Summary
Langerin is a C‐type lectin receptor that is expressed on Langerhans cells and langerin‐positive dermal dendritic cells in the skin. Little is known about the function of langerin+ cells in wound healing. In this study, the effects of ablation of langerin+ cells on healing of a full‐thickness excision wound were investigated using the langerin‐DTR depletable mouse. Strikingly, depletion of langerin+ cells resulted in more rapid reduction in wound area. Accelerated wound healing in the langerin+‐cell‐depleted group was characterized by enhanced neo‐epidermis and granulation tissue formation, and increased cellular proliferation within the newly formed tissues. Accelerated healing in the absence of langerin+ cells was associated with increased levels of granulocyte–macrophage colony‐stimulating factor, F4/80+ cells and blood vessels within the granulation tissue. These data support an inhibitory role for langerin+ cells during wound healing. Therapies that suppress langerin+ cells or their function may therefore have utility in progressing the healing of wounds in humans.
Keywords: angiogenesis, epithelialization, Langerhans cells, langerin‐positive dermal dendritic cells, mouse, skin, wound healing
Ablation of langerin‐positive cells in the langerin‐DTR depletable mouse accelerates healing of a full‐thickness cutaneous wound. Neo‐epidermis and granulation tissue formation was increased, associated with increased cellular proliferation, F4/80‐positive cell infiltration, GM‐CSF production and granulation tissue vascularization. These data provide evidence of a diverse role for langerin‐positive cells in the regulation of wound healing.

Abbreviations
- CD
cluster of differentiation
- Col IV
collagen IV
- DAPI
4,6‐diamidino‐2‐phenolindole
- DC
dendritic cell
- dDC
dermal dendritic cell
- DT
diphtheria toxin
- DTR
diphtheria toxin receptor
- GM‐CSF
granulocyte–macrophage colony‐stimulating factor
- IL
interleukin
- LC
Langerhans cell
- LangDTR
langerin diphtheria toxin receptor
- Lyve‐1
lymphatic vessel endothelial hyaluronan receptor 1
- MSB
Martius Scarlet Blue
- PBS
phosphate‐buffered saline
- PC
panniculus carnosus
- TBS
Tris‐buffered saline
- TBST
Tris‐buffered saline containing Tween‐20
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand factor
INTRODUCTION
The skin acts as the primary barrier to foreign invasion, and as such has a diverse complement of immune cell populations. 1 Langerhans cells (LCs) are the only antigen‐presenting cells that reside in normal epidermis, whereas a range of different myeloid cells are present in the dermis, including dendritic cells (DCs), mast cells, macrophages and monocytes. These cells contribute to innate immunity through release of cytokines and phagocytosis upon activation, and LCs and DCs are critical for antigen uptake and processing for initiation of the adaptive immune response.
Langerin is a C‐type lectin expressed in two populations of antigen‐presenting cells in the skin, LCs and a subpopulation of dermal dendritic cells (dDCs). The LCs populate the basal and suprabasal layers of the epidermis, and are distinguished by the presence of tennis racquet‐like Birbeck granules. 2 They express CD11c, MHC class II, DEC‐205/CD205 and the endocytic receptor langerin/CD207. 3 Upon activation with antigenic stimuli, LCs mature and migrate from the epidermis to regional lymph nodes and activate naive T lymphocytes. The LCs not only take part in antigen uptake and presentation but also play a role in maintaining peripheral tolerance against self‐antigens. 4 However, the primary function of LCs as antigen‐presenting cells that initiate immunity has been repeatedly challenged, and it is more likely that these cells are multifunctional. Langerin‐positive dDCs are a rare subset of dermal DCs that were initially thought to be LCs that were in transit through the dermis on their way to the lymph nodes. 5 Similar to classical DCs, langerin+ dDCs are derived from bone marrow precursors and depend on FMS‐like tyrosine kinase 3 ligand for their differentiation. 6 In contrast to LCs, DCs have a short half‐life and are replenished by a circulating pool of bone‐marrow‐derived committed precursors. 6 Langerin+ dDCs are potent cross priming cells whereas LCs are considered more likely to induce CD8+ T‐cell tolerance. 7
Wound healing is a complex process that can be divided into three overlapping phases: inflammation, tissue formation and tissue remodelling. 8 In acute wounds, such as the excisional punch biopsy model, the initial inflammatory phase commences immediately on wounding, and lasts for around 5 days. 9 There is overlapping commencement of the proliferative tissue formation phase from around day 1, where keratinocytes proliferate and migrate to cover the wound surface and newly formed granulation tissue starts to cover the wound area. 9 Remodelling commences after closure on day 7–8, and may continue for up to 1 year. 8 , 10 When one or more of these phases is delayed or impaired, the wound can fail to heal and becomes chronic, 11 , 12 as observed in humans. 13 Chronic wounds, including conditions such as diabetic foot ulcers, pressure ulcers and other wounds resulting from venous insufficiency, are a significant global health burden.
The success of wound healing largely depends on a complex interplay between different cell types and matrix components. These are mediated to a great extent by growth factors, cytokines and chemokines. Granulocyte–macrophage colony‐stimulating factor (GM‐CSF) is a versatile cytokine secreted by keratinocytes and macrophages shortly after wounding. GM‐CSF functions include mediation of wound contraction, 14 stimulation of proliferation of keratinocytes 15 and recruitment of immune cells, such as LCs and macrophages. 16 Constitutive expression of GM‐CSF in murine skin increases wound closure 17 while knocking out GM‐CSF impairs significantly wound healing. 18
The role of langerin+ cells, especially langerin+ dDCs, in wound healing has not yet been extensively explored. There are some reports describing changes and the role of LCs in healing. Densities of LCs are increased in chronic wounds, 19 within hypertrophic scars, 20 and are also associated with the regression of diabetic foot ulcers. 21 Hyperproliferation of keratinocytes is a key hallmark of chronic wounds, 22 and over‐production of cytokines by hyperproliferative cells may inhibit the migration of LCs. 23 Those LCs with improved motility, because of an increase in the number of dendrites, are associated with more rapid healing. 24 In a SCID (severe combined immunodeficient) mouse model, LCs migrate out of the wound and the epidermis is repopulated with LC progenitors during early re‐epithelialization, 25 suggesting that LCs and their migration might be playing a role in chronic wound progression. In this study, we show that langerin+ cells have repopulated the neo‐epidermis by day 9, during re‐epithelialization of a full‐thickness excision cutaneous wound. We find that langerin+ cell‐depleted mouse wounds exhibit accelerated healing, characterized by increased re‐epithelialization and granulation tissue formation. We attribute the enhanced epidermal and dermal proliferation in the absence of langerin+ cells to increases in the production of GM‐CSF and in the number of blood vessels and macrophages in the granulation tissue. We propose that langerin+ cells may impede healing processes induced by GM‐CSF. These data support a suppressive role for LCs in the regulation of cutaneous wound healing.
METHODS
Acute skin wound murine model
Specific pathogen‐free female C57BL/6 and transgenic Langerin DTR 26 mice were used for this skin wound model at 10–14 weeks of age. Mice were kept in a specific pathogen‐free environment at the Hercus Taieri Research Unit of the University of Otago under ethical approval AEC 20/13.
The transgenic mice were separated into control and experimental groups, and given intra‐peritoneal injections of phosphate‐buffered saline (PBS) or diphtheria toxin (DT; 1 μg of DT in 100 μl of PBS), 27 respectively. These injections were administered at 4 days and 1 day before wounding, and 6 days after wounding (for day 9 and day 16 wounds only) to deplete mice of LCs in the experimental groups.
Full thickness wounds (4 mm thick) were created on each shaved hind limb of anaesthetized mice (ketamine 75 mg/kg; Domitor 1 mg/kg) using a sterile biopsy punch (Shoof International, Cambridge, New Zealand). Before wounding, pain relief was administered to the site by subcutaneous injections of bupivacaine (2 mg/kg). A subcutaneous injection of Amphoprim (10·2 mg/kg) was administered in the scruff of the neck to prevent infection. Once the procedure was completed, Antisedan (5 mg/kg) was given by subcutaneous injection to the inguinal region to reverse the effects of the anaesthesia.
Photographs of each wound were taken using an SLR camera (Nikon, Tokyo, Japan), and a ruler for scale, immediately after wounding and daily until day 16. Wound tissue was harvested after killing of the mice by CO2 asphyxiation at either day 1, 3, 6, 9 or 16. Wound biopsies were excised (1 cm × 1·5 cm) and fixed in 0·5 % zinc/salt fixative and transferred into 70% ethanol for 4 hr. The tissues were dehydrated with xylene and graded ethanol water baths to displace the water. The tissues were then embedded in paraffin. Serial 4‐μm sections were cut and one section from the centre of each wound was used for Martius Scarlet Blue (MSB) staining, following a standard protocol. 28 All embedding, section cutting and MSB staining were performed at the Histology Services Unit, Department of Pathology, Dunedin School of Medicine.
Immunofluorescence and histochemical analyses
Antigen‐presenting cells were stained using a fluorescein isothiocyanate‐conjugated anti‐MHC class II monoclonal antibody (Cat. no. ab93560; clone M5/114.15.2; 1:400 dilution; Abcam, Cambridge, UK) and supernatant from cells expressing an anti‐Langerin/CD207 monoclonal antibody, in conjunction with an Alexa Fluor 594 goat anti‐rat IgG2a (clone 929F3, 1:5 dilution). Proliferating cells in the dermis and granulation tissue were detected using monoclonal rabbit anti‐Ki67 (Ki67) antigen antibody (Cat. no. ab16667; 1:200 dilution; Abcam). Tissues were stained for GM‐CSF using a rabbit monoclonal anti‐GM‐CSF antibody (Cat. no. ab54429; clone 30‐4; Abcam). Rat monoclonal anti‐F4/80 antibody (Cat. no. ab6640; clone CI:A3‐1; Abcam) was used to stain for F4/80+ immune cells. Blood vessels were stained using a polyclonal rabbit anti‐collagen IV (Col IV) antibody (Cat. no. ab6585; 1:200 dilution; Abcam), von Willebrand factor (vWF) antibody (Cat. no. A0082; 1:50; DAKO) and CD31 antibody (Cat. no. ab28364; 1:200: Abcam). Lymphatic vessels were stained with a polyclonal rabbit anti‐lymphatic vessel endothelial hyaluronan receptor‐1 (Lyve‐1) antibody (Cat. no. ab14917; 1:100; Abcam). Cell nuclei were stained using 4,6‐diamidino‐2‐phenolindole (DAPI; 1:50 dilution; Invitrogen, Carlsbad, CA).
Wounds were bisected through the centre of the wound along the medial–lateral axis, and one half of the wound was sectioned for immunofluorescence analysis, in accordance with the recommended protocol by Ansell et al. 29 Slides were used sequentially, commencing from the middle of the wound. Slides were incubated at 37° overnight before staining to improve adhesion of the tissues onto the slide. Sections were de‐paraffinized and taken to water, and antigen retrieval was achieved by incubating the sections in 37° Tris‐buffered saline, pH 7·4 (TBS) for 20 min for the MHCII and Langerin staining and microwave retrieval using sodium citrate buffer, pH 6·0 for the other antibodies, then at room temperature for a further 20 min, followed by three 5‐min washes in TBS containing 10% Tween‐20 (TBST). Non‐specific antigen‐binding sites were blocked by incubation with 10% normal sheep serum in TBS containing Fc block (rat anti‐mouse CD16/CD32 (1:700; BD Biosciences, Franklin Lakes, NJ) and rat IgG (1:500; Jackson ImmunoResearch, West Grove, PA, USA) for 1 hr at room temperature. The sections were then incubated with primary antibodies diluted in antibody diluent (1% bovine serum albumin, 10% Triton × 100 in TBS) overnight at 4°, followed by three 5‐min washes in TBST. When necessary, the section was incubated with the corresponding secondary antibody – either goat anti‐rat IgG H&L Alexa Fluor® 594 (Cat. No. ab150160; 1:200 dilution; Abcam) or F(ab’)2 ‐ Goat anti‐rabbit IgG (H + L) cross adsorbed, Alexa Fluor 488 (Cat. no. A11070; 1:200 dilution; Invitrogen) diluted in assay diluent for 1 hr at room temperature. For the final 30 min of antibody incubation, DAPI (1:50) was added to counter‐stain the nuclei. Sections were washed three times for 5 min in TBST, mounted with SlowFade™ Diamond anti‐fade reagent (Invitrogen), and stored overnight at 4°. Tissues were stained with primary antibody only and secondary antibody only antibodies to serve as negative controls (data not shown). Images of the entire tissue section were taken using a fluorescence microscope (Olympus BX51 TRF) under the 40× objective lens. Photographs were merged and converted into panoramas using imagej (http://rsbweb.nih.gov/ij).
Morphometric analyses
The numbers of CD207+ stained cells within the epidermis (avoiding glands and hair follicles) were quantified in the immunofluorescence histochemistry sections. The skin within each section was divided into different zones: wound region [between the cut edges of the panniculus carnosus (PC)], adjacent region (adjacent to the wound) and periphery (peripheral to the wound edge) with the latter two zones measuring 700 μm in width.
New epidermis formation within the wound (defined as between the edges of the PC) was quantified using the panoramas generated of MSB‐stained sections. The length in μm of the neo‐epidermis was measured using the free‐hand line tool to trace the lower edge of the neo‐epidermis. The mean thickness of the neo‐epidermis was measured in triplicate on each side at equidistant regions from the cut edges of the PC. The areas in μm2 of the neo‐epidermis and granulation tissue were measured using imagej by setting the scale, then using the free‐hand polygon tool to trace the neo‐epidermis and granulation tissue and selecting the ‘measure’ key from the ‘analyse’ menu. All analyses were conducted on sections from the middle of the wound.
The total numbers of proliferating cells and macrophages were quantified in the epidermis and granulation tissue by selecting the ‘Cell counter’ option in the ‘Analyze’ option of the ‘Plugins’ tab. The total numbers of proliferating cells and macrophages were presented per mm2 of area. The total number of Col IV+ blood vessels in the granulation tissue was quantified by selecting the ‘Cell counter’ option in the ‘Analyze’ option of the ‘Plugins’ tab.
GM‐CSF is a secreted cytokine, hence the area of expression was measured rather than the total number of positive cells. The area of GM‐CSF production in the epidermis and dermis per mm2 was calculated using imagej. The GM‐CSF staining was analysed by selecting 1‐mm2 samples across the total area of the neo‐epidermis and granulation tissue, between the two cut edges of the PC using the polygon drawing tool. The colour threshold tool was set to include the positively staining cells on the first sample, and then consistently applied to all samples. The ‘Measure’ option from the ‘Analyze’ tab was used to measure the total area of GM‐CSF expression within the selected area. Individual samples for each tissue were then averaged to give the final result, presented as the GM‐CSF‐positive area (mm2) per mm2.
Statistical analyses
All data are presented as the mean ± standard error of the mean, and statistical analyses were conducted in graphpad prism7 and performed using Mann–Whitney U‐test for comparison between two groups or two‐way analysis of variance for time–course analysis across groups with significant points of difference (P ≤ 0·05) determined by using the Bonferroni method.
RESULTS
Langerin/CD207+ cells populate the neo‐epidermis during wound re‐epithelialization
The purpose of this study was to assess the contribution of langerin+ cells to wound healing. Initial studies were carried out to establish how langerin+ cell numbers and localization were affected by administration of full‐thickness wounding of cutaneous skin on C57BL/6 mice. Wounds were sampled at a range of time‐points from days 1 to 16, stained (see Supplementary material, Fig. S1), and the langerin/CD207‐positive cells were enumerated in the epidermis of the wound bed (region between the PC), in the adjacent epidermis (the 700‐µm region out from the wound boundary), and in the epidermis peripheral to the wound (the 700‐µm region of epidermis next to the adjacent epidermis), as shown in Fig. 1(a).
Figure 1.
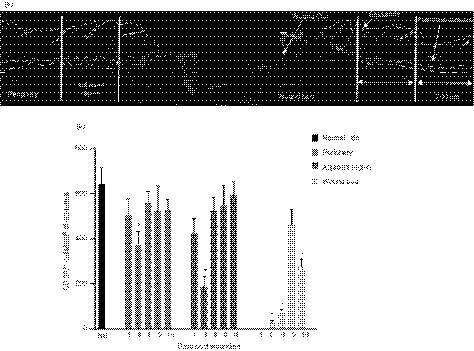
Localization of Langerin/CD207+ in the murine skin during normal wound healing. (a) Representative image of a wound section showing histological features and zones in which the cells were quantified. The wound is defined as the region between the cut edges of the panniculus carnosus (PC). The adjacent region is within 700 µm of the PC and the periphery is between 700 µm and 1400 µm from the PC. (b) The number of CD207+ cells per mm2 of epidermis in the wound, adjacent to, and peripheral to the wound were counted at days 1, 3, 6, 9 and 16 post‐wounding and compared against normal unwounded skin (NS, Day 0). Data presented as mean ± SEM from n = 8 wounds. Statistical significance (P < 0·05) is indicated by two‐way analysis of variance with Bonferroni correction.
Stained cells were not detected at day 1 post wounding, but were present at day 3 and increased over time, peaking at day 9 (Fig. 1b). The number of CD207+ cells dropped to around half that of normal skin at day 16, indicating that langerin+ cell numbers were not stably restored to normal levels by that time, even after wound closure. There was no difference in the number of CD207+ cells in the peripheral or adjacent epidermis when compared with normal skin at any of the time‐points tested, with the exception of day 3 post wounding. At that time‐point there was more than a 50% reduction in the number of langerin+ cells in the adjacent epidermis, and to a lesser extent also a reduction in the number of langerin+ cells in the peripheral epidermis (Fig. 1b). These data indicate that the numbers of langerin+ cells are decreased to at least as far as 1400 µm out from the wound, with the effect becoming less apparent as the distance from the wound increased.
Depletion of langerin+ cells accelerates the rate of wound healing
The effect of langerin+ cells on the rate of wound healing was measured in experiments using the LangDTR mouse, which is selectively depletable of langerin+ cells following DT treatment. LangDTR mice injected with DT at days −4, and −1 pre‐wounding, then again at day 6 post‐wounding, were compared with control LangDTR mice injected with PBS (Fig. 2a). Measurements of the wound over time in the LangDTR‐treated and untreated mice showed that the wound area was reduced as early as 24 hr after wounding in the absence of CD207+ cells (Fig. 2b). As early as day 1 post‐wounding, depletion of CD207+ cells resulted in around a 40% reduction in wound area relative to the area of the original wound, compared with around 22% in controls (Fig. 2c, P < 0·05). The wound area of the mice depleted of CD207+ cells continued to be significantly smaller up to and including day 7, when compared with the control mice (Fig. 2c, P < 0·079).
Figure 2.
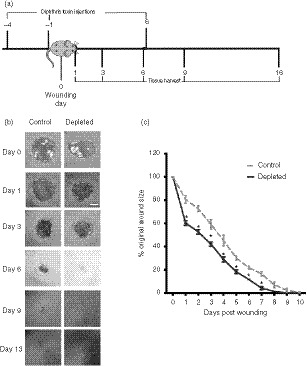
Effect of Langerin/CD207+ cell depletion on wound size during the course of healing. (a) Schematic diagram showing the timing of diphtheria toxin (DT) injections, wounding and wound harvesting in LangDTR mice. (b) Macroscopic observation of healing of 4‐mm full‐thickness punch biopsy skin wounds in LangDTR mice after PBS (control) or DT (depleted) injection, respectively. Representative images with 2‐mm scale bar indicated. Wounds are outlined by dashed lines. (c) Kinetics of wound closure in the control and depleted groups of mice (n = 8 wounds). The change in wound size over time was calculated as a percentage of the original wound size, and is presented as the mean ± SEM. Significant differences between groups determined by two‐way analysis of variance with Bonferroni correction are indicated with an asterisk (*P < 0·05).
To confirm if the improved healing was not due to a direct or indirect effect of DT treatment, we performed the same wounding experiment on wild‐type C57BL/6 mice treated with either PBS or DT (see Supplementary material, Fig. S1a). There was no significant difference in the rate of wound closure following the treatment of DT at any of the time‐points tested (see Supplementary material, Fig. S1b).
Tissues from the control and depleted group were stained for langerin/CD207 to confirm the depletion of langerin+ cells in the depleted group. The number of langerin+ cells in the wound epidermis was measured in sections derived from the depleted and control groups throughout the course of healing using the same zones as mentioned in Fig. 1(a), and representative examples of the staining are shown in the Supplementary material (Fig. S2). Langerin+ cells were readily detectable at all time‐points in the control mice, but were not detected in peripheral epidermis in the DT‐treated group at any time‐point. The exception being at day 9, where a single langerin+ cell was identified in the epidermis of one sample. Langerin+ cells also were not detected adjacent to the wound or in the wound neo‐epidermis at any time‐point, except at day 9. At that time, CD207+ cells were observed in low numbers, mostly in the wound neo‐epidermis (see Supplementary material, Fig. S2). There were also no morphological changes observed between the langerin+ cells observed in the depleted group at day 9 when compared with the control group.
Faster repopulation of langerin+ cells was observed in the dermis of the depleted mice, as early as day 1 in the periphery. There was nearly half the number of langerin+ cells in the depleted mice in the periphery at day 1 after wounding (Fig. 3c, P = 0·0135). There was a significant difference in the number of langerin+ cells in the epidermis (P = 0·0001) and dermis (P = 0·0001) in the adjacent region at day 6 after wounding. The numbers fluctuated on the other days but there was no significant difference between the two groups.
Figure 3.

Effect of diphtheria toxin (DT) treatment on mouse skin. Representative images of LangDTR mouse tissues treated with (a) PBS (Control) and (b) DT (Depleted) (Days 1−16 post‐wounding) stained with antibodies against Langerin/CD207+ (red) and nuclear stain DAPI (blue). The lines indicate the dermal–epidermal junction. The numbers of CD207+ cells per mm2 of epidermis in the (c) periphery, (d) adjacent region and (e) wound bed were counted at days 1, 3, 6 and 9 post‐wounding and compared against each other in both the control and depleted groups. The number of CD207+ cells per mm2 of the dermis in the (f) periphery, (g) adjacent region and (h) wound bed were counted at days 1, 3, 6 and 9 post‐wounding and compared between control and depleted groups. Data presented as mean ± SEM from n = 8 wounds. Statistical differences between the groups over time were determined by two‐way analysis of variance with Bonferroni correction and indicated with an asterisk (*P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001).
Depletion of langerin+ cells does not alter wound contraction
To assess whether the accelerated reduction in wound area in the depleted group could be a result of contraction, the distance between the cut edges of the subcutaneous muscle, the PC, was measured in MSB‐stained sections as shown in Fig. 3(a). There was a substantial difference in PC distance in the control mice between day 1 and days 6, 9 and 16, showing that as expected the distance between the two sides of the PC gradually reduced over time as the wound healed (Fig. 3b). By day 16, the wound in the control mice had contracted to half the original size (Fig. 3b). Importantly, there was no significant difference in wound contraction between the depleted and control groups at any time‐point (Fig. 3b).
Depletion of langerin+ cells increases wound re‐epithelialization
To investigate whether wound re‐epithelialization contributed to the accelerated rate of healing in the depleted group, changes in the area of the wound neo‐epidermis (as indicated in Fig. 3a) were measured. In the control mice, there was very little neo‐epidermis at 1 day post‐wounding; however, by day 3 there was an expansion of the neo‐epidermis, and the area was maximal at day 6 (Fig. 3c). By day 16, the area of neo‐epidermis was substantially reduced and resembled that observed in the periphery (Fig. 3c). These changes were also reflected in both the length and thickness of the neo‐epidermis (Fig. 3d‐e).
Interestingly, in langerin+ cell‐depleted mice there was nearly a twofold increase in the neo‐epidermal area when compared with control mice at both days 3 and 6 (Fig. 3c, P < 0·0212). This difference was reflected in a significant increase in the length (Fig. 3d, P < 0·043), but not the thickness (Fig. 3e) of the neo‐epidermis at those times.
Depletion of langerin+ cells increases granulation tissue formation
To investigate whether increased granulation tissue formation contributed to the accelerated rate of wound healing in the langerin+ cell‐depleted mice, the fibrin‐rich (red‐stained) area within the neo‐dermis above the PC was measured in MSB‐stained tissue over time, as shown in Fig. 3(a). In control wounds, the granulation tissue was measurable at 1 day post‐wounding, peaked at day 9 (Fig. 3f), and decreased again at day 16. In contrast, the granulation tissue area peaked earlier (day 6) in the absence of langerin+ cells, and was significantly increased compared with langerin+‐cell‐containing skin at that time‐point (Fig. 3f, P < 0·0069).
Langerin+ cell depletion increases cell proliferation in the neo‐epidermis and granulation tissue
As significant changes in tissue formation were measured at days 3 and 6, we studied these time‐points in more detail. To establish if the increased neo‐epidermis in the depleted group is a consequence of keratinocyte proliferation, immunofluorescence staining for the proliferation marker Ki67 was carried out. Overall there was an increase in the number of the Ki67‐positive cells in the langerin+‐cell‐depleted epidermis (Fig. 4a), and following enumeration, there was significantly increased proliferation (proliferating cells per mm2) in samples without langerin+ cells relative to controls at both time‐points (Fig. 4b, P < 0·0003).
Figure 4.
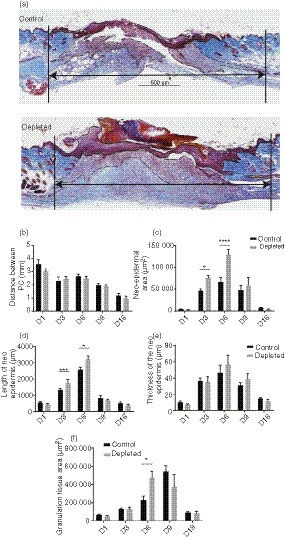
Effect of the Langerin/CD207+ depletion on wound contraction, re‐epithelialization and granulation tissue formation. (a) Representative images of MSB‐stained sections from control or langerin+‐cell‐depleted LangDTR mouse wounds harvested at day 6 (7 days after PBS/diphtheria toxin (DT) injection). Wound contraction (distance between PC) is indicated by the black arrows and the neo‐epidermal and granulation tissue area were measured as outlined and indicated by arrows. Scale bar represents 500 μm. (b) The distance between the panniculus carnosus (PC), (c) neo‐epidermal area, (d) length and (e) thickness of the epidermis (in triplicate on each side of the neo‐epidermis) and (f) granulation tissue area were measured in MSB‐stained sections and are expressed as the mean ± SEM (n = 8 wounds). Significant differences between the groups were determined by two‐way analysis of variance with Bonferroni correction are indicated with an asterisk (*P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001).
To determine if there was a difference in the amount of proliferation of cells in the granulation tissue, we enumerated the Ki67‐positive cells at days 3 and 6, and calculated the number of proliferating cells per mm2 (Fig. 4c). There was a significant increase in proliferation in the granulation tissue at both time‐points in the langerin+‐cell‐depleted mice relative to controls (Fig. 4d, P < 0·0008).
Depletion of langerin+ cells increases GM‐CSF production
We then focused on day 6, which was the time‐point with the most significant changes in tissue formation overall. As GM‐CSF has been previously associated with accelerated wound closure and the proliferation of both epidermal and dermal cells, we examined the levels of this cytokine in the depleted and control wound sections using an anti‐GM‐CSF antibody (Fig. 5a). GM‐CSF‐stained images of the wound beds of all control and depleted sections have been shown in the Supplementary material (Fig. S2). We observed a significant, around twofold, increase in mean GM‐CSF levels in the epidermis (P = 0·0281, Fig. 5b), and around threefold increase in the granulation tissue in langerin+‐cell‐depleted mice, relative to controls (Fig. 5b, P < 0·0015).
Figure 5.
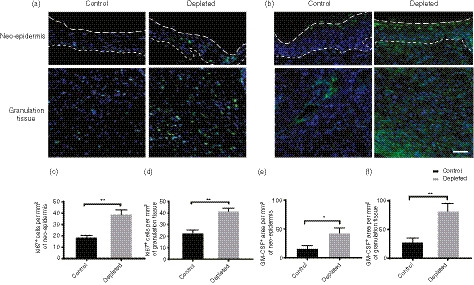
Effect of Langerin/CD207+ depletion on cell proliferation and granulocyte–macrophage colony‐stimulating factor (GM‐CSF) production in the neo‐epidermis and granulation tissue. Representative images of sections from control or langerin+‐cell‐depleted LangDTR mouse wounds, harvested day 6 [D6; 7 days after PBS/diphtheria toxin (DT) injection], which were stained with an antibody against Ki67 showing the (a) epidermis granulation tissue. Scale bar represents 100 μm. The dashed lines indicate the edge of the epidermis (upper) and epidermal–dermal junction (lower) in (a), while the lines indicate the epidermal–dermal junction in (c). Ki67 positive DAPI‐stained cells within the (b) neo‐epidermis or (c) granulation tissue were counted per mm2 area and are presented as mean ± SEM (n = 8). (d) Representative images of sections harvested at day 6 from control or langerin+‐cell‐depleted LangDTR mouse wounds that were stained with antibody against GM‐CSF. The dashed lines demarcate the edge of the epidermis (upper) and epidermal/dermal junction (lower) respectively. Scale bar represents 250 μm. (e) The total area of GM‐CSF staining within the neo‐epidermis and (f) granulation tissue between the two cut edges of panniculus carnosus (PC) was measured and is presented as the mean ± SEM (n = 8 wounds). Significant differences between the groups were determined by two‐way analysis of variance with Bonferroni correction are indicated with an asterisk (*P < 0·05; **P < 0·01).
Depletion of langerin+ cells increases wound re‐vascularization
GM‐CSF is reported to induce re‐vascularization, so it might be expected that there would be increased re‐vascularization in the langerin+‐cell‐depleted mice, compared with control mice. To establish if this was the case, the total number of vessels in the granulation tissue was quantified in control and langerin+‐cell‐depleted tissues at day 6 using Col IV staining (Fig. 6a). Col IV is a protein expressed on the basement membrane of vessels. The staining showed that there was twice the number of vessels per mm2 at day 6 in the depleted group compared with the control mice (P < 0·0333).
Figure 6.
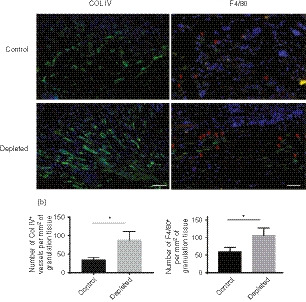
Effect of Langerin/CD207+ depletion on vessel formation and F4/80+ cell recruitment in the granulation tissue. Representative images of sections harvested at day 6 [7 days after PBS/diphtheria toxin (DT) injection] from control and langerin+‐cell‐depleted LangDTR mouse wounds stained with antibodies against (a) Collagen IV (Col IV) or (b) F4/80 in the granulation tissue area. F4/80+ cells are indicated by the arrows. Scale bar represents 150 μm. The total number of Col IV‐ or F4/80‐positive blood vessels per mm2 granulation tissue was quantified. Scale bar represents 100 μm. All data are presented as the mean ± SEM (n = 8 wounds). Significant differences between the groups determined by a Mann–Whitney U‐test are indicated with an asterisk (*P < 0·05).
Col IV stains for the basement membrane of lymphatic and blood vessels alike, hence the tissues were stained with vWF (Fig. 7a), CD31 (Fig. 7c) and Lyve‐1 markers (Fig. 7e) to distinguish the two vessel types. There was a significant increase in the number of vWF+ vessels in the granulation tissue of depleted mice, consistent with the Col IV staining (Fig. 7b; P < 0·0286), and the number of CD31+ vessels in the depleted mice approached significance (Fig. 7d; P = 0·0571). In contrast, the number of lymphatic vessels staining for Lyve‐1+ vessels was highly variable in the depleted mice, and was not significantly different between the two groups (Fig. 7e; P = 0·6982).
Figure 7.
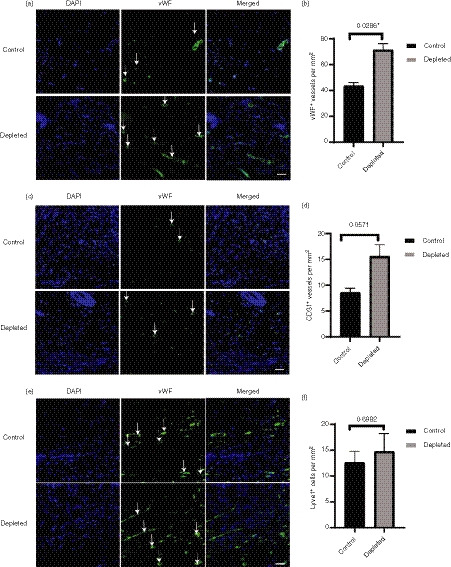
Effect of depletion of langerin+ cells on blood and lymphatic vessels in the granulation tissue. The day 6 tissues were stained with vessel marker [von Willebrand factor (vWF), CD31 or Lyve‐1 – green] and nuclear stain DAPI (blue). Representative images of (a) vWF+, (c) CD31+ and (E) Lyve‐1+ vessels (not the cells) in the granulation tissue of control and depleted groups at day 6 post‐wounding (n = 8 wounds). The scale bar represents 50 μm. The total numbers of (b) vWF+, (d) CD31+ and (f) Lyve‐1+ per mm2 were quantified and presented as mean ± SEM (n = 8 wounds). Significant differences between the groups determined by Mann–Whitney U‐test are indicated with an asterisk (*P < 0·05).
Depletion of langerin+ cells increases F4/80+ cells infiltration
We hypothesized that there would be increased recruitment of F4/80+ cells (macrophages/DCs) associated with the increased GM‐CSF in the absence of langerin+ cells. To determine if this was the case, on day 6 post‐wounding, sections for the control and depleted groups were stained for F4/80 and positive cells were quantified (Fig. 6b). There was clear evidence of an increase in the number of F4/80+ cells in depleted mice, with a significant twofold increase in the number of F4/80+ cells in the granulation tissue of the depleted mice relative to controls (P < 0·05).
DISCUSSION
Langerhans cells are epidermal dendritic cells that reside in the basal layer of the skin and are involved in antigen presentation, as well as the maintenance of tolerance in the skin. 30 Previous studies showed that the number of LCs changed upon tissue injury and during tissue damage‐associated diseases, 19 , 25 , 31 suggesting a potential role for LCs during wound healing. However, the data in some cases are contradictory, and the role and importance of LCs during cutaneous wound healing has not been rigorously examined. In addition, the role of dermal langerin+ cells in wound healing has not been explored.
Consistent with the reports of others, 25 , 32 , 33 we show that langerin+ LCs repopulate the newly formed epidermis in the wound region during the proliferative phase of cutaneous wound healing. LC activation and emigration can be profoundly modified by barrier disruption through changes in cytokine signals. 34 , 35 , 36 In particular, tumour necrosis factor‐α provides signal for LC migration during cutaneous immune and inflammatory responses. 37 , 38 , 39 Local availability of interleukin‐1β and interleukin‐18 also causes the migration of LCs away from the epidermis. 40 , 41 All of these cytokines that are produced early in the wound healing process, 42 , 43 , 44 are likely to be contributing to the emigration of the langerin+ LCs from the wound.
Langerin‐positive cell numbers within the neo‐epidermis of the wound peaked at day 9 in both C57BL/6 and LangDTR mouse, with CD207+ cells observed at day 9 in the neo‐epidermis even in mice depleted of langerin+ cells. Depletion studies have shown >95% of LCs are lost for a period of at least 7 days following DT injection of the mouse, 45 even though the B subunit of DT is only stable for 2·5 hr in vivo. 46 Langerin+ dDCs repopulate the dermis faster than epidermal LCs after depletion. On that basis, the CD207+ cells that are transiently present at day 9 in the wound neo‐epidermis of the LangDTR‐depleted mice (3 days after the third DT injection), are unlikely to be skin‐resident LCs that have repopulated from the stem cell precursors residing around the hair follicle. The more likely explanation for the origin of these cells is an infiltration of a transient population of monocyte‐derived inflammatory LCs that have up‐regulated CD207 expression on their surface following their migration into the skin. 47 These cells are no longer detected at day 16, suggesting that CD207 expression is lost, the cells have died, or they have migrated out of the wound neo‐epidermis. The chemokines or other specific signals that drive the immigration and emigration of these cells is yet to be determined.
To understand the function of langerin+ cells in wound healing, key features of this process were compared in skin wounds in the presence and absence of langerin+ cells. Strikingly, depletion of langerin+ cells resulted in a decrease in wound size at each time‐point, as well as faster closure compared with control mice. Histologically, the differences in tissue formation between wounds with or without langerin+ cells were most evident at day 6 post‐wounding. The accelerated wound closure in the depleted group was probably contributed to by increased cellular proliferation within the neo‐epidermis and granulation tissue at later time‐points. The early difference that we observed in wound area between the groups at 24 hr was not the result of wound contraction, and a limitation of this study is that we have not been able to explain why this occurs. Irrespective, the findings from this study are the first to indicate that langerin+ cells have an inhibitory effect on the proliferative phase of wound healing. The human wound repair process is primarily mediated by re‐epithelialization and granulation tissue formation, and the data presented here may therefore translate to humans. 48
Our findings suggest that the langerin+ cells may act directly on the epithelium to increase the proliferation and/or migration of the keratinocytes. The relative frequency of epidermal LCs is inversely proportional to keratinocyte proliferation, 49 suggesting that LCs inhibit proliferation in the epidermis. Although increased proliferation is important to fill the defect, a lack of appropriate signals to down‐regulate proliferation can cause a delay in wound healing. This is evident in chronic wounds for which a common hallmark is their highly proliferative wound edge. Research has also indicated a relationship between the number of epidermal LCs and proliferative carcinoma transformation in the epidermis, 50 suggesting that langerin+ cells might be playing an important role in the prevention of carcinogenesis.
Accelerated formation of granulation tissue was also evident in the absence of langerin+ cells, with an increased number of proliferating cells in the granulation tissue. As far as we are aware, no studies have explored the link between langerin+ cells and granulation tissue formation. To form granulation tissue, inflammatory macrophage fibroblasts, and endothelial cells need to proliferate and migrate into the blood clot that occupies the wound space. 51 It is possible that depletion of langerin+ cells, particularly in the dermis, causes a change in the pool of cells recruited to the wound bed, altering the cytokine profile to favour increased proliferation.
Complex crosstalk between chemokines, interleukins and growth factors is needed to support the formation of the epidermis and granulation tissue, 52 , 53 , 54 and langerin+ cells in the epidermis and dermis could suppress one or more of these wound mediators. GM‐CSF is a key cytokine known to mediate these processes during wound healing. 17 GM‐CSF is also important for the proliferation, differentiation and activation of normal epidermal LCs. 55 It induces changes in phenotype and distribution of LCs consistent with LC functional maturation and exit from the epidermis to dermis. 16 Additionally, GM‐CSF has been shown to exert a maturation effect on LC function, which is distinct from the maintenance. 56 Expression of GM‐CSF was increased in the absence of langerin+ cells, suggesting that enhanced GM‐CSF expression by keratinocytes could be accelerating wound healing in the depleted group. Increased GM‐CSF has been associated with increased proliferation in epidermal carcinomas, 57 suggesting that they play a role in the proliferation of keratinocytes. Constitutively expressing keratinocyte‐derived GM‐CSF levels increase proliferation of epidermal keratinocytes and improve wound re‐epithelialization and granulation tissue formation, 17 similar to the phenotype we observed with the depletion model. Another study, which used a knockout mouse model of GM‐CSF, showed that wound healing was significantly impaired in the absence of GM‐CSF. 18 Topically applied recombinant GM‐CSF has been reported to successfully treat a range of acute and chronic wounds in human patients, including burns, venous leg ulcers and pressure ulcers. 58 This evidence suggests that GM‐CSF could be contributing to the accelerated wound healing in the depleted group.
GM‐CSF has been reported to recruit macrophages and improve the neo‐vasculature during wound healing. 18 Mice over‐expressing an antagonist of GM‐CSF exhibit reduced microvessel formation. 59 GM‐CSF has been shown to be an important factor for endothelial cell proliferation and survival. 60 , 61 We used the F4/80 marker for the detection of macrophages and dendritic cells. This marker is reported to co‐stain with M2 macrophage activation markers and vascular endothelial growth factor (VEGF), which contributes to angiogenesis, 64 it could be likely that there is an increase in the number of macrophages. Macrophages are usually found in close proximity to blood vessels and produce VEGF, a key cytokine required for new vessel formation, during wound healing. 62 , 63 Consistent with the increase in GM‐CSF observed in this study, F4/80+ infiltration was greater in langerin+‐cell‐depleted wounds, as was angiogenesis, which likely results from VEGF produced by macrophages. 65 , 66 Production of VEGF by keratinocytes has also been shown to modulate the migration of LCs to the lymph nodes. 67 In vivo experiments for epithelial tumours that produce high levels of tumour necrosis factor‐α and VEGF showed a decrease in LC numbers. 68 It will therefore be critical to examine the effects of LC/langerin+ cells on VEGF levels within cutaneous wounds, and the impact of VEGF levels on LC trafficking during the course of healing.
An optimal marker for blood vessels should be specific, independent of pathological changes in the tissue and open to detection of variety of sizes and ages of vessels. Endothelial cells have been shown to modify their antigens depending on the pathological or physiological condition. 69 Col IV stains for the basement membrane of vessels, detecting a range of vessels including blood and lymphatic vessels. vWF (factor VIII‐related antigen) is a glycoprotein that appears to be expressed exclusively on endothelial cells. 69 CD31, transmembrane glycoprotein, plays a major role in the adhesion cascade between endothelial cells and the inflammatory cells during inflammation in facilitating leucocyte migration. This marker stains for the new and proliferating micro‐vessels, while showing more discontinuous staining for larger, mature vessels. 69 In the skin, CD31 and vWF showed similar patterns of staining for small arteries, venules and capillaries. 69 Across different tissues, vWF showed a pattern of staining that was similar to but less intense than that of CD31. 69 Most available histochemical markers do not meet all the criteria, hence angiogenesis in the wound was validated with multiple markers in this study.
Evidence suggests that increased angiogenesis is not always necessary for adequate tissue oxygenation and nutrient support during wound regression, 70 , 71 and that excessive angiogenesis can lead to poor healing outcomes, exacerbating scarring. 72 , 73 It is therefore possible that LCs play a role in maintaining the level of angiogenesis in the wound and thus may help limit scar formation. However, human hypertrophic scars are associated with increased numbers of LCs, 29 whereas human fetal skin, which exhibits scarless wound healing, contains fewer LCs compared with adult skin. 74 Several studies have reported that reduction in DCs resulted in impaired angiogenesis in burn models; 75 however, the exact role of langerin+ dDCs in scarring and angiogenesis warrants further research.
Here, we have carried out an extensive histological analysis of a range of markers to describe the events in the context of the healing wound, using matched sectioned tissue from eight wounds for each condition (±DT). Fixed, paraffin‐embedded tissue sections retain the architecture of the wound and allow for the determination of location of cells and proteins. However, a limitation of this approach is that the entire wound is not sampled for each of the markers that we tested. Studies using whole‐mount histology or flow cytometry would add additional data as they allow for whole‐wound representation.
The healing process differs in male and females because of differences in skin structure 76 and the effects of sex hormones. 77 , 78 , 79 The female skin also has been reported to have a higher density of LCs in the epidermis. 80 The changes we report here in the absence of LCs were assessed only in female mice. It is yet to be determined if these effects are retained in male epidermis, or if instead there are gender‐specific differences.
It has been shown that skin inflammation is not induced by DT treatment in LangDTR mice. 81 , 82 , 83 DT induces apoptosis rather than necrotic death, by inhibition of protein synthesis and activating components of the death receptor pathway, and apoptotic death is not typically associated with inflammation. 83 However, others have demonstrated that there is modulation of inflammation following administration of DT in CD11b and CD11c DTR mice. 84 , 85 , 86 This suggests that DT‐induced inflammation can occur, but in a model‐specific manner.
The pain relief/anaesthesia/recovery drugs were administered for the welfare of the animals in both the control and depleted groups. Bupivacaine has been shown to influence proteolytic factors at later time‐points during wound healing 87 but does not affect healing at clinically relevant doses. 88 Furthermore, there is a possibility that DT interacts with drugs such as bupivacaine to influence wound healing. However, DT has a short half‐life 46 and was administered 24 hr before any of the other drugs were given. The timing of the administration of the drugs does not support any direct interaction between the DT and the other drugs that were given that might impact on the healing process. In addition, to the best of our knowledge, no such affect between DT, drugs and wound healing has been previously reported.
In summary, depletion of langerin+ cells enhances keratinocyte proliferation in the epidermis, F4/80+ cell recruitment, angiogenesis in the granulation tissue and ultimately skin repair through the induction of GM‐CSF, suggesting a sentinel suppressive role for langerin+ cells in the skin. In the event of an injury, langerin+ cells migrate out of the wound, relieving this suppressive regulation, and allowing the inflammatory and proliferative phases of healing to initiate. The specific roles of epidermal LCs and langerin+ dDCs are still to be determined. Further understanding as to how langerin+ cells regulate these processes in the steady state and during wound closure may open up new avenues for the treatment of patients suffering from wound healing impairments.
Author contributions
AR, GS, NR and JA performed the experiments, LW, MH and AR designed the study, AR, MH, LW and AT wrote the paper.
Disclosure
The authors have no conflicts of interest to declare.
Supporting information
Figure S1. (a) Representative images of wound healing in C57BL/6 mice treated with PBS (mock) or diphtheria toxin (DT) from day 0 (D0) to D9 (b) Graph showing the percentage of wound closure in C57BL/6 mock and DT groups (n = 4) over time.
Figure S2. Images of all (a) control and (b) depleted tissues stained with anti‐GM‐CSF antibody at day 6 post working (AF488 green channel only).
Acknowledgements
We acknowledge the kind gift of the Langerin DTR mice from Dr Bernard Malissen. This work was supported by grants from the Otago Medical Research Foundation and the University of Otago. Ms Aarthi Rajesh is the recipient of a postgraduate scholarship from the University of Otago.
†L.W. and M.H. are joint senior authors, contributing equally to this study.
References
- 1. Nestle FO, Di Meglio P, Qin J‐Z, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol 2009; 9:679–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Birbeck MS, Breathnach AS, Everall JD. An electron microscope study of basal melanocytes and high‐level clear cells (Langerhans cells) in vitiligo. J Invest Dermatol 1961; 37:51–64. [Google Scholar]
- 3. Romani N, Holzmann S, Tripp CH, Koch F, Stoitzner P. Langerhans cells – dendritic cells of the epidermis. APMIS 2003; 111:725–40. [DOI] [PubMed] [Google Scholar]
- 4. Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc the Natl Acad Sci U S A 2002; 99:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Romani N, Ratzinger G, Pfaller K, Salvenmoser W, Stossel H, Koch F et al Migration of dendritic cells into lymphatics the Langerhans cell example: routes, regulation, and relevance. Int Rev Cytol 2001; 207:237–70. [DOI] [PubMed] [Google Scholar]
- 6. Merad M, Manz MG. Dendritic cell homeostasis. Blood 2009; 113:3418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flacher V, Tripp CH, Mairhofer DG, Steinman RM, Stoitzner P, Idoyaga J et al Murine Langerin+ dermal dendritic cells prime CD8+ T cells while Langerhans cells induce cross‐tolerance. EMBO Mol Med 2014; 6:1191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999; 341:738–46. [DOI] [PubMed] [Google Scholar]
- 9. Wise LM, Stuart GS, Real NC, Fleming SB, Mercer AA. Orf virus IL‐10 accelerates wound healing while limiting inflammation and scarring. Wound Repair Regen 2014; 22:356–67. [DOI] [PubMed] [Google Scholar]
- 10. Eming SA, Martin P, Tomic‐Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 2014; 6:265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goren I, Allmann N, Yogev N, Schurmann C, Linke A, Holdener M et al A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin‐driven lysozyme M‐specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol 2009; 175:132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao G, Hochwalt PC, Usui ML, Underwood RA, Singh PK, James GA et al Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: a model for the study of chronic wounds. Wound Repair Regen 2010; 18:467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mustoe TA, O'Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg 2006; 117:35S–41S. [DOI] [PubMed] [Google Scholar]
- 14. Rubbia‐Brandt L, Sappino AP, Gabbiani G. Locally applied GM‐CSF induces the accumulation of alpha‐smooth muscle actin containing myofibroblasts. Virchows Arch B Cell Pathol Incl Mol Pathol 1991; 60:73–82. [DOI] [PubMed] [Google Scholar]
- 15. Kaplan G, Walsh G, Guido LS, Meyn P, Burkhardt RA, Abalos RM et al Novel responses of human skin to intradermal recombinant granulocyte/macrophage‐colony‐stimulating factor: Langerhans cell recruitment, keratinocyte growth, and enhanced wound healing. J Exp Med 1992; 175:1717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith CH, Allen MH, Groves RW, Barker JN. Effect of granulocyte macrophage‐colony stimulating factor on Langerhans cells in normal and healthy atopic subjects. Br J Dermatol 1998; 139:239–46. [DOI] [PubMed] [Google Scholar]
- 17. Mann A, Breuhahn K, Schirmacher P, Blessing M. Keratinocyte‐derived granulocyte‐macrophage colony stimulating factor accelerates wound healing: stimulation of keratinocyte proliferation, granulation tissue formation, and vascularization. J Invest Dermatol 2001; 117:1382–90. [DOI] [PubMed] [Google Scholar]
- 18. Fang Y, Gong SJ, Xu YH, Hambly BD, Bao S. Impaired cutaneous wound healing in granulocyte/macrophage colony‐stimulating factor knockout mice. Br J Dermatol 2007; 157:458–65. [DOI] [PubMed] [Google Scholar]
- 19. Galkowska H, Olszewski WL, Wojewodzka U. Expression of natural antimicrobial peptide β‐defensin‐2 and Langerhans cell accumulation in epidermis from human non‐healing leg ulcers. Folia Histochem Cytobiol 2005; 43:133–6. [PubMed] [Google Scholar]
- 20. Niessen FB, Schalkwijk J, Vos H, Timens W. Hypertrophic scar formation is associated with an increased number of epidermal Langerhans cells. J Pathol 2004; 202:121–9. [DOI] [PubMed] [Google Scholar]
- 21. Stojadinovic O, Yin N, Lehmann J, Pastar I, Kirsner RS, Tomic‐Canic M. Increased number of Langerhans cells in the epidermis of diabetic foot ulcers correlates with healing outcome. Immunol Res 2013; 57:222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andriessen MP, van Bergen BH, Spruijt KI, Go IH, Schalkwijk J, van de Kerkhof PC. Epidermal proliferation is not impaired in chronic venous ulcers. Acta Dermato‐Venereologica 1995; 75:456–62. [DOI] [PubMed] [Google Scholar]
- 23. Low QEH, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ et al Wound healing in MIP‐1α −/− and MCP‐1−/− mice. Am J Pathol 2001; 159:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kohn S, Kohn D, Schiller D. Effect of zinc supplementation on epidermal Langerhans' cells of elderly patients with decubital ulcers. J Dermatol 2000; 27:258–63. [DOI] [PubMed] [Google Scholar]
- 25. Juhász I, Simon M, Herlyn M, Hunyadi J. Repopulation of Langerhans cells during wound healing in an experimental human skin/SCID mouse model. Immunol Lett 1996; 52:125–8. [DOI] [PubMed] [Google Scholar]
- 26. Kissenpfennig A, Henri S, Dubois B, Laplace‐Builhe C, Perrin P, Romani N et al Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 2005; 22:643–54. [DOI] [PubMed] [Google Scholar]
- 27. Pappenheimer AM, Harper AA, Moynihan M, Brockes JP. Diphtheria toxin and related proteins: effect of route of injection on toxicity and the determination of cytotoxicity for various cultured cells. J Infect Dis 1982; 145:94–102. [DOI] [PubMed] [Google Scholar]
- 28. Lendrum AC, Fraser DS, Slidders W, Henderson R. Studies on the character and staining of fibrin. J Clin Pathol 1962; 15:401–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ansell DM, Campbell L, Thomason HA, Brass A, Hardman MJ. A statistical analysis of murine incisional and excisional acute wound models. Wound Repair Regen 2014; 22:281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin‐expressing dendritic cell subsets in the skin. Immunol Rev 2010; 234:120–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taguchi K, Fukunaga A, Ogura K, Nishigori C. The role of epidermal Langerhans cells in NB‐UVB‐induced immunosuppression. Kobe J Med Sci 2013; 59:E1–9. [PubMed] [Google Scholar]
- 32. Helfman T, Streilein JW, Eaglstein WH, Mertz PM. Studies on the repopulation of langerhans cells in partial‐thickness wounds: air exposed and occlusively dressed. Arch Dermatol 1993; 129:592–5. [PubMed] [Google Scholar]
- 33. Ouwehand K, Spiekstra SW, Waaijman T, Breetveld M, Scheper RJ, de Gruijl TD et al CCL5 and CCL20 mediate immigration of Langerhans cells into the epidermis of full thickness human skin equivalents. Eur J Cell Biol 2012; 91:765–73. [DOI] [PubMed] [Google Scholar]
- 34. van den Berg LM, de Jong MA, Witte L, Ulrich MM, Geijtenbeek TB. Burn injury suppresses human dermal dendritic cell and Langerhans cell function. Cell Immunol 2011; 268:29–36. [DOI] [PubMed] [Google Scholar]
- 35. Proksch E, Brasch J. Influence of epidermal permeability barrier disruption and Langerhans' cell density on allergic contact dermatitis. Acta Derm Venereol 1997; 77:102–4. [DOI] [PubMed] [Google Scholar]
- 36. Lateef Z, Stuart G, Jones N, Mercer A, Fleming S, Wise L. The cutaneous inflammatory response to thermal burn injury in a murine model. Int J Mol Sci 2019; 20:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Groves RW, Allen MH, Ross EL, Barker JN, MacDonald DM. Tumour necrosis factor α is pro‐inflammatory in normal human skin and modulates cutaneous adhesion molecule expression. Br J Dermatol 1995; 132:345–52. [DOI] [PubMed] [Google Scholar]
- 38. Kimber I, Cumberbatch M. Stimulation of Langerhans cell migration by tumor necrosis factor α (TNF‐α). J Invest Dermatol 1992; 99:48S–50S. [DOI] [PubMed] [Google Scholar]
- 39. Cumberbatch M, Kimber I. Dermal tumour necrosis factor‐α induces dendritic cell migration to draining lymph nodes, and possibly provides one stimulus for Langerhans' cell migration. Immunology 1992; 75:257–63. [PMC free article] [PubMed] [Google Scholar]
- 40. Cumberbatch M, Dearman RJ, Antonopoulos C, Groves RW, Kimber I. Interleukin (IL)‐18 induces Langerhans cell migration by a tumour necrosis factor‐α‐ and IL‐1β‐dependent mechanism. Immunology 2001; 102:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Antonopoulos C, Cumberbatch M, Dearman RJ, Daniel RJ, Kimber I, Groves RW. Functional caspase‐1 is required for Langerhans cell migration and optimal contact sensitization in mice. J Immunol 2001; 166:3672–7. [DOI] [PubMed] [Google Scholar]
- 42. Rapala K. The effect of tumor necrosis factor‐α on wound healing. An experimental study. Ann Chir Gynaecol Suppl 1996; 211:1–53. [PubMed] [Google Scholar]
- 43. Thomay AA, Daley JM, Sabo E, Worth PJ, Shelton LJ, Harty MW et al Disruption of interleukin‐1 signaling improves the quality of wound healing. Am J Pathol 2009; 174:2129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kampfer H, Muhl H, Manderscheid M, Kalina U, Kauschat D, Pfeilschifter J et al Regulation of interleukin‐18 (IL‐18) expression in keratinocytes (HaCaT): implications for early wound healing. Eur Cytokine Netw 2000; 11:626–33. [PubMed] [Google Scholar]
- 45. Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH et al Identification of a novel population of Langerin+ dendritic cells. J Exp Med 2007; 204:3147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamaizumi M, Mekada E, Uchida T, Okada Y. One molecule of diphtheria toxin fragment a introduced into a cell can kill the cell. Cell 1978; 15:245–50. [DOI] [PubMed] [Google Scholar]
- 47. Sere K, Baek JH, Ober‐Blobaum J, Muller‐Newen G, Tacke F, Yokota Y et al Two distinct types of Langerhans cells populate the skin during steady state and inflammation. Immunity 2012; 37:905–16. [DOI] [PubMed] [Google Scholar]
- 48. Grillo HC, Watts GT, Gross J. Studies in wound healing: I. Contraction and the wound contents. Ann Surg 1958; 148:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Potten CS, Allen TD. A model implicating the Langerhans cell in keratinocyte proliferation control. Differentiation 1976; 5:43–7. [DOI] [PubMed] [Google Scholar]
- 50. Han CS, Park YN, Lee KG, Choi IJ. The epidermal proliferation and the number of Langerhans cells in 7,12‐dimethylbenzanthracene induced epidermal changes. J Pathol Transl Med 1993; 27:590–604. [Google Scholar]
- 51. Häkkinen L, Larjava H, Koivisto L. Granulation tissue formation and remodeling. Endod Topics 2011; 24:94–129. [Google Scholar]
- 52. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic‐Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008; 16:585–601. [DOI] [PubMed] [Google Scholar]
- 53. Wu L, Diny NL, Ong S, Barin JG, Hou X, Rose NR et al Pathogenic IL‐23 signaling is required to initiate GM‐CSF‐driven autoimmune myocarditis in mice. Eur J Immunol 2016; 46:582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roupé KM, Nybo M, Sjöbring U, Alberius P, Schmidtchen A, Sørensen OE. Injury is a major inducer of epidermal innate immune responses during wound healing. J Invest Derm 2010; 130:1167–77. [DOI] [PubMed] [Google Scholar]
- 55. Ushach I, Zlotnik A. Biological role of granulocyte macrophage colony‐stimulating factor (GM‐CSF) and macrophage colony‐stimulating factor (M‐CSF) on cells of the myeloid lineage. J Leukoc Biol 2016; 100:481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Heufler C, Koch F, Schuler G. Granulocyte/macrophage colony‐stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med 1988; 167:700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mueller MM, Peter W, Mappes M, Huelsen A, Steinbauer H, Boukamp P et al Tumor progression of skin carcinoma cells in vivo promoted by clonal selection, mutagenesis, and autocrine growth regulation by granulocyte colony‐stimulating factor and granulocyte‐macrophage colony‐stimulating factor. Am J Pathol 2001; 159:1567–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hu X, Sun H, Han C, Wang X, Yu W. Topically applied rhGM‐CSF for the wound healing: a systematic review. Burns 2011; 37:729–41. [DOI] [PubMed] [Google Scholar]
- 59. Mann A, Niekisch K, Schirmacher P, Blessing M. Granulocyte–macrophage colony‐stimulating factor is essential for normal wound healing. J Investig Dermatol Symp Proc 2006; 11:87–92. [DOI] [PubMed] [Google Scholar]
- 60. Bussolino F, Ziche M, Wang JM, Alessi D, Morbidelli L, Cremona O et al In vitro and in vivo activation of endothelial cells by colony‐stimulating factors. J Clin Invest 1991; 87:986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ebner K, Bandion A, Binder BR, de Martin R, Schmid JA. GMCSF activates NF‐κB via direct interaction of the GMCSF receptor with IκB kinase β . Blood 2003; 102:192–9. [DOI] [PubMed] [Google Scholar]
- 62. Ogle ME, Segar CE, Sridhar S, Botchwey EA. Monocytes and macrophages in tissue repair: implications for immunoregenerative biomaterial design. Exp Biol Med 2016; 241:1084–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ferrara N, Davis‐Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 1997; 18:4–25. [DOI] [PubMed] [Google Scholar]
- 64. Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W et al Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010; 184:3964–77. [DOI] [PubMed] [Google Scholar]
- 65. Stockmann C, Kirmse S, Helfrich I, Weidemann A, Takeda N, Doedens A et al A wound size‐dependent effect of myeloid cell‐derived vascular endothelial growth factor on wound healing. J Invest Dermatol 2011; 131:797–801. [DOI] [PubMed] [Google Scholar]
- 66. Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I et al CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 2012; 120:613–25. [DOI] [PubMed] [Google Scholar]
- 67. Staquet MJ, Godefroy S, Jacquet C, Viac J, Schmitt D. Vascular endothelial growth factor (VEGF) induces human Langerhans cell migration. Arch Dermatol Res 2001; 293:26–8. [DOI] [PubMed] [Google Scholar]
- 68. Viac J, Palacio S, Schmitt D, Claudy A. Expression of vascular endothelial growth factor in normal epidermis, epithelial tumors and cultured keratinocytes. Arch Dermatol Res 1997; 289:158–63. [DOI] [PubMed] [Google Scholar]
- 69. Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli‐1 in normal human tissues. J Histochem Cytochem 2006; 54:385–95. [DOI] [PubMed] [Google Scholar]
- 70. Haroon ZA, Amin K, Saito W, Wilson W, Greenberg CS, Dewhirst MW. SU5416 delays wound healing through inhibition of TGF‐β1 activation. Cancer Biol Ther 2002; 1:121–6. [DOI] [PubMed] [Google Scholar]
- 71. Koh W, Stratman AN, Sacharidou A, Davis GE. Chapter 5 In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Method Enzymol 2008; 443: 83–101. [DOI] [PubMed] [Google Scholar]
- 72. Davis GE, Koh W, Stratman AN. Mechanisms controlling human endothelial lumen formation and tube assembly in three‐dimensional extracellular matrices. Birth Defects Res C Embryo Today 2007; 81:270–85. [DOI] [PubMed] [Google Scholar]
- 73. Hunt S. Increased dietary intake of ω‐3‐PUFA reduces pathological retinal angiogenesis. Ophthalmologe 2007; 104:727–9. [DOI] [PubMed] [Google Scholar]
- 74. Walraven M, Talhout W, Beelen RH, van Egmond M, Ulrich MM. Healthy human second‐trimester fetal skin is deficient in leukocytes and associated homing chemokines. Wound Repair Regen 2016; 24:533–41. [DOI] [PubMed] [Google Scholar]
- 75. Vinish M, Cui W, Stafford E, Bae L, Hawkins H, Cox R et al Dendritic cells modulate burn wound healing by enhancing early proliferation. Wound Repair Regen 2016; 24:6–13. [DOI] [PubMed] [Google Scholar]
- 76. Azzi L, El‐Alfy M, Martel C, Labrie F. Gender differences in mouse skin morphology and specific effects of sex steroids and dehydroepiandrosterone. J Invest Dermatol 2005; 124:22–7. [DOI] [PubMed] [Google Scholar]
- 77. Gilliver SC, Ruckshanthi JP, Hardman MJ, Nakayama T, Ashcroft GS. Sex dimorphism in wound healing: the roles of sex steroids and macrophage migration inhibitory factor. Endocrinology 2008; 149:5747–57. [DOI] [PubMed] [Google Scholar]
- 78. Hardman MJ, Ashcroft GS. Estrogen, not intrinsic aging, is the major regulator of delayed human wound healing in the elderly. Genome Biol 2008; 9:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Romana‐Souza B, Assis de Brito TL, Pereira GR, Monte‐Alto‐Costa A. Gonadal hormones differently modulate cutaneous wound healing of chronically stressed mice. Brain Behav Immun 2014; 36:101–10. [DOI] [PubMed] [Google Scholar]
- 80. Koyama Y, Nagao S, Ohashi K, Takahashi H, Marunouchi T. Sex differences in the densities of epidermal Langerhans cells of the mouse. J Invest Dermatol 1987; 88:541–4. [DOI] [PubMed] [Google Scholar]
- 81. Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML et al Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol 2005; 169:569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Morimoto H, Bonavida B. Diphtheria toxin‐ and Pseudomonas A toxin‐mediated apoptosis. ADP ribosylation of elongation factor‐2 is required for DNA fragmentation and cell lysis and synergy with tumor necrosis factor‐α . J Immunol 1992; 149:2089–94. [PubMed] [Google Scholar]
- 83. Thorburn J, Frankel AE, Thorburn A. Apoptosis by leukemia cell‐targeted diphtheria toxin occurs via receptor‐independent activation of Fas‐associated death domain protein. Clin Cancer Res 2003; 9:861–5. [PubMed] [Google Scholar]
- 84. Frieler RA, Nadimpalli S, Boland LK, Xie A, Kooistra LJ, Song J et al Depletion of macrophages in CD11b diphtheria toxin receptor mice induces brain inflammation and enhances inflammatory signaling during traumatic brain injury. Brain Res 2015; 1624:103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Roberts LM, Ledvina HE, Tuladhar S, Rana D, Steele SP, Sempowski GD et al Depletion of alveolar macrophages in CD11c diphtheria toxin receptor mice produces an inflammatory response. Immun Inflamm Dis 2015; 3:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Westermark L, Fahlgren A, Fallman M. Immune response to diphtheria toxin‐mediated depletion complicates the use of the CD11c‐DTR(tg) model for studies of bacterial gastrointestinal infections. Microb Pathog 2012; 53:154–61. [DOI] [PubMed] [Google Scholar]
- 87. Kesici S, Kesici U, Ulusoy H, Erturkuner P, Turkmen A, Arda O. Effects of local anesthetics on wound healing. Rev Bras Anestesiol 2018; 68:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Waite A, Gilliver SC, Masterson GR, Hardman MJ, Ashcroft GS. Clinically relevant doses of lidocaine and bupivacaine do not impair cutaneous wound healing in mice. Br J Anaesth 2010; 104:768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (a) Representative images of wound healing in C57BL/6 mice treated with PBS (mock) or diphtheria toxin (DT) from day 0 (D0) to D9 (b) Graph showing the percentage of wound closure in C57BL/6 mock and DT groups (n = 4) over time.
Figure S2. Images of all (a) control and (b) depleted tissues stained with anti‐GM‐CSF antibody at day 6 post working (AF488 green channel only).


