Abstract
Background: Recent research indicates that shift work is associated with neurocognitive function. However, studies that examine the association between shift work and neurocognitive function in firefighters have not yet been performed. We examined the effect of shift work on neurocognitive function in firefighters by measuring and comparing neurocognitive function before and after night shift. Methods: 352 firefighters from eight fire stations in South Korea were included in this study. We performed neurocognitive function test using central nervous system vital signs (CNSVS) during daytime work and on the next day after night work. We performed paired t-tests to assess differences between neurocognitive function before and after night work. We also compared neurocognitive function in insomnia and depression. We used a general linear model to analyze the associations between shiftwork schedule and the changes in neurocognitive function. Results: The neurocognitive function significantly decreased in six domains (composite memory, verbal memory, visual memory, complex attention, psychomotor speed, and motor speed) as did the neurocognitive index on the next day after night work compared with during day work. These decreased domains were the same following night work regardless of the type of shift work. Conclusion: Night work in firefighters may cause neurocognitive decline.
Keywords: firefighter, shift work, neurocognitive function, sleep deprivation, CNSVS
1. Introduction
Firefighters in South Korea are responsible not only for fire suppression but also for various accident-related rescue and emergency activities and preparation for large-scale disasters and on-site response [1]. Firefighters have an increased risk of exposure to toxic gases (e.g., carbon monoxide and phosgene) and heat stress at fire sites [2]. Firefighters also suffer from high levels of job stress due to the mental tension caused by waiting time in the field and the sleep chronic deprivation caused by shift work. In fact, firefighters have higher risks of psychological problems, such as depression [3] and post-traumatic stress disorder (PTSD) [4,5,6] compared with the general population. Many firefighters suffer from sleep disorders due to exposure to shift work [7,8].
These risk factors in firefighters may also be related to neurocognitive function. Both the heat stress in a hot environment [9,10,11] and the exposure to higher stress levels [12,13] can affect neurocognitive function. Firefighters are at high-risk of PTSD [14] and depression [15,16,17], and study results indicate that these conditions are associated with cognitive impairment. In particular, results of recent studies suggest that shift work can also affect neurocognitive function [18,19,20,21]. Shift work-associated disruptions in circadian rhythms are associated with neurodegeneration [22,23]. In support of this hypothesis, one animal model experiment found that deletion of the master clock gene Bmal1 in the mouse brain results in an increase in neuronal oxidative damage [24]. A firefighter who is engaged in long-term shift work may be at risk of cognitive decline. An experimental study performed by Rodrigues et al. found that cognitive performance in firefighters decreases after exposure to increased levels of stress [25]. However, the effects of shift work on neurocognitive function in firefighters have not yet been studied.
This study aimed to investigate the effects of shift work on neurocognitive function in firefighters by measuring and comparing cognitive functions before and after they worked the night shift.
2. Materials and Methods
2.1. Study Subjects
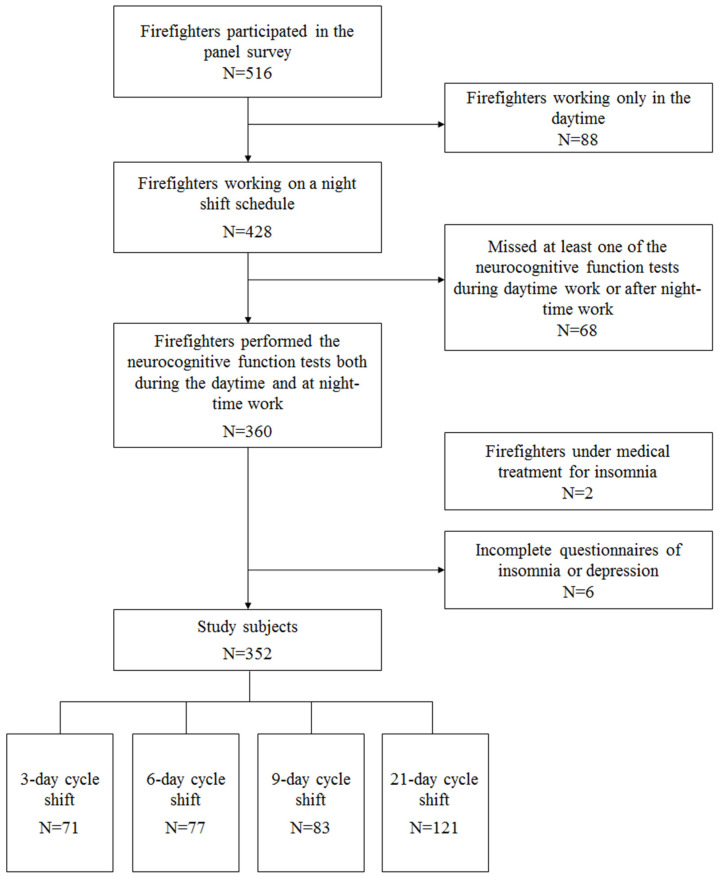
This study was included in the SLEep Panel Study (SLEPS). The purpose of SLEPS was to investigate sleep problems among Korean firefighters. Using the two means formula for continuous variables, we calculated the sample size before conducting the SLEPS. The calculation was carried out using G*power version 3.1.9.7. When the α error was set to 0.05 and the statistical power (1–β error) to 80%, the calculated sample size was 124. In order to perform a stratified analysis in respect to sex, age group, department, job position, and shift work schedule, a sample size of 8928 was required; however, the final targeted sample size needed to be determined considering the budget constraint and time needed to construct the panel. A final target sample size of 500 was decided upon, and 516 firefighters were recruited to participate in the SLEPS. From 2017 to 2018, we conducted the questionnaire survey on a panel of 516 firefighters from eight fire stations in South Korea and performed neurocognitive function tests. Among the 516 firefighters in the panel, 88 who worked only in the daytime (i.e., no night shift work) were excluded from the analysis. We also excluded 68 firefighters who missed at least one of the neurocognitive function tests during daytime work or after nighttime work. Two firefighters being treated for insomnia by a physician were excluded because this condition could affect the results of neurocognitive function tests. Six firefighters who did not complete the insomnia or depression questionnaires were also excluded from the study. Finally, the data from 352 subjects who were currently working on a night shift schedule were included in the analysis (Figure 1).
Figure 1.
Flow chart of the study subjects.
2.2. Type of Shift Work
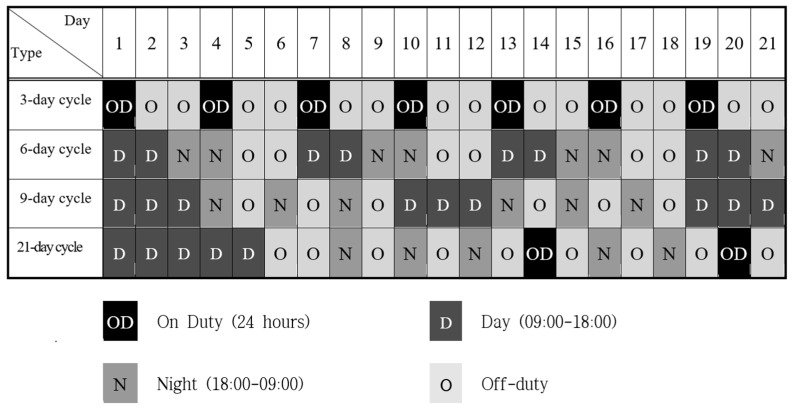
Firefighters in South Korea are typically subjected to 3-, 6-, 9-, or 21-day cycle shift work. In this study, the 3-day cycle shift work schedule consisted of a full day (24 h) of work, followed by 2 days off-duty. The 6-day cycle shift work schedule consisted of 2 days of daytime work (9:00 a.m.–6:00 p.m.), followed by 2 days of nighttime work (6:00 p.m.–9:00 a.m.) and 2 days off-duty. The 9-day cycle shift work schedule consisted of 3 days of daytime work (9:00 a.m.–6:00 p.m.), followed by three consecutive sets of nighttime work and off-duty. The 21-day schedule consisted of 5 days of daytime work (9:00 a.m.–6:00 p.m.), followed in order by 2 days off-duty, three consecutive sets of nighttime work and off-duty, a full day (24 h) of work, two consecutive sets of off-duty and nighttime work, 1 day off-duty, a full day (24 h) of work, and 1 day off-duty (Figure 2).
Figure 2.
Shift work cycles of firefighters in South Korea.
2.3. Questionnaire
During the panel survey, each subject was asked to complete a self-reported questionnaire. Data on age, sex, department and job position, monthly income, education level, task, type of shift work, history of illness, family medical history, history of smoking and drinking, physical activity, and symptoms related to the target organs were collected.
2.4. Sleep Disorder and Depression Evaluation Tools
The Insomnia Severity Index (ISI) [26,27] was used to measure insomnia. The ISI is a self-reported questionnaire that consists of seven questions used to evaluate degrees of difficult sleep onset and sleep maintenance, satisfaction with current sleep patterns, interference with daily functioning, noticeability of impairments attributed to sleep problems, and degrees of distress or concern caused by the sleep problem [28]. Each question was scored between 0 and 4. A higher score indicated a more severe status. An ISI score ≥8 indicated the presence of a mild sleep disorder and an ISI score ≥15 indicated the presence of sleep disorder [29]. Patient Health Questionnaire-9 (PHQ-9) [30] was used to evaluate each subject’s depressive symptoms. Each item scores between 0 and 3 points for 9 depressive symptoms during the last 2 weeks. A PHQ-9 score ≥5 indicated the presence of mild depression and a score ≥10 indicated the presence of depression [31]. The Korean versions of the ISI and PHQ-9 have been validated by past studies [32,33].
2.5. Neurocognitive Function Testing
Neurocognitive function testing was based on the use of central nervous system vital signs (CNSVS). The CNSVS is a computerized neurocognitive test battery that was developed as a screening instrument for neurocognitive impairment; test validity and reliability have been verified [34]. CNSVS calculates standardized scores for the domains of composite memory, verbal memory, visual memory, complex attention, psychomotor speed, motor speed, processing speed, reaction time, cognitive flexibility, executive functioning, and neurocognitive index [35]. The standardized CNSVS score is calculated via a process of data standardization; the data are stratified by 10 age groups, and an average value of 100 and standard deviation of 15 are used.
To evaluate the change in scores due to shift work, the CNSVS assessments were performed during daytime work and on the next day after nighttime work. The measurement of daytime work was performed on the last day of the daytime work; it was measured as firefighters went to work. Measurement of the nighttime work was performed once on the next day after the second or third nighttime work shift, and on the next day after the full day of work. Since the measurement of the nighttime work occurred as night shift was over, both measurements of the daytime and nighttime work were taken between 9 a.m. and 10 a.m.
The CNSVS test was designed to be given within a 30-min period to increase the probability of subject compliance. To avoid the possible effects of differences between computers, all tests were performed using the same kind of computers.
2.6. Study Endpoints
The primary endpoint was a change in neurocognitive function after night shift; the secondary endpoint was a change in neurocognitive function after night shift according to status of depression and insomnia. The exploratory endpoint was whether there was a difference in cognitive function changes depending on the type of shift work.
2.7. Statistical Analysis
We performed descriptive statistical analyses for demographic and socioeconomic characteristics, and health characteristics (lifestyle habits, and status of insomnia and depression symptoms). Among the socioeconomic characteristics, monthly income was divided into high, middle, and low with 3 million KRW (2,500 USD) and 5 million KRW (4,167 USD), respectively, separating the categories. Analysis of variance (ANOVA) and chi-square tests were used to assess differences in these general characteristics between types of shift work. Paired t-tests were used to assess differences between the standard scores measured during daytime work and on the next day after nighttime work for each category of the CNSVS assessment. Paired t-tests were also performed using stratification according to the subcategories within the insomnia and depressive symptoms categories. In the stratification analysis for depressive symptoms, mild depression was included in the depression category because the number of firefighters classified as having depression was small. We used a general linear model (GLM) to analyze the association between type of shift work and the change in CNSVS score and identified whether there were differences in CNSVS score changes during daytime work and on the next day after nighttime work, according to type of shift work. To adjust for confounding variables, we used four GLM models with different variables and calculated the least square mean (LSmean) value for each type of shift work for each category of CNSVS. Model 1 did not adjust other variables. Model 2 adjusted sex, age, and educational level. Model 3 included the Model 2 adjusted variables and further adjusted the job and income variables, and the ISI/PHQ-9 scores. Multiple comparisons were also performed to determine whether the LSmean values among the types of shift work were significantly different. All analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC, USA).
2.8. Ethics Statement
This study was performed after obtaining approval from the Institutional Review Board (IRB) of Yonsei University Wonju Severance Christian Hospital (IRB no. CR318031).
3. Results
3.1. General Characteristics of Subjects
The 3-day cycle shift group was the oldest; a significant difference in the proportion of age groups was present across different shift groups. There were no between-group differences in sex ratios. There were statistically significant differences between the groups in socioeconomic status, such as income, highest level of education, and proportion of fire suppression work. Coffee consumption was also significantly different between groups. Health behaviors such as smoking, drinking, and exercise did not significantly differ between groups. There were no statistically significant between-group differences in mean scores of ISI and PHQ-9 scores for insomnia and depression. The ISI and PHQ-9 results indicated that there were no statistically significant differences in the proportion of insomnia and depression status among the groups of different shift work schedules (Table 1).
Table 1.
General characteristics of the subjects.
| Characteristics | Shift Work Schedule | All Subjects N = 352 |
p-Value | |||
|---|---|---|---|---|---|---|
| 3-Day Cycle N = 71 |
6-Day Cycle N = 77 |
9-Day Cycle N = 83 |
21-Day Cycle N = 121 |
|||
| Age (years) | 0.043 3–6 | |||||
| 20–29 | 4 (5.6%) | 11 (14.3%) | 11 (13.3%) | 14 (11.6%) | 40 (11.4%) | |
| 30–39 | 21 (29.6%) | 35 (45.5%) | 29 (34.9%) | 47 (38.8%) | 132 (37.5%) | |
| 40–49 | 30 (42.3%) | 19 (24.7%) | 24 (28.9%) | 48 (39.7%) | 121 (34.4%) | |
| 50–59 | 16 (22.5%) | 12 (15.6%) | 19 (22.9%) | 12 (9.9%) | 59 (16.8%) | |
| Mean ± SD | 42.5 ± 8.1 | 38.4 ± 9.3 | 41.2 ± 9.0 | 39.1 ± 8.0 | 40.1 ± 8.7 | 0.012 3–6,3–21 |
| Sex | 0.051 | |||||
| Men | 61 (85.9%) | 73 (94.8%) | 80 (96.4%) | 114 (94.2%) | 328 (93.2%) | |
| Women | 10 (14.1%) | 4 (5.2%) | 3 (3.6%) | 7 (5.8%) | 24 (6.8%) | |
| Job | 0.009 3–21,6–21 | |||||
| Fire suppression | 14 (19.7%) | 21 (27.3%) | 25 (30.1%) | 51 (42.1%) | 111 (31.5%) | |
| Others | 57 (80.3%) | 56 (72.7%) | 58 (69.9%) | 70 (57.9%) | 241 (68.5%) | |
| Education | 0.046 3–6,6–21 | |||||
| High school | 10 (14.1%) | 24 (31.2%) | 18 (21.7%) | 18 (14.9%) | 70 (19.9%) | |
| 2-year degree | 23 (32.4%) | 23 (29.9%) | 18 (21.7%) | 36 (29.7%) | 100 (28.4%) | |
| 4-year degree | 38 (53.5%) | 30 (39.0%) | 47 (56.6%) | 67 (55.4%) | 182 (51.7%) | |
| Monthly income | <0.001 3–6,3–21,6–9,9–21 | |||||
| Missing | 0 | 0 | 2 | 0 | 2 | |
| Low | 11 (15.5%) | 33 (42.9%) | 12 (14.8%) | 37 (30.6%) | 93 (26.6%) | |
| Middle | 32 (45.1%) | 32 (41.6%) | 42 (51.9%) | 62 (51.2%) | 168 (48.0%) | |
| High | 28 (39.4%) | 12 (15.6%) | 27 (33.3%) | 22 (18.2%) | 89 (25.4%) | |
| Smoking | 0.576 | |||||
| Missing | 0 | 0 | 0 | 1 | 1 | |
| Never | 29 (40.8%) | 37 (48.0%) | 33 (39.8%) | 40 (33.3%) | 139 (39.6%) | |
| Past smoker | 24 (33.8%) | 22 (28.6%) | 21 (25.3%) | 45 (37.5%) | 112 (31.9%) | |
| Current light smoker | 10 (14.1%) | 10 (13.0%) | 17 (20.5%) | 21 (17.5%) | 58 (16.5%) | |
| Current heavy smoker | 8 (11.3%) | 8 (10.4%) | 12 (14.5%) | 14 (11.7%) | 42 (12.0%) | |
| Alcohol | 0.996 | |||||
| Missing | 2 | 3 | 1 | 2 | 8 | |
| No | 15 (21.7%) | 17 (23.0%) | 16 (19.5%) | 24 (20.2%) | 72 (20.9%) | |
| Normal drinking | 41 (59.4%) | 41 (55.4%) | 48 (58.5%) | 69 (58.0%) | 199 (57.9%) | |
| Heavy drinking | 13 (18.8%) | 16 (21.6%) | 18 (22.0%) | 26 (21.8%) | 73 (21.2%) | |
| Regular exercise | 0.636 | |||||
| No | 33 (46.5%) | 36 (46.8%) | 44 (53.0%) | 53 (43.8%) | 166 (47.2%) | |
| Yes | 38 (53.5%) | 41 (53.2%) | 39 (47.0%) | 68 (56.2%) | 186 (52.8%) | |
| Caffeine | 0.045 3–6,3–9 | |||||
| Missing | 5 | 7 | 2 | 4 | 18 | |
| No | 8 (12.1%) | 16 (22.9%) | 15 (18.5%) | 18 (15.4%) | 57 (17.1%) | |
| Light coffee drinking | 25 (37.9%) | 35 (50.0%) | 45 (55.6%) | 61 (52.1%) | 166 (49.7%) | |
| Moderate to heavy coffee drinking | 33 (50.0%) | 19 (27.1%) | 21 (25.9%) | 38 (32.5%) | 111 (33.2%) | |
| Insomnia | 0.148 | |||||
| Normal (≤ 7) | 43 (60.6%) | 31 (40.3%) | 44 (53.0%) | 68 (56.2%) | 186 (52.8%) | |
| Mild Insomnia (8–14) | 21 (29.6%) | 40 (51.9%) | 32 (38.6%) | 41 (33.9%) | 134 (38.1%) | |
| Insomnia (≥ 15) | 7 (9.9%) | 6 (7.8%) | 7 (8.4%) | 12 (9.9%) | 32 (9.1%) | |
| Mean ± SD | 7.3 ± 2.2 | 8.8 ± 2.4 | 7.7 ± 4.7 | 7.2 ± 5.3 | 7.7 ± 5.0 | 0.151 |
| Depression | 0.896 | |||||
| Normal (≤ 4) | 60 (84.5%) | 64 (83.1%) | 71 (85.5%) | 103 (85.1%) | 298 (84.7%) | |
| Mild depression (5–9) | 9 (12.7%) | 9 (11.7%) | 11 (13.3%) | 14 (11.6%) | 43 (12.2%) | |
| Depression (≥ 10) | 2 (2.8%) | 4 (5.2%) | 1 (1.2%) | 4 (3.3%) | 11 (3.1%) | |
| Mean ± SD | 2.2 ± 2.8 | 2.4 ± 3.1 | 2.1 ± 2.3 | 2.0 ± 2.9 | 2.1 ± 2.8 | 0.771 |
Values are presented as means ± standard deviation (SD) or number (%). Analyzed by ANOVA or chi-square test. Types of shift work with significant differences in multiple comparisons are listed with a superscript (3: 3-day cycle, 6: 6-day cycle, 9: 9-day cycle, 21: 21-day cycle). Heavy smoker: smoking of ≥15 cigarettes per day. Heavy drinking: consumption of ≥7 glasses per day at least two times per week in men, ≥5 glasses per day at least two times per week in women. Moderate to heavy coffee drinking: consumption of ≥3 cups per day (moderate: 3–4 cups, heavy: ≥5 cups). Regular exercise: exercise above moderate intensity for more than 1 hour a week.
3.2. Neurocognitive Function
The results of neurocognitive function testing measured using CNSVS assessment indicated that the neurocognitive scores decreased on the next day after nighttime work, compared with during the daytime work, for all domains except reaction time and executive function. The scores for composite memory, verbal memory, visual memory, complex attention, psychomotor speed, motor speed, and neurocognitive index were significantly decreased on the next day after nighttime work (Table 2).
Table 2.
Comparison of the neurocognitive function during the daytime work and after the night shift work of firefighters.
| Domain | Mean ± SD (N = 352) | p-Value | |
|---|---|---|---|
| During Day Work | After Night Work | ||
| Composite memory | 90.6 ± 19.1 | 84.7 ± 19.7 | <0.001 |
| Verbal memory | 87.7 ± 20.0 | 81.3 ± 21.9 | <0.001 |
| Visual memory | 97.1 ± 16.3 | 94.0 ± 16.6 | 0.001 |
| Complex attention | 97.8 ± 18.2 | 93.3 ± 32.4 | 0.007 |
| Psychomotor speed | 112.4 ± 15.4 | 110.1 ± 15.2 | <0.001 |
| Motor speed | 111.0 ± 15.1 | 108.7 ± 14.1 | <0.001 |
| Processing speed | 107.6 ± 15.7 | 107.4 ± 17.1 | 0.860 |
| Reaction time | 92.4 ± 15.0 | 92.7 ± 17.1 | 0.711 |
| Cognitive flexibility | 106.2 ± 16.9 | 105.8 ± 19.1 | 0.671 |
| Executive functioning | 107.0 ± 16.5 | 107.2 ± 18.5 | 0.799 |
| Neurocognitive index | 99.9 ± 11.6 | 97.4 ± 13.4 | <0.001 |
All subgroups were analyzed by paired t-test.
3.3. Stratifying Analysis Based on the ISI
We compared neurocognitive function during daytime work and on the next day after nighttime work by stratifying according to the ISI questionnaire results for insomnia. The composite memory, verbal memory, visual memory, and neurocognitive index in the normal group were significantly decreased on the next day after nighttime work. In the mild insomnia group, the composite memory, verbal memory, complex attention, psychomotor speed, motor speed, and neurocognitive index were significantly decreased on the day after night shift work. In the insomnia group, composite memory, verbal memory, and motor speed were significantly decreased on the next day after nighttime work. Composite memory and verbal memory were significantly decreased in all groups, regardless of insomnia status. Complex attention, psychomotor speed, and motor speed were not significantly different in the normal group but were significantly decreased on the next day after nighttime work in the mild insomnia group (Table 3).
Table 3.
Comparison of the neurocognitive function during the daytime work and after the night shift work of firefighters according to the Insomnia Severity Index (ISI) category.
| Domain | ISI Category | N | Mean ± SD (n = 352) | p-Value | |
|---|---|---|---|---|---|
| During Daytime Work | Post Nighttime Work | ||||
| Composite memory | Normal | 186 | 90.2 ± 20.1 | 84.8 ± 19.5 | <0.001 |
| Mild insomnia | 134 | 90.6 ± 17.2 | 85.4 ± 19.4 | 0.002 | |
| Insomnia | 32 | 92.8 ± 21.6 | 81.5 ± 21.9 | 0.012 | |
| Verbal memory | Normal | 186 | 87.7 ± 20.6 | 82.7 ± 22.5 | <0.001 |
| Mild insomnia | 134 | 87.4 ± 19.1 | 80.4 ± 20.3 | <0.001 | |
| Insomnia | 32 | 89.7 ± 20.1 | 77.2 ± 24.6 | 0.001 | |
| Visual memory | Normal | 186 | 96.6 ± 16.8 | 92.8 ± 15.8 | 0.006 |
| Mild insomnia | 134 | 97.6 ± 14.9 | 95.8 ± 17.1 | 0.269 | |
| Insomnia | 32 | 98.3 ± 18.9 | 92.8 ± 18.6 | 0.110 | |
| Complex attention | Normal | 186 | 96.1 ± 20.3 | 94.3 ± 23.7 | 0.260 |
| Mild insomnia | 134 | 100.1 ± 15.0 | 92.2 ± 43.3 | 0.027 | |
| Insomnia | 32 | 98.2 ± 17.0 | 92.7 ± 22.4 | 0.227 | |
| Psychomotor speed | Normal | 186 | 111.7 ± 16.5 | 110.2 ± 15.9 | 0.056 |
| Mild insomnia | 134 | 114.1 ± 14.5 | 111.5 ± 14.5 | 0.008 | |
| Insomnia | 32 | 109.3 ± 11.3 | 104.3 ± 13.5 | 0.069 | |
| Motor speed | Normal | 186 | 110.1 ± 15.8 | 108.6 ± 14.7 | 0.053 |
| Mild insomnia | 134 | 112.2 ± 14.8 | 108.3 ± 13.5 | 0.001 | |
| Insomnia | 32 | 111.4 ± 12.1 | 104.8 ± 13.6 | 0.007 | |
| Processing speed | Normal | 186 | 107.7 ± 15.9 | 106.5 ± 16.9 | 0.298 |
| Mild insomnia | 134 | 109.1 ± 15.9 | 110.2 ± 17.4 | 0.341 | |
| Insomnia | 32 | 100.8 ± 11.3 | 101.4 ± 15.0 | 0.845 | |
| Reaction time | Normal | 186 | 92.4 ± 15.2 | 92.3 ± 16.8 | 0.931 |
| Mild insomnia | 134 | 93.5 ± 15.2 | 93.3 ± 17.3 | 0.872 | |
| Insomnia | 32 | 88.5 ± 12.3 | 93.0 ± 19.2 | 0.190 | |
| Cognitive flexibility | Normal | 186 | 104.8 ± 18.8 | 105.1 ± 18.9 | 0.809 |
| Mild insomnia | 134 | 108.4 ± 14.0 | 106.8 ± 20.1 | 0.306 | |
| Insomnia | 32 | 105.3 ± 15.4 | 106.0 ± 15.8 | 0.821 | |
| Executive functioning | Normal | 186 | 105.6 ± 18.3 | 106.6 ± 18.3 | 0.426 |
| Mild insomnia | 134 | 109.2 ± 13.8 | 108.0 ± 19.8 | 0.414 | |
| Insomnia | 32 | 105.8 ± 15.2 | 108.0 ± 14.7 | 0.397 | |
| Neurocognitive index | Normal | 186 | 99.0 ± 13.0 | 97.3 ± 12.7 | 0.009 |
| Mild insomnia | 134 | 101.4 ± 9.6 | 97.9 ± 14.9 | 0.001 | |
| Insomnia | 32 | 98.9 ± 10.9 | 95.4 ± 10.3 | 0.089 | |
All subgroups were analyzed by paired t-test.
3.4. Stratifying Analysis Based on the PHQ-9
We compared neurocognitive function during daytime work and on the next day after nighttime work; we stratified according to the PHQ-9 results for symptoms of depression. In both the normal group and the depression group including those with mild depressive symptoms, composite memory, verbal memory, visual memory, complex attention, psychomotor speed, motor speed, and neurocognitive index were significantly decreased on the next day after nighttime work. There was no difference in the domain in which the neurocognitive functions were significantly decreased between normal and depression group (Table 4).
Table 4.
Comparison of the neurocognitive function during the daytime work and after the night shift work of firefighters according to the Patient Health Questionnaire-9 (PHQ-9) category.
| Domain | PHQ-9 Category | N | Mean ± SD (n = 352) | p-Value | |
|---|---|---|---|---|---|
| During Daytime Work | Post Nighttime Work | ||||
| Composite memory | Normal | 298 | 90.1 ± 19.1 | 84.6 ± 19.7 | <0.001 |
| Depression | 54 | 93.3 ± 19.4 | 85.3 ± 19.7 | 0.003 | |
| Verbal memory | Normal | 298 | 87.3 ± 19.8 | 81.1 ± 22.0 | <0.001 |
| Depression | 54 | 90.1 ± 20.9 | 82.7 ± 21.3 | <0.001 | |
| Visual memory | Normal | 298 | 96.8 ± 16.3 | 94.0 ± 16.3 | 0.010 |
| Depression | 54 | 99.1 ± 16.4 | 93.8 ± 18.4 | 0.040 | |
| Complex attention | Normal | 298 | 97.2 ± 19.0 | 93.1 ± 34.0 | 0.030 |
| Depression | 54 | 101.0 ± 12.7 | 94.5 ± 22.1 | 0.017 | |
| Psychomotor speed | Normal | 298 | 112.3 ± 15.8 | 111.0 ± 15.0 | 0.035 |
| Depression | 54 | 112.9 ± 13.5 | 105.4 ± 15.9 | <0.001 | |
| Motor speed | Normal | 298 | 110.6 ± 15.5 | 108.6 ± 14.1 | 0.002 |
| Depression | 54 | 113.2 ± 12.7 | 105.8 ± 14.3 | <0.001 | |
| Processing speed | Normal | 298 | 108.1 ± 15.9 | 108.6 ± 16.9 | 0.626 |
| Depression | 54 | 104.5 ± 14.2 | 101.2 ± 16.8 | 0.143 | |
| Reaction time | Normal | 298 | 92.7 ± 15.1 | 92.5 ± 17.3 | 0.808 |
| Depression | 54 | 90.8 ± 14.5 | 93.7 ± 16.4 | 0.142 | |
| Cognitive flexibility | Normal | 298 | 106.0 ± 17.4 | 105.9 ± 19.0 | 0.921 |
| Depression | 54 | 107.6 ± 14.1 | 105.6 ± 19.7 | 0.471 | |
| Executive functioning | Normal | 298 | 106.8 ± 16.9 | 107.4 ± 18.4 | 0.545 |
| Depression | 54 | 108.2 ± 13.7 | 106.6 ± 19.4 | 0.544 | |
| Neurocognitive index | Normal | 298 | 99.7 ± 12.0 | 97.4 ± 13.6 | <0.001 |
| Depression | 54 | 101.1 ± 9.4 | 96.9 ± 12.1 | 0.003 | |
All subgroups were analyzed by paired t-test.
3.5. Multivariate Analysis for the Changes of Neurocognitive Function
The GLM analysis found that in the unadjusted model, type of shift work was significantly related to the domains of verbal memory, processing speed, cognitive flexibility, and executive functioning. However, the analyses using Model 2 and Model 3, which were adjusted for confounding variables, found that type of shift work was not significantly associated with verbal memory domain. The statistical significance of all domains, except verbal memory, was maintained after adjusting for confounding variables. The composite memory, verbal memory, visual memory, complex attention, psychomotor speed, motor speed, and neurocognitive index domains, which showed statistically significant neurocognitive function decreases before and after nighttime work, were not significantly associated with type of shift work (Table 5).
Table 5.
Comparison of the changes of neurocognitive function according to the type of shift work.
| Domain | Type of Shift Work | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| LSmeans | p-Value | LSmeans | p-Value | LSmeans | p-Value | ||
| Composite memory | 3-day cycle | 8.38 | 0.503 | 9.26 | 0.493 | 8.68 | 0.483 |
| 6-day cycle | 4.74 | 5.06 | 4.59 | ||||
| 9-day cycle | 3.81 | 4.82 | 4.18 | ||||
| 21-day cycle | 6.50 | 7.35 | 7.07 | ||||
| Verbal memory | 3-day cycle | −20.17 | 0.030 | −15.78 | 0.060 | −15.92 | 0.071 |
| 6-day cycle | −15.49 9 | −12.13 | −12.07 | ||||
| 9-day cycle | −25.95 6,21 | −13.01 | −21.21 | ||||
| 21-day cycle | −17.19 9 | −15.78 | −12.68 | ||||
| Visual memory | 3-day cycle | 6.20 | 0.327 | 6.39 | 0.246 | 5.34 | 0.313 |
| 6-day cycle | 1.30 | 0.61 | 0.12 | ||||
| 9-day cycle | 1.49 | 1.30 | 0.69 | ||||
| 21-day cycle | 3.79 | 3.59 | 3.29 | ||||
| Complex attention | 3-day cycle | 0.21 | 0.563 | 0.29 | 0.499 | −0.15 | 0.547 |
| 6-day cycle | 7.03 | 7.75 | 6.86 | ||||
| 9-day cycle | 4.11 | 4.53 | 4.29 | ||||
| 21-day cycle | 5.63 | 6.50 | 6.32 | ||||
| Psychomotor speed † | 3-day cycle | 2.04 | 0.307 | 2.40 | 0.215 | 1.81 | 0.279 |
| 6-day cycle | 0.31 | 0.10 | −0.41 | ||||
| 9-day cycle | 3.66 | 3.96 | 3.15 | ||||
| 21-day cycle | 2.71 | 2.68 | 2.15 | ||||
| Motor speed ‡ | 3-day cycle | 4.21 | 0.131 | 3.25 | 0.075 | 3.20 | 0.112 |
| 6-day cycle | 3.14 | 1.92 | 1.69 | ||||
| 9-day cycle | 4.31 | 3.29 | 2.81 | ||||
| 21-day cycle | 1.00 | −0.39 | −0.50 | ||||
| Processing speed †† | 3-day cycle | −2.66 21 | <0.001 | −0.23 21 | <0.001 | −1.47 21 | <0.001 |
| 6-day cycle | −4.21 9,21 | −2.37 9,21 | −2.93 9,21 | ||||
| 9-day cycle | 1.12 6 | 3.60 6 | 2.64 6 | ||||
| 21-day cycle | 3.88 3,6 | 6.36 3,6 | 5.50 3,6 | ||||
| Reaction time | 3-day cycle | −0.69 | 0.986 | 1.64 | 0.907 | 2.52 | 0.860 |
| 6-day cycle | −0.56 | 0.71 | 1.47 | ||||
| 9-day cycle | 0.04 | 2.41 | 3.50 | ||||
| 21-day cycle | −0.09 | 1.57 | 2.41 | ||||
| Cognitive flexibility | 3-day cycle | −3.24 21 | <0.001 | −3.02 21 | <0.001 | −2.88 21 | <0.001 |
| 6-day cycle | −3.74 21 | −4.53 21 | −4.62 21 | ||||
| 9-day cycle | 0.20 21 | −0.36 21 | −0.05 21 | ||||
| 21-day cycle | 5.25 3,6,9 | 4.90 3,6,9 | 5.18 3,6,9 | ||||
| Executive functioning | 3-day cycle | −3.52 21 | <0.001 | −3.21 21 | <0.001 | −3.10 21 | <0.001 |
| 6-day cycle | −4.78 21 | −5.51 21 | −5.39 21 | ||||
| 9-day cycle | −0.46 21 | −0.83 21 | −0.59 21 | ||||
| 21-day cycle | 4.77 3,6,9 | 4.42 3,6,9 | 4.80 3,6,9 | ||||
| Neurocognitive index | 3-day cycle | 1.46 | 0.291 | 2.19 | 0.269 | 1.52 | 0.225 |
| 6-day cycle | 1.57 | 1.77 | 2.92 | ||||
| 9-day cycle | 2.35 | 3.00 | 4.55 | ||||
| 21-day cycle | 3.99 | 4.53 | 2.07 | ||||
Analyzed by general linear model. Types of shift work with significant differences in multiple comparisons are listed with a superscript (3: 3-day cycle, 6: 6-day cycle, 9: 9-day cycle, 21: 21-day cycle). Model 1: unadjusted. Model 2: adjustment for age, sex, and education level. Model 3: Model 2 + adjustments for income, job, and ISI/PHQ-9 scores. LSmeans: least square means. † PHQ-9 score (β: 1.05, 95% confidence interval (CI) 0.54 to 1.56) was significantly associated with change in the domain of psychomotor speed. ‡ Age was significantly associated with change in the domain of reaction time in Model 2 (β: −0.30, 95% CI −0.48 to −0.12) and Model 3 (β: −0.26, 95% CI −0.48 to −0.03). †† ISI (β: −0.58, 95% CI −0.94 to −0.21) and PHQ-9 (β: 1.32, 95% CI 0.67 to −1.98) scores were significantly associated with change in the domain of processing speed.
Among the variables that were used as covariates other than the type of shift work, age was significantly related to neurocognitive function after nighttime work in the domain of retention time. The older firefighters had less change in neurocognitive function. The PHQ-9 score was significantly associated with neurocognitive function after nighttime work for the psychomotor speed and processing speed domains. As PHQ-9 scores increased, neurocognitive function after nighttime work was significantly more decreased. ISI score was significantly associated with changes in neurocognitive function after nighttime work for the psychomotor speed domain. However, as the ISI score increased, the change in neurocognitive function after nighttime work was significantly decreased contrary to the PHQ-9 score.
4. Discussion
The results of the neurocognitive function testing using the CNSVS assessment showed a significant decrease on the next day after nighttime work compared with during daytime work in the scores for six domains (composite memory, verbal memory, visual memory, complex attention, psychomotor speed, and motor speed) and the score of neurocognitive index, respectively. There was no statistically significant improvement for any domain after nighttime work.
In the multivariate analysis using GLM, the changes in neurocognitive function were associated with the type of shift work for the processing speed, cognitive flexibility, and executive functioning domains. The neurocognitive index and the domains with significant decreases after nighttime work did not show significant associations between the type of shift work and neurocognitive function change. In other words, the results confirmed that the decreases in significant neurocognitive function scores after nighttime work were the same regardless of the type of shift work.
When the data were stratified according to degree of insomnia, we found that the domains of complex attention, psychomotor speed, and motor speed score, which were not significantly decreased after nighttime work in the normal group, were significantly decreased in the mild insomnia group. Because there were differences in only some domains and there were no differences in the insomnia group except for the domain of motor speed, the generalizability of the results is limited. However, there were only 32 firefighters classified as having insomnia, so the statistical power required to identify differences was lacking. Therefore, taken together, these results suggest that even mild insomnia made the firefighters more vulnerable to the effects of nighttime work. Greater reductions in neurocognitive function were likely possible in these firefighters, compared with those without insomnia.
A firefighter’s sleep during the night shift can be disturbed by fire and emergency calls. These events may lead to sleep deprivation, which in turn can lead to poor short-term memory, slow response times, lapses in attention or concentration, and mood changes [36]. Several studies comparing the results of functional imaging of the brain for the sleep-deprived brain versus the well-rested brain have consistently found decreases in working memory in the sleep-deprived brain [37]. The results of cognitive and motor performance tests of participants with moderate sleep deprivation are affected not only by cognitive function but also by motor speed, accuracy, coordination, and attention [38]. In this study, the functions of memory domains such as composite memory, verbal memory, and visual memory, and domains in complex attention, psychomotor speed, and motor speed, were decreased after nighttime work. These changes may have been due to the sleep deprivation caused by nighttime work, resulting in a decrease in attention, and its effects on working memory, short-term memory, and motor function.
Firefighters repeatedly experience this sleep deprivation during long and repeated shift work cycles. Chronic sleep deprivation affects neurobehavioral function and shows a dose–response relationship [39]. Chronic sleep deprivation also induces changes in brain metabolism and neural activation [40]. Therefore, the deterioration in cognitive function due to the sleep deprivation of firefighters is not a one-time change; it constantly affects the brain and nervous system of firefighters and decreases the individual’s neurobehavioral function. This change increases the risk of accidents for firefighters during fire suppression activities [41] and might be a risk factor for the development of central neurological diseases such as Alzheimer’s disease in the long term [42].
In firefighters, sleep deprivation due to night work increased level of stress. A study on Dutch police officers revealed that the level of salivary cortisol was significantly increased between 4–14 months after the transition from regular day work to rotating shift work [43]. In addition, a study on German physicians reported that the level of salivary cortisol was significantly increased compared to that of who did not work night shifts [44]. Since a cortisol response is known to be associated with work stress, it can be interpreted that stress level has increased due to shift work [45,46]. Stressful conditions can induce the activation of the neuroendocrine stress system and epigenetic changes in brain, which can drive neuroplastic changes in emotional and cognitive functions [47]. Thus, it could be understood that stress induced by sleep deprivation might have influenced neurocognitive decline in firefighters after a night shift.
Previous studies of neurocognitive function changes in firefighters mainly assessed the effects of high temperatures. There is a concern that firefighters involved in fire suppression activities are exposed to high temperature environments for long periods and that the physical factors could affect neurocognitive function. Recently, an experimental study of U.S. firefighters responsible for wildland fire suppression found that there is no significant difference in the results of cognitive function assessments in very hot (45 °C) conditions compared to temperate environment (18 °C) conditions [48]. Morley et al. [49] performed a neurocognitive function test immediately after giving a heat stress similar to that of a fire suppression; they exposed the firefighters to a 50-min continuous treadmill exercise while wearing thermal protective clothing. The neurocognitive test scores did not change immediately after exercise. Our study, and these previous studies, found that firefighters in charge of fire suppression did not differ in changes in neurocognitive function test scores compared to other firefighters.
Depression, as well as sleep, can also affect a firefighter’s neurocognitive function. In previous studies, depression has been consistently associated with cognitive decline [50,51]. Firefighters also have many risk factors for depression, such as physical environment, organizational culture, and job stress [3]. Although the prevalence of depression in firefighters is not precisely known, several epidemiological studies reported high rates of depressive symptoms among firefighters [52,53]. In this study, after the other factors were adjusted for using multivariate analysis, depression was significantly related to the score changes after nighttime work for the psychomotor speed and motor speed domains. There was no difference in cognitive function before and after nighttime work between the normal and depression groups. However, only 54 firefighters were classified as having depression by PHQ-9, even if mild depression was included in the depression category. Therefore, the number of subjects was too small to confirm the difference, and further studies are needed.
In addition, measures to prevent and manage a cognitive decline due to shift work are needed. Prevention and treatment for sleep disorders are essential in reducing cognitive decline. Administrative management including the adjustment of a shift work cycle and rotating work with day shifts helps to prevent sleep disorders caused by shift work. In addition, maintenance of a sound personal sleep hygiene is also crucial at the individual level. Non-pharmacological therapies are also useful, especially for firefighters with sleep disorders. It has been reported that melatonin improved sleep efficiency [54,55]; taking a melatonin supplement or eating foods rich in melatonin might assist sleeping in firefighters on a night shift. In addition, cognitive behavioral therapy could improve sleep quality in firefighters with sleep disorders. A proper use of these non-pharmacological therapies will help to prevent cognitive decline due to sleep deprivation [56,57,58].
This study had several limitations. The CNSVS testing was conducted directly during the daytime and immediately after the night shift, but most of the tests were performed only once or twice. It was not followed up longitudinally. In addition, the specific firefighting activities at the nighttime fire scenes, which occurred before measurements were taken, are important factors that affect cognitive function, but no detailed records and observations of these activities were available. These activities likely acted as unmeasured confounders and resulted in bias. Furthermore, although this study included firefighters involved in various occupations and from many fire stations, the numbers of participants available for the stratified analyses were relatively small. The statistical power was low when the effects of stratification were analyzed according to PHQ-9 category because only a small number of firefighters was classified as having depression. To overcome these limitations, a longitudinal follow-up study involving more participants is needed in the future.
5. Conclusions
We compared neurocognitive function using CNSVS testing before and after nighttime work performed by firefighters in South Korea who were working in shifts. We found that after nighttime work, neurocognitive functions were generally lower, compared with those during daytime work. In particular, neurocognitive function was decreased for the memory and attention domains; and psychomotor and motor function were also decreased. This result is consistent with the effects of sleep deprivation found by previous studies. Therefore, the results of this study suggested that working the night shift in firefighters is a risk factor for neurocognitive decline.
Acknowledgments
The authors acknowledge the firefighters who participated in this study.
Author Contributions
Conceptualization, K.S.J.; methodology, K.K., B.-K.K., C.S.S., Y.-S.A., K.-S.C., and K.S.J.; software, K.K.; validation, K.K. and T.-W.J.; formal analysis, K.K., B.-K.K., and K.S.J.; investigation, T.-W.J., C.S.S., Y.-S.A., K.-S.C., and K.S.J.; resources, Y.-S.A.; data curation, K.K.; writing—original draft preparation, K.K.; writing—review and editing, B.-K.K., T.-W.J., C.S.S., Y.-S.A., K.-S.C., and K.S.J.; visualization, K.-S.C.; supervision, K.S.J.; project administration, Y.-S.A.; funding acquisition, Y.-S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korean National Fire Agency, grant number 2017-NFA001-010-01010000-2020.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- 1.Lee N., Kim J.H., Kim J.Y., Kim S.S. Association between workplace discrimination and depressive symptoms among firefighters in South Korea. Am. J. Ind. Med. 2018;61:741–750. doi: 10.1002/ajim.22876. [DOI] [PubMed] [Google Scholar]
- 2.Guidotti T.L., Clough V.M. Occupational health concerns of firefighting. Annu. Rev. Public Health. 1992;13:151–171. doi: 10.1146/annurev.pu.13.050192.001055. [DOI] [PubMed] [Google Scholar]
- 3.An S.J., Chung Y.K., Kim B.H., Kwak K.M., Son J.S., Koo J., Ju Y.S., Kwon Y.J. The effect of organisational system on self-rated depression in a panel of male municipal firefighters. Ann. Occup. Envrion. Med. 2015;27:1. doi: 10.1186/s40557-014-0044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakuma A., Takahashi Y., Ueda I., Sato H., Katsura M., Abe M., Nagao A., Suzuki Y., Kakizaki M., Tsuji I., et al. Post-traumatic stress disorder and depression prevalence and associated risk factors among local disaster relief and reconstruction workers fourteen months after the Great East Japan Earthquake: A cross-sectional study. BMC Psychiatry. 2015;15:58. doi: 10.1186/s12888-015-0440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berninger A., Webber M.P., Niles J.K., Gustave J., Lee R., Cohen H.W., Kelly K., Corrigan M., Prezant D.J. Longitudinal study of probable post-traumatic stress disorder in firefighters exposed to the World Trade Center disaster. Am. J. Ind. Med. 2010;53:1177–1185. doi: 10.1002/ajim.20894. [DOI] [PubMed] [Google Scholar]
- 6.Del Ben K.S., Scotti J.R., Chen Y.C., Fortson B.L. Prevalence of posttraumatic stress disorder symptoms in firefighters. Work Stress. 2006;20:37–48. doi: 10.1080/02678370600679512. [DOI] [Google Scholar]
- 7.Carey M.G., Al-Zaiti S.S., Dean G.E., Sessanna L., Finnell D.S. Sleep problems, depression, substance use, social bonding, and quality of life in professional firefighters. J. Occup. Environ. Med. 2011;53:928–933. doi: 10.1097/JOM.0b013e318225898f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim D.K., Baek K.O., Chung I.S., Lee M.Y. Factors related to sleep disorders among male firefighters. Ann. Occup. Environ. Med. 2014;26:11. doi: 10.1186/2052-4374-26-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazloumi A., Golbabaei F., Khani S.M., Kazemi Z., Hosseini M., Abbasinia M., Dehghan S.F. Evaluating effects of heat stress on cognitive function among workers in a hot industry. Health Promot. Perspect. 2014;4:240–246. doi: 10.5681/hpp.2014.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock P.A., Vasmatzidis I. Effects of heat stress on cognitive performance: The current state of knowledge. Int. J. Hyperthermia. 2003;19:355. doi: 10.1080/0265673021000054630. [DOI] [PubMed] [Google Scholar]
- 11.Gaoua A. Cognitive function in hot environments: A question of methodology. Scand. J. Med. Sci. Sports. 2010;20:60–70. doi: 10.1111/j.1600-0838.2010.01210.x. [DOI] [PubMed] [Google Scholar]
- 12.McEwen B.S., Sapolsky R.M. Stress and cognitive function. Curr. Opin. Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-X. [DOI] [PubMed] [Google Scholar]
- 13.Marin M.F., Lord C., Andrews J., Juster R.P., Sindi S., Arsenault-Lapierre G., Fiocco A.J., Lupien S.J. Chronic stress, cognitive functioning and mental health. Neurobiol. Learn. Mem. 2011;96:583–595. doi: 10.1016/j.nlm.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Barrett D.H., Green M.L., Morris R., Giles W.H., Croft J.B. Cognitive functioning and posttraumatic stress disorder. Am. J. Psychiatry. 1996;153:1492–1494. doi: 10.1176/ajp.153.11.1492. [DOI] [PubMed] [Google Scholar]
- 15.Austin M.P., Ross M., Murray C., O’Caŕroll R.E., Ebmeier K.P., Goodwin G.M. Cognitive function in major depression. J. Affect. Disord. 1992;25:21–29. doi: 10.1016/0165-0327(92)90089-O. [DOI] [PubMed] [Google Scholar]
- 16.McDermott L.M., Ebmeier K.P. A meta-analysis of depression severity and cognitive function. J. Affect. Disord. 2009;119:1–8. doi: 10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Rock P.L., Roiser J.P., Riedel W.J., Blackwell A.D. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol. Med. 2014;44:2029–2040. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- 18.Titova O.E., Lindberg E., Elmståhl S., Lind L., Schiöth H.B., Benedict C. Association between shift work history and performance on the trail making test in middle-aged and elderly humans: The EpiHealth study. Neurobiol. Aging. 2016;45:23–29. doi: 10.1016/j.neurobiolaging.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Ansiau D., Wild P., Niezborala M., Rouch I., Marquie J.C. Effects of working conditions and sleep of the previous day on cognitive performance. Appl. Ergon. 2008;39:99–106. doi: 10.1016/j.apergo.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Machi M.S., Staum M., Callaway C.W., Moore C., Jeong K., Suyama J., Patterson P.D., Hostler D. The relationship between shift work, sleep, and cognition in career emergency physician. Acad. Emerg. Med. 2012;19:85–91. doi: 10.1111/j.1553-2712.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- 21.Marquie J.C., Tucker P., Folkard S., Gentil C., Ansiau D. Chronic effects of shift work on cognition: Findings from the VISAT longitudinal study. Occup. Environ. Med. 2015;72:258–264. doi: 10.1136/oemed-2013-101993. [DOI] [PubMed] [Google Scholar]
- 22.Kondratova A.A., Kondratov R.V. The circadian clock and pathology of the ageing brain. Nat. Rev. Neurosci. 2012;13:325–335. doi: 10.1038/nrn3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wulff K., Gatti S., Wettstein J.G., Foster R.G. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat. Rev. Neurosci. 2010;11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- 24.Musiek E.S., Lim M.M., Yang G., Bauer A.Q., Qi L., Lee Y., Roh J.H., Gonzalez X.O., Dearborn J.T., Culver J.P., et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Investig. 2013;123:5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigues S., Paiva J.S., Dias D., Pimentel G., Kaiseler M., Cunha J.P.S. Wearable Biomonitoring Platform for the Assessment of Stress and its Impact on Cognitive Performance of Firefighters: An Experimental Study. Clin. Pract. Epidemiol. Ment. Health. 2018;14:250–262. doi: 10.2174/1745017901814010250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bastien C.H., Vallières A., Morin C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 27.Ensrud K.E., Joffe H., Guthrie K.A., Larson J.C., Reed S.D., Newton K.M., Sternfeld B., LaCroix A.Z., Landis C.A., Woods N.F., et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy menopausal women with hot flashes: A randomized controlled trial. Menopause. 2012;19:848–855. doi: 10.1097/gme.0b013e3182476099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwak K.M., Ju Y.S., Kwon Y.J., Chung Y.K., Kim B.K., Kim H., Youn K. The effect of aircraft noise on sleep disturbance among the residents near a civilian airport: A cross-sectional study. Ann. Occup. Environ. Med. 2016;28:38. doi: 10.1186/s40557-016-0123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morin C.M., Colecchi C., Stone J., Sood R.M., Brink D. Behavioral and pharmacological therapies for late-life insomnia: A randomised controlled trial. JAMA. 1999;281:991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 30.Spitzer R.L., Kroenke K., Williams J.B.W. Patient Health Questionnaire Study Group. Validity and utility of a self-report version of PRIME-MD: The PHQ Primary Care Study. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 31.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho Y.W., Song M.L., Morin C.M. Validation of a Korean Version of the Insomnia Severity Index. J. Clin. Neurol. 2014;10:210–215. doi: 10.3988/jcn.2014.10.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han C., Jo S.A., Kwak J.H., Pae C.U., Steffens D., Jo I., Park M.H. Validation of the Patient Health Questionnaire-9 Korean version in the elderly population: The Ansan Geriatric study. Compr. Psychiatry. 2008;49:218–223. doi: 10.1016/j.comppsych.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Gualtieri C.T., Johnson L.G. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch. Clin. Neuropsychol. 2006;21:623–643. doi: 10.1016/j.acn.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 35.CNS Vital Signs CNS Vital Signs Brief Interpretation Guide. [(accessed on 19 July 2019)]; Available online: https://www.cnsvs.com/WhitePapers/CNSVS-BriefInterpretationGuide.pdf.
- 36.Bonnet M., Arand D. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med. Rev. 2003;7:297–310. doi: 10.1053/smrv.2001.0245. [DOI] [PubMed] [Google Scholar]
- 37.Chee M.W.L., Chuah L.Y.M. Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Curr. Opin. Neurol. 2008;21:417–423. doi: 10.1097/WCO.0b013e3283052cf7. [DOI] [PubMed] [Google Scholar]
- 38.Williamson A.M., Feyer A.M. Moderate sleep deprivation produces impairments in cognitive and motor performance equivalent to legally prescribed levels of alcohol intoxication. Occup. Environ. Med. 2000;57:649–655. doi: 10.1136/oem.57.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Dongen H., Maislin G., Mullington J.M., Dinges D.F. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 40.Basner M., Rao H., Goel N., Dinges D.F. Sleep deprivation and neurobehavioral dynamics. Curr. Opin. Neurobiol. 2013;23:854–863. doi: 10.1016/j.conb.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melamed S., Oksenberg A. Excessive daytime sleepiness and risk of occupational injuries in non-shift daytime workers. Sleep. 2002;25:315–322. doi: 10.1093/sleep/25.3.315. [DOI] [PubMed] [Google Scholar]
- 42.Geerlings M.I., Bouter L.M., Schoevers R.A., Beekman A.T.F., Jonker C., Deeg D.J.H., Van Tilburg W., Adèr H.J., Schmand B. Depression and risk of cognitive decline and Alzheimer’s disease: Results of two prospective community-based studies in The Netherlands. Br. J. Psychiatry. 2000;176:568–575. doi: 10.1192/bjp.176.6.568. [DOI] [PubMed] [Google Scholar]
- 43.Lammers-van der Holst H.M., Kerkhof G.A. Individual differences in the cortisol-awakening response during the first two years of shift work: A longitudinal study in novice police officers. Chronobiol. Int. 2015;32:1162–1167. doi: 10.3109/07420528.2015.1064130. [DOI] [PubMed] [Google Scholar]
- 44.Li J., Bidlingmaier M., Petru R., Gil F.P., Loerbroks A., Angerer P. Impact of shift work on the diurnal cortisol rhythm: A one-year longitudinal study in junior physicians. J. Occup. Med. Toxicol. 2018;13:23. doi: 10.1186/s12995-018-0204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chida Y., Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biol. Psychol. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Clow A., Hucklebridge F., Stalder T., Evans P., Thorn L. The cortisol awakening response: More than a measure of HPA axis function. Neurosci. Biobehav. Rev. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 47.Bottaccioli A.G., Bottaccioli F., Minelli A. Stress and the psyche-brain-immune network in psychiatric diseases based on psychoneuroendocrineimmunology: A concise review. Ann. N. Y. Acad. Sci. 2018;1437:31–42. doi: 10.1111/nyas.13728. [DOI] [PubMed] [Google Scholar]
- 48.Williams-Bell F.M., Aisbett B., Murphy B.A., Larsen B. The Effects of Simulated Wildland Firefighting Tasks on Core Temperature and Cognitive Function under Very Hot Conditions. Front. Physiol. 2017;8:815. doi: 10.3389/fphys.2017.00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morley J., Beauchamp G., Suyama J., Guyette F.X., Reis S.E., Clifton W., Callaway C.W., Hostler D. Cognitive function following treadmill exercise in thermal protective clothing. Eur. J. Appl. Physiol. 2012;112:1733–1740. doi: 10.1007/s00421-011-2144-4. [DOI] [PubMed] [Google Scholar]
- 50.Christensen H., Griffiths K., Mackinnon A., Jacomb P.A. A quantitative review of cognitive deficits in depression and Alzheimer-type dementia. J. Int. Neuropsychol. Soc. 1997;3:631–651. doi: 10.1017/S1355617797006310. [DOI] [PubMed] [Google Scholar]
- 51.Jorm A.F. Is depression a risk factor for dementia or cognitive decline? Gerontology. 2000;46:219–227. doi: 10.1159/000022163. [DOI] [PubMed] [Google Scholar]
- 52.Saijo Y., Ueno T., Hashimoto Y. Twenty-four-hour shift work, depressive symptoms, and job dissatisfaction among Japanese firefighters. Am. J. Ind. Med. 2008;51:380–391. doi: 10.1002/ajim.20571. [DOI] [PubMed] [Google Scholar]
- 53.Saijo Y., Ueno T., Hashimoto Y. Job stress and depressive symptoms among Japanese fire fighters. Am. J. Ind. Med. 2007;50:470–480. doi: 10.1002/ajim.20460. [DOI] [PubMed] [Google Scholar]
- 54.Brzezinski A., Vangel M.G., Wurtman R.J., Norrie G., Zhdanova I., Ben-Shushan A., Ford I. Effects of exogenous melatonin on sleep: A meta-analysis. Sleep Med. Rev. 2005;9:41–50. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Ferracioli-Oda E., Qawasmi A., Bloch M.H. Meta-analysis: Melatonin for the treatment of primary sleep disorders. PLoS ONE. 2013;8:e63773. doi: 10.1371/journal.pone.0063773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Zweerde T., Bisdounis L., Kyle S.D., Lancee J., van Straten A. Cognitive behavioral therapy for insomnia: A meta-analysis of long-term effects in controlled studies. Sleep Med. Rev. 2019;48:101208. doi: 10.1016/j.smrv.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Eidelman P., Talbot L., Ivers H., Bélanger L., Morin C.M., Harvey A.G. Change in dysfunctional beliefs about sleep in behavior therapy, cognitive therapy, and cognitive-behavioral therapy for insomnia. Behav. Ther. 2016;47:102–115. doi: 10.1016/j.beth.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Small G.W. Detection and prevention of cognitive decline. Am. J. Geriatr. Psychiat. 2016;24:1142–1150. doi: 10.1016/j.jagp.2016.08.013. [DOI] [PubMed] [Google Scholar]