Summary
The intestine is continuously exposed to an enormous variety and quantity of antigens and innate immune stimuli derived from both pathogens and harmless materials, such as food and commensal bacteria. Accordingly, the intestinal immune system is uniquely adapted to ensure appropriate responses to the different kinds of challenge; maintaining tolerance to harmless antigens in the steady‐state, whilst remaining poised to deal with potential pathogens. To accomplish this, leucocytes of the intestinal immune system have to adapt to a constantly changing environment and interact with many different non‐leucocytic intestinal cell types, including epithelial and endothelial cells, neurons, and a heterogenous network of intestinal mesenchymal cells (iMC). These interactions are intricately involved in the generation of protective immunity, the elaboration of inflammatory responses, and the development of inflammatory conditions, such as inflammatory bowel diseases. Here we discuss recent insights into the immunological functions of iMC under homeostatic and inflammatory conditions, focusing particularly on iMC in the mucosa and submucosa, and highlighting how an appreciation of the immunology of iMC may help understand the pathogenesis and treatment of disease.
Keywords: homeostasis, immune response, inflammation, intestine, mesenchymal stromal cell
Intestinal mesenchymal cells (iMC) help to maintain mucosal immune homeostasis, and play an active role in the induction and resolution of inflammatory responses. However, until recently, their immune regulatory roles have been largely overlooked, and a clear understanding of iMC heterogeneity and the discrete functions of individual subsets is lacking. Identifying which iMC are involved in these processes, and the mechanisms they use, could be an important advance in designing new therapies for treating intestinal diseases, such as inflammatory bowel disease.
Abbreviations
- ACKR
atypical chemokine receptor
- bFGF
basic fibroblast growth factor
- CD
Crohn’s disease
- COX2
cyclo‐oxygenase 2
- CTGF
connective tissue growth factor
- DAMP
damage‐associated molecular pattern
- DC
dendritic cell
- DSS
dextran sodium sulphate
- ECM
extracellular matrix
- EGF
epidermal growth factor
- Eos
eosinophils
- EPO
eosinophil peroxidase
- FDC
follicular dendritic cell
- GALT
gut‐associated lymphoid tissue
- GM‐CSF
granulocyte macrophage colony‐stimulating factor
- IBD
inflammatory bowel disease
- ICAM
intercellular adhesion molecule
- IGF‐1
insulin‐like growth factor‐1
- IGFBP3
IGF binding protein 3
- ILC
innate lymphoid cell
- iMC
intestinal mesenchymal cell
- LPS
lipopolysaccharide
- MAMP
microbe‐associated molecular pattern
- M‐CSF
myeloid colony‐stimulating factor
- MLN
mesenteric lymph node
- MMP
matrix metalloproteinase
- NLR
NOD‐like receptor
- PDGF
platelet‐derived growth factor
- PDL
programmed death ligand
- PGE2
prostaglandin E2
- PMN
polymorphonuclear cells
- PRR
pattern recognition receptor
- RA
retinoic acid
- RALDH
retinaldehyde dehydrogenase
- ROS
reactive oxygen species
- SCFA
short‐chain fatty acid
- scRNAseq
single‐cell RNA sequencing
- SMA
smooth muscle actin
- TACI
transmembrane activator and CAML interactor
- TIMP‐1
tissue inhibitor of metalloproteinase‐1
- TLR
toll‐like receptor
- TNSF
TNF superfamily
- UC
ulcerative colitis
- VCAM
vascular cell adhesion protein
Introduction
Mesenchymal cells (MCs) are non‐epithelial, non‐endothelial, non‐haematopoietic cells that differentiate from mesenchymal stem cells and form the framework on which all mammalian tissues are built. However, this is no silent scaffolding. MCs are also able to act as sentinels, poised to provide physiological and immunological support in response to environmental cues. In the intestine, MCs are found in all anatomical compartments, including the mucosa, submucosa and muscle layers of the intestinal wall (Fig. 1). MCs are also abundant in the associated secondary lymphoid tissues, where the priming of local immune responses takes place. These include the isolated lymphoid follicles (ILF) and Peyer’s patches of the gut‐associated lymphoid tissue (GALT), as well as the draining mesenteric lymph nodes (MLN). Although MCs in the GALT and MLN play crucial roles in the development, structure and immunological function of these organs, these properties are similar to those of MCs in other secondary lymphoid tissues that have been reviewed extensively elsewhere. 1
Figure 1.
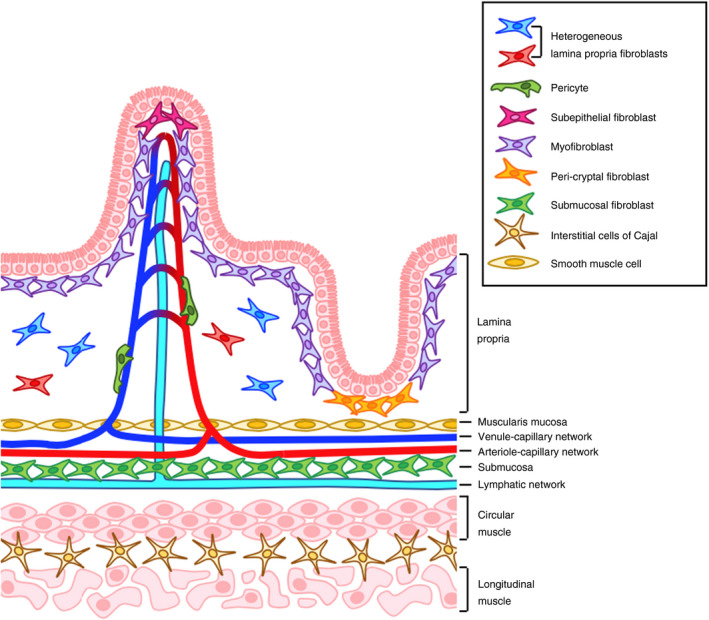
Mesenchymal stromal cells in the intestine. Several populations of fibroblasts exist in the lamina propria, where they secrete the extracellular matrix (ECM) components that are essential for maintaining the structural integrity of the gut. The true extent of lamina propria intestinal mesenchymal cell (iMC) heterogeneity remains to be fully established. Adjacent to the mucosal barrier, myofibroblasts, subepithelial and crypt‐associated fibroblasts regulate the turnover, differentiation and movement of epithelial cells, 2 , 22 while pericytes and smooth muscle cells maintain the growth and function of vascular and lymphatic endothelial cells. Submucosal fibroblasts reside in close proximity to, and physically interact with, collecting lymphatic vessels. 6 These fibroblasts specifically express genes encoding endothelial regulators, so may play a role in lymphangiogenesis. Smooth muscle cells and the interstitial cells of Cajal drive peristalsis and gut motility.
Different anatomical locations contain phenotypically and functionally distinct MC populations, and substantial MC heterogeneity exists within tissues. This is particularly true in the intestine, where numerous populations of intestinal mesenchymal cells (iMC) occupy distinct niches and perform site‐specific physiological functions. As well as secreting the extracellular matrix (ECM) components that maintain intestinal architecture, myofibroblasts, subepithelial and crypt‐associated fibroblasts regulate epithelial cell function. Pericytes and smooth muscle cells sustain blood and lymphatic vessels, while a novel group of submucosal fibroblasts may play a role in lymphangiogenesis. Smooth muscle cells and the interstitial cells of Cajal drive peristalsis and gut motility (Fig. 1; Table 1). We have long known that iMC populations play fundamental roles in the maintenance of intestinal architecture and function, but until recently their contribution to immune responses has largely been overlooked. Furthermore, we have lacked knowledge of the specific markers required to fully explore iMC heterogeneity and the functions of individual subsets. However, this is now changing, and single‐cell RNA sequencing (scRNA seq) technology is beginning to enhance our understanding of these cells. 2 , 3 Here we will focus on the immunological roles of iMC, specifically fibroblasts, myofibroblasts, pericytes and smooth muscle cells. Moreover, we will address how phenotypically and spatially distinct iMC populations might perform unique immune regulatory functions in the intestinal mucosa and submucosa, before considering how these properties may contribute to inflammatory responses in the gut, including inflammatory bowel diseases (IBD). Finally, we will discuss how iMC may be modulated by the biggest environmental factor in their locale, the microbiome.
Table 1.
Physiological functions of iMC in mucosa and submucosa
| iMC subset | Markers used for classification | Function |
|---|---|---|
| Myofibroblasts | α‐SMA+, Vimentin+, PDGFRα+, CD34−, Myosin‐11+, CD90+ |
Contractile function supports enterocyte movement along crypt‐villus axis 109 Production of the basement membrane 109 Epithelial support |
| Lamina propria fibroblasts | α‐SMA−, Vimentin+, PDGFRα+, CD34+, ICAM+/−, CD90+/−, CD55+/− |
ECM production and remodelling Heterogeneous population of cells 2 , 3 May include precursors 2 |
| Subepithelial iMC | α‐SMA−, PDGFRα+, CD34+, F3+, SOX6hi |
Production of hedgehog molecules 110 |
| Peri‐cryptal iMC | α‐SMA−, PDGFRα+, CD34+, F3+, SOX6+, Wnt‐2b+, Wnt‐5a+ | Maintain epithelial regeneration via trophic effects on epithelial step cell niche 2 , 7 , 22 |
| Submucosal fibroblasts | PDGFRα+, CD34+, ACKR4+, CD55+, CD90+ (col), CD90− (SI) |
Modulation of lymphangiogenesis 6 Maintenance of chemokine gradients 6 |
| Pericytes | α‐SMA+/−, PDGFRα−, CD146hi, ESAMhi, CD34−, CD36+, α7 integrin+ | Vascular contraction and support |
| Smooth muscle cells | α‐SMA+, PDGFRα−, Desmin+, Vimentinlo |
Smooth muscle contraction Mechanical support |
ACKR, atypical chemokine receptor; ECM, extracellular matrix; ICAM, intercellular adhesion molecule; iMC, intestinal mesenchymal cells; PDGF, platelet‐derived growth factor; SMA, smooth muscle actin.
Immunological functions of iMC in steady‐state
Intestinal mesenchymal cells are not simply bystanders in intestinal immune responses, and even in the steady‐state they are involved in active, two‐way communication with neighbouring leucocytes (Fig. 2). Here we will discuss the evidence that iMC can orchestrate leucocyte migration into, within and out of the intestine by producing and/or displaying chemokines, or by scavenging them from the extracellular environment. We will then describe how iMC help to maintain a tolerogenic environment in the steady‐state intestine.
Figure 2.
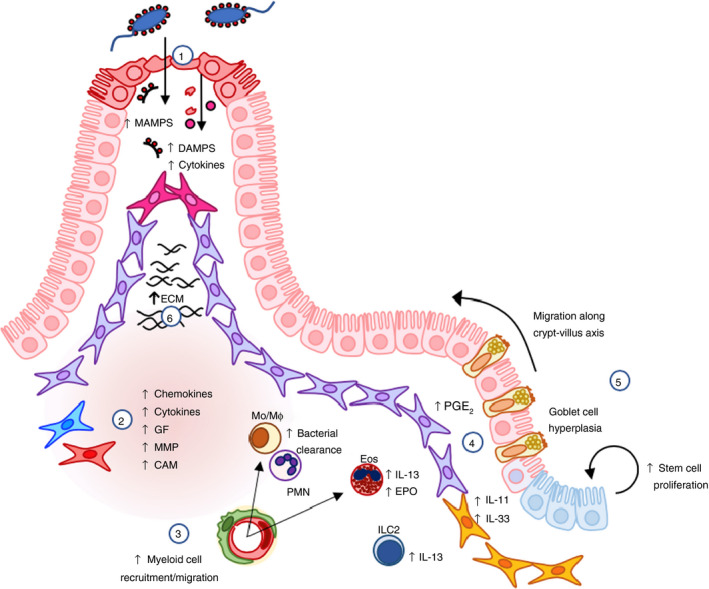
Role of intestinal mesenchymal cells (iMC) in intestinal inflammation and repair. 1. Following mechanical injury, infection or inflammation, iMC become activated by pro‐inflammatory cytokines and pattern recognition receptor (PRR) agonists, such as microbe‐associated molecular proteins (MAMPs; from the resident microbiota) and damage‐associated molecular patterns (DAMPs; from the damaged epithelium). 2. This results in increased production of cytokines, such as interleukin (IL)‐6, IL‐11 and IL‐33; prostaglandin E2 (PGE2); myeloid cell chemoattractants, such as CXCL1, CXCL2, IL‐8/CXCL8, CCL2 and CCL11; growth factors (GF) myeloid colony‐stimulating factor (M‐CSF) and granulocyte macrophage colony‐stimulating factor (GM‐CSF); cellular adhesion molecules (CAM); and matrix metalloproteinases (MMPs). 41 , 42 , 43 , 44 , 46 , 47 , 48 , 49 3. This enhances recruitment and migration of monocytes/macrophages (Mo/MØ), polymorphonuclear cells (PMN) and eosinophils (Eos), which facilitate bacterial clearance and bolster innate immune responses in the gut. 4. IL‐11, IL‐33 and PGE2 may contribute to local inflammatory responses, but can also promote barrier repair through their pro‐survival and proliferative effects on the epithelial stem cell niche. 47 , 68 , 108 5. IL‐33 also increases IL‐13 production by innate lymphoid cells (ILC)2s and eosinophils, driving goblet cell hyperplasia and enterocyte migration up the crypt‐villus axis. 70 , 71 IL‐33‐mediated induction of IL‐13 and eosinophil peroxidase (EPO) can amplify cytokine production by iMC. 6. Cytokines in the inflamed mucosa increase the production of extracellular matrix (ECM) components, contributing to normal wound healing.
Regulation of leucocyte migration
Fibroblasts, myofibroblasts and pericytes have all been implicated in driving leucocyte migration into and within the gut in response to insult or injury, and they are thought to play a similar role in the steady‐state. At the most basic level, ECM produced by iMC acts as a scaffold that leucocytes adhere to and crawl along after extravasating from the blood. 4 These fibres are rich in glycosaminoglycans that can bind extracellular chemokines and present them to patrolling leucocytes to trigger motility. 5 iMC can also directly contribute to leucocyte migration by producing chemokines, and they express numerous chemokine genes in the steady‐state intestine of both humans and mice. 2 , 6 , 7 These include the genes encoding CXCL13, the B‐cell and lymphoid tissue inducer cell chemoattractant; 6 , 7 the myeloid cell chemoattractants CCL2, CCL8, CCL11 and CCL13; 2 , 3 , 7 and the pleiotropic primordial chemokines CXCL12 and CXCL14. 2 , 6 , 7 Lamina propria iMC in the steady‐state human colon have also been reported to express genes encoding the neutrophil chemoattractants CXCL1 and CXCL2, 2 despite the fact that neutrophils are rarely present in the healthy gut. Although few studies have examined chemokine production by iMC at the protein level, the steady‐state intestine is highly enriched with eosinophils (Eos), 8 and is one of the few tissues where resident macrophages are continuously replenished from circulating monocytes. 9 Thus, it seems likely that the production and presentation of chemoattractants by iMC helps regulate the turnover of these populations in situ.
In addition to orchestrating leucocyte migration, some of the chemokines associated with resting iMC are reported to have bactericidal properties. The most well characterized is CXCL14, which is structurally similar to antimicrobial β‐defensins due to its positive charge at neutral pH and presence of anti‐parallel β‐sheets, a C‐terminal α‐helix and several cationic residues. 10 , 11 Fibroblast‐specific CXCL14 production has been suggested to provide protection against pathogens at other epithelial sites, such as the skin and lungs, 12 , 13 and known bacterial targets of CXCL14 include the intestinal pathobionts Escherichia coli and Candida albicans. 10 , 12 The chemokine concentrations required for antimicrobial action are high. Nevertheless, the constitutive expression of the gene encoding CXCL14 by subepithelial fibroblasts 2 , 6 may provide rapid bactericidal support in the event of bacterial translocation.
In addition to producing chemokines, some iMC express atypical chemokine receptors (ACKR), which are known to scavenge extracellular chemokines and target them for intracellular degradation. ACKR4 binds and internalizes the CCR7 ligands CCL19 and CCL21, 14 , 15 which are crucial for the mobilization of mature dendritic cells (DCs) from the intestine to the draining MLN. 16 ACKR4 also scavenges CCL25, 15 the only known CCR9 ligand, which is produced constitutively by the small intestinal epithelium, and is a key player in the recruitment of T and B lymphocytes and plasmacytoid DCs to the tissue. 17 , 18 , 19 In recent studies, we found that in the colon and small intestine of mice, ACKR4 is exclusively expressed selectively by a population of CD34+ fibroblasts in the submucosa. 6 We have also observed that a large proportion of iMC express Ackr3, which encodes an ACKR specific for CXCL11 and 12 (Carolyn Thomson, unpublished data). The functional significance of ACKR expression by iMC subsets remains unclear, and there were no obvious immunological defects in the intestine of steady‐state Ackr4‐deficient mice. 6 However, it is possible that by scavenging chemokines, iMCs modulate chemokine gradients in vivo to finely tune leucocyte recruitment to, positioning within, or egress from the intestine, as has been described for ACKR4 in the skin and skin‐draining lymph nodes where it is expressed by keratinocytes and lymphatic endothelial cells. 20 , 21 Alternatively, the immunological effects of ACKR4 in the intestine may only become more apparent following an infectious or inflammatory insult, which are known to be associated with increased CCR7‐ and CCR9‐dependent leucocyte migration and de novo transcription of Ccl19 by CD34+ and CD34− iMC subsets. 22
Regulation of leucocyte activation and differentiation
It has been reported that iMC are capable of expressing both MHC class I and II molecules, but there are conflicting reports about whether iMC express MHCII molecules at rest. 23 , 24 As well as finding that some subepithelial myofibroblasts can express HLA‐DR in situ in the resting colon, Saada and colleagues showed that primary cultures of these cells could induce MHCII‐dependent proliferation of allogeneic CD4+ T‐cells in vitro. This required myofibroblast expression of the co‐stimulatory molecules CD80 and CD86, which were induced by MHCII‐mediated interaction with allogeneic T‐cells. 24 As interferon (IFN)γ is a major driver of MHCII expression by human intestinal myofibroblasts, 25 it is possible that MHCII expression by iMC in vivo is dependent on the local levels of IFNγ. In turn, this may be determined by the exact composition of local microbiota, thus accounting for discrepancies in reports of MHCII expression by iMC in situ. Alternatively, MHCII expression may be lost following isolation and culture. Indeed, the earlier experiments that failed to find steady‐state expression of MHCII on iMC 23 used cells that had been cultured for several weeks in vitro, whereas Saada and colleagues assessed MHCII expression in situ and on freshly isolated cells. 24
It is not clear what impact cognate interactions between MHCII‐expressing myofibroblasts and CD4+ T‐cells would have in the steady‐state mucosa, where the rarity of naïve T‐cells means that presentation of antigen by iMC is unlikely to be important in initiating immune responses. However, as non‐professional antigen‐presenting cells are normally found to induce T‐cell tolerance, it is possible that iMC contribute to the tolerogenic environment of the steady‐state intestine. Via their expression of the programmed death ligands PDL1 and PDL2, iMC can also regulate the function of the fully differentiated PD‐1+ effector/memory T‐cells that dominate the lamina propria. 26 , 27 , 28 CD90+ colonic iMC use this mechanism to drive Treg polarization, suppress T effector cell proliferation and dampen IFNγ production in vitro. 26 , 27 , 29 A similar antiproliferative phenotype has been described for tissue‐resident fibroblasts in other organs, including skin and lung 30 and, interestingly, severe T‐cell‐mediated autoimmune enteritis is a significant side‐effect of PDL1 antibody blockade. 31
Another way that iMC may regulate local T‐cell responses is through their production of retinoic acid (RA) via retinaldehyde dehydrogenase (RALDH)‐mediated metabolism of dietary vitamin A. iMC‐derived RA can drive expression of the P2‐purinoceptor component P2X7 by CD4+ memory/effector T‐cells in the lamina propria, rendering them more sensitive to nicotinamide adenine dinucleotide‐induced apoptosis. 32 This may enhance the ability of iMC to promote local tolerance. Furthermore, iMC‐mediated production of RA in combination with granulocyte macrophage colony‐stimulating factor (GM‐CSF) induces the expression of RALDH2 by intestinal DCs, 33 and plays a crucial role in the development and transcriptional programming of the CD103+CD11b+ DCs that are unique to the intestinal mucosa. 33 , 34 However, iMC are not the only source of these immunoregulatory mediators in the mucosa, as epithelial cells express RALDH1 and contribute to DC imprinting, 35 while innate lymphoid cell (ILC)3 can produce GM‐CSF in response to microbial colonization. 36 RA from colonic iMC can also induce upregulation of P2X7 in bone marrow‐derived mast cells, together with several genes associated with mucosal mast cells. Thus, contact between local iMC and mast cell precursors may be involved in mucosal mast cell development in the intestine. 37
Recent evidence suggests that small intestinal iMC may be unique in their ability to drive the differentiation of B‐cells into IgA‐secreting plasma cells. This was mediated by B‐cell‐activating factor (BAFF) as well as other unidentified soluble mediators. 38 It is possible that RALDH activity in iMC plays a role in this, as has been shown for RALDH+ mucosal DCs in the draining MLN. 39
Together these findings indicate that iMC have a number of properties that allow them to contribute to the maintenance of homeostasis in the steady‐state intestine. However, the importance of iMC in steady‐state leucocyte trafficking and immune homeostasis in vivo is unclear, and the precise roles of individual subsets of iMC remain to be determined.
Immunological functions of iMC in intestinal infection and inflammation
Following mechanical injury or exposure to pathogens and their products, leucocytes and tissue‐resident stromal cells in the intestine undergo a series of changes designed to facilitate pathogen clearance, protect the epithelial barrier and promote wound repair (Fig. 2). Important components of these responses include amplification of local inflammatory responses, reorganization of the ECM and modulation of the villus‐crypt unit structure in response to insult or injury. These innate and adaptive responses are tightly regulated and, in most circumstances, self‐limiting. Failure to control intestinal inflammation and fully repair the epithelial barrier can result in the development of IBD, such as Crohn’s disease (CD) and ulcerative colitis (UC). Prolonged colonic inflammation also increases susceptibility to colorectal cancer but, as the role that iMC play in tumours has been reviewed extensively elsewhere, 40 we will not consider it here. Instead we will focus on the role of iMC‐derived cytokines, chemokines and growth factors in intestinal injury, infection, inflammation and repair.
Pro‐inflammatory effects of iMC
As in the steady‐state, the lack of specific markers has meant that there is little definitive information on how individual subsets of iMC contribute to intestinal inflammation. Nevertheless, iMC as a whole can express a number of cytokine and chemokine receptors, as well as pattern recognition receptors (PRRs) such as Toll‐like receptors (TLRs) and NOD‐like receptors (NLRs), indicating that they have the potential to react to changes in their environment.
Because of their proximity to the lumen, many studies have concentrated on the ability of subepithelial myofibroblasts to respond to microbe‐ and danger‐associated molecular patterns (MAMPs and DAMPs). 41 , 42 Indeed, transcriptional profiling has revealed that cultured primary αSMA+ iMC from the human colon express mRNA encoding TLR1−9, as well as NOD1 and NOD2. 41 , 42 , 43 , 44 , 45 Although the exact range of PRR expressed by other subsets of iMC remains unclear, cultured human and murine myofibroblasts produce the inflammatory cytokine IL‐6, upregulate the neutrophil chemoattractants IL‐8/CXCL8 or CXCL1, and upregulate cyclo‐oxygenase 2 (COX2) to produce prostaglandin E2 (PGE2) in response to lipopolysaccharide (LPS). 41 , 42 , 46 LPS also stimulates murine iMC to upregulate Cxcl2, Cxcl5, Ccl2 and Ccl7 transcripts in vitro. 43 Less is known about if and how iMC respond to PRR ligands other than LPS, although murine iMC can produce IL‐6 in response to synthetic lipoproteins, including Pam3CSK4, heat‐killed Listeria monocytogenes and FSL‐1, indicating that they can respond to ligation of TLRs 1, 2 and 6. 43 Furthermore, colonic iMCs from mice infected with Citrobacter rodentium produce CCL2 in response to NOD2 ligands. 44
Interestingly, iMC and leucocytes may respond differently to PRR ligation. For instance, TLR2 expression by non‐haematopoietic cells is required for clearance of C. rodentium and, rather than amplifying local inflammatory responses, this helps maintain mucosal integrity, reduces bacterial dissemination and limits intestinal inflammation. 47 In contrast, activation of TLR2 on leucocytes amplifies the inflammatory response in this model, leading to increased morbidity and mortality. 47 Although it was not shown directly that iMC were the tissue‐resident cells responding to TLR2 ligands during C. rodentium infection, IL‐11 was upregulated by α‐SMA+ iMC in the muscularis mucosa in a TLR2‐dependent manner, and protection against C. rodentium could be restored by treating TLR2‐deficient mice with recombinant IL‐11. 47 Thus, although iMC and other intestinal stromal cells are known to express TLR2, their downstream response appears to be distinct from that of leucocytes.
The induction of pro‐inflammatory cytokines and chemokine production by iMC is not limited to ligation of PRR, and they can also respond to other extracellular stimuli such as cytokines. Interestingly, current evidence seems to indicate that iMC may be hard‐wired to produce a similar repertoire of inflammatory mediators regardless of the exogenous stimulus. Thus stimulation of human colonic iMC with IL‐1α, IL‐1β, TNFα or IL‐17 in vitro triggers the production of the same mediators that are produced following TLR ligation; 42 , 43 , 46 namely IL‐6, chemokines CXCL1, IL‐8/CXCL8 and CCL2, and growth factors myeloid colony‐stimulating factor (M‐CSF) and GM‐CSF. 46 , 48 , 49 A similar phenomenon has been observed in mice, where iMC stimulated with IL‐36γ, or isolated following acute dextran sodium sulphate (DSS)‐mediated colitis in vivo, produced IL‐6, CXCL1, CXCL2, CCL2 and GM‐CSF. 22 , 50 Thus, iMC may contribute to host defence and inflammation via generic, pre‐programmed production of cytokines and the release of chemokines that recruit innate immune cells such as monocytes, neutrophils and Eos. Migration of leucocytes through the tissue may be aided by the upregulation of adhesion molecules intercellular adhesion molecule (ICAM) and vascular cell adhesion protein (VCAM) by iMC, as has been observed at a transcript level during DSS‐mediated colitis. 22 Furthermore, the growth factors and chemokines produced by iMC may trigger the mobilization of innate leucocytes from the bone marrow, as indicated by the GM‐CSF‐driven expansion of Eos in experimental IBD. 51
iMC and trained immunity
Altered iMC responses during inflammation may have long‐term consequences for mucosal immunity, due to the phenomenon of ‘trained immunity’ in which a primary innate stimulus alters subsequent responses by leucocytes and stromal cells. 52 Although often transient, trained immunity can also be a long‐lasting consequence of epigenetic modification, and it has been shown that pro‐inflammatory cytokines can induce histone acetylation in synovial fibroblasts, resulting in a heightened inflammatory response to secondary stimulation in vitro. 53 , 54 These long‐lived changes to fibroblast function may contribute to the perpetuation of synovial pathology, and it would be interesting to determine whether similar processes occur during chronic intestinal inflammation. Indeed, epigenetic modifications have been observed in mucosal fibroblasts isolated from patients with fibrostenotic CD, with resulting changes in pathogenic gene expression. 55 , 56 Furthermore, early‐life lymphotoxin‐β receptor signalling has a life‐long impact on the ability of MCs to promote IgA class‐switching in the MLN. 57 Understanding whether trained immunity in iMC contributes to ongoing inflammation in the gut may be crucial for the development of novel therapeutics to tackle IBD.
Role of iMC in inflammatory bowel diseases
Mesenchymal cells are increasingly implicated in the pathogenesis and treatment of chronic inflammatory diseases with, for example, synovial fibroblasts thought to be central to the initiation and maintenance of pathology in rheumatoid arthritis. 58 , 59 Analogous processes of relapsing and remitting immunopathology are found in the IBDs CD and UC, in which inappropriate immune responses directed against harmless commensal microbes. This causes damage to the mucosal epithelium, impaired wound healing, and the formation of mucosal ulcers and strictures. Elegant studies using scRNAseq have shown that these features are accompanied by dramatic changes in colonic iMC, including the acquisition of a transcriptional profile consistent with a role in inflammation and in enhancing leucocyte recruitment to the mucosa. 2 , 3 As suggested from the in vitro studies described above, 40 , 42 , 46 , 48 , 49 this is likely mediated by inflammatory cytokines present in the mucosa and the increased abundance of endotoxin resulting from increased barrier permeability. Pericytes and putative peri‐cryptal WNT2B hi fibroblasts from the colon of children with CD and UC showed increased expression of CCL2 and CCL8, while lamina propria and WNT2B hi fibroblasts expressed elevated levels of CXCL1 and CCL11. 3 In addition, a novel population of pro‐inflammatory fibroblasts was found in UC, which was characterized by high levels of expression of the neutrophil chemoattractant CXCL5, as well as MMP3, IL1B, IL24 and IL6. 3 Again, it is interesting to note that the chemokine profile associated with iMC is highly selective for myeloid cells, underlining the possibility that iMC may be pre‐primed to mobilize this particular class of innate immune cells in response to an inflammatory insult. It has also been proposed that the pro‐inflammatory iMC, which increase in numbers in IBD, may contribute to barrier damage by inhibiting the stem cell niche‐associated iMC that promote epithelial renewal and differentiation. 2 , 3
As well as altering the mucosal milieu in IBD, iMC may also drive the generation of tertiary lymphoid follicles, as indicated by increased numbers of FRC‐like stromal cells in adults with UC. These cells expressed elevated levels of CCL19, as well as genes encoding CCL21, TNF superfamily (TNFSF) member 14 (LIGHT) and follicular DC secreted protein (FDC‐SP). 2 A similar enrichment of these genes, as well as TNFSF13b (TACI), was observed in FRC‐like fibroblasts isolated from children with IBD. 3 All these molecules are involved in the development, organization and immune function of tertiary lymphoid tissue, including naïve lymphocyte recruitment, modulation of germinal centre responses, and IgA class‐switching. 60 , 61 , 62
Thus, transcriptional analyses suggest that IBD is associated with expansion of pro‐inflammatory and immunomodulatory stromal cells in both the LP and organized lymphoid structures. An important goal of future studies will be to identify exactly where these populations are located and the nature of the immune cells they interact with.
IMC‐derived immunomodulators in intestinal repair and fibrosis
Mucosal inflammation is pivotal for host defence and pathogen clearance, but it comes at a potential cost, because the production of cytokines, matrix metalloproteinases (MMPs), reactive oxygen species (ROS) and nitrogen intermediates can lead to pathological ECM remodelling, epithelial cell death and ulceration. As a result, would healing and tissue repair play critical roles in ensuring healthy termination of acute inflammatory responses, and MCs are centrally involved in these processes. iMC are well known to promote wound healing and barrier repair via the production of ECM components, and via their trophic effects on epithelial cells and the stem cell niche (Fig. 2). These processes need to be tightly controlled, because excessive ECM production and iMC activation can lead to the development of fibrosis, scarring and stricture formation (Fig. 3). The important contributions iMC play in normal and pathological wound healing are largely physiological, and have been reviewed extensively elsewhere. 63 We will focus on the pathological roles that iMC‐derived immunomodulators can play in intestinal inflammation, repair and fibrosis.
Figure 3.
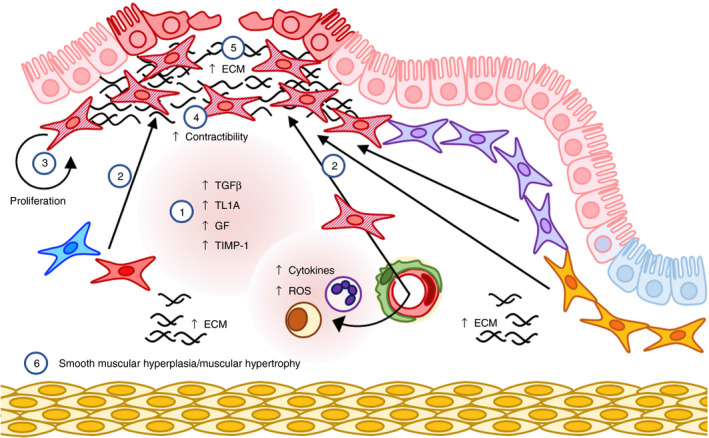
Fibrosis and pathological response to wounding. Dysregulated wound healing can lead to fibrosis and fibrostenosis, two major complications of inflammatory bowel disease (IBD). 1. Transforming growth factor (TGF)‐β and TL1A, reactive oxygen species (ROS), basic fibroblast growth factor (bFGF), insulin‐like growth factor‐1 (IGF‐I), platelet‐derived growth factor (PDGF) and epidermal growth factor (EGF) are released by leucocytes and intestinal mesenchymal cells (iMC) during chronic inflammation. 2–4. These mediators drive the accumulation of numerous populations of iMC, 77 , 78 , 92 which then differentiate into contractile, pathologically activated myofibroblasts. 63 5. Fibrosis‐associated myofibroblasts lose their migratory potential and produce excess extracellular matrix (ECM) proteins resulting in pathological ECM deposition. 63 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 6. Ultimately this leads to fibrosis, smooth muscle cell hyperplasia and hypertrophy of the muscularis.
Some of the characteristic cytokines produced by iMC during inflammation, such as IL‐11 and IL‐33, can have pro‐repair and pro‐inflammatory functions depending on the context. As noted above, IL‐11 produced by subepithelial myofibroblasts and α‐SMA+ iMC in the muscularis mucosa protects against the intestinal inflammation caused by C. rodentium. 47 This effect is mediated by STAT3 phosphorylation in neighbouring epithelial cells driving the expression of genes associated with survival and proliferation, thus promoting wound repair and preventing epithelial damage. 47 , 64 IL‐11 also modulates cytokine production by mononuclear phagocytes, downregulating production of Th1‐associate cytokines IL‐12 and IFNγ. 65 This skews CD4+ T‐cell polarization towards a Th2 phenotype, rather than a more inflammatory Th1 response. Recombinant IL‐11 ameliorates inflammation and tissue destruction in several animal models of colitis, 47 , 64 and has subsequently been used with some success in clinical trials for CD. 66 , 67
Interleukin‐33 is expressed by numerous stomal cell populations in the steady‐state intestine, including subepithelial and peri‐cryptal iMC, 68 , 69 and it plays an important role in maintaining epithelial barrier integrity in the intestine. 68 IL‐33 is upregulated by peri‐cryptal iMC during Salmonella typhimurium infection in mice and, by binding to its ST2 receptor on epithelial progenitor cells, it helps protective immunity by driving expansion of secretory epithelial cells and promoting epithelial antimicrobial defence. 68 These direct effects of IL‐33 on epithelial stem cells are complemented by its ability to induce the production of IL‐13 by type 2 ILC2. This drives goblet cell hyperplasia and enhances epithelial cell migration from crypt to villus, both important aspects of barrier repair and protection against intestinal helminth infection. 70 , 71 IL‐33 is also upregulated in the inflamed mucosa of patients with UC, predominantly by the α‐SMA+ iMC that accumulate within lesions. 69 , 72 However, ST2 expression on epithelial cells is downregulated in IBD, possibly negating any protective effects of IL‐33 under these circumstances. 73 It is important to note that IL‐33 drives and amplifies type 2 inflammation and pathology, 74 and its expression correlates with disease severity in mixed Th1/Th2‐mediated colitis associated with SAMP1/YitFc mice. 73 Furthermore, IL‐33 increases the expression of IL‐13 and Eos peroxidase by Eos, and this can lead to increased production of TNFα, IL‐1β and IL‐6 by IL‐33‐primed intestinal fibroblasts in vitro. 75 Therefore, whether iMC‐derived IL‐33 plays a harmful or beneficial role needs to be explored in individual forms of intestinal inflammation.
Dysregulated wound healing can lead to fibrosis and fibrostenosis, which are major complications of IBD (Fig. 3). iMC such as fibroblasts, myofibroblasts and pericytes accumulate in chronically inflamed sites 63 and, in contrast to the relative lack of knowledge of their role in primary immune responses, there is much clearer evidence that iMC are centrally involved in IBD‐associated fibrosis. In this context, iMC lose migratory capacity, differentiate into contractile myofibroblast‐like cells, produce excess ECM proteins, and release inhibitors of the MMPs that control tissue remodelling. This disrupts the normal balance between ECM deposition and degradation, leading to fibrosis, smooth muscle cell hyperplasia, hypertrophy of the muscularis and eventually stricture formation. 76 Identifying which iMC are involved in these processes, and the mechanisms they use, could be an important advance in designing new therapies for treating IBD‐associated fibrosis.
Transforming growth factor‐β is a well‐known product of MCs that can drive fibrosis by inducing the migration, differentiation and proliferation of myofibroblasts, as well as increasing collagen production by numerous iMC populations. 63 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 TGF‐β also enhances the production of MMP inhibitors, such as tissue inhibitor of metalloproteinase‐1 (TIMP‐1), 80 and induces the expression of other profibrogenic molecules, such as fibronectin, connective tissue growth factor, platelet‐derived growth factor (PDGF), basic fibroblast growth factor (bFGF), IL‐11 and IGF binding protein 3 (IGFBP3). 78 , 85 , 86 , 87 , 88 Although TGF‐β is expressed constitutively in the steady‐state mucosa, overexpression of TGF‐β isoform 1 (TGF‐β1) drives progressive fibrosis and stricture formation in mice. 81 TGF‐β expression is greatly increased in the stricture‐associated and inflamed mucosa of patients with CD and UC, respectively, 80 , 89 , 90 and this production has been attributed to myofibroblasts and lamina propria lymphocytes. 80 , 91
Other profibrogenic factors elevated in the mucosa of IBD patients include insulin‐like growth factor‐1 (IGF‐I), PDGF, epidermal growth factor (EGF), bFGF and fibronectin, as well as inflammatory cytokines such as tumour necrosis factor (TNF)α, IFNγ, IL‐13, IL‐17A and IL‐33. Fibronectin, IGF‐I, PDGF and EGF can all stimulate myofibroblast migration in vitro, 77 , 78 , 92 whereas TNFα and IFNγ reduce their migratory potential. 93 These dichotomous roles could account for the accumulation and subsequent retention of activated myofibroblasts within a fibrotic site in vivo. Importantly, these cytokines and growth factors can also drive fibroblast production of ECM proteins, predominantly collagen. 75 , 94 , 95 , 96 , 97 Although TNFα can increase the production of potentially protective MMPs, 98 it also induces the expression of TIMP‐1 by myofibroblasts. 95 Moreover, TNFα‐induced MMP production is inhibited in the presence of IL‐13, which is overexpressed in fibrotic CD tissue, resulting in excessive collagen deposition. 98 Another novel player involved in inflammation‐mediated fibrosis is TNFSF member TL1A. IBD development has been associated with variants of the TL1A coding gene TNFSF15 that result in increased TL1A protein expression, 99 , 100 and TL1A can drive the expression of the profibrogenic factors TGF‐β1, connective tissue growth factor (CTGF) and IGF‐I. 97 TL1A inhibition reverses established fibrosis in two different murine models of colitis, 97 whereas transgenic overexpression drives the development of fibrostenosis in experimental colitis. 101 Collectively, myofibroblasts and smooth muscle cells responding to local mediators in intestinal inflammation play important roles in fibrosis when inflammation does not resolve.
What role do microbes play in iMC responses?
The intestine is a prime example of a tissue whose cellular components are exposed to and imprinted by a highly complex microenvironment. Several local factors are known to be involved in these processes, including the microbiota, dietary products and metabolites, epithelial cell‐derived mediators and neurological factors. Of these, only the microbiota has been explored to any extent for an effect on iMC. Importantly, this ‘forgotten organ’, constituting trillions of microbial cells from thousands of different species of bacteria, viruses, protozoa and archaea, has major effects on shaping immune function throughout life. The maternal microbiota can even shape fetal immune development in utero. Most studies of microbiota‐driven imprinting have focused on cells of haematopoietic origin. Interestingly, however, human fetal myofibroblasts have a more inflammatory phenotype than those isolated from infants, perhaps indicating that acquisition of the microbiota at birth may dampen iMC activity. 25 The mechanisms and pathways underlying these effects are largely unknown. However, microbial signals are reportedly required for RALDH activity and RA production in iMC so this pathway might make a contribution. 33 Furthermore, microbially derived short‐chain fatty acids (SCFA) dampen chemokine production by myofibroblasts, and attenuate their production of MMPs in response to cytokine stimulation in vitro. 102 , 103 , 104 Thus, regulation of iMC inflammatory processes by microbial mediators may help maintain a tolerogenic environment in the steady‐state gut.
Microbial metabolites can also regulate other aspects of iMC function. For instance, SCFA and urolithins can modulate prostaglandin synthesis by human myofibroblasts in culture, suggesting that the microbiome may contribute to how iMC influence barrier function and mucus production in vivo. 105 , 106 Evidence is also beginning to emerge that the microbiota may play a role in iMC‐mediated pathologies. For example, the ability of transgenic overexpression of TL1A to induce intestinal fibrosis requires an intact microbiome, and fibroblasts isolated from germ‐free mice showed impaired migration and collagen production in response to TL1A or bacterial products. 107 Thus, microbial priming of iMC may shape their responsiveness to certain stimuli, shifting them from a pro‐inflammatory to a more tolerogenic, wound‐healing phenotype.
Future perspectives
Studies in recent years have revealed some of the roles that specific populations of iMC play in intestinal immunity, both in steady‐state and during infection and inflammation. However, our knowledge is far from complete and stems predominantly from observations in vitro. Recent studies using scRNAseq have characterized iMC heterogeneity in unprecedented detail, and will no doubt help allow the function of specific iMC populations to be investigated extensively in vivo. Furthermore, this recent work suggests that pro‐inflammatory populations of iMC expand in IBD, and further studies are now required to establish how these discrete populations collaborate with the immune system during homeostasis and inflammation, and whether novel targeted therapeutics can be developed for intestinal diseases, such as IBD, that are characterized by persistent iMC activation and defective function. Moreover, it will be key to understand how the microbiota impacts iMC phenotype and function because, by educating the stromal cell compartment, microbe−iMC interactions could have important implications for the development of IBD and other intestinal pathologies.
Disclosures
The authors have no competing interests to declare.
References
- 1. Perez‐Shibayama C, Gil‐Cruz C, Ludewig B. Fibroblastic reticular cells at the nexus of innate and adaptive immune responses. Immunol Rev 2019; 289:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kinchen J, Chen HH, Parikh K, Antanaviciute A, Jagielowicz M, Fawkner‐Corbett D et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell 2018; 175:372–86. e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang B, Chen Z, Geng L, Wang J, Liang H, Cao Y et al. Mucosal profiling of pediatric‐onset colitis and ibd reveals common pathogenics and therapeutic pathways. Cell 2019; 179:1160–76. e24. [DOI] [PubMed] [Google Scholar]
- 4. Vaday GG, Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J Leukoc Biol 2000; 67:149–59. [DOI] [PubMed] [Google Scholar]
- 5. Proudfoot AEI, Handel TM, Johnson Z, Lau EK, LiWang P, Clark‐Lewis I et al. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci USA 2003; 100:1885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomson CA, van de Pavert SA, Stakenborg M, Labeeuw E, Matteoli G, Mowat AM et al Expression of the atypical chemokine receptor ACKR4 identifies a novel population of intestinal submucosal fibroblasts that preferentially expresses endothelial cell regulators. J Immunol 2018; 201:215–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karpus ON, Westendorp BF, Vermeulen JLM, Meisner S, Koster J, Muncan V et al. Colonic CD90+ crypt fibroblasts secrete semaphorins to support epithelial growth. Cell Rep 2019; 26:3698–708. e5. [DOI] [PubMed] [Google Scholar]
- 8. Jung Y, Rothenberg ME. Roles and regulation of gastrointestinal eosinophils in immunity and disease. J Immunol 2014; 193:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bain CC, Bravo‐Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol 2014; 15:929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang D, Chen Q, Hoover DM, Staley P, Tucker KD, Lubkowski J et al. Many chemokines including CCL20/MIP‐3α display antimicrobial activity. J Leukoc Biol 2003; 74:448–55. [DOI] [PubMed] [Google Scholar]
- 11. Lu J, Chatterjee M, Schmid H, Beck S, Gawaz M. CXCL14 as an emerging immune and inflammatory modulator. J Inflamm 2016; 13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maerki C, Meuter S, Liebi M, Mühlemann K, Frederick MJ, Yawalkar N et al. Potent and broad‐spectrum antimicrobial activity of CXCL14 suggests an immediate role in skin infections. J Immunol 2009; 182:507–14. [DOI] [PubMed] [Google Scholar]
- 13. Dai C, Basilico P, Cremona TP, Collins P, Moser B, Benarafa C et al. CXCL14 displays antimicrobial activity against respiratory tract bacteria and contributes to clearance of Streptococcus pneumoniae pulmonary infection. J Immunol 2015; 194:5980–89. [DOI] [PubMed] [Google Scholar]
- 14. Comerford I, Milasta S, Morrow V, Milligan G, Nibbs R. The chemokine receptor CCX‐CKR mediates effective scavenging of CCL19 in vitro. Eur J Immunol 2006; 36:1904–16. [DOI] [PubMed] [Google Scholar]
- 15. Gosling J, Dairaghi DJ, Wang Y, Hanley M, Talbot D, Miao Z et al. Cutting edge: identification of a novel chemokine receptor that binds dendritic cell‐ and T cell‐active chemokines including ELC, SLC, and TECK. J Immunol 2000; 164:2851–56. [DOI] [PubMed] [Google Scholar]
- 16. Jang MH, Sougawa N, Tanaka T, Hirata T, Hiroi T, Tohya K et al. CCR7 is critically important for migration of dendritic cells in intestinal lamina propria to mesenteric lymph nodes. J Immunol 2006; 176:803–10. [DOI] [PubMed] [Google Scholar]
- 17. Wendland M, Czeloth N, Mach N, Malissen B, Kremmer E, Pabst O et al. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci USA 2007; 104:6347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pabst O, Ohl L, Wendland M, Wurbel M‐A, Kremmer E, Malissen B et al Chemokine receptor CCR9 contributes to the localization of plasma cells to the small intestine. J Exp Med 2004; 199:411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Svensson M, Marsal J, Ericsson A, Carramolino L, Brodén T, Márquez G et al CCL25 mediates the localization of recently activated CD8αβ+ lymphocytes to the small‐intestinal mucosa. J Clin Invest 2002; 110:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bryce SA, Wilson RAM, Tiplady EM, Asquith DL, Bromley SK, Luster AD et al ACKR4 on stromal cells scavenges CCL19 to enable CCR7‐dependent trafficking of APCs from inflamed skin to lymph nodes. J Immunol 2016; 196:3341–53. [DOI] [PubMed] [Google Scholar]
- 21. Ulvmar MH, Werth K, Braun A, Kelay P, Hub E, Eller K et al The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat Immunol 2014; 15:623–30. [DOI] [PubMed] [Google Scholar]
- 22. Stzepourginski I, Nigro G, Jacob J‐M, Dulauroy S, Sansonetti PJ, Eberl G et al CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci USA 2017; 114:E506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Signore M, Cerio AM, Boe A, Pagliuca A, Zaottini V, Schiavoni I et al Identity and ranking of colonic mesenchymal stromal cells. J Cell Physiol 2012; 227:3291–300. [DOI] [PubMed] [Google Scholar]
- 24. Saada JI, Pinchuk IV, Barrera CA, Adegboyega PA, Suarez G, Mifflin RC et al Subepithelial myofibroblasts are novel nonprofessional APCs in the human colonic mucosa. J Immunol 2006; 1:5968–79. [DOI] [PubMed] [Google Scholar]
- 25. Zawahir S, Li G, Banerjee A, Shiu J, Blanchard TG, Okogbule‐Wonodi AC. Inflammatory and immune activation in intestinal myofibroblasts is developmentally regulated. J Interferon Cytokine Res 2015; 35:634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beswick EJ, Grim C, Singh A, Aguirre JE, Tafoya M, Qiu S et al Expression of programmed death‐ligand 1 by human colonic CD90+ stromal cells differs between ulcerative colitis and crohn’s disease and determines their capacity to suppress Th1 cells. Front Immunol 2018; 9:1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beswick EJ, Johnson JR, Saada JI, Humen M, House J, Dann S et al TLR4 activation enhances the PD‐L1–mediated tolerogenic capacity of colonic CD90 + stromal cells. J Immunol 2014; 193:2218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pinchuk IV, Saada JI, Beswick EJ, Boya G, Qiu SM, Mifflin RC et al PD‐1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T‐cell activity. Gastroenterology 2008; 135:1228–37. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pinchuk IV, Beswick EJ, Saada JI, Boya G, Schmitt D, Raju GS et al Human colonic myofibroblasts promote expansion of CD4+ CD25high Foxp3+ regulatory T Cells. Gastroenterology 2011; 140:2019–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol 2007; 179:2824–31. [DOI] [PubMed] [Google Scholar]
- 31. Reynoso ED, Elpek KG, Francisco L, Bronson R, Bellemare‐Pelletier A, Sharpe AH et al Intestinal tolerance is converted to autoimmune enteritis upon PD‐1 ligand blockade. J Immunol 2009; 182:2102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hashimoto‐Hill S, Friesen L, Kim M, Kim CH. Contraction of intestinal effector T cells by retinoic acid‐induced purinergic receptor P2X7. Mucosal Immunol 2017; 10:912–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vicente‐Suarez I, Larange A, Reardon C, Matho M, Feau S, Chodaczek G et al Unique lamina propria stromal cells imprint the functional phenotype of mucosal dendritic cells. Mucosal Immunol 2015; 8:141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng R, Bscheider M, Lahl K, Lee M, Butcher EC. Generation and transcriptional programming of intestinal dendritic cells: essential role of retinoic acid. Mucosal Immunol 2016; 9:183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis‐protective regulatory T‐cell differentiation through dendritic cell conditioning. Mucosal Immunol 2009; 2:340–50. [DOI] [PubMed] [Google Scholar]
- 36. Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y et al Microbiota‐dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 2014; 343:1 249 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kurashima Y, Amiya T, Fujisawa K, Shibata N, Suzuki Y, Kogure Y et al The enzyme Cyp26b1 mediates inhibition of mast cell activation by fibroblasts to maintain skin‐barrier homeostasis. Immunity 2014; 40:530–41. [DOI] [PubMed] [Google Scholar]
- 38. Cen SY, Moreau JM, Furlonger C, Berger A, Paige CJ. Differential regulation of IgA+ B cells in vitro by stromal cells from distinctive anatomical compartments. J Leukoc Biol 2019; 105:507–18. [DOI] [PubMed] [Google Scholar]
- 39. Mora JR, Iwata M, Eksteen B, Song S‐Y, Junt T, Senman B et al Generation of gut‐homing IgA‐secreting B cells by intestinal dendritic cells. Science 2006; 314:1157–60. [DOI] [PubMed] [Google Scholar]
- 40. Koliaraki V, Pallangyo CK, Greten FR, Kollias G. Mesenchymal cells in colon cancer. Gastroenterology 2017; 152:964–79. [DOI] [PubMed] [Google Scholar]
- 41. Zhang Z, Andoh A, Inatomi O, Bamba S, Takayanagi A, Shimizu N et al Interleukin‐17 and lipopolysaccharides synergistically induce cyclooxygenase‐2 expression in human intestinal myofibroblasts. J Gastroenterol and Hepatol 2005; 20:619–27. [DOI] [PubMed] [Google Scholar]
- 42. Walton KLW, Holt L, Sartor RB. Lipopolysaccharide activates innate immune responses in murine intestinal myofibroblasts through multiple signaling pathways. Am J Physiol Gastrointest Liver Physiol 2009; 296:G601–G611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koliaraki V, Chalkidi N, Henriques A, Tzaferis C, Polykratis A, Waisman A et al Innate sensing through mesenchymal TLR4/MyD88 signals promotes spontaneous intestinal tumorigenesis. Cell Rep 2019; 26:536–45. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim Y‐G, Kamada N, Shaw MH, Warner N, Chen GY, Franchi L et al The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2‐dependent recruitment of inflammatory monocytes. Immunity 2011; 34:769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Otte J‐M, Rosenberg IM, Podolsky DK. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology 2003; 124:1866–78. [DOI] [PubMed] [Google Scholar]
- 46. Rogler G, Gelbmann CM, Vogl D. Differential activation of cytokine secretion in primary human colonic fibroblast/myofibroblast cultures. Scand J Gastroenterol 2001; 36:389–98. [DOI] [PubMed] [Google Scholar]
- 47. Gibson DL, Montero M, Ropeleski MJ, Bergstrom KSB, Ma C, Ghosh S et al Interleukin‐11 reduces TLR4‐induced colitis in TLR2‐deficient mice and restores intestinal STAT3 signaling. Gastroenterology 2010; 139:1277–88. [DOI] [PubMed] [Google Scholar]
- 48. Hata K, Andoh A, Shimada M, Fujino S, Bamba S, Araki Y et al IL‐17 stimulates inflammatory responses via NF‐κB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol 2002; 282:G1035–44. [DOI] [PubMed] [Google Scholar]
- 49. Okuno T, Andoh A, Bamba S, Araki Y, Fujiyama Y, Fujimiya M et al Interleukin‐1β and tumor necrosis factor‐α induce chemokine and matrix metalloproteinase gene expression in human colonic subepithelial myofibroblasts. Scand J Gastroenterol 2002; 37:317–24. [DOI] [PubMed] [Google Scholar]
- 50. Scheibe K, Backert I, Wirtz S, Hueber A, Schett G, Vieth M et al IL‐36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut 2017; 66:823–38. [DOI] [PubMed] [Google Scholar]
- 51. Griseri T, Arnold IC, Pearson C, Krausgruber T, Schiering C, Franchini F et al Granulocyte macrophage colony‐stimulating factor‐activated eosinophils promote interleukin‐23 driven chronic colitis. Immunity 2015; 43:187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hamada A, Torre C, Drancourt M, Ghigo E. Trained immunity carried by non‐immune cells. Front Microbiol 2019; 9:3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Crowley T, O’Neil JD, Adams H, Thomas AM, Filer A, Buckley CD et al Priming in response to pro‐inflammatory cytokines is a feature of adult synovial but not dermal fibroblasts. Arthritis Res Ther 2017; 19:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sohn C, Lee A, Qiao Y, Loupasakis K, Ivashkiv LB, Kalliolias GD. Prolonged tumor necrosis factor α primes fibroblast‐like synoviocytes in a gene‐specific manner by altering chromatin: prolonged TNFα priming of FLS via chromatin alteration. Arthritis Rheumatol. 2015; 67:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sadler T, Bhasin JM, Xu Y, Barnholz‐Sloan J, Chen Y, Ting AH et al Genome‐wide analysis of DNA methylation and gene expression defines molecular characteristics of Crohn’s disease‐associated fibrosis. Clin Epigenet 2016; 8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li Yim AYF, de Bruyn JR, Duijvis NW, Sharp C, Ferrero E, de Jonge WJ et al A distinct epigenetic profile distinguishes stenotic from non‐inflamed fibroblasts in the ileal mucosa of Crohn’s disease patients. PLoS ONE 2018; 13:e0209656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li C, Lam E, Perez‐Shibayama C, Ward LA, Zhang J, Lee D et al Early‐life programming of mesenteric lymph node stromal cell identity by the lymphotoxin pathway regulates adult mucosal immunity. Sci Immunol 2019; 4:eaax1027. [DOI] [PubMed] [Google Scholar]
- 58. Dakin SG, Coles M, Sherlock JP, Powrie F, Carr AJ, Buckley CD. Pathogenic stromal cells as therapeutic targets in joint inflammation. Nat Rev Rheumatol 2018; 14:714–26. [DOI] [PubMed] [Google Scholar]
- 59. Croft AP, Campos J, Jansen K, Turner JD, Marshall J, Attar M et al Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 2019; 570:246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schneider K, Potter KG, Ware CF. Lymphotoxin and LIGHT signaling pathways and target genes. Immunol Rev 2004; 202:49–66. [DOI] [PubMed] [Google Scholar]
- 61. Cerutti A. The regulation of IgA class switching. Nat Rev Immunol 2008; 8:421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hou S, Landego I, Jayachandran N, Miller A, Gibson IW, Ambrose C et al Follicular dendritic cell secreted protein FDC‐SP controls IgA production. Mucosal Immunol 2014; 7:948–57. [DOI] [PubMed] [Google Scholar]
- 63. Rieder F, Fiocchi C. Intestinal fibrosis in IBD—a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol 2009; 6:228–35. [DOI] [PubMed] [Google Scholar]
- 64. Nguyen PM, Putoczki TL, Ernst M. STAT3‐activating cytokines: a therapeutic opportunity for inflammatory bowel disease? J Interferon Cytokine Res 2015; 35:340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bozza M, Bliss JL, Dorner AJ, Trepicchio WL. Interleukin‐11 modulates Th1/Th2 cytokine production from activated CD4 + T cells. J Interferon Cytokine Res 2001; 21:21–30. [DOI] [PubMed] [Google Scholar]
- 66. Sands BE, Winston BD, Salzberg B, Safdi M, Barish C, Wruble L et al Randomized, controlled trial of recombinant human interleukin‐11 in patients with active Crohn’s disease. Aliment Pharmacol Ther 2002; 16:399–406. [DOI] [PubMed] [Google Scholar]
- 67. Herrlinger KR, Witthoeft T, Raedler A, Bokemeyer B, Krummenerl T, Schulzke JD et al Randomized, double blind controlled trial of subcutaneous recombinant human interleukin‐11 versus prednisolone in active Crohn’s disease. Am J Gastroenterol 2006; 101:793–7. [DOI] [PubMed] [Google Scholar]
- 68. Mahapatro M, Foersch S, Hefele M, He G‐W, Giner‐Ventura E, Mchedlidze T et al Programming of intestinal epithelial differentiation by IL‐33 derived from pericryptal fibroblasts in response to systemic infection. Cell Rep 2016; 15:1743–56. [DOI] [PubMed] [Google Scholar]
- 69. Sponheim J, Pollheimer J, Olsen T, Balogh J, Hammarström C, Loos T et al Inflammatory bowel disease‐associated interleukin‐33 is preferentially expressed in ulceration‐associated myofibroblasts. Am J Pathol 2010; 177:2804–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H et al Innate production of TH2 cytokines by adipose tissue‐associated c‐Kit+Sca‐1+ lymphoid cells. Nature 2010; 463:540–4. [DOI] [PubMed] [Google Scholar]
- 71. Cliffe LJ. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science 2005; 308:1463–5. [DOI] [PubMed] [Google Scholar]
- 72. Kobori A, Yagi Y, Imaeda H, Ban H, Bamba S, Tsujikawa T et al Interleukin‐33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol 2010; 45:999–1007. [DOI] [PubMed] [Google Scholar]
- 73. Pastorelli L, Garg RR, Hoang SB, Spina L, Mattioli B, Scarpa M et al Epithelial‐derived IL‐33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci USA 2010; 107:8017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK et al IL‐33, an interleukin‐1‐like cytokine that signals via the IL‐1 receptor‐related protein ST2 and induces T helper type 2‐associated cytokines. Immunity 2005; 23:479–90. [DOI] [PubMed] [Google Scholar]
- 75. Masterson JC, Capocelli KE, Hosford L, Biette K, McNamee EN, de Zoeten EF et al Eosinophils and IL‐33 perpetuate chronic inflammation and fibrosis in a pediatric population with stricturing Crohnʼs Ileitis. Inflamm Bowel Dis 2015; 21:2429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen W, Lu C, Hirota C, Iacucci M, Ghosh S, Gui X. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn’s fibrostenosing bowel strictures: a semiquantitative analysis by using a novel histological grading scheme. J Crohns Colitis 2017; 11:92–104. [DOI] [PubMed] [Google Scholar]
- 77. Leeb SN, Vogl D, Falk W, Schölmerich J, Rogler G, Gelbmann CM. Regulation of migration of human colonic myofibroblasts. Growth Factors 2002; 20:81–91. [DOI] [PubMed] [Google Scholar]
- 78. Brenmoehl J, Miller SN, Hofmann C, Vogl D, Falk W, Schölmerich J et al Transforming growth factor‐β1 induces intestinal myofibroblast differentiation and modulates their migration. World J Gastroenterol 2009; 15:1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Simmons JG, Pucilowska JB, Keku TO, Lund PK. IGF‐I and TGF‐β1 have distinct effects on phenotype and proliferation of intestinal fibroblasts. Am J Physiol Gastrointest Liver Physiol 2002; 283:G809–G818. [DOI] [PubMed] [Google Scholar]
- 80. Di Sabatino A, Jackson CL, Pickard KM, Buckley M, Rovedatti L, Leakey NAB et al Transforming growth factor signalling and matrix metalloproteinases in the mucosa overlying Crohn’s disease strictures. Gut 2009; 58:777–89. [DOI] [PubMed] [Google Scholar]
- 81. Vallance BA, Gunawan MI, Hewlett B, Bercik P, Van Kampen C, Galeazzi F et al TGF‐β1 gene transfer to the mouse colon leads to intestinal fibrosis. Am J Physiol Gastrointest Liver Physiol 2005; 289:G116–G128. [DOI] [PubMed] [Google Scholar]
- 82. Flynn RS, Mahavadi S, Murthy KS, Grider JR, Kellum JM, Akbari H et al Endogenous IGFBP‐3 regulates excess collagen expression in intestinal smooth muscle cells of Crohnʼs disease strictures. Inflamm Bowel Dis 2011; 17:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM et al Transforming growth factor type beta: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA 1986; 83:4167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Graham F, Bryson GR. Transforming growth factor beta selectively augments collagen synthesis by human intestinal smooth muscle cells. Gastroenterology 1990; 99:447–53. [DOI] [PubMed] [Google Scholar]
- 85. Bamba S, Andoh A, Yasui H, Makino J, Kim S, Fujiyama Y. Regulation of IL‐11 expression in intestinal myofibroblasts: role of c‐Jun AP‐1‐ and MAPK‐dependent pathways. Am J Physiol Gastrointest Liver Physiol 2003; 285:G529–G538. [DOI] [PubMed] [Google Scholar]
- 86. Grotendorst GR. Connective tissue growth factor: a mediator of TGF‐β action on fibroblasts. Cytokine Growth Factor Rev 1997; 8:171–9. [DOI] [PubMed] [Google Scholar]
- 87. Taylor LM, Khachigian LM. Induction of platelet‐derived growth factor B‐chain expression by transforming growth factor‐beta involves transactivation by Smads. J Biol Chem 2000; 275:16 709–16. [DOI] [PubMed] [Google Scholar]
- 88. Martin JL, Ballesteros M, Baxter RC. Insulin‐like growth factor‐I (IGF‐I) and transforming growth factor‐beta 1 release IGF‐binding protein‐3 from human fibroblasts by different mechanisms. Endocrinology 1992; 131:1703–10. [DOI] [PubMed] [Google Scholar]
- 89. Li C, Flynn RS, Grider JR, Murthy KS, Kellum JM, Akbari H et al Increased activation of latent TGF‐β1 by αVβ3 in human Crohnʼs disease and fibrosis in TNBS colitis can be prevented by cilengitide. Inflamm Bowel Dis 2013; 19:2829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lawrance IC, Maxwell L, Doe W. Inflammation location, but not type, determines the increase in TGF‐β1 and IGF‐1 expression and collagen deposition in IBD intestine. Inflamm Bowel Dis 2001; 7:16–26. [DOI] [PubMed] [Google Scholar]
- 91. Del Zotto B, Mumolo G, Pronio AM, Montesani C, Tersigni R, Boirivant M. TGF‐beta1 production in inflammatory bowel disease: differing production patterns in Crohn’s disease and ulcerative colitis. Clin Exp Immunol 2003; 134:120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Leeb SN, Vogl D, Grossmann J, Falk W, Schölmerich J, Rogler G et al Autocrine fibronectin‐induced migration of human colonic fibroblasts. Am J Gastroenterol 2004; 99:335–40. [DOI] [PubMed] [Google Scholar]
- 93. Leeb SN, Vogl D, Gunckel M, Kiessling S, Falk W, Ke MG et al Reduced migration of fibroblasts in inflammatory bowel disease: role of inflammatory mediators and focal adhesion kinase. Gastroenterology 2003; 125:1341–54. [DOI] [PubMed] [Google Scholar]
- 94. Lawrance IC, Maxwell L, Doe W. Altered response of intestinal mucosal fibroblasts to profibrogenic cytokines in inflammatory bowel disease. Inflamm Bowel Dis 2001; 7:226–36. [DOI] [PubMed] [Google Scholar]
- 95. Theiss AL, Simmons JG, Jobin C, Lund PK. Tumor necrosis factor (TNF) α increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J Biol Chem 2005; 280:36 099–109. [DOI] [PubMed] [Google Scholar]
- 96. Biancheri P, Pender SL, Ammoscato F, Giuffrida P, Sampietro G, Ardizzone S et al The role of interleukin 17 in Crohn’s disease‐associated intestinal fibrosis. Fibrogenesis Tissue Repair 2013; 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Shih DQ, Zheng L, Zhang X, Zhang H, Kanazawa Y, Ichikawa R et al Inhibition of a novel fibrogenic factor Tl1a reverses established colonic fibrosis. Mucosal Immunol 2014; 7:1492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bailey JR, Bland PW, Tarlton JF, Peters I, Moorghen M, Sylvester PA et al IL‐13 promotes collagen accumulation in Crohn’s disease fibrosis by down‐regulation of fibroblast MMP synthesis: a role for innate lymphoid cells? PLoS ONE 2012; 7:e52332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. The Wellcome Trust Case Control Consortium . Genome‐wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 2007; 447:661–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Michelsen KS, Thomas LS, Taylor KD, Yu QT, Mei L, Landers CJ et al IBD‐associated TL1A gene (TNFSF15) haplotypes determine increased expression of TL1A protein. PLoS ONE 2009; 4:e4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Barrett R, Zhang X, Koon HW, Vu M, Chang J‐Y, Yeager N et al Constitutive TL1A expression under colitogenic conditions modulates the severity and location of gut mucosal inflammation and induces fibrostenosis. Am J Pathol 2012; 180:636–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kawamura T, Andoh A, Nishida A, Shioya M, Yagi Y, Nishimura T et al Inhibitory effects of short‐chain fatty acids on matrix metalloproteinase secretion from human colonic subepithelial myofibroblasts. Dig Dis Sci 2009; 54:238–45. [DOI] [PubMed] [Google Scholar]
- 103. Inatomi O, Andoh A, Kitamura K, Yasui H, Zhang Z, Fujiyama Y. Butyrate blocks interferon‐γ‐inducible protein‐10 release in human intestinal subepithelial myofibroblasts. J Gastroenterol 2005; 40:483–89. [DOI] [PubMed] [Google Scholar]
- 104. Gruchlik A, Chodurek E, Zajdel A, Wilczok A, Glarz AW, Dzierøewicz Z. Influence of troglitazone, sodium butyrate, 5‐aminosalicylic acid and BAY 11–7082 on the chemokine ENA‐78/CXCL5 secretion in the intestinal subepithelial myofibroblasts. Acta Pol Pharm 2010; 67:690–5. [PubMed] [Google Scholar]
- 105. Willemsen LEM. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003; 52:1442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. González‐Sarrías A, Larrosa M, Tomás‐Barberán FA, Dolara P, Espín JC. NF‐κB‐dependent anti‐inflammatory activity of urolithins, gut microbiota ellagic acid‐derived metabolites, in human colonic fibroblasts. Br J Nutr 2010; 104:503–12. [DOI] [PubMed] [Google Scholar]
- 107. Jacob N, Jacobs JP, Kumagai K, Ha CWY, Kanazawa Y, Lagishetty V et al Inflammation‐independent TL1A‐mediated intestinal fibrosis is dependent on the gut microbiome. Mucosal Immunol 2018; 11:1466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Roulis M, Nikolaou C, Kotsaki E, Kaffe E, Karagianni N, Koliaraki V et al Intestinal myofibroblast‐specific Tpl2‐Cox‐2‐PGE2 pathway links innate sensing to epithelial homeostasis. Proc Natl Acad Sci USA 2014; 111:E4658–E4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, Myofibroblasts WAB et al. Intestinal subepithelial myofibroblasts. Am J Physiol Cell Physiol 1999; 277:C183–C201. [DOI] [PubMed] [Google Scholar]
- 110. Kurahashi M, Nakano Y, Peri LE, Townsend JB, Ward SM, Sanders KM. A novel population of subepithelial platelet‐derived growth factor receptor α‐positive cells in the mouse and human colon. Am J Physiol Gastrointest Liver Physiol 2013; 304:G823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


