Abstract
Preparations of comfrey (Symphytum officinale L.) roots are used topically to reduce inflammation. Comfrey anti-inflammatory and analgesic properties have been proven in clinical studies. However, the bioactive compounds associated with these therapeutic activities are yet to be identified. An LC–ESI–Orbitrap–MSn metabolite profile of a hydroalcoholic extract of comfrey root guided the identification of 20 compounds, including a new arylnaphthalene lignan bearing a rare δ-lactone ring, named comfreyn A. Its structure was determined using extensive 2D NMR and ESI–MS experiments. Additionally, the occurrence of malaxinic acid, caffeic acid ethyl ester, along with the lignans ternifoliuslignan D, 3-carboxy-6,7-dihydroxy-1-(3′,4′-dihydroxyphenyl) -naphthalene, globoidnan A and B, and rabdosiin was reported in S. officinale for the first time. These results helped to redefine the metabolite profile of this medicinal plant. Finally, caffeic acid ethyl ester and comfreyn A were found to significantly inhibit E-selectin expression in IL-1β stimulated human umbilical vein endothelial cells (HUVEC), with EC values of 64 and 50 µM, respectively.
Keywords: Symphytum officinale, comfrey roots, LC–ESI–Orbitrap–MS, phenylpropanoids, comfreyn A
1. Introduction
Symphytum officinale L. (Boraginaceae) known as comfrey, is a perennial herbaceous plant commonly found in Europe, Asia, and North America [1,2]. Comfrey is used in folk medicine for the treatment of diarrhea, bronchitis, tuberculosis, ulcers, and hemorrhoids [3]. Nowadays, clinical trials have demonstrated the efficacy and safety of its topical preparations [2,4]. Comfrey roots contain pyrrolizidine alkaloids (PAs) with 1,2 unsaturated necine structures, including lycopsamine, intermedine, their acetyl, and N-oxide derivatives, together with the diester symphytine [2,5,6]. Therefore, care is taken to deplete the PA presence through extraction, and strict limits must be met for the placement on the market of PA containing herbal medicines [7]. PA-depleted extracts are used in over-the-counter topical medicines to reduce inflammation and for the treatment of broken bones, tendon damages, painful joints, and muscles [3]. Indeed, a liquid hydroalcoholic extract of comfrey root was clinically proven to be effective for the treatment of acute upper and lower back pain, gonarthrosis, and blunt injuries [8,9,10].
It was reported that the main constituents of comfrey root include allantoin, polyphenols such as rosmarinic acid, lithospermic acid, ellagic acid, caffeic acid, and abundant mucilage and polysaccharides composed of fructose and glucose units [1,2,3,11]. Moreover, comfrey roots have yielded a number of oleanolic and bidesmosidic triterpene saponins [12,13]. Recently, a phenolic profile of a hydroalcoholic extract from the roots of Symphytum officinale L. was reported to contain salvianolic acid isomers [2,14].
At present, medicinal products from comfrey root commercialized on the European market contain only extracts from pyrrolizidine-depleted plant material or are obtained from special cultivars that do not synthesize pyrrolizidine alkaloids [2].
As part of our ongoing work that aims at better understanding the chemical composition of comfrey roots and obtaining preliminary insights on the contribution of its specialized metabolites to the anti-inflammatory activity, we performed an LC–MSn guided fractionation of a mucilage- and PA-depleted extract of comfrey root that showed inhibition of interleukin-1β-induced expression of E-selectin in HUVEC cells, at a concentration of 20 µg/mL [4]. Through these means, 20 compounds were identified, including a new arylnaphthalene lignan, named comfreyn A. Its structure was fully characterized by extensive NMR and ESI–MS analysis.
Moreover, malaxinic acid, caffeic acid ethyl ester, along with the lignans ternifoliuslignan D, 3-carboxy-6,7-dihydroxy-1-(3′,4′-dihydroxyphenyl)-naphthalene, globoidnan A and B, and rabdosiin were reported for the first time in S. officinale.
2. Results and Discussion
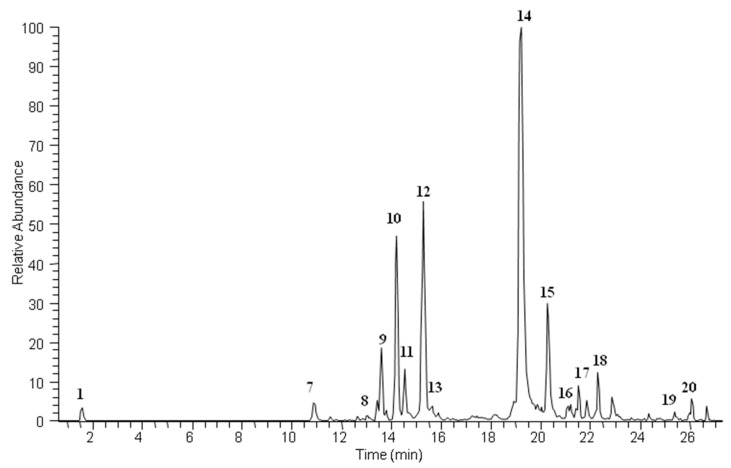
A hydroalcoholic liquid extract of S. officinale root was analyzed by LC–ESI/HR/MS, using the “data dependent scan” mode in which precursor ions corresponding to the most intense peaks were selected. A preliminary metabolite profiling showed 20 main compounds corresponding to different structural classes (Figure 1 and Figure 2).
Figure 1.
LC–MS profile in negative ion mode of Symphytum officinale ethanol extract.
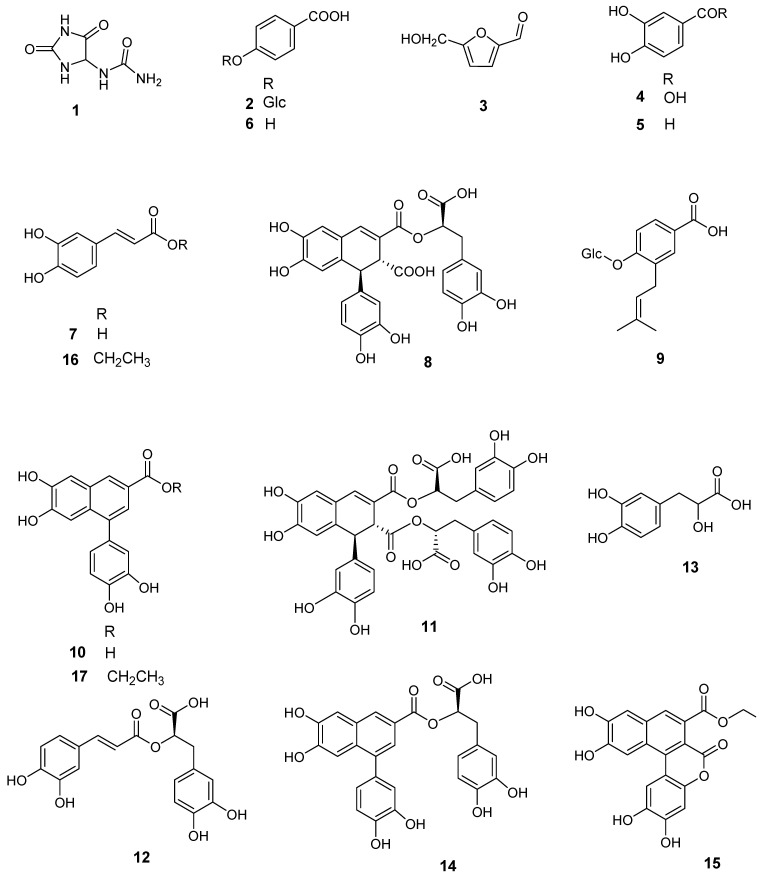
Figure 2.
Compounds isolated from the roots of Symphytum officinale.
Some of these metabolites were identified by a comparison of their accurate masses, characteristic fragmentation patterns, and retention times to those of authentic standards. On the basis of the above approach, the occurrence of allantoin (1), protocatechuic acid (4), protocatechuic aldehyde (5), p-hydroxy benzoic acid (6), caffeic acid (7) and its ethyl ester derivative (16), and rosmarinic acid (12), along with the fatty acids linolenic (18), linoleic (19), and hydroxypalmitic (20) was reported. The remaining compounds could not be unambiguously assigned by HR–ESI–MS, either because their accurate masses did not match any of the compounds reported from S. officinale in the literature or because their preliminary NMR data did not support the compounds reported in S. officinale, with the same molecular weights. In particular, compound 15 showed an [M-H]− ion at m/z 381.0601 in the LC–MS profile, suggesting a molecular formula of C20H14O8, which did not correspond to any molecule reported in the scientific literature. To fully characterize compounds 2–3, 8–11, and 13–17, we first removed the mucilage fraction of the extract by partitioning the liquid extract with ethyl acetate. Then, the organic phase was subjected to size exclusion chromatography on a Sephadex LH-20 column. Subsequently, fractions containing the above-mentioned compounds were purified by reverse-phase HPLC. Their structures were fully characterized by extensive 1D- and 2D-NMR experiments, as well as MS/MS analysis (see Table 1).
Table 1.
Secondary metabolites identified by LC–ESI–HR–MS and LC–ESI–HR–MS/MS.
| N° | Rt | [M-H]− | [M+H]+ | Molecular Formula | Δ ppm | MS/MS | Identity |
|---|---|---|---|---|---|---|---|
| 1 | 1.54 | 157.0362 | C4H6O3N4 | 3.8 | 140.01/114.03/97.00 | allantoin | |
| 2 | 2.42 | 299.0764 | C13H16O8 | 0.9 | 137.02 | p-hydroxybenzoic acid glucoside | |
| 3 | 3.17 | - | 127.0388 | C6H6O3 | 1 | - | 5-hydroxymethyl-2-furfural |
| 4 | 5.25 | 153.0196 | C7H6O4 | 0.5 | - | protocatechuic acid | |
| 5 | 6.79 | 137.0247 | C7H6O3 | 0.8 | - | protocatechuic aldehyde | |
| 6 | 8.07 | 137.0245 | C7H6O3 | 0.8 | 93.03 | p-hydroxybenzoic acid | |
| 7 | 10.88 | 179.0343 | C9H8O4 | 2.3 | - | caffeic acid | |
| 8 | 13.41 | 537.1033 | C27H22O12 | 1.08 | 339.05/493.10 | globoidnan B | |
| 9 | 13.60 | 367.1384 | C18H24O8 | 2.6 | 205.09 | malaxinic acid | |
| 10 | 14.21 | 311.0547 | C17H12O6 | −0.85 | 267.06/108.90 | 3-carboxy-6,7-dihydroxy-1-(3′,4′-dihydroxyphenyl)-naphthalene | |
| 11 | 15.06 | 717.1449 | C36H30O16 | −0.15 | 519.09/475.10/339.05 | (+)-rabdosiin | |
| 12 | 15.28 | 359.0764 | C18H16O8 | 0.6 | 161.02 | rosmarinic acid | |
| 13 | 15.65 | 197.0448 | C9H10O5 | 1.7 | 179.03 | α-hydroxyhydrocaffeic acid | |
| 14 | 19.21 | 491.0974 | C26H20O10 | 0.2 | 311.05/267.06/197.85 | globoidnan A | |
| 15 | 20.27 | 381.0601 | C20H14O8 | −1.06 | 353.02/309.10/265.10 | comfreyn A | |
| 16 | 21.18 | 207.0654 | C11H12O4 | 0.1 | 179.03/135.04/161.02 | caffeic acid ethyl ester | |
| 17 | 22.31 | 339.0863 | C19H16O6 | 0.05 | 311.05/229.01 | ternifoliuslignan D | |
| 18 | 25.56 | 277.2159 | C18H30O2 | −1.28 | 233.22 | linolenic acid | |
| 19 | 26.08 | 279.2315 | C18H32O2 | −1.14 | 261.22 | linoleic acid | |
| 20 | 28.50 | 271.2263 | C16H32O3 | −1.5 | 225.22 | hydroxy-palmitic acid |
Our NMR analysis confirmed 2 and 3 as p-hydroxy benzoic acid glucoside [15] and 5-hydroxymethyl-2-furfural [16], respectively. However, it is worth mentioning that 3 could be an artefact of the extract preparation due to the use of a cation-exchange resin to deplete the PAs [17]. Indeed, the resin might cause degradation of sugar residues (e.g., fructose, glucose, sucrose) to form 5-hydroxymethyl-2-furfural [16]. Compound 9 showed a major ion peak at m/z 367.1384 [M-H]−, supporting the molecular formula C18H24O8. Its MS/MS spectrum showed a fragment ion at m/z 205.09 [M-H-162]− suggesting the presence of a hexose unit. Its structure was established through NMR as malaxinic acid [18]. Interestingly, this was the first report of this glucosylated and prenylated phenolic acid (9), in S. officinale.
The HR–ESI–MS spectra of 8, 11, and 14 supported the molecular formulae of C27H22O12, C36H30O16, and C26H20O10, respectively. Analysis of their NMR and MS/MS data allowed us to identify 14 and 8 as globoidnan A [19] and B [20], respectively, whereas compound 11 was established as (+)-rabdosiin in agreement with its optical rotation [21] (see Table S1). To our knowledge, this is the first report of these lignans in roots of S. officinale. Interestingly, the molecular formulae and fragmentation patterns of 8, 11, and 14 perfectly matched those of lithospermic acid, salvianolic acid B, and salvianolic acid C, respectively. These lithospermic acid isomers were previously identified in roots of S. officinale by LC–MS or UV and IR spectroscopy, however, their structures were not confirmed by NMR experiments [2,22]. The molecular formula of compound 13 was established as C9H10O5 and the structural elucidation afforded by the NMR analysis, allowed the identification of compound 13 as α-hydroxyhydrocaffeic acid [23].
MS/MS fragmentation patterns suggested that 10 and 17 bear a similar structural framework as that of globoidnan A (14). Indeed, interpretation of the NMR data confirmed this hypothesis establishing the structure of 10 as 3-carboxy-6,7-dihydroxy-1-(3′,4′-dihydroxyphenyl)-naphthalene and 17 as its ethyl ester derivative ternifoliuslignan D [24].
The HR–ESI–MS spectrum of compound 15 (m/z 381.0601 [M-H]−, calculated for C20H13O8, 381.0610) supported a molecular formula of C20H14O8. The ESI/MS/MS spectra of 15 displayed an intense ion at m/z 353.02 ([M-H-28]−), suggesting the presence of an ethyl group. The MS3 spectra obtained from this product ion displayed fragment ions at m/z 309.10 ([M-H-44]−) and 265.10 ([M-H-44-44]−), corresponding to the loss of two CO2 molecules. The analysis of the 13C and HSQC NMR data for 15 (Table 2) suggested a highly unsaturated molecule bearing 16 aromatic carbons, whereas its 1H NMR spectrum displayed five aromatic singlets at δ 8.30 (H-8), 8.02 (H-6′), 7.67 (H-4), 7.34 (H-5), 6.91 (H-3′). Additionally, 1H NMR of 15 showed characteristic signals of an ethoxy group at δ 4.43 (2H, q, J = 7.0 Hz) and 1.41 (3H, t, J = 7.0 Hz). Moreover, a detailed analysis of the HMBC spectrum confirmed the presence of two main fragments. In particular, key long-range correlations between the aromatic proton H-4 and the carbon resonances at δ 172.2 (C-9), 130.5 (C-3), 113.4 (C-2), 133.7 (C-4a), 112.4 (C-5), and 124.5 (C-8a), H-5 and the carbon resonances at δ 133.7, 151.3 (C-6), 150.3 (C-7), and 124.5, and H-8, and the carbon resonances at δ 136.1 (C-1), 151.3, and 133.7, established the occurrence of a 1,2-disubstituted 6,7-dihydroxy-naphthalene-3-carboxylic acid. The structure of the second fragment was established as 1-substituted benzene-2,4,5-triol based on HMBC correlations from protons H-3′ and H-6′ to the carbon resonances at δ 111.5 (C-1′), 143.8 (C-2′), 149.6 (C-4′), and 147.1 (C-5′). In turn, connectivity between these two fragments was deduced from the HMBC correlation from H-6′ to C-1. In addition, an HMBC correlation from the equivalent methylene at δ 4.43 (H-1′’) to the carbonyl at δ 172.2 linked the ethoxy residue to the naphthalene moiety. Inspection of this partial structure revealed that it contained 13 of the required 14 degrees of unsaturation and only one of the two carbonyls was assigned. Consequently, the phenol ring and the 6,7-dihydroxy-naphthalene-3-carboxylic acid residue had to connect via formation of a lactone ring to satisfy the unsaturation index and molecular formula. HMBC experiments with long-range delays ranging from 2.75 to 10 Hz were performed. The HMBC spectrum, optimized for the nJ(C,H) coupling of 5 Hz, corresponding to a delay time Δ2 = 100 ms, showed four-bonds of long-range correlations and stronger two-bond long-range correlations. Finally, the structure of 15 was assembled as follows.
Table 2.
13C (150 MHz) and 1H NMR (600 MHz) data of compound 15.
| δC | δH (J in Hz) | HMBC (H→C) Correlations | |
|---|---|---|---|
| 1 | 136.2 | - | |
| 2 | 113.8 | - | |
| 3 | 130.5 | - | |
| 4 | 126.6 | 7.67, s | C-1, C-2, C-4, C-4a, C-8a, C-9, C-10 |
| 4a | 133.6 | - | |
| 5 | 112.4 | 7.34, s | C-4, C-8a, C-6, C-7 |
| 6 | 151.3 | - | |
| 7 | 150.2 | - | |
| 8 | 111.5 | 8.30, s | C-1, C-4a, C-6, C-7 |
| 8a | 124.5 | - | |
| 9 | 172.1 | ||
| 10 | 162.4 | - | |
| 1′ | 111.5 | - | |
| 2′ | 144.0 | - | |
| 3′ | 104.5 | 6.91, s | C-1, C-1′, C-2′, C-4′, C-5′ |
| 4′ | 149.6 | - | |
| 5′ | 147.1 | - | |
| 6′ | 113.8 | 8.02, s | C-1, C-1, C-2′, C-4′, C-5′ |
| 1″ | 62.8 | 4.43, q (7.0) | C-9, C-2″ |
| 2″ | 14.2 | 1.41, t (7.0) | C-1″ |
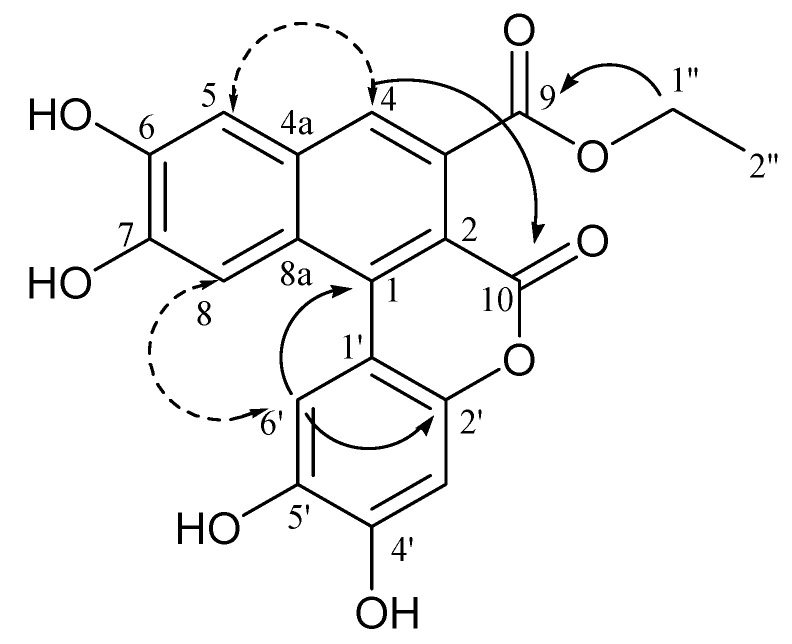
A 4JCH HMBC correlation from H-4 to the carbon resonance at δ 162.5 (C-10) allowed the deduction of the linkage of the carbonyl group to C-2, whereas an ROE correlation between H-6′ and H-8, along with a 4JCH HMBC correlation from H-6′ to C-2 indicated the esterification site at C-2 (Figure 3). Therefore, comfreyn A (15) was established as an arylnaphthalene lignan bearing an unusual δ-lactone ring, corresponding to a coumarin skeleton.
Figure 3.
Key HMBC ( ) and ROESY (
) and ROESY ( ) correlations of compound 15.
) correlations of compound 15.
It is worth mentioning that the natural occurrence of the ethyl esters 15–17 was ascertained by HPLC-UV analysis of the methanol extract of dried roots, to prove their presence in comfrey not as artefacts due to the use of ethanol but as extraction solvent (Figure S1). Figures S2–S18 (NMR spectra of compounds 8, 11, 14 and 15) and Table S1 (13C and 1H NMR data of compounds 8, 11 and 14) are reported in the Supplementary Materials.
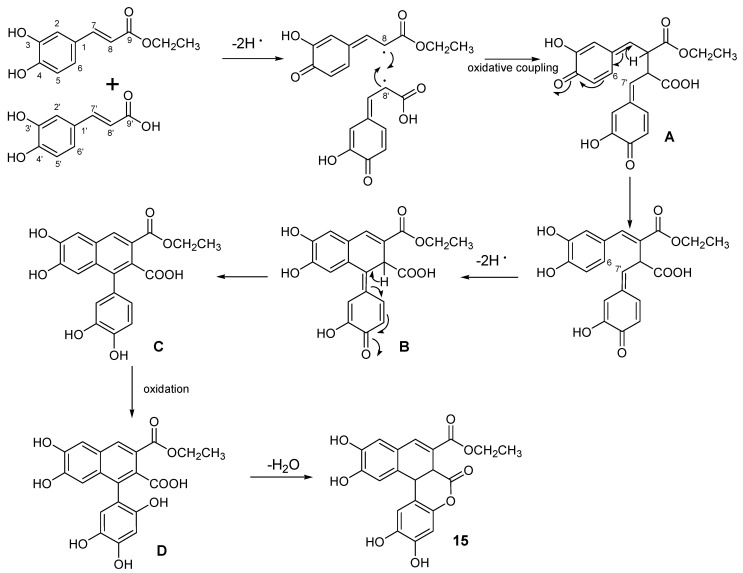
From a biosynthetic point of view, this work highlighted comfrey roots as a great source of phenylpropanoids, including monomeric phenylpropanoids (7, 13, and 16), dimeric phenylpropanoids with the two C6C3 moieties linked via an esther function (12) and lignans (8, 10, 11, 14, 15, and 17). The latter are a class of secondary metabolites derived from two phenylpropanoid units, linked by a C–C bond between C8 and C8ʹ, which could show a remarkable structural diversity [25]. Indeed, the putative biosynthetic pathway of comfreyn A probably originated from caffeic acid and its ethyl ester analogue (Figure 4).
Figure 4.
Proposed biosynthetic pathway for compound 15.
As previously reported by Daquino et al., removal of a hydrogen atom from both the p-phenolic group of caffeic acid and its ethyl ester analogue originated phenoxy radicals with the unpaired electron at the β position, which might couple to each other (8–8′coupling), generating a reactive bis-quinonemethide (A) [25]. Its tautomer underwent an oxidative intramolecular cyclization (6–7′) (B). Then, the subsequent oxidative step introducing a phenolic function on the tautomer C produced the intermediate D. Finally, the structure of 15 was completed by a condensation step that led to the formation of a lactone ring.
It was reported that interleukin-1β (IL-1β) induced E-selectin mRNA levels were inhibited by comfrey extract by approximately 70% at the starting concentration of 20 µg/mL [4]. E-selectin is a NF-κB-dependent target gene and IL-1β is a well-described pro-inflammatory mediator that exerts an important role in the regulation of immune and inflammatory responses, in particular those involving sterile insults, such as trauma and blunt injuries. Thus, the anti-inflammatory activity of compounds 1, 5, 9, 12, 14, 15, and 16 was tested using IL-1-stimulated human umbilical vein endothelial cells (HUVEC) and analyzed by real time PCR, determining the fold change in mRNA expression of E-selectin. The results are shown in Table 3. Caffeic acid ethyl ester (16) was the most active compound inhibiting E-selectin expression by 79.6 ± 4%, at a concentration of 64 µM, followed by comfreyn A (15), which inhibited E-selectin expression by 51.5 ± 5.3% at 50 µM. Globoidnan A (14), protocatechuic aldehyde (5), and malaxinic acid (9) were significantly inhibited E-selectin expression inhibited but with less potency, while allantoin (1) and rosmarinic acid (12) did not show any activity at concentrations as high as 250 µM.
Table 3.
Inhibitory effect of compounds 1, 5, 9, 12, 14, 15, and 16 on IL-1β induced E-selectin expression.
| Compound | EC [µM] | Max. Inhibition (%) |
|---|---|---|
| 1 | >250 | ND |
| 5 | 120 | 59.1 ± 16.7 |
| 9 | 108 | 56.2 ± 12.1 |
| 12 | >250 | ND |
| 14 | 40 | 35.1 ± 10.1 |
| 15 | 50 | 51.5 ± 5.3 |
| 16 | 64 | 79.6 ± 4 |
EC: Effective concentration is based on at least on three independent experiments. Tested compounds: allantoin (1), protocatechuic aldehyde (5), malaxinic acid (9), rosmarinic acid (12), globoidnan A (14), comfreyn A (15), and caffeic acid ethyl ester (16).
Surprisingly, the anti-inflammatory activity of the tested compounds did not support the activity displayed by the mucilage- and PA-depleted extract of comfrey root at 20 µg/mL, in our in vitro anti-inflammatory model [4]. These results might suggest that the activity of the extract could be due to a combined or synergistic effects of the compounds herein occurring in the extract, belonging to the phenolic class [26]. Indeed, our phytochemical investigation allowed us to determine the chemical composition of the comfrey root, highlighting the presence of natural compounds previously reported to show anti-inflammatory activity, which could partially explain the biological activity shown by the extract. Among these compounds, caffeic acid ethyl ester (16) was reported to suppress in vitro or in mouse skin NF-κB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2 [27], and ternifoliuslignan D (17) was shown to inhibit NO, TNF-α, and PGE2 production and also to suppress the expression of iNOS, NF-κB/p65, and COX-2 in LPS-induced RAW 264.7 cells [24]. Additionally, human peripheral blood mononuclear cells (PBMCs) stimulated by lipopolysaccharide (LPS), rabdosiin (11), and rosmarinic acid (12) significantly decreased secretion of TNF-α, IL-1b, IL-2, and IL-6, at a concentration of 100 µM. Globoidnan A (14) significantly reduced the production of only TNF- α [28].
3. Materials and Methods
3.1. General Procedures
Optical rotation of compound 11 was measured on an Autopol IV (Rudolph Research Analytical, Hackettstown, NJ, USA) polarimeter. IR measurements were carried out on a Bruker IFS-48 spectrometer. UV spectra were obtained on a Beckman DU 670 spectrometer. NMR experiments were acquired in methanol-d4 (99.95%, Sigma-Aldrich, Milan, Italy) on a Bruker DRX-600 spectrometer (Bruker BioSpin GmBH, Rheinstetten, Germany), equipped with a Bruker 5 mm TCI CryoProbe at 300 K. Data processing was carried out with Topspin 3.2 software (Bruker BioSpin, Rheinstetten, Germany). The ROESY spectra were acquired with tmix = 400 ms. Size exclusion chromatography was performed using Sephadex LH-20 (GE Healthcare, Sigma Aldrich, Milan, Italy).
3.2. Reagents and Solvents
Ethanol and methanol for extraction were purchased from VWR international PBI S.r.l. (Milan, Italy). Water and Acetonitrile for HPLC were purchased from VWR international PBI S.r.l. (Milan, Italy). Water, acetonitrile, and formic acid (all of LC–MS grade) were purchased from Merck (Darmastadt, Germany). Allantoin standard was purchased from Sigma Aldrich (Milan, Italy).
3.3. Sample Extraction
Comfrey roots were harvested in Serbia (region Vojvodina) in 2016. The botanical identification of the roots was done in accordance with the Symphytum officinale monograph of the German homeopathic pharmacopeia. The comfrey roots were extracted with ethanol 60% (v/v) and PAs were removed with a cation exchange resin, and a PA-depleted hydroalcoholic (20% EtOH w/w) liquid extract (DER 1:2) was obtained. The extract was supplied by Procter Health Care International & P&G Health Austria GmbH & Co. OG. To remove the mucilage, the ethanol content of 500 g of liquid extract was evaporated and the aqueous phase was partitioned with ethylacetate (1:1), obtaining 50 g of ethyl acetate extract. A total of 3 g of ethyl acetate extract were fractionated on a Sephadex LH-20 (GE Healthcare, Sigma Aldrich, Milan, Italy) column (100 × 5 cm), using methanol as the mobile phase, affording 80 fractions (8 mL), monitored by LC-ESI-HR-MS (Thermo Fisher Scientific, Bremen, Germany) analysis.
3.4. LC–ESI/LTQOrbitrap/MS Analysis
The secondary metabolite profile of the hydroalcoholic extract of comfrey roots was obtained using an HPLC method, coupled with a hybrid mass spectrometer, which combined the linear trap quadrupole (LTQ) and Orbitrap mass analyzer. All experiments were carried out using a Thermo scientific liquid chromatography system constituted of a quaternary Accela 600 pump and an Accela autosampler, connected to a linear Trap-Orbitap hybrid mass spectrometer (LTQ-Orbitrap XL, Thermo Fisher Scientific, Bremen, Germany), with electrospray ionization (ESI). Separation was performed on a Kinetex EVO C18 5 µm (150 mm × 2.10 mm) column (Phenomenex Aschaffenburg, Germany), using a flow rate of 0.2 mL/min and a mobile phase consisting of a combination of A (0.1% formic acid in water, v/v) and B (0.1% formic acid in acetonitrile, v/v). A linear gradient program starting with isocratic at 5% B in 5 min, then from 5 to 23% B in 5 min, held at 23% B for 5 min, from 23 to 55% B in 7 min, from 55 to 95% B in 5 min, held at 95% B for 5 min was used. The mass spectrometer operated in negative ion mode. The ESI source parameters were as follows—capillary voltage –48V; tube lens voltage –176.47; capillary temperature 280 °C; sheath and auxiliary gas flow (N2), 15 (arbitrary units) and 5 (arbitrary units), and sweep gas 0 spray voltage 5 kV. The mass range was from 120 to 1600 m/z with a resolution of 30,000. For the fragmentation study, a data dependent scan was performed, selecting precursor ions corresponding to the most intense peaks in the LC–MS analysis. Xcalibur software version 2.1 was used for instrument control, data acquisition, and data analysis.
3.5. Isolation Procedure
On the basis of the results obtained from the LC–ESI–HR–MS analyses, Sephadex fractions were further purified by semipreparative HPLC–UV. Indeed, the obtained dried fractions were diluted in acetonitrile (at a concentration of 10 mg dry residue per 100 µL), and submitted to semipreparative HPLC–UV separations, using a Phenomenex C18 Synergi-Hydro-RP (250 mm × 10 mm, 10 μm) column on an Agilent 1260 Infinity system (Agilent Technologies, Palo Alto, CA, USA), equipped with a binary pump (G-1312C), and a UV detector (G-1314B). The mobile phase consisted of solvent A (H2O + 0.1 % formic acid) and solvent B (CH3CN + 0.1% formic acid). The analyses were performed at room temperature without thermostating. The peaks collected at HPLC–UV were evaporated to dryness in vacuum and were freeze-dried before NMR analysis. Fraction 23–26 (10 mg) corresponded to compound 12; fraction 40–42 (10 mg) corresponded to compound 14. Fractions 19–22 (51.6 mg) were purified using a linear gradient program at a flow rate of 2 mL/min at a wavelength of 254 nm (from 0 to 20% B in 15 min; from 20 to 40% B in 10 min; from 40 to 60% B in 10 min; from 60 to 100% B in 11 min, and then followed by 6 min at 100% B), to afford compounds 2 (1.2 mg, Rt = 10.3 min), 3 (1.3 mg, Rt = 13.5 min), 6 (1.5 mg, Rt = 11.2 min), and 9 (2.2 mg, Rt = 27.3). Fractions 27–39 (42.3 mg), 43–54 (7.1 mg), and 55–80 (22.2 mg) were purified in the same elution conditions at a flow rate of 2 mL/min and at a wavelength of 280 nm: (0–5 min held at 5% B, from 5% to 20% B in 15 min; from 20% to 50% B in 20 min, from 50% to 100% B in 10 min, and held at 100% B for 5 min). In these conditions, compounds 4 (1.1 mg, Rt = 22.60 min), 5 (1.2 mg, Rt = 27.9min), 7 (1.5 mg, Rt = 29.4min), 8 (1.3 mg, Rt = 31.7 min), 11 (1.6 mg, Rt = 34.3), 13 (1.7 mg, Rt = 35.1 min), and 10 (4.4 mg, Rt = 35.3) were isolated from fractions 27–39; compound 15 (1.6 mg Rt = 41.10) was isolated from fractions 43–54, and finally compounds 16 (1.2 mg, Rt = 42.2), and 17 (1.0 mg, Rt = 43.3) were isolated from fractions 55–80. Moreover, 3 g of raw material were extracted with 50 mL of methanol to obtain the methanol extract.
3.6. Spectroscopic Data of Compound 15
Amorphous yellow solid; C20H14O8; IR νKBrmax cm−1: 3420, 1660, 1615, 1600; 1H and 13C NMR (methanol-d4, 600 MHz and 150 MHz) data, see Table 2; ESI-HR-MS m/z 381.0601 [M-H]− (calcd. for C20H13O8, 381.0610).
3.7. Cell Culture
Primary human umbilical vein endothelial cells (HUVEC) were isolated from umbilical cords, as described previously [29] and maintained in M199 medium (Lonza, Basel, Switzerland) supplemented with 20% FCS (Sigma), 2 mM L-glutamine (Sigma), penicillin (100 units/mL), streptomycin (100 mg/mL), 5 units/mL heparin, and 25 mg/mL ECGS (Promocell, Heidelberg, Germany), and were used up to 5 passages. HUVEC cells were pre-incubated with various concentrations of purified compounds (1, 5, 9, 12, 14, 15, and 16) for 30 min, following stimulation with 5 ng/mL IL-1β (R&D Systems) for 90 min. Cells were then analyzed for the change in mRNA expression of E-Selectin (SELE).
3.8. Real-Time PCR
Total RNA was isolated using the PeqGold Total RNA Isolation Kit (VWR International, Vienna, Austria), according to the manufacturer’s instructions. A total of 1 µg RNA was reverse transcribed using random hexamers (Fermentas) and murine leukemia virus reverse transcriptase (Thermo Scientific Fisher, Vienna, Austria). Primers were designed using the software “Primer3”. Following primer sequences were used for qPCR: glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward, 5′-AGAAGGCTGGGGCTCATTT-3′and reverse, 5′-CTAAGCAGTTGGTGGTGCAG-3′; E-Selectin (SELE) forward, 5′-CCTGTGAAGCTCCCACTGA-3′, and reverse 5′- GGCTTTTGGTAGCTTCCATCT-3′. Real-time PCR was done with the SsoAdvanced Universal SYBR Green Supermix (BioRad), using the StepOnePlus instrument (Applied Biosystems, Foster City, CA, USA), and relative mRNA expression was normalized to GAPDH. Fold changes in the mRNA expression were calculated according to the 2-ΔΔCT method. Observed effective concentration and the corresponding inhibition of three independent experiments are given as the percentage of maximum inhibition of the E-Selectin expression, compared to the untreated IL-1β control (Table 3).
4. Conclusions
The present study provided a detailed information about the chemical composition of a hydroalcoholic extract of comfrey roots. A new arylnaphthalene lignan, named comfreyn A was isolated and characterized by the extensive use of 1D and 2D-NMR in combination with mass spectrometry. Moreover, this was the first occurrence of malaxinic acid, caffeic acid ethyl ester, along with the lignans ternifoliuslignan D, 3-carboxy-6,7-dihydroxy-1-(3′,4′-dihydroxyphenyl)- naphthalene, globoidnan A and B, and rabdosiin in S. officinale. These results, together with the fact that lithospermic acid and the salvianolic acid were not detected, might contribute to redefine the metabolite profile of this medicinal plant. In addition, the anti-inflammatory activity of compounds 1, 5, 9, 12, and 14–16 was tested using IL-1-stimulated human umbilical vein endothelial cells (HUVEC) and were analyzed by real-time PCR, determining the fold change in the mRNA expression of E-selectin. The obtained results showed a weak activity for each pure compound but a good anti-inflammatory activity for the extract. This interesting activity might be due to combined or synergistic effects of the compounds herein occurring in the extract belonging to the phenolic class. These results extend and reinforce the use of Symphytum PA-depleted extracts in the preparation of pharmaceutical formulations.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/13/4671/s1. Figure S1: HPLC–UV profiles of the methanol and ethanol extracts at wavelength 280nm. Figure S2: 1H NMR Spectrum (600 MHz, CD3OD) of compound 15. Figure S3: 13C NMR Spectrum (600 MHz, CD3OD) of compound 15. Figure S4: HSQC Spectrum (CD3OD) of compound 15. Figure S5: HMBC Spectrum (CD3OD) of compound 15. Figure S6: ROESY Spectrum (CD3OD) of compound 15. Figure S7: Globoidnan B (8). Figure S8: 1H NMR Spectrum (600 MHz, CD3OD) of compound 8. Figure S9: HSQC Spectrum (CD3OD) of compound 8. Figure S10: HMBC Spectrum (CD3OD) of compound 8. Figure S11: Rabdosiin (11). Figure S12: 1H NMR Spectrum (600 MHz, CD3OD) of compound 11. Figure S13: HSQC Spectrum (CD3OD) of compound 11. Figure S14: HMBC Spectrum (CD3OD) of compound 11. Figure S15: Globoidnan A (14). Figure S16: 1H NMR Spectrum (600 MHz, CD3OD) of compound 14. Figure S17: HSQC Spectrum (CD3OD) of compound 14. Figure S18: HMBC Spectrum (CD3OD) of compound 14. Table S1: 13C and 1H NMR data (J in Hz) of compounds 8, 11, and 14 (600 MHz, δ ppm, in CD3OD).
Author Contributions
Conceptualization, A.P. and S.P.; methodology, S.P., G.D., and M.M.; formal analysis, G.D. and M.M.; investigation, G.D. and M.M.; resources, A.P.; writing—original draft preparation, G.D. and M.M.; writing—review and editing, S.P. and A.P.; supervision, S.P. and A.P.; project administration, S.P. and A.P.; funding acquisition, A.P.; Design and methodology for the evaluation of the anti-inflammatory activity, Y.M.H.-S., R.d.M., and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was in part financially supported by P&G Health Germany GmbH; Alberto Plaza is an employee of P&G Health Germany GmbH and Yvonne M. Holper-Schichl is an employee of The Procter & Gamble Company—Austria.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Staiger C. Comfrey: A Clinical Overview. Phytother. Res. 2012;26:1441–1448. doi: 10.1002/ptr.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trifan A., Opitz S.E., Josuran R., Grubelnik A., Esslinger N., Peter S., Bräm S., Meier N., Wolfram E. Is comfrey root more than toxic pyrrolizidine alkaloids? Salvianolic acids among antioxidant polyphenols in comfrey (Symphytum officinale L.) roots. Food Chem. Toxicol. 2018;112:178–187. doi: 10.1016/j.fct.2017.12.051. [DOI] [PubMed] [Google Scholar]
- 3.Sowa I., Paduch R., Strzemski M., Zielińska S., Rydzik-Strzemska E., Sawicki J., Kocjan R., Polkowski J., Matkowski A., Latalski M., et al. Proliferative and antioxidant activity of Symphytum officinale root extract. Nat. Prod. Res. 2017;32:605–609. doi: 10.1080/14786419.2017.1326492. [DOI] [PubMed] [Google Scholar]
- 4.Seigner J., Junker-Samek M., Plaza A., D’Urso G., Masullo M., Piacente S., Holper-Schichl Y.M., de Martin R. A Symphytum officinale Root Extract Exerts Anti-inflammatory Properties by Affecting Two Distinct Steps of NF-κB Signaling Front. Pharmacology. 2019;10:289. doi: 10.3389/fphar.2019.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coulombe R.A. Pyrrolizidine alkaloids in foods. Adv. Food Nutr. Res. 2003;45:61–99. doi: 10.1016/s1043-4526(03)45003-1. [DOI] [PubMed] [Google Scholar]
- 6.Liu F., Wan S.Y., Jiang Z., Li S.F.Y., Ong E.S., Castaño-Osorio J.C. Determination of pyrrolizidine alkaloids in comfrey by liquid chromatography–electrospray ionization mass spectrometry. Talanta. 2009;80:916–923. doi: 10.1016/j.talanta.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency . Assessment Report on Symphytum Officinale L., Radix. EMA/HMPC; London, UK: 2009. 572844/2009. [Google Scholar]
- 8.Grube B., Grünwald J., Krug L., Staiger C. Efficacy of a comfrey root (Symphyti offic. radix) extract ointment in the treatment of patients with painful osteoarthritis of the knee: Results of a double-blind, randomised, bicenter, placebo-controlled trial. Phytomedicine. 2007;14:2–10. doi: 10.1016/j.phymed.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Koll R., Buhr M., Dieter R., Pabst H., Predel H.-G., Petrowicz O., Giannetti B., Klingenburg S., Staiger C. Efficacy and tolerance of a comfrey root extract (Extr. Rad. Symphyti) in the treatment of ankle distorsions: Results of a multicenter, randomized, placebo-controlled, double-blind study. Phytomedicine. 2004;11:470–477. doi: 10.1016/j.phymed.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Predel H.-G., Giannetti B., Koll R., Bulitta M., Staiger C. Efficacy of a Comfrey root extract ointment in comparison to a Diclo-fenac gel in the treatment of ankle distortions: Results of an observer-blind, randomized, multicenter study. Phytomedicine. 2005;12:707–714. doi: 10.1016/j.phymed.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Grabias B., Swiatek L. Phenolic acids in Symphytum officinale. Pharm. Pharmacol. Lett. 1998;8:81–83. [Google Scholar]
- 12.Ahmad V.U., Noorwala M., Mohammad F.V., Şener B., Gilani A.H., Aftab K. Symphytoxide A, A triterpenoid saponin from the roots of Symphytum officinale. Phytochemistry. 1993;32:1003–1006. doi: 10.1016/0031-9422(93)85244-L. [DOI] [PubMed] [Google Scholar]
- 13.Mohammad F.V., Noorwala M., Ahmad V.U., Şener B. A bidesmosidic hederagenin hexasaccharide from the roots of Symphytum officinale. Phytochemistry. 1995;40:213–218. doi: 10.1016/0031-9422(95)00246-4. [DOI] [PubMed] [Google Scholar]
- 14.Nastić N., Borrás-Linares I., Lozano-Sánchez J., Švarc-Gajić J., Segura-Carretero A. Comparative Assessment of Phytochemical Profiles of Comfrey (Symphytum officinale L.) Root Extracts Obtained by Different Extraction Techniques. Molecules. 2020;25:837. doi: 10.3390/molecules25040837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heide L., Floss H.G., Tabata M. Incorporation of shikimic acid into p-hydroxybenzoic acid in Lithospermum erythrorhizon cell cultures. Phytochemistry. 1989;28:2643–2645. doi: 10.1016/S0031-9422(00)98058-0. [DOI] [Google Scholar]
- 16.Chen P.X., Tang Y., Zhang B., Liu R., Marcone M., Li X., Tsao R. 5-Hydroxymethyl-2-furfural and Derivatives Formed during Acid Hydrolysis of Conjugated and Bound Phenolics in Plant Foods and the Effects on Phenolic Content and Antioxidant Capacity. J. Agric. Food Chem. 2014;62:4754–4761. doi: 10.1021/jf500518r. [DOI] [PubMed] [Google Scholar]
- 17.Pérez-Maqueda J., Arenas-Ligioiz I., López Ó., Fernandez-Bolaños J.G. Eco-friendly preparation of 5-hydroxymethylfurfural from sucrose using ion-exchange resins. Chem. Eng. Sci. 2014;109:244–250. doi: 10.1016/j.ces.2014.01.037. [DOI] [Google Scholar]
- 18.Bottone A., Masullo M., Montoro P., Pizza C., Piacente S. HR-LC-ESI-Orbitrap-MS based metabolite profiling of Prunus dulcis Mill. (Italian cultivars Toritto and Avola) husks and evaluation of antioxidant activity. Phytochem. Anal. 2019;30:415–423. doi: 10.1002/pca.2824. [DOI] [PubMed] [Google Scholar]
- 19.Ovenden S.P.B., Yu J., Wan S.S., Sberna G., Tait R.M., Rhodes D., Cox S., Coates J., Walsh N.G., Meurer-Grimes B.M. Globoidnan A: A Lignan from Eucalyptus globoidea Inhibits HIV Integrase. Phytochemistry. 2005;36:3255–3259. doi: 10.1002/chin.200519236. [DOI] [PubMed] [Google Scholar]
- 20.Basli A., Delaunay J.-C., Pedrot E., Bernillon S., Madani K., Monti J.-P., Mérillon J.-M., Chibane M., Richard T. New Cyclolignans from Origanum glandulosum Active Against β-amyloid Aggregation. Rec. Nat. Prod. 2014;8:208–216. [Google Scholar]
- 21.Zhang J.-L., Yan R.-J., Yu N., Zhang X., Chen D.-J., Wu T., Xin J.-G. A new caffeic acid tetramer from the Dracocephalum moldavica L. Nat. Prod. Res. 2017;32:370–373. doi: 10.1080/14786419.2017.1359168. [DOI] [PubMed] [Google Scholar]
- 22.Wagner H., Hörhammer L., Frank U. [Lithospermic acid, the antihormonally active principle of Lycopus europaeus L. and Symphytum officinale. Ingredients of medicinal plants with hormonal and antihormonal-like effect] Arzneimittelforschung. 1970;20:705–713. [PubMed] [Google Scholar]
- 23.Wang X., Li W., Ma X.-H., Yan K., Chu Y., Han M., Li S., Zhang H., Zhou S., Zhu Y., et al. Identification of a major metabolite of danshensu in rat urine and simultaneous determination of danshensu and its metabolite in plasma: Application to a pharmacokinetic study in rats. Drug Test. Anal. 2014;7:727–736. doi: 10.1002/dta.1750. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y., Wang K., Chen H., He R., Cai R., Li J., Zhou D., Liu W., Huang X., Yang R., et al. Anti-inflammatory lignans and phenylethanoid glycosides from the root of Isodon ternifolius (D.Don) Kudô. Phytochemistry. 2018;153:36–47. doi: 10.1016/j.phytochem.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Daquino C., Rescifina A., Spatafora C., Tringali C. Biomimetic Synthesis of Natural and “Unnatural” Lignans by Oxidative Coupling of Caffeic Esters. Eur. J. Org. Chem. 2009:6289–6300. doi: 10.1002/ejoc.200900804. [DOI] [Google Scholar]
- 26.Balestrieri C., Felice F., Piacente S., Pizza C., Montoro P., Oleszek W., Visciano V., Balestrieri M.L. Relative effects of phenolic constituents from Yucca schidigera Roezl. bark on Kaposi’s sarcoma cell proliferation, migration, and PAF synthesis. Biochem. Pharmacol. 2006;71:1479–1487. doi: 10.1016/j.bcp.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Chiang Y.-M., Lo C.-P., Chen Y.-P., Wang S.-Y., Yang N.-S., Kuo Y.-H., Shyur L.-F. Ethyl caffeate suppresses NF-kappaB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2 in vitro or in mouse skin. Br. J. Pharmacol. 2005;146:352–363. doi: 10.1038/sj.bjp.0706343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bedane K.G., Zuhlke S., Spiteller M. Bioactive constituents of Lobostemon fruticosus: Anti-inflammatory properties and quantitative analysis of samples from different places in South Africa. S. Afr. J. Bot. 2020;131:174–180. doi: 10.1016/j.sajb.2020.02.016. [DOI] [Google Scholar]
- 29.Hoeth M., Niederleithner H., Hofer-Warbinek R., Bilban M., Mayer H., Resch U., Lemberger C., Wagner O., Hofer E., Petzelbauer P., et al. The Transcription Factor SOX18 Regulates the Expression of Matrix Metalloproteinase 7 and Guidance Molecules in Human Endothelial Cells. PLoS ONE. 2012;7:e30982. doi: 10.1371/journal.pone.0030982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.