Summary
Psoriasis is a chronic inflammatory skin disease with unclear pathogenesis. Interleukin‐33 (IL‐33) is highly expressed in patients with psoriasis, but its role in psoriasis is unknown. The aim of this study was to investigate the possible role of IL‐33 in the pathogenesis and treatment of psoriasis. IL‐33 expression was determined using enzyme‐linked immunosorbent assay, real‐time fluorescent quantitative polymerase chain reaction and immunohistochemical staining. CD4+ T cells were sorted using magnetic beads and treated with or without IL‐33. Imiquimod (IMQ) was used to induce psoriatic inflammation in mice. The frequency of immune cells was determined using flow cytometry. The cytokine level in mouse skin was measured using cytometric bead array. Our results showed that IL‐33 was highly expressed in the lesional skin and serum of patients with moderate‐to‐severe plaque psoriasis. IL‐33 inhibited the expression of IL‐17 in CD4+ T cells of psoriasis patients. Subcutaneous injection of IL‐33 alleviated the IMQ‐induced psoriatic inflammation in mice, reduced tumor necrosis factor‐α and IL‐23 expression, and decreased the proportion of T helper type 17 (Th17) cells in the skin‐draining lymph nodes in the mice. Our results suggest that IL‐33 plays a protective role in the pathogenesis of psoriasis by suppressing Th17 cell differentiation and function. The potential therapeutic effect of IL‐33 in treating psoriasis warrants further investigation.
Keywords: psoriasis, interleukin‐17, interleukin‐33, T helper type 17 cells
Interleukin‐33 is highly expressed in the lesional skin of psoriasis patients. IL‐33 can inhibit the differentiation and function of Th17 cells from psoriasis patients. Subcutaneous injection of IL‐33 can alleviate imiquimod‐induced mouse psoriatic inflammation.
Abbreviations
- αGalCer
α‐galactosylceramide
- CBA
cytometric bead array
- IFN
‐γ
interferon‐γ
- IgG
immunoglobulin G
- IL
interleukin
- IMQ
imiquimod
- iNKT
invariant natural killer T
- mAb
monoclonal antibodies
- PBMCs
peripheral blood mononuclear cells
- PV
psoriasis vulgaris
- STAT3
signal transducer and activator of transcription 3
- Th
T helper
- TNF
tumor necrosis factor
- Treg
T regulatory
Introduction
Psoriasis is a common, genetically determined, environment‐triggered chronic inflammatory autoimmune disease predominantly affecting the skin and joints. The prevalence of psoriasis is 2–3% worldwide 1 and 0·47% in China. 2 Psoriasis has been validated as a bona fide T‐cell‐mediated disorder characterized by the T helper type 1 (Th1)/Th2 paradigm and Th17/T regulatory (Treg) cell imbalance, with the tumor necrosis factor‐α (TNF‐α)–interleukin‐23 (IL‐23)–IL‐17 axis being central to the disease immunopathogenesis. 3 Emerging biological therapies targeting these inflammatory cytokines have demonstrated remarkable efficacy and safety profiles in individuals with refractory psoriasis who fail to respond to conventional treatments. 4 , 5
A recently identified member of the IL‐1 superfamily, IL‐33 is a nuclear cytokine expressed in epithelial cells, endothelial cells, and fibroblast‐like cells at barrier surfaces under both steady‐state and inflamed‐state conditions. 6 Through interaction with its receptor ST2, IL‐33 mediates versatile functions in a variety of ST2‐expressing immune cells, such as mast cells, Treg cells, Th2 cells and invariant natural killer T (iNKT) cells. 6 Accordingly, IL‐33 elicits pleiotropic activities in the innate and adaptive immune responses, and therefore plays vital roles in tissue homeostasis and infectious, inflammatory, metabolic, and neoplastic diseases. 7
Compared with perilesional and normal healthy skin, human psoriatic plaques have considerably enhanced IL‐33 and ST2 expression, 8 , 9 , 10 , 11 whereas serum IL‐33 estimates are paradoxical. 11 , 12 Therapeutic modalities such as methotrexate, 13 narrowband ultraviolet B radiation, 13 and TNF‐α inhibitors 10 , 12 differentially alter lesional and circulating IL‐33 levels. IL‐33 has also been correlated with positive Köbner reaction in patients with psoriasis vulgaris (PV), 14 and accelerates atherosclerosis and osteoporosis in patients with psoriasis arthritis. 15 However, Athari et al. recently reported that the development of imiquimod (IMQ) ‐induced psoriasis‐like skin inflammation was not impaired in IL‐33‐deficient mice, whereas Duan et al. reported that IL‐33 aggravated psoriatic inflammation by inhibiting autophagy and promoting tyrosyl phosphorylation of signal transducer and activator of transcription 3 (STAT3) in keratinocytes. 16 , 17 Nevertheless, the role of IL‐33 in psoriatic T cells, especially Th17 cells, remains unknown.
Here, we sought to address the role of IL‐33 in Th17 cells from individuals with psoriasis. We demonstrate, for the first time, that in vitro IL‐33 treatment inhibits IL‐17 expression by CD4+ T cells isolated from the peripheral blood mononuclear cells (PBMCs) of patients with PV while enhancing the proportion of iNKT cells. Moreover, in contrast to Duan et al., we found that subcutaneous injection of IL‐33 alleviated IMQ‐induced psoriatic inflammation in mice.
Materials and methods
Patients
The study was approved by the ethics committees of the Shanghai Tenth People’s Hospital and was performed in accordance with the Declaration of Helsinki. Patients with moderate‐to‐severe PV (Psoriasis Area and Severity Index score ≥10) were enrolled in the study. Exclusion criteria were topical treatment within 2 weeks or systemic therapy within 4 weeks. Healthy volunteers without a family history of psoriasis were enrolled as healthy controls. All participants had no other systemic diseases, active infections, or autoimmune diseases. Ten patients were treated with anti‐TNF‐α therapy (Remicade; Janssen Biotech, Horsham, PA; 5 mg/kg, intravenously) at weeks 0, 2, and 6, and then every 8 weeks. Skin and blood samples were collected after enrollment and at week 10. Written informed consent was obtained from all patients and controls.
Enzyme‐linked immunosorbent assay
Serum IL‐33 levels were measured using a human IL‐33 ELISA Kit (eBioscience, San Diego, CA) according to the manufacturer’s instructions.
Histology and immunohistochemistry
Skin tissue sections were stained with hematoxylin & eosin. Peroxidase inactivation in 3% H2O2 and heat retrieval in 1 mm EDTA buffer (pH 8·0) were followed by incubation in 5% bovine serum. The sections were stained overnight with mouse anti‐human IL‐33 monoclonal antibodies (mAb) (1:30; Abcam, Cambridge, UK) at 4° and incubated with rabbit anti‐mouse immunoglobulin G (IgG) at room temperature for 1 hr, which was visualized with diaminobenzidine and counterstained with hematoxylin. Images were captured using a Zeiss Axioscope.
RNA extraction and real‐time quantitative PCR
RNA was extracted from human skin biopsy samples using TRIzol (Invitrogen, Carlsbad, CA). Total RNA was reverse‐transcribed to complementary DNA with a reverse transcription kit (TaKaRa Biotechnology, Shiga, Japan). RNA expression levels were detected using SYBR Green quantitative PCR (KAPA). The primer sequences were as follows: β‐actin [forward: 5′‐TGGCACCCAGCACAATGAA‐3′, reverse: 5′‐TAAGTCATAGTCCGCCTAGAAGCA‐3′) and IL‐33 (forward: 5′‐CTGGTACTCGCTGCCTGTCAAC‐3′, reverse: 5′‐ACCATCAACACCGTCACCTGATTC‐3′).
Mice and treatment
Wild‐type C57BL/6 mice were purchased from the Beijing Vital River Laboratory Animal Technology. The mice were housed in a specific pathogen‐free barrier unit. Experiments were conducted when the mice were about 7 weeks old. The mouse handling and experimental procedures were in accordance with the requirements of the Institutional Animal Care and Use Committee of Tongji University. For IMQ‐induced psoriasis‐like skin inflammation, a daily dose of 60 mg 5% IMQ cream (Mingxin Pharmaceuticals, Sichuan, China) was topically applied on the shaved back skin of the mice for 6 consecutive days, and the mice were killed on day 7. Control mice were treated with the same amount of Vaseline. Mouse recombinant IL‐33 (rIL‐33, 1 µg in 300 μl PBS; PeproTech, Rocky Hill, NJ) or PBS was injected subcutaneously daily into the back skin of the mice. Two researchers independently assigned a clinical score of 0–4 (0, none; 1, mild; 2, moderate; 3, severe; 4, very severe) for erythema, scaling, and thickness. Serum was collected, and dorsal skin was harvested, fixed, and paraffin‐embedded. Skin‐draining lymph nodes were collected, ground, and harvested through a 70‐μm cell strainer to obtain single‐cell suspensions.
PBMC isolation
Peripheral blood mononuclear cells were freshly separated from human peripheral blood using Ficoll‐Paque Plus (GE Healthcare, Chalfont St Giles, UK) according to the manufacturer’s recommendations. The PBMC survival rate was evaluated using trypan blue (Sangon Biotech, Shanghai, China). CD4+ T cells were isolated from the PBMCs. To expand iNKT cells, the PBMCs were stimulated with α‐galactosylceramide (αGalCer, 2 μg/ml, a kind gift from Professor Qing‐Sheng Mi of the Henry Ford Health System).
CD4+ T‐cell separation
CD4+ T cells were purified from the human PBMCs according to an EasySep Human CD4+ T Cell Iso Kit (Stemcell Technologies, Vancouver, Canada) user manual. Flow cytometry showed that the CD4+ cell purity was >98%. The purified cells were suspended in RPMI‐1640 medium (Gibco, Grand Island, NY) containing 10% fetal bovine serum (Gibco), seeded on 96‐well plates (1 × 106 cells per well) with plate‐bound anti‐CD3 (1 µg/ml, BioGems, Westlake Village, CA), plate‐bound anti‐CD28 (0·5 µg/ml, BioGems), recombinant IL‐2 (20 ng/ml, PeproTech), and transforming growth factor‐β (20 ng/ml, PeproTech) with or without IL‐33 (50 ng/ml, PeproTech).
Flow cytometric analysis
CD4+ T cells were treated in vitro with Cell Stimulation Cocktail (eBioscience) for 5 hr to detect cytokine secretion. Single‐cell suspensions were pre‐incubated with Fc Receptor Blocking Solution (BioLegend, San Diego, CA) for 10 min at room temperature. To identify dead cells, the cells were first stained with Fixable Viability Stain 780 (BD Biosciences, Franklin Lakes, NJ) for 30 min at 4°. Subsequently, the cells were stained for 30 min with surface marker mAb in PBS containing 2% fetal bovine serum at 4°. For detecting intracytoplasmic cytokines (IC), the cells were fixed with IC Fixation Buffer (eBioscience) for 30 min at 4°. For analyzing intranuclear transcription factors, the cells were fixed with Fixation/Permeabilization Diluent and Concentrate (eBioscience) at 4° for 40 min. After fixation, the cells were stained with intracellular mAb in Permeabilization Buffer (eBioscience) at 4° for 30 min. The following human mAb were used: fluorescein isothiocyanate‐conjugated anti‐CD4 (Clone: OKT4, eBioscience), phycoerythrin (PE) ‐conjugated anti‐IL‐17A (Clone: eBio64DEC17, eBioscience), allophycocyanin (APC) ‐conjugated anti‐interferon‐γ (Clone: 4S.B3, eBioscience), PE/cyanine 7 (Cy7)‐conjugated anti‐IL‐10 (Clone: JES3‐9D7, eBioscience), PE‐conjugated anti‐CD25 (Clone: BC96, eBioscience), APC‐conjugated anti‐forkhead box P3 (FOXP3) (Clone: 236A/E7, eBioscience), PE/Cy7‐conjugated anti‐CD3 (Clone: UCHT1, BioLegend), and APC‐conjugated αGalCer:CD1d complex (a kind gift from Professor Qing‐Sheng Mi of Henry Ford Health System). The following mouse mAb were used: APC‐conjugated anti‐CD3 (Clone: 17A2, BioLegend), Peridinin chlorophyll protein/Cy5.5‐conjugated anti‐CD4 (Clone: RM4‐5, BioLegend), FITC‐conjugated anti‐CD4 (Clone: GK1.5, BioLegend), PE‐conjugated anti‐IL‐17A (Clone: eBio17B7, eBioscience), PE/Cy7‐conjugated anti‐CD25 (Clone: PC61, BD Biosciences), PE‐conjugated anti‐FOXP3 (Clone: MF23, BD Biosciences), and PE‐conjugated αGalCer:CD1d complex (eBioscience). Data were acquired on a facscanto II (BD Biosciences) and analyzed using flowjo software (Tree Star, Ashland, OR).
Cytometric bead array
Cytometric bead array (CBA) was used to detect the levels of IL‐4 in the supernatant of cultured CD4+ T cells and the cytokines in the skin and serum of mice. Mouse skin tissues were lyzed with radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China). Protein levels were determined using CBA (BD Pharmingen) according to the manufacturer’s protocols. Data were acquired on a facscanto II (BD Biosciences) and analyzed using FCAP array software (BD Biosciences).
Statistical analysis
Statistical significance was assessed by two‐tailed paired or unpaired Student’s t‐test or one‐way analysis of variance with Bonferroni multiple comparisons t‐test as required. All analyses were performed using graphpad prism software (GraphPad, San Diego, CA). Significant differences were considered when P < 0·05.
Results
Patients with moderate‐to‐severe psoriasis had increased IL‐33 expression
Interleukin‐33 is highly expressed in individuals with psoriasis. However, it remains unclear whether its expression is upregulated in Chinese patients with psoriasis. Therefore, we first detected IL‐33 expression in the lesional skin and serum of patients with moderate‐to‐severe plaque psoriasis and in people without psoriasis, i.e. the controls. Consistent with previous studies, IL‐33 and IL‐17A were highly expressed in the lesional skin of the patients at mRNA level (Fig. 1a,b). The mRNA expression of IL‐33 was positively correlated with that of IL‐17A (Fig. 1c). The results of immunohistochemistry staining showed that the expression of IL‐33 was also increased at protein level (Fig. 1d,e). Furthermore, IL‐33 was expressed not only in the epidermis (Fig. 1d), but also in the dermis (Fig. 1e), indicating that it was expressed by not only keratinocytes, but also other cells in the patients. The patients also had increased serum IL‐33 levels (Fig. 1f). We also found that although IL‐17A mRNA level was dramatically decreased after successful anti‐TNF therapy, IL‐33 mRNA expression was not altered (Fig. 1g), while the serum level of IL‐33 tended to be increased after therapy (Fig. 1h). Taken together, IL‐33 is highly expressed in Chinese patients with moderate‐to‐severe plaque psoriasis, suggesting its role in the pathogenesis of psoriasis.
Figure 1.
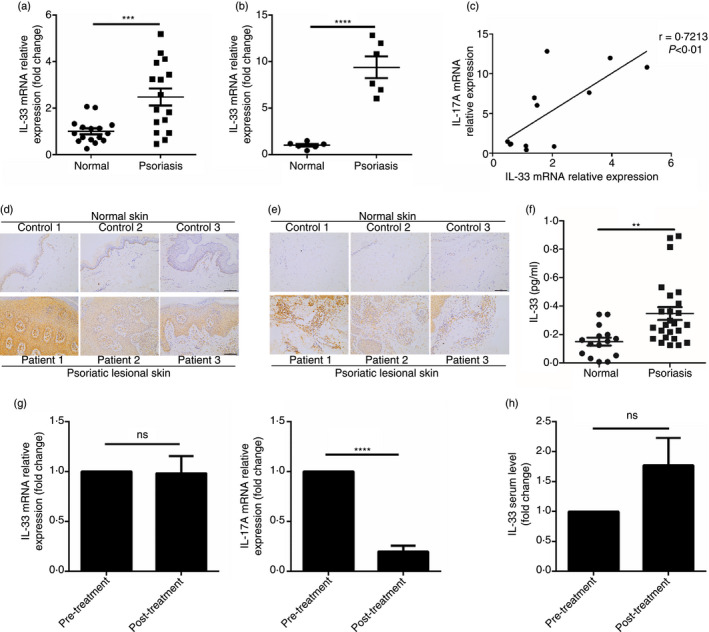
Interleukin‐33 (IL‐33) expression is markedly increased in individuals with moderate‐to‐severe psoriasis. (a) mRNA expression of IL‐33 in the skin of normal people (n = 16) and lesional skin of psoriasis patients (n = 16). (b) mRNA expression of IL‐17A in the skin of normal people (n = 6) and lesional skin of psoriasis patients (n = 6). (c) The correlation of the mRNA expression of IL‐33 and IL‐17A in the skin (n = 12). (d,e) Immunohistochemical staining of IL‐33 in the epidermis (d) and dermis (e) of skin of normal people (n = 10) and lesional skin of psoriasis patients (n = 10). (f) Serum level of IL‐33 of normal people (n = 15) and psoriasis patients (n = 25). (g) mRNA expression of IL‐33 and IL‐17A in the lesional of psoriasis patients before and after anti‐tumor necrosis factor‐α (TNF‐α) therapy (n = 5). (h) Serum level of IL‐33 of psoriasis patients before and after anti‐TNF‐α therapy (n = 10). Data show mean + SEM or mean ± SEM. P‐values were determined by unpaired Student’s t‐test. Correlation of the mRNA expression of IL‐33 and IL‐17A was determined by Pearson coefficient. **P < 0·01, ***P < 0·001 and ****P < 0·0001
IL‐33 inhibited IL‐17A expression while promoting IL‐4 production; it had no effect on interferon‐γ and FOXP3 expression in patients’ CD4+ T cells
Given that Th17 cells are the major source of IL‐17A in individuals with psoriasis, we investigated the effect of IL‐33 on CD4+ T cells, particularly Th17 cells. CD4+ T cells were isolated from patients’ PBMCs using magnetic beads, and then treated with or without IL‐33. After IL‐33 treatment, the proportion of IL‐17A+ cells was decreased, as was the geometric mean fluorescence intensity of IL‐17A (Fig. 2a,b), indicating that IL‐33 can inhibit Th17 cell differentiation and function. In contrast, interferon‐γ (IFN‐γ) expression was not altered, nor was its geometric mean fluorescence intensity, after IL‐33 treatment (Fig. 2a,b). Intriguingly, the proportion of IL‐17A+ IFN‐γ + cells was reduced in the IL‐33 treatment group as well (Fig. 2a,b), suggesting that IL‐33 can further inhibit the formation of pathogenic Th17 cells. Nevertheless, the role of CD4+ IL‐17A+ IFN‐γ + T cells in psoriasis remains unknown, but appears more important than that of IL‐17A single‐positive CD4+ T cells in other autoimmune diseases. 18 , 19 , 20 We also found an increased proportion of IL‐17A+ IFN‐γ + cells in the CD4+ T cells of the patients (data not shown). Given that IL‐33 is a Th2‐related cytokine, we tested the IL‐4 levels in the supernatant of cultured CD4+ T cells, and found increased IL‐4 expression in the supernatant of IL‐33‐treated cells (Fig. 2c). As it was reported that IL‐33 promoted IL‐10 production in Th17 cells, 21 we investigated whether IL‐33 could also induce IL‐10 production in Th17 cells from the patients. Inconsistent with that previous study, IL‐33 did not enhance IL‐10 production in Th17 cells (Fig. 2d). Considering that IL‐33 can promote Treg cell differentiation and proliferation, we investigated whether the proportion of Treg cells would increase after IL‐33 treatment. Inconsistent with previous studies, IL‐33 did not promote Treg cell differentiation and proliferation in the CD4+ T cells isolated from the patients (Fig. 2f). We hypothesize that this may be because of the inactivation of Treg cells in psoriasis or the indirect effect of IL‐33 on Treg cells. 22 Finally, because iNKT cells can inhibit Th17 cell differentiation and IL‐33 can promote iNKT cell proliferation, 23 we investigated whether IL‐33 could also enhance αGalCer‐induced iNKT cell proliferation. Consistent with that previous study, the proportion of iNKT cells was increased after IL‐33 treatment (Fig. 2f). Taken together, these data demonstrate that IL‐33 can inhibit Th17 cell differentiation and function in patients with psoriasis, therefore IL‐33 may play an anti‐inflammatory role in the pathogenesis of psoriasis.
Figure 2.
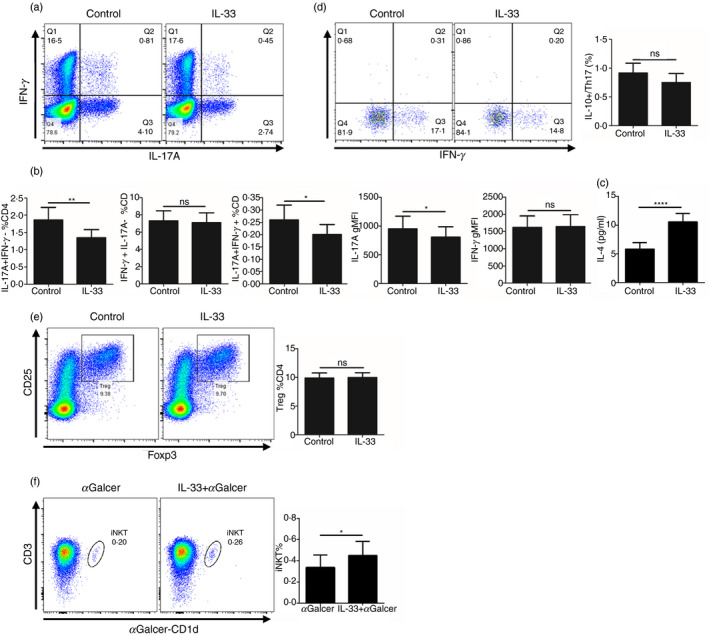
Interleukin‐33 (IL‐33) suppresses T helper type 17 (Th17) cells in individuals with moderate‐to‐severe psoriasis. (a,b) The proportion of IL‐17A+ IFN‐γ –, IL‐17A+ IFN‐γ + and IL‐17A– IFN‐γ + cells and the geometric mean fluorescence intensity (gMFI) of IL‐17A and interferon‐γ (IFN‐γ) in CD4+ T cells from psoriasis patients (n = 17) treated with or without IL‐33 (50 ng/ml) for 72 hr. (c) The supernatant level of IL‐4 in CD4+ T cells from psoriasis patients (n = 10) treated with or without IL‐33 (50 ng/ml) for 72 hr. (d) The proportion of IL‐10+ and IFN‐γ + cells in Th17 (CD4+ and IL‐17A+) cells from psoriasis patients (n = 10) treated with or without IL‐33 (50 ng/ml) for 72 hr. (e) The proportion of regulatory T (Treg) (CD25+ and Foxp3+) cells in CD4+ T cells from psoriasis patients (n = 10) treated with or without IL‐33 (50 ng/ml) for 72 hr. (f) The proportion of invariant natural killer T (iNKT) (CD3+ and αGalCer‐CD1d+) cells in the peripheral blood mononuclear cells (PBMCs) from psoriasis patients (n = 10) treated with αGalCer and with or without IL‐33 (50 ng/ml) for 72 hr. Data show mean + SEM. P‐values were determined by paired Student’s t‐test. ns, no significance, *P < 0·05, **P < 0·01, ***P < 0.001 and ****P < 0.0001
IL‐33 alleviated psoriatic inflammation in IMQ‐treated mice
Given that IL‐33 could inhibit Th17 cell differentiation and promote iNKT cell proliferation in vitro, we hypothesized that IL‐33 plays an anti‐inflammatory role in the development of psoriasis in vivo. To confirm this, we investigated the effect of exogenous IL‐33 on the development of IMQ‐induced psoriatic inflammation. IMQ was applied topically to C57BL/6 mice for 6 days. Each mouse was injected subcutaneously with rIL‐33 (1 µg/300 µl) or PBS for 6 consecutive days (Fig. 3a). At day 7, IL‐33 treatment reduced skin inflammation and epidermal thickness markedly compared with the PBS control treatment (Fig. 3b,c). Hematoxylin & eosin staining confirmed the phenotype that was observed in the mice (Fig. 3d,e). Taken together, these data demonstrate that exogenous IL‐33 can attenuate the development of IMQ‐induced psoriatic inflammation in mice.
Figure 3.
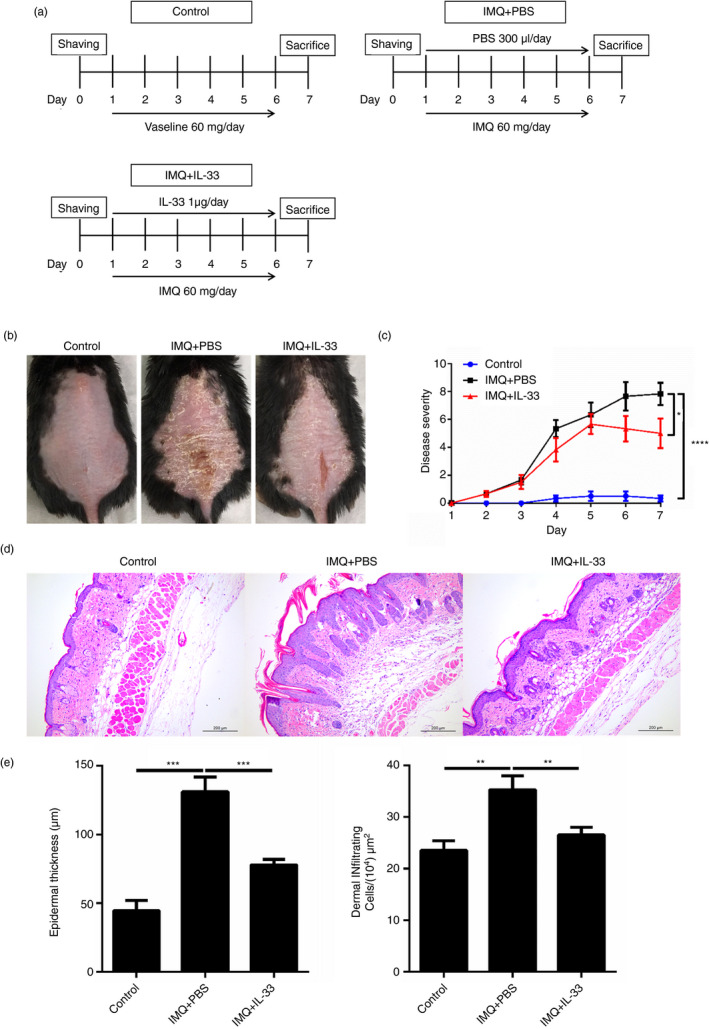
Subcutaneous injection of interleukin‐33 (IL‐33) alleviates imiquimod (IMQ) ‐induced murine psoriatic inflammation. (a) Schematic representation of the plan with the injection of PBS or IL‐33 on mice treated with IMQ. (b) Representative photos of the lesional skin of mice from each group. (c) The disease severity scoring of the mice from each group based on scaling, erythema, and skin thickness from day 1 to day 7. (d) Representative hematoxylin & eosin staining of skin sections of mice from each group on day 7 (bar = 200 um). (e) Epidermal thickness and infiltrating inflammatory cells of the skin sections of mice from each group on day 7. Each group contains six mice and all experiments were repeated at least twice. Data show mean + SEM. P‐values were determined by one‐way analysis of variane with Bonferroni multiple‐comparisons t‐test. *P < 0·05, **P < 0·01 and ***P < 0·001
IL‐33 altered cytokine production and cellular phenotype in the IMQ‐induced psoriasis mouse model
To investigate the immunological mechanisms by which IL‐33 alleviated IMQ‐induced mouse psoriatic inflammation, we collected mouse skin‐draining lymph node and skin samples to test the cytokine levels and immune cell phenotypes. Consistent with the decreased skin inflammation, the levels of two key pro‐inflammatory cytokines, i.e. TNF‐α and IL‐23, were significantly reduced in the lesional skin of IL‐33‐treated mice (Fig. 4a). Furthermore, serum TNF‐α levels were decreased in the IL‐33‐treated mice (Fig. 4b). Nevertheless, we could not detect IL‐17A from all mouse skin samples and serum (data not shown). Interleukin‐10 could not be detected in any mice of the IMQ + PBS group and although IL‐10 levels were increased in the IL‐33‐treated mice, there was no significant difference (Fig. 4a). We also examined the cellular phenotypes. Single‐cell suspensions were prepared from the skin‐draining lymph nodes and stained for cell surface and intracellular markers by flow cytometry. IL‐17+ cells were decreased in the CD4+ T cells from the IL‐33‐treated mice, whereas no difference was found in the γδT cells, which were thought to be the main source of IL‐17A in the psoriasis mouse model (Fig. 4c,d). In contrast to the in vitro study, FOXP3+ cells were increased in CD4+ T cells from the skin‐draining lymph nodes of IL‐33‐treated mice (Fig. 4c,d). Furthermore, the proportion of iNKT cells was significantly increased in the IL‐33‐treated mice (Fig. 4d). In addition, skin‐infiltrating T cells and epidermal proliferated keratinocytes were decreased in the IL‐33‐treated mice (Fig. 4e,f). Collectively, these results indicate that IL‐33 attenuates IMQ‐induced psoriatic inflammation by suppressing the Th17 response.
Figure 4.
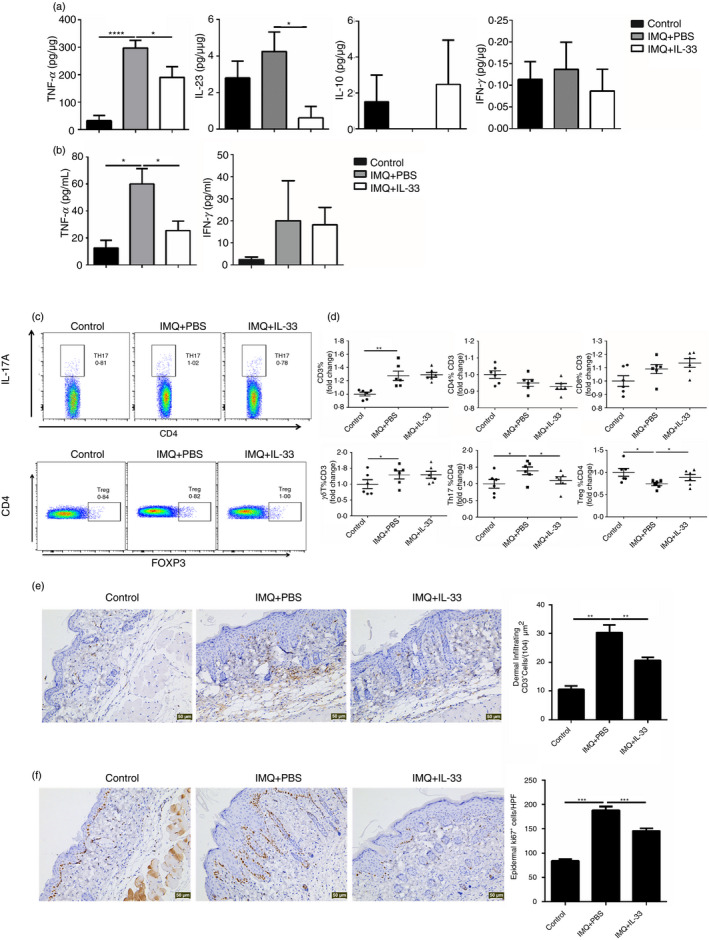
Interleukin‐33 (IL‐33) altered the expression of inflammatory cytokines and T‐cell subpopulations of skin‐draining lymph nodes in imiquimod (IMQ) ‐treated mice. (a) The protein level of tumor necrosis factor‐α (TNF‐α), IL‐23, IL‐10 and interferon‐γ (IFN‐γ) of the skin of mice from each group. (b) The serum level of TNF‐α and IFN‐γ of the mice from each group. (c,d) The proportion of T‐cell subpopulations in the skin‐draining lymph nodes of mice from each group. (e) Immunohistochemical staining of CD3 in the skin of mice from each group. (f) Immunohistochemical staining of Ki67 in the skin of mice from each group. Each group contains six mice and all experiments were repeated at least twice. Data show mean + SEM. P‐values were determined by one‐way analysis of variance with Bonferroni multiple‐comparisons t‐test. *P < 0·05, **P < 0·01, ***P < 0·001 and ****P < 0·0001
Discussion
In the present study, IL‐33 was highly expressed in both the lesional skin and the serum of patients with psoriasis, which is consistent with previous studies. 12 , 24 , 25 It has long been suspected that IL‐33 participates in the pathogenesis of psoriasis, given the increased IL‐33 expression in patients and the effect of IL‐33 on various immune cells. 12 , 24 , 25 However, the possible role of IL‐33 in psoriasis remains unclear.
The Th17 cells are some of the most important cells that participate in the pathogenesis of psoriasis. 26 They can secrete various pro‐inflammatory cytokines, such as IL‐17A and IL‐22, to promote the inflammatory responses, particularly in psoriasis. 26 Biological agents targeting the upstream and downstream cytokines (e.g. IL‐23 and IL‐17A) of Th17 cells have achieved great success for treating psoriasis, further proving the vital role of Th17 cells in psoriasis. 27 , 28 , 29 , 30 , 31 Therefore, we investigated the effect of IL‐33 on the Th17 cells of patients with psoriasis. Intriguingly, our results revealed a suppressive role of IL‐33 in Th17 cell differentiation and function, indicating that IL‐33 might also play an anti‐inflammatory role in psoriasis.
Imbalance of Th17 and Treg cells is present in many autoimmune diseases, including psoriasis. 32 Treg cells perform their suppressive function both by cell‐contact mechanisms that involve specific cell‐surface receptors and by secreting inhibitory cytokines such as IL‐10 and transforming growth factor‐β. 33 Interleukin‐33 can enhance Treg cell differentiation and proliferation, 34 , 35 , 36 in contrast, our in vitro study showed that IL‐33 had no effect on the Treg cells of individuals with psoriasis. One possible explanation is the Treg cell dysfunction in psoriasis, and another explanation is that the previous studies were in vivo studies, suggesting that IL‐33 may act indirectly on Treg cells.
Other than Th17 cells and Treg cells, other immune cells are involved in the pathogenesis of psoriasis. Invariant NKT cells are a subpopulation of T cells with both T cell and natural killer cell characteristics. The expression levels of IFN‐γ and CCR5 in iNKT cells of psoriatic lesional skin have been correlated positively with the progression of psoriasis, 37 whereas others have shown that iNKT cells can suppress Th17 cell differentiation, 38 suggesting an anti‐inflammatory role of iNKT cells in psoriasis. As IL‐33 can enhance iNKT cell proliferation, 23 we wanted to explore whether this was also the case in psoriasis. Consistent with that previous study, IL‐33 increased the proportion of iNKT cells in PBMCs, further suggesting the anti‐inflammatory role of IL‐33 in psoriasis, although further studies are needed.
Herein, we investigated the effect of IL‐33 on a psoriatic mouse model. Given that IMQ‐induced psoriatic inflammation was not altered in IL‐33‐deficient mice, 16 we wanted to know whether exogenous IL‐33 treatment would have an effect on this model. Although it has been indicated that subcutaneous injection of low‐concentrations of IL‐33 aggravates IMQ‐induced mouse psoriatic inflammation, 17 and IL‐33 is considered a pro‐inflammatory cytokine in psoriasis based on its enhancement of pro‐inflammatory cytokine expression in mast cells 8 or promotion of keratinocyte proliferation, 39 our results indicate that treatment with high concentrations of IL‐33 markedly decreased disease severity in IMQ‐induced mouse psoriasis. Moreover, the cytokine profile of the lesional skin confirmed the observation of the phenotype. This finding proves our in vitro results. As to why psoriatic inflammation was not altered in the IL‐33‐deficient mice, 16 we believe that IL‐33 balances its anti‐inflammatory and pro‐inflammatory roles during the pathogenesis of psoriasis (Fig. 5).
Figure 5.
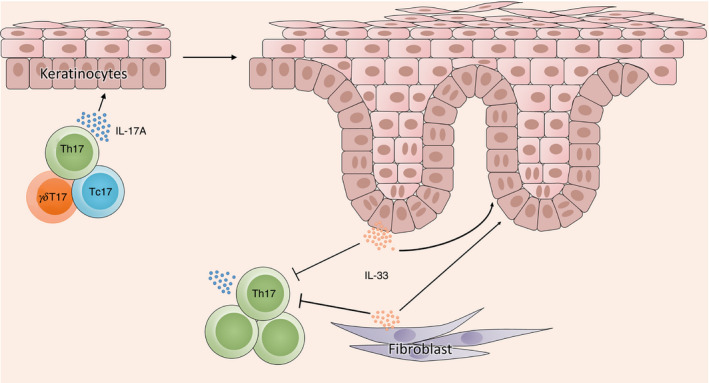
Summary of interleukin‐33 (IL‐33) actions in psoriatic inflammation. IL‐17‐producing T cells [T helper type 17 (Th17), IL‐17‐producing γδT (γδT17) and IL‐17‐producing CD8+ T (Tc17) cells] produce and secrete large amounts of IL‐17A under the inflammatory microenvironment of psoriasis. Then IL‐17A acts on keratinocytes to promote the IL‐33 expression. On the one hand, the increased IL‐33 from keratinocytes or other cells in the dermis, such as fibroblasts, can promote the proliferation of keratinocytes to aggravate the psoriatic inflammation, but on the other hand, inhibit the differentiation and function of Th17 cells to remit the psoriatic inflammation
In conclusion, our data suggest that IL‐33 also participates in the pathogenesis of psoriasis in an anti‐inflammatory role through its suppressive effect on Th17 cells. Our results suggest that IL‐33 may be a potential therapeutic target in psoriasis.
Disclosure
The authors declare no commercial or financial conflict of interest.
Acknowledgments
Zeyu Chen, Yifan Hu, Xilin Zhang, Lian Cui, Rongfen Chen, Yingyuan Yu, Yu Gong, Qian Yu, Youdong Chen, Hongyue Diao, Jia Chen, Yuanyuan Wang and Zengyang Yu conducted the experiments. Zeyu Chen and Yifan Hu analyzed the results. Yuling Shi planned the study and evaluated the results. Zeyu Chen wrote the paper. Yuling Shi edited the paper. This work was supported by grants from the National Natural Science Foundation of China (No. 81673050, 81872522), the Innovation Program of Shanghai Municipal Education Commission (No. 2019‐01‐07‐00‐07‐E00046), the Program of Science and Technology Commission of Shanghai Municipality (No. 18140901800), the Excellent Subject Leader Program of Shanghai Municipal Commission of Health and Family Planning (No. 2018BR30), and the Clinical Research Program of Shanghai Hospital Development Center (No. SHDC12018X06).
References
- 1. Srivastava A, Nikamo P, Lohcharoenkal W, Li D, Meisgen F, Xu Landen N et al MicroRNA‐146a suppresses IL‐17‐mediated skin inflammation and is genetically associated with psoriasis. J Allergy Clin Immunol 2017; 139:550–61. [DOI] [PubMed] [Google Scholar]
- 2. Ding X, Wang T, Shen Y, Wang X, Zhou C, Tian S et al Prevalence of psoriasis in China: a population‐based study in six cities. Euro J Dermatol 2012; 22:663–7. [DOI] [PubMed] [Google Scholar]
- 3. Hirahara K, Nakayama T. CD4+ T‐cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int Immunol 2016; 28:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campanati A, Molinelli E, Brisigotti V, Offidani A. Biologic therapy in psoriasis (Part I): efficacy and safety of tumor necrosis factor‐α inhibitors. Curr Pharm Biotechnol 2017; 18:945–63. [DOI] [PubMed] [Google Scholar]
- 5. Molinelli E, Campanati A, Brisigotti V, Offidani A. Biologic therapy in psoriasis (Part II): efficacy and safety of new treatment targeting IL23/IL‐17 pathways. Curr Pharm Biotechnol 2017; 18:964–78. [DOI] [PubMed] [Google Scholar]
- 6. Cayrol C, Girard JP. Interleukin‐33 (IL‐33): a nuclear cytokine from the IL‐1 family. Immunol Rev 2018; 281:154–68. [DOI] [PubMed] [Google Scholar]
- 7. Liew FY, Girard JP, Turnquist HR. Interleukin‐33 in health and disease. Nat Rev Immunol 2016; 16:676–89. [DOI] [PubMed] [Google Scholar]
- 8. Theoharides TC, Zhang B, Kempuraj D, Tagen M, Vasiadi M, Angelidou A et al IL‐33 augments substance P‐induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc Natl Acad Sci USA 2010; 107:4448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hueber AJ, Alves‐Filho JC, Asquith DL, Michels C, Millar NL, Reilly JH et al IL‐33 induces skin inflammation with mast cell and neutrophil activation. Eur J Immunol 2011; 41:2229–37. [DOI] [PubMed] [Google Scholar]
- 10. Balato A, Di Caprio R, Canta L, Mattii M, Lembo S, Raimondo A et al IL‐33 is regulated by TNF‐α in normal and psoriatic skin. Arch Dermatol Res 2014; 306:299–304. [DOI] [PubMed] [Google Scholar]
- 11. Sehat M, Talaei R, Dadgostar E, Nikoueinejad H, Akbari H. Evaluating serum levels of IL‐33, IL‐36, IL‐37 and gene expression of IL‐37 in patients with psoriasis vulgaris. Iran J All Asthma Immunol 2018; 17:179–87. [PubMed] [Google Scholar]
- 12. Mitsui A, Tada Y, Takahashi T, Shibata S, Kamata M, Miyagaki T et al Serum IL‐33 levels are increased in patients with psoriasis. Clin Exp Dermatol 2016; 41:183–9. [DOI] [PubMed] [Google Scholar]
- 13. Meephansan J, Subpayasarn U, Ponnikorn S, Chakkavittumrong P, Juntongjin P, Komine M et al Methotrexate, but not narrowband ultraviolet B radiation, suppresses interleukin‐33 mRNA levels in psoriatic plaques and protein levels in serum of patients with psoriasis. J Dermatol 2018; 45:322–5. [DOI] [PubMed] [Google Scholar]
- 14. Suttle MM, Nilsson G, Snellman E, Harvima IT. Experimentally induced psoriatic lesion associates with interleukin (IL)‐6 in mast cells and appearance of dermal cells expressing IL‐33 and IL‐6 receptor. Clin Exp Immunol 2012; 169:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen J, Shang Q, Wong CK, Li EK, Kun EW, Cheng IT et al Carotid plaque and bone density and microarchitecture in psoriatic arthritis: the correlation with soluble ST2. Sci Rep 2016; 6:32116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Athari SK, Poirier E, Biton J, Semerano L, Herve R, Raffaillac A et al Collagen‐induced arthritis and imiquimod‐induced psoriasis develop independently of interleukin‐33. Arthritis Res Ther 2016; 18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duan Y, Dong Y, Hu H, Wang Q, Guo S, Fu D et al IL‐33 contributes to disease severity in psoriasis‐like models of mouse. Cytokine 2019; 119:159–67. [DOI] [PubMed] [Google Scholar]
- 18. Duhen R, Glatigny S, Arbelaez CA, Blair TC, Oukka M, Bettelli E. Cutting edge: the pathogenicity of IFN‐γ‐producing Th17 cells is independent of T‐bet. J Immunol 2013; 190:4478–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Awasthi A, Riol‐Blanco L, Jager A, Korn T, Pot C, Galileos G et al Cutting edge: IL‐23 receptor gfp reporter mice reveal distinct populations of IL‐17‐producing cells. J Immunol 2009; 182:5904–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jain R, Chen Y, Kanno Y, Joyce‐Shaikh B, Vahedi G, Hirahara K et al Interleukin‐23‐induced transcription factor blimp‐1 promotes pathogenicity of T helper 17 cells. Immunity 2016; 44:131–42. [DOI] [PubMed] [Google Scholar]
- 21. Pascual‐Reguant A, Bayat Sarmadi J, Baumann C, Noster R, Cirera‐Salinas D, Curato C et al TH17 cells express ST2 and are controlled by the alarmin IL‐33 in the small intestine. Mucosal Immunol 2017; 10:1431–42. [DOI] [PubMed] [Google Scholar]
- 22. Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR et al Dysfunctional blood and target tissue CD4+ CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol 2005; 174:164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S et al The pro‐Th2 cytokine IL‐33 directly interacts with invariant NKT and NK cells to induce IFN‐γ production. Eur J Immunol 2009; 39:1046–55. [DOI] [PubMed] [Google Scholar]
- 24. Meephansan J, Komine M, Tsuda H, Karakawa M, Tominaga S, Ohtsuki M. Expression of IL‐33 in the epidermis: the mechanism of induction by IL‐17. J Dermatol Sci 2013; 71:107–14. [DOI] [PubMed] [Google Scholar]
- 25. Balato A, Lembo S, Mattii M, Schiattarella M, Marino R, De Paulis A et al IL‐33 is secreted by psoriatic keratinocytes and induces pro‐inflammatory cytokines via keratinocyte and mast cell activation. Exp Dermatol 2012; 21:892–4. [DOI] [PubMed] [Google Scholar]
- 26. Dainichi T, Kitoh A, Otsuka A, Nakajima S, Nomura T, Kaplan DH et al The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat Immunol 2018; 19:1286–98. [DOI] [PubMed] [Google Scholar]
- 27. Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K et al Secukinumab in plaque psoriasis – results of two phase 3 trials. N Engl J Med 2014; 371:326–38. [DOI] [PubMed] [Google Scholar]
- 28. Georgakopoulos JR, Ighani A, Zhou LL, Yeung J. Efficacy and safety of secukinumab in treating moderate to severe plaque psoriasis in two real‐world Canadian dermatology clinics: a multicenter retrospective study. JEADV 2018; 32:e32–e4. [DOI] [PubMed] [Google Scholar]
- 29. Momose M, Asahina A, Umezawa Y, Nakagawa H. Long‐term clinical efficacy and safety of secukinumab for Japanese patients with psoriasis: a single‐center experience. J Dermatol 2018; 45:318–21. [DOI] [PubMed] [Google Scholar]
- 30. Cui L, Chen R, Subedi S, Yu Q, Gong Y, Chen Z et al Efficacy and safety of biologics targeting IL‐17 and IL‐23 in the treatment of moderate‐to‐severe plaque psoriasis: a systematic review and meta‐analysis of randomized controlled trials. Int Immunopharmacol 2018; 62:46–58. [DOI] [PubMed] [Google Scholar]
- 31. Chen Z, Gong Y, Shi Y. Novel biologic agents targeting interleukin‐23 and interleukin‐17 for moderate‐to‐severe psoriasis. Clin Drug Invest 2017; 37:891–9. [DOI] [PubMed] [Google Scholar]
- 32. Ma L, Xue H, Gao T, Gao M, Zhang Y. Notch1 signaling regulates the Th17/Treg immune imbalance in patients with psoriasis vulgaris. Mediators Inflamm 2018; 2018:3069521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dominguez‐Villar M, Hafler DA. Regulatory T cells in autoimmune disease. Nat Immunol 2018; 19:665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bruhs A, Proksch E, Schwarz T, Schwarz A. Disruption of the epidermal barrier induces regulatory T cells via IL‐33 in mice. J Invest Dermatol 2018; 138:570–9. [DOI] [PubMed] [Google Scholar]
- 35. Peine M, Marek RM, Lohning M. IL‐33 in T cell differentiation, function, and immune homeostasis. Trends Immunol 2016; 37:321–33. [DOI] [PubMed] [Google Scholar]
- 36. Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA et al The alarmin IL‐33 promotes regulatory T‐cell function in the intestine. Nature 2014; 513:564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kono F, Honda T, Aini W, Manabe T, Haga H, Tsuruyama T. Interferon‐γ/CCR5 expression in invariant natural killer T cells and CCL5 expression in capillary veins of dermal papillae correlate with development of psoriasis vulgaris. Br J Dermatol 2014; 170:1048–55. [DOI] [PubMed] [Google Scholar]
- 38. Oh K, Byoun OJ, Ham DI, Kim YS, Lee DS. Invariant NKT cells regulate experimental autoimmune uveitis through inhibition of Th17 differentiation. Eur J Immunol 2011; 41:392–402. [DOI] [PubMed] [Google Scholar]
- 39. Wu Y, Quan Y, Liu Y, Liu K, Li H, Jiang Z et al Hyperglycaemia inhibits REG3A expression to exacerbate TLR3‐mediated skin inflammation in diabetes. Nat Commun 2016; 7:13393. [DOI] [PMC free article] [PubMed] [Google Scholar]


