD (HD070855; Hershey, PI) and supported by CTSA (UL1 RR024992) and Diabetes Research Center (DK 020579), the Jack and J.T. Snow Foundation, American Diabetes Association (Permutt/Urano), the George Decker and Julio V. Santiago Pediatric Diabetes Research Fund, and the Mallinckrodt Institute of Radiology Pilot Fund (Hershey). This work was also supported by the Washington University Wolfram Syndrome Research Group.
Abbreviations
- BBD
bowel and bladder dysfunction
- DDVAP
desmopressin acetate
- EBC
estimated bladder capacity
- FBC
functional bladder capacity
- MRI
magnetic resonance imaging
- PinQ
Pediatric Incontinence Quality of Life questionnaire
- PROs
patient-reported outcomes
- PVR
post-void residual
- WFS
Wolfram Syndrome
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolfram DJ: Diabetes mellitus and simple optic atrophy among siblings: report of four cases. Proc Staff Meet Mayo Clin 1938; 13: 715. [Google Scholar]
- 2.Marshall BA, Permutt MA, Paciorkowski AR, et al. : Phenotypic characteristics of early Wolfram syndrome. Orphanet J Rare Dis 2013; 8: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett TG, Bundey SE, Fielder AR, et al. : Optic atrophy in Wolfram (DIDMOAD) syndrome. Eye (Lond) 1997; 11: 882–888. [DOI] [PubMed] [Google Scholar]
- 4.Fowler CJ, Griffiths D and de Groat WC: The neural control of micturition. Nat Rev Neurosci 2008; 9: 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Groat WC and Wickens C: Organization of the neural switching circuitry underlying reflex micturition. Acta Physiol 2012; 207: 66–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hershey T, Lugar HM, Shimony JS, et al. : Early Brain Vulnerability in Wolfram Syndrome. Edited byR Linden. PLoS ONE 2012; 7: e40604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue H, Tanizawa Y, Wasson J, et al. : A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat Genet 1998; 20: 143–148. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Inoue H, Tanizawa Y, et al. : WFS1 (Wolfram syndrome 1) gene product: predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Hum Mol Genet 2001; 10: 477–484. [DOI] [PubMed] [Google Scholar]
- 9.Ishihara H, Takeda S, Tamura A, et al. : Disruption of the WFS1 gene in mice causes progressive beta-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum Mol Genet 2004; 13: 1159–1170. [DOI] [PubMed] [Google Scholar]
- 10.Yamada T, Ishihara H, Tamura A, et al. : WFS1 -deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic beta-cells. Hum Mol Genet 2006; 15: 1600–1609. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto H, Hofmann S, Hamasaki DI, et al. : Wolfram syndrome 1 (WFS1) protein expression in retinal ganglion cells and optic nerve glia of the cynomolgus monkey. Exp Eye Res 2006; 83: 1303–1306. [DOI] [PubMed] [Google Scholar]
- 12.Rigoli L, Lombardo F and Di Bella C: Wolfram syndrome and WFS1 gene. Clin Genet 2010; 79: 103–117. [DOI] [PubMed] [Google Scholar]
- 13.Najjar SS, Saikaly MG, Zaytoun GM, et al. : Association of diabetes insipidus, diabetes mellitus, optic atrophy, and deafness. The Wolfram or DIDMOAD syndrome. Arch Dis Child 1985; 60: 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu P, Staff WG, Morris JA, et al. : DIDMOAD syndrome with megacystis and megaureter. Postgrad Med J 1986; 62: 859–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wragg R, Dias RP, Barrett T, et al. : Bladder dysfunction in Wolfram syndrome is highly prevalent and progresses to megacystis. J Ped Surg 2017; 53: 321–325. [DOI] [PubMed] [Google Scholar]
- 16.Tekgül S, Oge O, Simşek E, et al. : Urological manifestations of the Wolfram syndrome: observations in 14 patients. J Urol 1999; 161: 616–617. [PubMed] [Google Scholar]
- 17.Lugar HM, Koller JM, Rutlin J, et al. : Neuroimaging evidence of deficient axon myelination in Wolfram syndrome. Scientific Reports 2016 6 2016; 6: 21167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bower WF, Sit FKY, Bluyssen N, et al. : PinQ: a valid, reliable and reproducible quality-of-life measure in children with bladder dysfunction. J Pediatr Urol 2006; 2: 185–189. [DOI] [PubMed] [Google Scholar]
- 19.Farhat W, Bägli DJ, Capolicchio G, et al. : The dysfunctional voiding scoring system: quantitative standardization of dysfunctional voiding symptoms in children. J Urol 2000; 164: 1011–1015. [DOI] [PubMed] [Google Scholar]
- 20.Sletten DM, Suarez GA, Low PA, et al. : COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc 2012; 87: 1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koff SA: Estimating bladder capacity in children. Urology 1983; 21: 248. [DOI] [PubMed] [Google Scholar]
- 22.Franco I, Shei-Dei Yang S, Chang S-J, et al. : A quantitative approach to the interpretation of uroflowmetry in children. Neurourol Urodyn 2016; 35: 836–846. [DOI] [PubMed] [Google Scholar]
- 23.Fischl B, Salat DH, Busa E, et al. : Whole brain segmentation: automated labeling of neuro-anatomical structures in the human brain. Neuron 2002; 33: 341–355. [DOI] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis SJ and Heaton KW: Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997; 32: 920–924. [DOI] [PubMed] [Google Scholar]
- 26.Aboseif S, Gasparini M, Schmidt R, et al. : Wolfram’s (DIDMOAD) Syndrome and its Urological Manifestation. Br J Urol 1993. 72: 106–111. [DOI] [PubMed] [Google Scholar]
- 27.Simşek E, Simsek T, TekgüT S, et al. : Wolfram (DIDMOAD) syndrome: a multidisciplinary clinical study in nine Turkish patients and review of the literature. Acta Paediatrica 2007; 92: 55–61. [DOI] [PubMed] [Google Scholar]
- 28.Ribière C, Kaboré FA, Chaussenot A, et al. : Troubles vésicosphinctériens au cours du syndrome de Wolfram. Prog Urol 2013; 23: 519–523. [DOI] [PubMed] [Google Scholar]
- 29.Barrett TG, Bundey SE and Macleod AF: Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet 1995; 346: 1458–1463. [DOI] [PubMed] [Google Scholar]
- 30.Santos Dos J, Varghese A, Williams K, et al. : Recommendations for the management of bladder bowel dysfunction in children. Pediatr Ther 2014: 1–11. [Google Scholar]