Abstract
The present is a comprehensive review of the immunopathology of Covid-19. The immune reaction to SARS-CoV-2 infection is characterized by differentiation and proliferation of a variety of immune cells with immune mediator production and release, and activation of other pathogen resistance mechanisms. We fully address the humoral and cellular immune changes induced by the virus, with particular emphasis on the role of the “cytokine storm” in the evolution of the disease. Moreover, we also propose some immune alterations (i.e., inflammatory parameters, cytokines, leukocytes and lymphocyte subpopulations) as prognostic markers of the disease. Furthermore, we discuss how immune modifying drugs, such as tocilizumab, chloroquine, glucocorticoids and immunoglobulins, and blood purification therapy, can constitute a fundamental moment in the therapy of the infection. Finally, we made a critical analysis of a number of substances, not yet utilized, but potentially useful in SARS-CoV-2 patients, such as IFN lambda, TNF blockers, ulinastatin, siponimod, tacrolimus, mesenchymal stem cells, inhibitors of mononuclear macrophage recruitment, IL-1 family antagonists, JAK-2 or STAT-3 inhibitors.
Keywords: SARS-CoV-2, immune response, cytokine storm, IL-6, prognostic factor, T lymphocyte
1. Introduction
In December 2019, an epidemic provoked by Coronavirus disease 2019 (COVID-19) arose in Wuhan, Hubei Province, China. As of June 20, 8,700,000 COVID-19 cases were reported worldwide. More than 450,000 patients died from infection with this new virus called Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).
SARS-CoV-2 belongs to the Coronaviridae family and is correlated to the subgenus Sarbecovirus. This is an enveloped virus comprising a single-stranded positive sense RNA viral genome. Virions are spherical, with the spiked glycoprotein inserted in the envelope [1]. In other viruses of the same family, this protein has been demonstrated to connect to host cellular receptors and to facilitate membrane fusion [2].
After entering the lungs by respiration, SARS-CoV-2- stimulates the activity of immune cells, increases cytokine production, and actives other pathogen resistance mechanisms.
Viral RNAs are identified by the innate immune system via three groups of pattern recognition receptors: RIG-I-like receptors (RLRs), NOD-like receptors (NLRs), and Toll-like receptors (TLRs), which stimulate the production of interferon (IFN) and trigger anti-viral effectors such as T CD8 + cells, Natural Killer (NK) cells, and macrophages [3,4].
Cytotoxic T lymphocytes (CTLs) are stimulated after identifying infected cells presenting the viral antigens as portions of surface antigen-MHC-I complexes. Efficacious presentation is determined by the correct harboring of antigens by MHC-I molecules through hydrogen bonds and salt–bridge relations that permit great affinity with higher specificity [5]. An immunoinformatic method was employed to recognize major CTL and B cell epitopes in the SARS-CoV-2 surface glycoprotein. The authors recognized five different CTL epitopes, three sequential B cell epitopes and five discontinuous B cell epitopes in the viral surface glycoprotein [6].
SARS-CoV and MERS-CoV infections are characterized by fast and robust initial virus replication with late IFN generation, resulting in disproportionate inflammatory host responses provoking grave lung alterations [7,8]. In the fight between the virus and the human body, the immunity of the subjects reduces, and the virus virulence augments [9]. This causes edema and congestion of the lung, thickening of the interstitial tissue, and augmented exudation in the alveolar space able to cause respiratory failure.
The purpose of this review is to analyze cellular and humoral immune changes induced by SARS-CoV-2 and to propose the possibility that such immune changes could be used as prognostic markers of the disease. Finally, we critically consider the various immuno-modifying drugs useful in the treatment of Covid-19 and underline how the immunotherapeutic approach is of fundamental importance for SARS-CoV-2 infection.
2. Immunopathology of SARS-CoV-2 Infection
2.1. Lymphocyte Subpopulations
Subsets of CD4+ T cells, CD8+ T cells, B cells, and NK cells play a central role in the functioning of the immune system. Several reports have studied the diverse lymphocyte populations in subjects with SARS-CoV-2 infection.
Lymphocyte populations were studied in 44 subjects on admission. The total amount of T cells, B cells and NK cells was significantly reduced in infected group as T cells and NK cells were below the normal range, while B cells were within the lower quantity of the reference values. T cells are the most altered by the viral infection, approximately half the lower reference limit. However, the function of CD4+, CD8+ T cells, and NK cells was within normal limits in this study, as suggested by PMA/Ionomycin-triggered IFN-γ positive cells in these three populations. Moreover, examining the various subsets of T cells, the authors evidenced that both helper (CD3+CD4+) and cytotoxic T cells (CD3+CD8+) were below the normal range in subjects with COVID-19, with the T helper/suppressor ratio (Th/Ts) within normal limits. Furthermore, subjects with SARS-CoV-2 infection showed lower numbers of regulatory T cells (Treg) (CD3+CD4+CD25+CD127low+), and this reduction was especially evident in critical patients. A decrease in naïve (CD45RA+CD3+CD4+CD25+ CD127low+) and induced (CD45RO+CD3+CD4+CD25+CD127low+) regulatory T cells was also evident in critical patients, although the data did not reach statistical significance in comparison to other groups of patients [10].
These data have been substantially confirmed by other studies showing lymphocyte numbers below the normal limit in most subjects. CD+, CD8+, NK T cells and B lymphocytes were all reduced in infected subjects. The presence of comorbidities significantly modified the quantity of CD8+ T cells [11].
Indeed, all studies already agree in showing that T cell subset alterations strongly correlate with inflammatory condition. In particular, the quantity of CD8+ T cells was negatively correlated, while the CD4+/CD8+ ratio positively correlated with C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and IL-6.
Infected subjects who responded positively to the treatment showed an increase in CD8 + T cells and B cells, while no relevant changes in any lymphocyte subset were reported in unresponsive patients. Thus, lymphocytes and their subsets, mainly CD8+ T cells, might be a possible marker for clinical efficacy of treatments for SARS-CoV-2 infection [12].
Specific studies were performed for the analysis of CD4 + T lymphocytes. It is worth knowing that the differentiation of naïve CD4+ T-cells into effector and memory population is one of the most essential aspects of T-cell-mediated immunity [13], and the equilibrium between the naïve and memory CD4+ T cells is essential for maintaining an effective immune response. Such balance is altered in COVID-19 critical patients with expansion of naïve CD4+ T-cell subset and reduced memory cell percentage further showing the severity of the immune system impairment in these subjects. SARS-CoV-2 RNA load and lymphocyte amount and CD4+ T and CD8+ T lymphocyte quantity were observed to be negatively correlated [13]. These results suggest that the decrease in lymphocytes and their subsets, directly affected by the viral load, was closely correlated to disease evolution [14].
As for other lymphocyte subsets, a peripheral flow cytometry study demonstrated an increase in the number of Th17 cells, which differentiate from Th0 cells mainly stimulated by IL-6 and IL-23 [14].
Differently from other researchers, Cossarizza et al. demonstrated that the infection affects not only the number of lymphocytes but also their functions. They evidenced significant differences in the generation of cytokines between CD8 + T cells of patients with infectious pneumonia and healthy donors matched for age and gender. Indeed, most of the CD8+ T cells of these subjects were capable of generating Granzyme B but not INF-γ or TNF-α [15]. Moreover, Diao et al. demonstrated that the surviving T cells appear functionally exhausted [9].
Interesting data to explain the ultimate mechanism by which viral infection causes a reduction in various cell populations, apart from the obvious antigenic stimulation, come from transcriptomic studies. Xiong et al. demonstrated that, in bronchoalveolar lavage fluid, numerous significantly altered genes are related to apoptosis and P53 signaling pathways, comprising GTSE1, RRM2, CTSL, CTSB, DDIT4, CCNB1, RRAS, CDK1. CTSD, STEAP3, BIRC5, TNFSF10, CTSZ, NTRK1, IGFBP3, CCNB2, and TP53I3. Remarkably, TP53, an essential gene in the process of programmed cell death, exhibits an increasing trend, suggesting that peripheral blood mononuclear cells’ decrease may be due to an increase in programmed cell death [16].
2.2. Immune Mediators in Patients with SARS-CoV-2 Infection
Cytokines are known to have a crucial role in the immunopathology of viral infection. A ready and well-organized innate immune response represents the first protection against viruses. On the contrary, an altered and disproportionate immune response may provoke a severe immune-mediated injury [8,17,18].
Elevated concentrations of IL-1β, IFN-γ, IP-10, monocyte chemoattractant protein 1 (MCP-1) and IL-17 have been reported in SARS-CoV-2 subjects. These pro-inflammatory cytokines may stimulate the T-helper type 1 (Th1) cell response [19,20]. Moreover, serum concentrations of IL-2R and IL-6 in these subjects are positively correlated with the severity of the disease [21]. Furthermore, TNF-alpha, granulocyte colony-stimulating factor, MCP-1, IP-10, and macrophage inflammatory protein-1α were higher in patients in the intensive care unit (ICU) compared to infected subjects from general wards, confirming that cytokine generation is positively correlated with disease gravity [19].
Yang et al. studied clinically moderate and severe Covid-19 patients; they performed a multiplex screen for several cytokines and interrelated these data with viral loads and clinical features. They displayed a relevant increase of 14 cytokines in SARS-CoV-2-infected patients compared to healthy controls. Moreover, augmented concentrations of three of these proteins (IL-1 receptor antagonist, CCL7 and CXCL10) were positively correlated with viral load, the extent of lung damage and fatal prognosis [22].
Quantitative and qualitative differences in cytokine production characterizing different immune responses may justify different outcomes in specific categories of COVID-19 patients. Interestingly, it is now recognized that children and pregnant women generally have a mild disease after SARS-CoV-2 infection if not a fully asymptomatic one [23]. These patients are characterized by an immune response skewed toward a Th2 profile (ruled by the T-helper Type 2 cells), with specific generation of related cytokines like IL-4 and IL-10, while, the generation of Th1 pro-inflammatory cytokines typically characterizes SARS-CoV-2 infection. Therefore, it could be of interest to analyze whether profiling immune cells for their capability to generate Th1 or Th2 cytokines could be beneficial for the management of SARS-CoV-2-infected patients.
Several studies have carefully analyzed IL-6 plasma concentrations during COVID-19, also for possible immediate therapeutic implications. Actually, whether increased IL-6 concentrations are disadvantageous or favorable in infected patients is still unclear. Indeed, in experimental models, IL-6 can either reduce or increase viral proliferation [24]. However, most studies tend to consider increased IL-6 as a negative factor in patients with SARS-CoV-2 pneumonia. IL-6 can inhibit CD8+ cytotoxic T-cells by reducing their production of IFN-γ. Moreover, IL-6 can block the cell-mediated antiviral response during a cytokine storm by inhibiting specific cytokine signaling such as SOCS-3 and increasing PD-1 production [24].
IL-6 also plays an essential role in lung repair responses after viral injury, suggesting that the timing of administration of anti-IL6R could interfere in correct tissue remodeling. In human epithelial cells, SARS-CoV-2 causes a greater production of IL-6 compared to Influenza-A virus and human parainfluenza virus type 2, but with a significantly lower production of SOC3, indicating a possible basis for disproportionate IL-6 responses to this family of viruses [25].
2.3. Cytokine Storm
Most critically ill and deceased patients did not show serious clinical symptoms in the early stages of the infection. Most patients only displayed cough, mild fever, or muscle ache. Clinical conditions of these subjects worsened unexpectedly in the later stages of the disease. Acute respiratory distress syndrome (ARDS) and multiple-organ failure occurred precipitously, resulting in death in a short time. It has been speculated that when the body is unable to perform an adequate adaptive immune response against the infection, an innate relentless inflammation can then cause a cytokine storm with ARDS and organ failure [26].
Hence, cytokine storm has a critical role in the process of disease worsening. Therefore, controlling the cytokine storm is an essential method of avoiding the worsening of infected subjects and saving their lives [27,28,29].
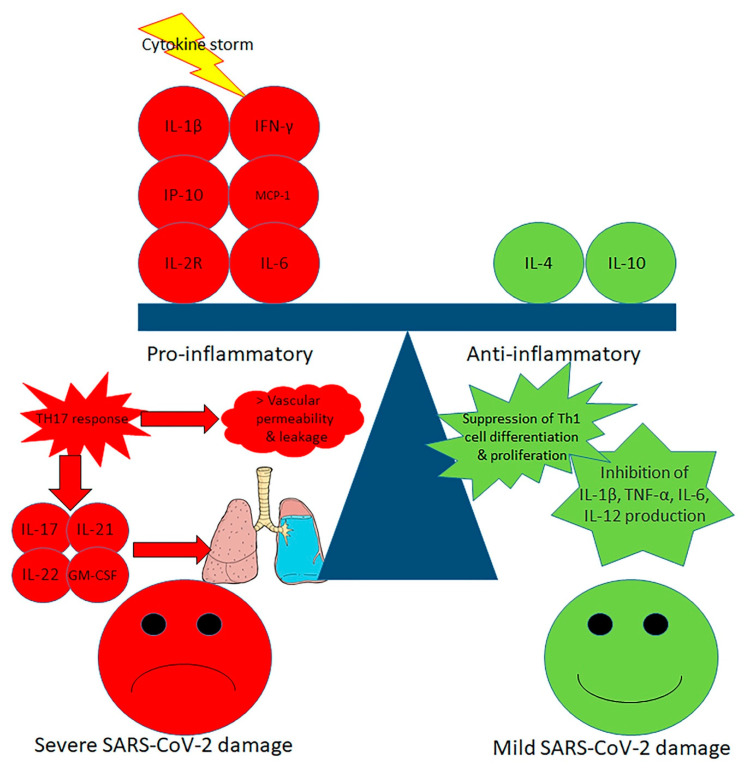
Apart from the increase in previously described cytokines, studies of particular interest are related to the cytokines implicated in Th17 responses. IL -1β and TNF-α both increase Th17 responses and vascular permeability and leakage. Th17 cells themselves generate IL-17, IL-21, IL-22 and GM-CSF. IL-17 exerts pro-inflammatory actions by promoting the production of inflammatory cytokines such as IL-1β, IL-6, G-CSF, TNF-α, and chemokines such as IL-8, IP10, KC, MIP2A, MIP3A capable of recruiting more immune cells, and matrix metalloproteinases that contribute to tissue injury and remodeling. IL-17 and TNF-α are able to increase the production of mucins, serum amyloid A, fibrinogen, and anti-apoptotic proteins [30]. The reason for the very high expression of IL-22 in infected 16HBE cells remains unclear. This interleukin has essential functions in tissue repair and immune regulation. Determining whether this high level of expression might explain why virus-infected cells maintain their structure and morphology or whether it might be part of the viral strategy to maintain its transmission ability needs further study [31,32].
Xu et al. reported that peripheral blood of a subject with grave SARS-CoV-2 infection had an extremely high amount of CCR6 Th17 cells, further sustaining a Th17-type cytokine storm in this infection [33]. Increased Th17 responses were also described in MERS-CoV and SARS-CoV-infected subjects, and in these diseases greater IL-17 concentration with lower IFNγ and IFNα favors worse prognosis [34]. H1N1 influenza virus also caused important Th17 and Th1 responses [35]. In an in vivo experimental model, H1N1 induced an acute IL-17-dependent lung injury [36,37]. Acting on the Th17 pathway may be a successful therapeutic strategy in the SARS-CoV-2-infected subjects with Th17-dominant immune profiles (Figure 1).
Figure 1.
Mechanisms of cytokine storm in severe SARS-CoV-2 infection. In severe Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), a pro-inflammatory cascade is prevalent, with T-helper type 1 (Th1) and T-helper type 17 (Th17) cytokine upregulation leading to increased vascular permeability and leakage with severe lung damage. In mild SARS-CoV-2, an anti-inflammatory behavior is prevalent, featuring the suppression of Th1 cell differentiation and proliferation with inhibition of IL-1β, TNF-α, IL-6 and IL-12 production.
Cytokine storm and in particular the huge local production of cytokines is the key element that determines the intensity of symptoms, the mortality rate and the onset of extrapulmonary involvement during SARS-CoV-2 infection [38,39,40]. Critically ill SARS-CoV-2-infected patients demonstrate features suggestive of a group of pathologies collected under the name of cytokine storm syndromes, in which multi-organ failure and hyperinflammation result from an exaggerated cytokine release from an uninhibited immune activation [41]. Comparative studies with these syndromes are stimulating and can offer useful pathogenetic information and effective therapeutic indications.
Actually, the cytokine storm of COVID-19 patients closely resembles the cytokine release syndrome (CRS), a widespread inflammatory condition, which can be induced by drugs and by infection [29,42,43] and is particularly frequent in the course of autoimmune diseases (i.e., juvenile idiopathic arthritis, adult-onset Still’s disease and systemic lupus erythematosus) and immune-related treatment, such as CAR-T cell therapy and organ transplantation [28,44]. Due to the action of pro-inflammatory proteins, vascular permeability augments and a great amount of fluid enters the alveoli, causing dyspnea and respiratory failure [45,46]. The therapeutic approaches used in the treatment of the various CRSs could be usefully evaluated for the cytokine storm of SARS-CoV-2 infection.
3. Immunological Alterations and Prognostic Factors
The possibility of estimating the evolutionary trend and the final outcome of the infection at an early stage of the disease and consequently starting an early and effective therapy for those who may progress into a more critical condition could reduce the mortality rate. Ascertaining trustworthy indicators of disease gravity and, above all, reliable markers of a possible negative progression is therefore essential.
It was demonstrated that the CD4+ T cell and CD8+ T cell amounts were strictly correlated to infection gravity and prognosis: the lower the amounts of T cell, CD4+ T cell, and CD8+ T cell at the time of hospitalization, the more negative the prognosis [11,13].
However, these findings were only partially confirmed by other groups reporting no significant variation of CD4+ T at the time of admission compared to before discharge, while CD8+ T cells significantly grew in patients with mild severity during hospitalization [47,48].
Other studies have tried to recognize suitable prognostic markers by assessing cytokine levels. High levels of IL-6 were found in severe subjects. Critical SARS-CoV-2 patients had significantly greater concentrations of Th1 cytokines (IL-6 and TNF-α) and greater percentages of ARDS, compared to less severe patients [49]. Significantly higher increases were reported for IL-6 and serum ferritin in non-survivors compared to survivors. These increases, along with the augment of CRP, could indicate the onset of a general inflammatory syndrome capable of causing an acute pulmonary damage that progresses to multiple organ failure [50].
Infected subjects with critical disease also have elevated IL-10 serum concentration. This could be a possible compensatory anti-inflammatory effect, in turn responsible for a greater number of secondary infections described in non-survivors [51].
Other parameters such as age, high body mass index, and the increase in transaminases, LDH, soluble IL-2 receptor, and D-Dimer [52,53,54,55] have been identified to be related to ICU admission or death. Severe SARS-CoV-2 infection is frequently complicated with coagulopathy and thrombo-embolic events [56]. Dehydration, multiple cardiovascular risk factors such as diabetes, obesity or hypertension, previous coronary artery disease, ischemic stroke, peripheral artery disease, and classical genetic thrombophilia, such as heterozygous Factor II and Factor V Leiden mutation, are frequent comorbidities in SARS-CoV-2-hospitalized subjects, which, along with the acute inflammatory condition and the protracted immobilization, possibly increase embolic risk. Some authors reported that severely ill COVID-19 patients with multisystem thrombosis and ischemic strokes were found to have antiphospholipid antibodies (aPLAb). However, aPLAb can transiently arise during acute infection, inflammation, or thrombosis. This is the reason for the 12-week interval recommended by ISTH guidelines for confirmatory laboratory testing to diagnose the antiphospholipid syndrome. Actually, there are only very limited data on aPLAb in COVID-19 and it is unclear if they are actually involved in any coagulopathies observed in COVID-19 or represent an epiphenomenon.
In a retrospective analysis, almost half of the patients with laboratory-confirmed SARS-CoV-2 infection had an increase in D-dimer, and the increase was more prominent in more serious patients. The authors suggest that D-dimer alteration can indicate the severity of the infection and an increased concentration is correlated with a poorer prognosis [57]. A different retrospective analysis performed in China demonstrated that prothrombin time (PT) and D-dimer concentrations were greater at the time of admittance of infected patients necessitating ICU assistance, whereas increased D-dimer concentrations were also connected with death in the multivariable analysis [58].
In the analysis performed by Tang et al., patients who died had substantively greater fibrin degradation products (FDP) concentrations, augmented PT and activated partial thromboplastin time (aPTT) at the time of admittance to hospital compared to survivors, with a reduction in fibrinogen and antithrombin (AT III) levels [59]. In a prospective study, D-dimer and FDP concentrations were significantly greater in patients with SARS-CoV-2 than in uninfected subjects, and patients with more serious disease had higher values than patients with mild symptoms [60].
In an ample meta-analysis, Henry et al. evaluated the prognostic value of hematologic or immunologic markers in subjects with different degrees of disease severity. Infected subjects with critical or fatal disease had elevated white blood cell (WBC) count, and low levels of lymphocytes and platelets compared to less serious infection and survivors. Markers of inflammation, and of cardiac, liver or kidney damage were also significantly higher in subjects with critical or fatal COVID-19 compared to mild disease [61].
Numerous reports have confirmed the number and type of leukocytes as appropriate prognostic factors. Indeed, the total WBC count positively correlated with the severity of the disease and the highest values were observed in subjects who died. The increase in WBCs was mainly caused by neutrophils, while a decreasing trend has been observed for lymphocytes, eosinophils and monocytes. Studies on SARS-CoV infection showed that lymphocytes are essential for virus clearance [62], therefore, the observed lymphopenia during COVID-19 infection is a negative prognostic factor, the survival of these patients being dependent on the capability to replace lymphocytes, which are destroyed by the virus [63]. As such, lymphocyte evaluation, specially CD4, may be useful as predictor of gravity and outcome, in fact, patients with more severe clinical illness, or patients who died, had significantly more profound CD4+ lymphopenia.
Mo et al. evaluated 155 SARS-CoV-2 subjects and confirmed that refractory subjects had a greater neutrophil count compared to the most severe patients [64]. Interestingly, some authors studied the clinical features of the SARS-CoV-2 reactivation showing that lymphopenia and neutrophilia are associated with this condition [65].
Neutrophil-to-Lymphocyte ratio (NLR) and Lymphocyte-to-C-reactive protein ratio (LCR) are also recognized inflammation indicators [66,67]. High NLR is a risk marker of mortality not only in infections but also in cancer, coronary syndrome, polymyositis and dermatomyositis [68,69,70,71].
The increased NLR is due to an increase in neutrophil count and a reduction in lymphocytes. Inflammation could be responsible for the increased production of neutrophils and might increase the programmed cell death of lymphocytes [72].
Multivariate analysis revealed that there was 8% greater risk in mortality for each unit increase in NLR. The NLR of subjects in the highest tertile had a 15.04-fold greater risk of death compared with subjects in the lowest tertile [73]. These data were confirmed by other authors [10,74].
A recent meta-analysis demonstrated that LCR might also be a useful marker of clinical severity in SARS-CoV-2 infection as LCR values were significantly decreased in serious SARS-CoV-2 patients [74].
A further prognostic factor for COVID-19 severity has been identified in the number of circulating platelets, which have an essential role in innate immunity and inflammatory response [75].
Platelets are generated by megakaryocytes in the bone marrow, and recent reports have demonstrated that several cytokines, comprising IL-3, IL-6, IL-9, IL-11, thrombopoietin (TPO), and Stem Cell Factor (SCF), can stimulate the generation of megakaryocytes. In an inflammatory condition, IL-6 can stimulate the production of megakaryocytes by increasing the production of TPO [76,77].
Platelets are present in an inactive form that can be quickly activated at the site of vascular damage, in response to proinflammatory cytokines or infection. Platelets activation is able to induce the release of other cytokines and chemokines leading to a perpetuation of inflammation [78].
Therefore, platelet count can be employed as a sensitive marker of infection and inflammation [79,80] and the platelet-to-lymphocyte ratio (PLR) indicates the intensity of systemic inflammation. Previous reports have shown that PLR is strictly correlated to cancer outcome and severity of diabetes and connective tissue diseases, and can be utilized as a potential inflammatory marker in patients with community-acquired pneumonia [81].
There are relevant connections between platelets and lymphocytes. In fact, platelet-released platelet factor-4 can inhibit agglutinin-A from inhibiting lymphocyte generation, and activated platelets increase lymphocyte adhesion to the endothelium, thus stimulating lymphocyte homing in endothelial veins and their passage to inflammatory sites. PLR mirrors both aggregation and inflammation and may be more suitable in predicting disease outcome than platelet or lymphocyte counts alone [82].
In any case, the use of biological parameters as prognostic factors for COVID-19 severity is a very demanding challenge. In a recent meta-analysis by Wynants et al., 4909 titles were screened, and 51 studies describing 66 prediction models were included [83]. Three different models were recognized for predicting hospital admission from SARS-CoV-2 pneumonia; 47 diagnostic models for identifying SARS-CoV-2 infection; and 16 prognostic models for predicting death risk, evolution to critical disease, or duration of hospital stay. The most stated markers of SARS-CoV-2 infection included fever, age, and symptoms. The most stated markers of bad outcome comprised sex, age, data from computed tomography scans, CRP, LDH and lymphocyte count.
However, all articles included in the meta-analysis were at high risk of bias, generally because of non-representative selection of control subjects, and at high risk of model overfitting. Moreover, the majority of studies did not contain a description of the study population and calibration of predictions was infrequently considered [83].
Prediction models for SARS-CoV-2 infection are rapidly made available to assist medical decisions. However, the paper of Wynants indicates that proposed models have to be confirmed and new studies are needed.
4. Immunological Approach to the Treatment of SARS-COv-2 Infection
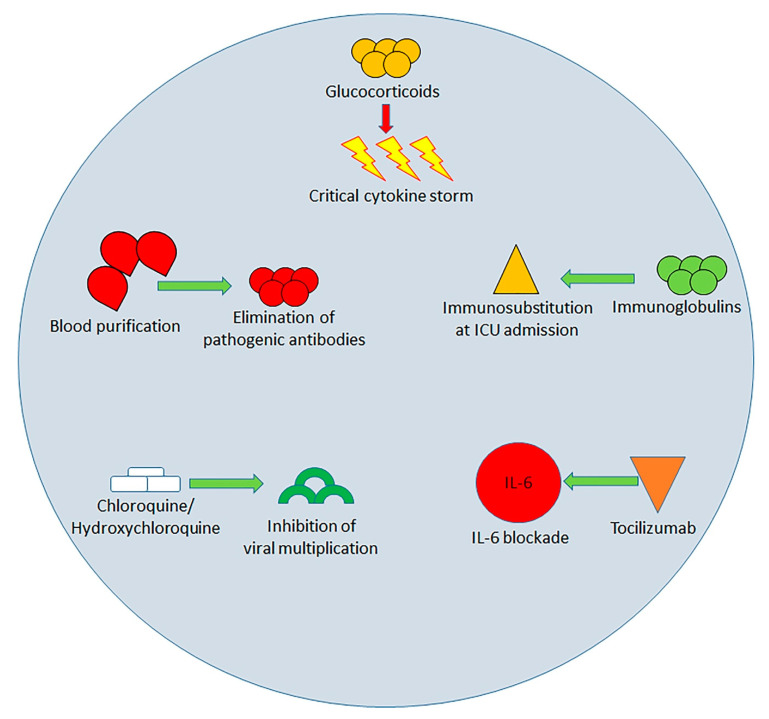
The identified immunological changes triggered by SARS-COv2 infection represent specific targets of therapies aimed at limiting the spread of the infection and the progress of organ damage. Numerous drugs or drug associations have been used or proposed (Figure 2).
Figure 2.
Possible treatment methods for SARS-CoV-2 until present (red arrows indicate inhibition, green arrows indicate promotion/enhancement of the related mechanism). Glucocorticoids limit the production and damaging effects of cytokines so avoiding acute lung injury induced by the cytokine storm; the blood purification removes pathogenic antibodies; Intravenous administration of immunoglobulins (IVIG) has the dual actions of immune substitution and immunomodulation, which are particularly useful during early phase of the cytokine storm; Chloroquine and hydroxychloroquine inhibit viral multiplication and restore the CD8+ cytotoxic viral response; Tocilizumab blocks IL-6; the preliminary results of clinical trials all demonstrate its clinical efficacy.
The use of glucocorticoids in treating COVID-19 has become a challenge for clinicians [84]. The scheduling of administration and the dose are critical in severe patients. A premature and/or abundant administration of steroids can inhibit T cells and block B cell antibody production, thus favoring an increased plasma viral load and causing relevant side effects. Therefore, glucocorticoids should be essentially employed in critical subjects experiencing a cytokine storm to limit the production and damaging effects of cytokines, thus avoiding the occurrence of ARDS. Administration of glucocorticoid for 3–5 days, at the dose equivalent to methylprednisolone 1–2mg/kg/day, is indicated for subjects with progressive respiratory failure and rapid imaging progression [85]. It has been shown that ARDS patients treated with methylprednisolone showed reduced mortality compared to those who did not receive glucocorticoids (46% vs. 62%) [86].
A different or concomitant therapeutic approach is the intravenous administration of immunoglobulins (IVIG). It was reported that 27% of 99 Wuhan patients received IVIG therapy. This treatment has the dual actions of immune substitution and immunomodulation. IVIG therapy performed within 48 h of entrance to the ICU has been reported to decrease the need for mechanical ventilation and mortality of patients with SARS-CoV-2 pneumonia [87]. However, its value in the therapy of SARS-CoV-2 needs further validation.
A promising therapeutic approach for patients with severe SARS-CoV-2 infection can be the use of drugs blocking IL-6. Tocilizumab definitely has this action [88]. Clinical evaluations performed in China showed that Tocilizumab is efficacious in critically ill subjects with diffuse bilateral lung alterations, who have increased IL-6 concentrations. The observation was confirmed by an Italian study reporting good results with the use of tocilizumab in patients with SARS-CoV-2 infection [89].
Chloroquine and its less toxic derivative hydroxychloroquine are additional drugs employed in the treatment of COVID-19. Their administration was followed by a reduction in viral proliferation [90]. These drugs act inhibiting viral multiplication in the endosome by avoiding endosomal acidification and endolysosomal fusion. Moreover, hydroxychloroquine stops the entrance of the virus into cells. Furthermore, chloroquine and hydroxychloroquine can inhibit TLR7 and TLR9 signaling and, thus, can restore the CD8+ cytotoxic viral response [91,92]. Adding azithromycin, able to block IL-6 and TNF-alpha [93], further reduces the nasopharyngeal SARS-CoV-2 presence.
Chloroquine phosphate has only been employed in adult patients [94,95]. However, Mehra et al. were unable to confirm a benefit of hydroxychloroquine or chloroquine, when used alone or with macrolide, on in-hospital outcomes for COVID-19 [96].
A completely different type of treatment of the cytokine storm is constituted by blood purification therapy. The capability of blood purification treatment in blocking IL-6/IL-6-receptor-eliminating pathogenic antibodies or cytokines has been demonstrated in this condition [97]. The blood purification therapy, comprising plasma exchange, perfusion, adsorption, and filtration, can be effective in eliminating inflammatory agents to a certain extent. This technique can stop the cytokine storm, thus decreasing the organ damage. It might be employed in critical subjects in the early and middle phases of the infection. The artificial liver treatment can remove inflammatory elements and was employed to block the cytokine storm of H7N9. Its use on SARS-CoV-2 has also attained evident effectiveness [98]. Early renal replacement therapy, which is analogous to the treatment with artificial liver technique, appears to be a useful method to treat cytokine storms [99].
5. Potential Targets for Therapeutic Intervention in SARS-COv2 Infection
Other treatments have been proposed but not yet utilized in SARS-COv-2 patients.
IFN-λ has antiviral function as it stimulates the expression of antiviral genes in epithelial cells and decreases the mononuclear macrophage-mediated proinflammatory action of IFN-αβ [100], without the inflammatory actions of Type I IFNs. In fact, IFN-λ blocks the passage of neutrophils to the sites of inflammation [101]. Early treatment with IFN had specific effects in decreasing viral load and reduced the clinical symptoms in the Middle East respiratory syndrome coronavirus infection, while, it failed in decreasing the mortality rate [102,103,104]. On the contrary, the late use of interferons did not cause more advantages than placebo [105].
Experiments in in vivo animal models have demonstrated that TNFs contribute considerably to lung damage and alter the T cell response in SARS-CoV-challenged mice. In animals, blocking TNF action or loss of TNF receptor reduced SARS-CoV-caused mortality [106]. However, TNF has not been identified in the serum of SARS-CoV-2 patients in the later phases of the disease and, at present, TNF blockers have not been proposed for subjects with SARS-CoV-2 infection.
Ulinastatin, a natural anti-inflammatory molecule, could have great prospects in the therapy of SARS-CoV-2 infection, as it protects the vascular endothelium by blocking the generation and the release of inflammatory substances. It decreases the concentration of TNF-α, IL-6, and IFN-γ, and augments the concentrations of the anti-inflammatory cytokine IL-10 [107]. Ulinastatin is commonly used to treat pancreatitis and acute circulatory failure.
Sphingosine-1-phosphate (S1P) is a bioactive signaling lysophospholipid that stimulates cytokine production and release [108]. The S1P receptor signaling has the ability to significantly limit immunopathologic injury caused by the host’s innate and adaptive immune response, thereby significantly reducing morbidity and mortality associated with influenza virus infection [109]. Agents able to block Sphingosine-1-phosphate receptor 1 (S1P1) reduce the disproportionate enrolment of inflammatory cells, decrease the amount of proinflammatory cytokines, and diminish the mortality of influenza virus infection [110]. Therefore, S1P1 agonist therapy, such as Siponimod, may be a possible therapeutic choice to block the cytokine storm and clinical trials should be performed to definitely evaluate its therapeutic effectiveness.
Carbajo-Lozoya et al. have reported that an active immunophilin pathway positively influences intracellular Coronavirus replication and that Tacrolimus, an agent that can inhibit calcineurin only when it binds with the immunophilin, intensely blocks the replication of SARS-CoV, HCoV-229E, and HCoV-NL63 at low concentrations in cell cultures [111]. Based on these results, investigating the therapeutic activity of low doses of tacrolimus towards SARS-CoV-2 can also be useful.
The immunomodulatory action of Mesenchymal stem cells (MSC) has been exploited recently for controlling diseases associated with inflammation. In fact, MSC can reduce the altered activation/maturation of T lymphocytes (in particular T-17) and macrophages and favor their differentiation into Treg cells and anti-inflammatory macrophages. They can also inhibit the production of pro-inflammatory cytokines, such as, IL-1, TNF-α, IL-6, IL-12, and IFN-γ, and therefore could be effective in conditions such as the cytokine storm [112,113]. Moreover, their ability to promote IL-10 production and to secrete multiple paracrine factors that regulate endothelial and epithelial permeability, inflammation and improve tissue repair, makes MSCs a possible therapeutic tool for ARDS [114].
A further therapeutic opportunity could be represented by the Inhibitors of mononuclear macrophage recruitment and function. Autopsy findings from patients who died of SARS-CoV2 showed a large amount of mononuclear inflammatory cells infiltrating the lung [33]. Therefore, a possible therapeutic attempt could be to reduce the enrolment of macrophages to the places of inflammation through small interfering RNA-mediated silencing of C-C chemokine receptor type 2, which has been proven to improve the prognosis of inflammatory diseases in animal experiments [115,116].
As reported in the previous sections, hyperinflammation is a characteristic of the most severe SARS-CoV 2 infections, therefore treating these patients with cytokine inhibitors to reduce the exaggerated immune response could give positive results [117]. Together with the use of IL-6 inhibitor, now in clinical practice, other anti-cytokine approaches have been evaluated including IL-1 and IL-18 antagonists.
The rationale for the use of these molecules during the cytokine storm of SARS-CoV-2 infection derives from the analogies with other conditions characterized by similar immune reactions. These include the hemophagocytic lymphohistiocytosis (HLH), a rare immune activation syndrome [118,119] and, in particular, the macrophage activation syndrome (MAS), a type of HLH, caused by viral infections in one third of affected patients [120]. MAS is a cytokine storm characterized by a huge release of proinflammatory mediators, with widespread hyperinflammation and progressive multiorgan failure. The suggested molecular mechanism hypothesizes alterations in lymphocytic cytolytic action triggering a pro-inflammatory cytokine cascade [121,122] with high concentrations of IL-1, IL-6, IL-18, soluble IL-2 receptor, TNF, and INF-γ [123] and activation of macrophages leading to tissue hemophagocytosis [124,125].
Anakinra, a recombinant interleukin-1 (IL-1) receptor antagonist, is presently FDA-accepted for the therapy of rheumatoid arthritis and neonatal-onset multi-organ inflammatory disease and off-label used for treating a variety of autoinflammatory diseases, including MAS, with favorable results. In a recent study, continuous i.v. anakinra infusions induced a rapid clinical response in 4/5 severely ill MAS patients with cytokine storm who were refractory to all other treatments [126].
The observation that patients with critical SARS-CoV-2 have shown signs of hemophagocytosis in lung tissues [127], and that Anakinra is effective in the treatment of cytokine storm caused by critical sepsis [128], justifies conducting trials investigating the use of Anakinra in patients with SARS-CoV-2 infection. At present, 15 trials have already been registered at www.ClinicalTrials.gov on the use of Anakinra alone or in combination with other medicines (e.g., ruxolitinib or baricitinib) in COVID-19 patients.
A further therapeutic option for treating the cytokine storm in SARS-CoV-2 patients is represented by the JAK-STAT inhibitors. It is well known that JAK1 and JAK3 pathways are involved in modulating the function of numerous cytokines that are implicated in antiviral responses and in the cytokine storm. Remarkably, IL-6 and GM- CSF, which are both stimulated by SARS- CoV-2, fully depend on JAK2 signaling. STAT3, a transcription factor, mediates IL-6 and IL-23 signals, while both IL-6 and IL-23 stimulate STAT3 via JAK2, and IL-21 stimulates STAT3 via JAK1 and JAK3.
It has been hypothesized that a JAK2 block can inhibit viral entry of SARS- CoV-2 and the subsequent inflammation [129]. The possible options are using the JAK inhibitors Fedratinib [130] and Baricitinib [129] that are associated with IRE1α Inositol-requiring transmembrane kinase/endoribonuclease 1α (IRE1α), or tylophorine-based compounds [131]. Baricitinib is a JAK inhibitor that has emerged as effective in the therapy of severe rheumatoid arthritis. IRE1α, an endoplasmic reticulum stress sensor, causes an augmented production of the negative controllers of JAK-STAT suppressor of cytokine signaling (SOCS)-1 and SOCS-3 [132]. Tylophorine-based compounds have powerful anti-coronaviral activities against SARS-CoV and MERS-CoV [133] and synergize with JAK2 inhibitors in inhibiting NF-κB activation [134].
6. Conclusions
Despite the numerous studies produced in a very short time on the pathophysiology and therapy of SARS-COv-2 infection, there are still many shadow areas. Particular attention should be paid to factors that promote/facilitate infection, for example, the age of the infected subjects. Indeed, immune senescence appears to have an essential role in disease progression [135,136].
Aging of the human immune system is characterized by an increase in innate immune responses and chronic inflammation (inflammaging), and a decrease in adaptive responses (immunosenescence). The inflammaging can lead to tissue damage and therefore reduce their ability to resist infections. Immunosenescence limits the ability to respond to new infections like this new coronavirus. This also limits the ability to respond to the vaccine with age.
Treatment is another area of uncertainty: when to perform it, with which drugs, and for how long? The possible risks and benefits of cytokine blockade require a cautious assessment to decide whether and when to start, continue or stop such treatments. Although the reduction in cytokines could be considered “immune suppression” and therefore dangerous in the context of an infection, several molecules, controlling the inflammation induced by the cytokine storm, could be decisive for the survival of the patients [137].
Knowing how to control and modulate the immune response can be a vital factor in controlling disease progression in SARS-CoV-2 infection.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Magrone T., Magrone M., Jirillo E. Focus on Receptors for Coronaviruses with Special Reference to Angiotensin-converting Enzyme 2 as a Potential Drug Target—A Perspective. Endocr. Metab. Immune Disord. Drug Targets. 2020 doi: 10.2174/1871530320666200427112902. [Epub ahead of print, 27 April 2020] [DOI] [PubMed] [Google Scholar]
- 2.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelemans T., Kikkert M. Viral innate immune evasion and the pathogenesis of emerging RNA virus infections. Viruses. 2019;11:961. doi: 10.3390/v11100961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fehr A.R., Channappanavar R., Perlman S. Middle East respiratory syndrome: Emergence of a pathogenic human coronavirus. Annu. Rev. Med. 2017;68:387–399. doi: 10.1146/annurev-med-051215-031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rognan D., Zimmermann N., Jung G., Folkers G. Molecular dynamics study of a complex between the human histocompatibility antigen HLA-A2 and the IMP58-66 nonapeptide from influenza virus matrix protein. Eur. J. Biochem. 1992;208:101–113. doi: 10.1111/j.1432-1033.1992.tb17163.x. [DOI] [PubMed] [Google Scholar]
- 6.Baruah V., Bose S. Immunoinformatics-aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019-nCoV. J. Med. Virol. 2020;92:495–500. doi: 10.1002/jmv.25698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kindler E., Thiel V. SARS-CoV and IFN: Too little, too late. Cell Host Microbe. 2016;19:139–141. doi: 10.1016/j.chom.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diao B., Wang C., Chen Y.T.X., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., et al. Reduction and functional exhaustion of t cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020 doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020:ciaa248. doi: 10.1093/cid/ciaa248. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z., Long W., Tu M., Chen S., Huang Y., Wang S., Zhou W., Chen D., Zhou L., Wang M., et al. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.054. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., Song S., Ma Z., Mo P., Zhang Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020:jiaa150. doi: 10.1093/infdis/jiaa150. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y., Liao W., Wan L., Xiang T., Zhang W. Correlation between relative nasopharyngeal virus RNA load and lymphocyte count disease severity in patients with COVID-19. Viral Immunol. 2020 doi: 10.1089/vim.2020.0062. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.He R., Lu Z., Zhang L., Fan T., Xiong R., Shen X., Feng H., Meng H., Lin W., Jiang W., et al. The clinical course and its correlated immune status in COVID-19 pneumonia. J. Clin. Virol. 2020;127:104361. doi: 10.1016/j.jcv.2020.104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cossarizza A., Gibellini L., De Biasi S., Lo Tartaro D., Mattioli M., Paolini A., Fidanza L., Bellinazzi C., Borella R., Castaniere I., et al. Handling and processing of blood specimens from patients with COVID-19 for safe studies on cell phenotype and cytokine storm. Cytometry A. 2020 doi: 10.1002/cyto.a.24009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson S., Maini M.K., Wack A. Disease-promoting effects of type I interferons in viral, bacterial, and coinfections. J. Interferon Cytokine Res. 2015;35:252–264. doi: 10.1089/jir.2014.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw A.C., Goldstein D.R., Montgomery R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao X., Yi Z., et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) medRxiv. 2020 Preprint. [Google Scholar]
- 21.Chen L., Liu H.-G., Liu W., Liu J., Liu K., Shang J., Deng Y., Wei S. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Chin. J. Tuberc. Respir. Dis. 2020;43:203–208. doi: 10.3760/cma.j.issn.1001-0939.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y., Shen C., Li J., Yuan J., Yang M., Wang F., Wei J. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. medRxiv. 2020 doi: 10.1101/2020.03.02.20029975. Preprint. [DOI] [Google Scholar]
- 23.Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X., Ma H., Chen W., Lin Y., Zheng Y., et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. China Life Sci. 2020;63:706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velazquez-Salinas L., Verdugo-Rodriguez A., Rodriguez L.L., Borca M.V. The role of interleukin 6 during viral infections. Front. Microbiol. 2019;10:1057. doi: 10.3389/fmicb.2019.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okabayashi T., Kariwa H., Yokota S., Iki S., Indoh T., Yokosawa N., Takashima I., Tsutsumi H., Fujii N. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J. Med. Virol. 2006;78:417–424. doi: 10.1002/jmv.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teijaro J.R., Walsh K.B., Rice S., Rosen H., Oldstone M.B. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc. Natl. Acad. Sci. USA. 2014;111:3799–3804. doi: 10.1073/pnas.1400593111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Special Expert Group for Control of the Epidemic of Novel Coronavirus Pneumonia of the Chinese Preventive Medicine Association An update on the epidemiological characteristics of novel coronavirus pneumonia (COVID-19) Chin. J. Epidemiol. 2020;41:139–144. doi: 10.3760/cma.j.issn.0254-6450.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 29.Shimabukuro-Vornhagen A., Gödel P., Subklewe M., Stemmler H.J., Schlößer H.A., Schlaak M., Kochanek M., Böll B., von Bergwelt-Baildon M.S. Cytokine release syndrome. J. Immunother. Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zenewicz L.A. IL-22: There is a gap in our knowledge. Immunohorizons. 2018;2:198–207. doi: 10.4049/immunohorizons.1800006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tse G.M., To K.F., Chan P.K., Lo A.W., Ng K.C., Wu A., Lee N., Wong H.C., Mak S.M., Chan K.F., et al. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS) J. Clin. Pathol. 2004;57:260–265. doi: 10.1136/jcp.2003.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao Y., Li X., Mou T., Zhou X., Li D., Wang L., Zhang Y., Dong X., Zheng H., Guo L., et al. Distinct infection process of SARS-CoV-2 in human bronchial epithelial cells line. J. Med. Virol. 2020 doi: 10.1002/jmv.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faure E., Poissy J., Goffard A., Fournier C., Kipnis E., Titecat M., Bortolotti P., Martinez L., Dubucquoi S., Dessein R., et al. Distinct immune response in two MERS-CoV-infected patients: Can we go from bench to bedside? PLoS ONE. 2014;9:e88716. doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Josset L., Menachery V.D., Gralinski L.E., Agnihothram S., Sova P., Carter V.S., Yount B.L., Graham R.L., Baric R.S., Katze M.G. Cell host response to infection with novel human coronavirus EMC predicts potential antivirals and important differences with SARS coronavirus. mBio. 2013;4:e00165-13. doi: 10.1128/mBio.00165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bermejo-Martin J.F., Ortiz de Lejarazu R., Pumarola T., Rello J., Almansa R., Ramírez P., Martin-Loeches I., Varillas D., Gallegos M.C., Serón C., et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13:R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li C., Yang P., Sun Y., Li T., Wang C., Wang Z., Zou Z., Yan Y., Wang W., Wang C., et al. IL-17 response mediates acute lung injury induced by the 2009 pandemic influenza A (H1N1) virus. Cell Res. 2012;22:528–538. doi: 10.1038/cr.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douda D.N., Jackson R., Grasemann H., Palaniyar N. Innate immune collectin surfactant protein D simultaneously binds both neutrophil extracellular traps and carbohydrate ligands and promotes bacterial trapping. J. Immunol. 2011;187:1856–1865. doi: 10.4049/jimmunol.1004201. [DOI] [PubMed] [Google Scholar]
- 39.Parsons P.E., Eisner M.D., Thompson B.T., Matthay M.A., Ancukiewicz M., Bernard G.R., Wheeler A.P., NHLBI Acute Respiratory Distress Syndrome Clinical Trials Network Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit. Care Med. 2005;33:1–6. doi: 10.1097/01.CCM.0000149854.61192.DC. [DOI] [PubMed] [Google Scholar]
- 40.Wang H., Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am. J. Emerg. Med. 2008;26:711–715. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 41.Henderson L.A., Canna S.W., Schulert G.S., Volpi S., Lee P.Y., Kernan K.F., Caricchio R., Mahmud S., Hazen M.M., Halyabar O., et al. On the alert for cytokine storm: Immunopathology in COVID-19. Arthritis Rheumatol. 2020 doi: 10.1002/art.41285. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norelli M., Camisa B., Barbiera G., Falcone L., Purevdorj A., Genua M., Sanvito F., Ponzoni M., Doglioni C., Cristofori P., et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 43.Teijaro J.R. Cytokine storms in infectious diseases. Semin Immunopathol. 2017;39:501–503. doi: 10.1007/s00281-017-0640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allegra A., Innao V., Gerace D., Vaddinelli D., Musolino C. Adoptive immunotherapy for hematological malignancies: Current status and new insights in chimeric antigen receptor T cells. Blood Cells Mol. Dis. 2016;62:49–63. doi: 10.1016/j.bcmd.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Leiva-Juarez M.M., Kolls J.K., Evans S.E. Lung epithelial cells: Therapeutically inducible effectors of antimicrobial defense. Mucosal Immunol. 2018;11:21–34. doi: 10.1038/mi.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knudsen L., Ochs M. The micromechanics of lung alveoli: Structure and function of surfactant and tissue components. Histochem. Cell. Biol. 2018;150:661–676. doi: 10.1007/s00418-018-1747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brune K., Frank J., Schwingshackl A., Finigan J., Sidhaye V.K. Pulmonary epithelial barrier function: Some new players and mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;308:L731–L745. doi: 10.1152/ajplung.00309.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z., et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br. J. Haematol. 2020 doi: 10.1111/bjh.16659. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., Shi J., Zhou M., Wu B., Yang Z., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.04.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meduri G.U., Headley S., Kohler G., Stentz F., Tolley E., Umberger R., Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 51.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World Health Organization-China Joint Mission Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Geneva 2020. [(accessed on 1 March 2020)]; Available online: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf.
- 53.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lippi G., Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chim. Acta. 2020;505:190–191. doi: 10.1016/j.cca.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snijders D., Schoorl M., Schoorl M., Bartels P.C., van der Werf T.S., Boersma W.G. D-dimer levels in assessing severity and clinical outcome in patients with community-acquired pneumonia. A secondary analysis of a randomised clinical trial. Eur. J. Intern. Med. 2012;23:436–441. doi: 10.1016/j.ejim.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 58.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020:e201585. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han H., Yang L., Liu R., Liu F., Wu K.-L., Li J., Liu X.-H., Zhu C.-L. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 61.Henry B., de Oliveira M., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0369. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 62.He Z., Zhao C., Dong Q., Zhuang H., Song S., Peng G., Dwyer D.E. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int. J. Infect. Dis. 2005;9:323–330. doi: 10.1016/j.ijid.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henry B. COVID-19, ECMO, and lymphopenia: A word of caution. Lancet Respir. Med. 2020;8:e24. doi: 10.1016/S2213-2600(20)30119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H., Xiong Y., Cheng Z., Gao S., Liang K., et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis. 2020:ciaa270. doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ye G., Pan Z., Pan Y., Deng Q., Chen L., Li J., Li Y., Wang X. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J. Infect. 2020;80:e14–e17. doi: 10.1016/j.jinf.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meng L.-B., Yu Z.-M., Guo P., Wang Q.Q., Qi R.M., Shan M.J., Lv J., Gong T. Neutrophils and neutrophil-lymphocyte ratio: Inflammatory markers associated with intimal-media thickness of atherosclerosis. Thromb. Res. 2018;17:45–52. doi: 10.1016/j.thromres.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 67.Okugawa Y., Toiyama Y., Yamamoto A., Shigemori T., Ide S., Kitajima T., Fujikawa H., Yasuda H., Hiro J., Yoshiyama S., et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann. Surg. 2019 doi: 10.1097/SLA.0000000000003239. [DOI] [PubMed] [Google Scholar]
- 68.Azab B., Zaher M., Weiserbs K.F., Torbey E., Lacossiere K., Gaddam S., Gobunsuy R., Jadonath S., Baldari D., McCord D., et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am. J. Cardiol. 2010;106:470–476. doi: 10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- 69.Guthrie G.J., Charles K.A., Roxburgh C.S., Horgan P.G., McMillan D.C., Clarke S.J. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 70.Giede-Jeppe A., Bobinger T., Gerner S.T., Sembill J.A., Sprügel M.I., Beuscher V.D., Lücking H., Hoelter P., Kuramatsu J.B., Huttner H.B. Neutrophil-to-lymphocyte ratio is an independent predictor for in-hospital mortality in spontaneous intracerebral hemorrhage. Cerebrovasc. Dis. 2017;44:26–34. doi: 10.1159/000468996. [DOI] [PubMed] [Google Scholar]
- 71.Ha Y.J., Hur J., Go D.J., Kang E.H., Park J.K., Lee E.Y., Shin K., Lee E.B., Song Y.W., Lee Y.J. Baseline peripheral blood neutrophil-to-lymphocyte ratio could predict survival in patients with adult polymyositis and dermatomyositis: A retrospective observational study. PLoS ONE. 2018;13:e19041. doi: 10.1371/journal.pone.0190411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H.X., Luo M., Chen L., Zhao Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.04.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25819. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jenne C.N., Kubes P. Platelets in inflammation and infection. Platelets. 2015;26:286–292. doi: 10.3109/09537104.2015.1010441. [DOI] [PubMed] [Google Scholar]
- 76.Kumar P.S., Chandrasekhar C., Srikanth L., Sarma P.V.G.K. In vitro large scale production of megakaryocytes to functional platelets from human hematopoietic stem cells. Biochem. Biophys. Res. Commun. 2018;505:168–175. doi: 10.1016/j.bbrc.2018.09.090. [DOI] [PubMed] [Google Scholar]
- 77.Behrens K., Alexander W.S. Cytokine control of megakaryopoiesis. Growth Factors. 2018;36:89–103. doi: 10.1080/08977194.2018.1498487. [DOI] [PubMed] [Google Scholar]
- 78.Rayes J., Bourne J.H., Brill A., Watson S.P. The dual role of platelet-innate immune cell interactions in thrombo-inflammation. Res. Pract. Thromb. Haemost. 2019;4:23–35. doi: 10.1002/rth2.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ramana C.V., De Berge M.P., Kumar A., Alia C.S., Durbin J.E., Enelow R.I. Inflammatory impact of IFN-γ in CD8+T cell mediated lung injury is mediated by both Stat1-dependent and independent pathways. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;308:L650–L657. doi: 10.1152/ajplung.00360.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020;7:taaa021. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;82:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qu R., Ling Y., Zhang Y.H., Wei L.Y., Chen X., Li X.M., Liu X.Y., Liu H.M., Guo Z., Ren H., et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25767. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wynants L., Van Calster B., Bonten M.M.J., Riley R.D., Heinze G., Schuit E., Bonten M.M.J., Damen J.A.A., Debray T.P.A., De Vos M., et al. Prediction models for diagnosis and prognosis of covid-19 infection: Systematic review and critical appraisal. Br. Med. J. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao J., Hu Y., Du R., Chen Z., Jin Y., Zhou M., Zhang J., Qu J.M., Cao B. Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia. Chin. J. Tuberc. Respir. Dis. 2020;43:183–184. doi: 10.3760/cma.j.issn.1001-0939.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 85.Qin Y.Y., Zhou Y.H., Lu Y.Q., Sun F., Yang S., Harypursat V., Chen Y.K. Effectiveness of glucocorticoid therapy in patients with severe novel coronavirus disease 2019: Protocol of a randomized controlled trial. Chin. Med. J. 2020;133:1080–1086. doi: 10.1097/CM9.0000000000000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xie Y., Cao S., Dong H., Li Q., Chen E., Zhang W., Yang L., Fu S., Wang R. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.044. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 89.Piva S., Filippini M., Turla F., Cattaneo S., Margola A., De Fulviis S., Nardiello I., Beretta A., Ferrari L., Trotta R., et al. Clinical presentation and initial management critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Brescia, Italy. J. Crit. Care. 2020;58:29–33. doi: 10.1016/j.jcrc.2020.04.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-labeled non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int. J. Antimicrob. Agents. 2020:105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou D., Dai S.M., Tong Q. COVID-19: A recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 2020:dkaa114. doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schultz M.J. Macrolide activities beyond their antimicrobial effects: Macrolides in diffuse panbronchiolitis and cystic fibrosis. J. Antimicrob. Chemother. 2004;54:21–28. doi: 10.1093/jac/dkh309. [DOI] [PubMed] [Google Scholar]
- 94.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 95.Multicenter collaboration group of department of science and technology of Guangdong province and health commission of Guangdong province for chloroquine in the treatment of novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:185–188. doi: 10.3760/cma.j.issn.1001-0939.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 96.Mehra M.R., Ruschitzka F., Patel A.N. Retraction-Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis. Lancet. 2020;395:1820. doi: 10.1016/S0140-6736(20)31324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ma J., Xia P., Zhou Y., Liu Z., Zhou X., Wang J., Li T., Yan X., Chen L., Zhang S., et al. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin. Immunol. 2020;214:108408. doi: 10.1016/j.clim.2020.108408. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu K., Cai H., Shen Y., Ni Q., Chen Y., Hu S., Li J., Wang H., Yu L., Huang H., et al. Management of corona virus disease-19 (COVID-19): The Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zuccari S., Damiani E., Domizi R., Scorcella C., D’Arezzo M., Carsetti A., Pantanetti S., Vannicola S., Casarotta E., Ranghino A., et al. Changes in cytokines, haemodynamics and microcirculation in patients with sepsis/septic shock undergoing continuous renal replacement therapy and blood purification with CytoSorb. Blood. Purif. 2020;49:107–113. doi: 10.1159/000502540. [DOI] [PubMed] [Google Scholar]
- 100.Andreakos E., Tsiodras S. COVID-19: Lambda interferon against viral load and hyperinflammation. EMBO Mol Med. 2020 doi: 10.15252/emmm.202012465. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davidson S., McCabe T.M., Crotta S., Gad H.H., Hessel E.M., Beinke S., Hartmann R., Wack A. IFNλ is a potent anti-influenza therapeutic without the inflammatory side effects of IFNα treatment. EMBO Mol Med. 2016;8:1099–1112. doi: 10.15252/emmm.201606413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blazek K., Eames H.L., Weiss M., Byrne A.J., Perocheau D., Pease J.E., Doyle S., McCann F., Williams R.O., Udalova I.A. IFN-λ resolves inflammation via suppression of neutrophil infiltration and IL-1β production. J. Exp. Med. 2015;212:845–853. doi: 10.1084/jem.20140995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arabi Y.M., Shalhoub S., Mandourah Y., Al-Hameed F., Al-Omari A., Al Qasim E., Jose J., Alraddadi B., Almotairi A., Al Khatib K., et al. Ribavirin and interferon therapy for critically ill patients with middle east respiratory syndrome: A multicenter observational study. Clin. Infect. Dis. 2019;70:1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y., Almakhlafi G.A., Albarrak M.M., Memish Z.A., Albarrak A.M. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: A retrospective cohort study. Lancet Infect. Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zumla A., Chan J.F.W., Azhar E.I., Hui D.S.C., Yuen K.-Y. Coronaviruses -drug discovery and therapeutic options. Nature Rev. Drug. Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McDermott J.E., Mitchell H.D., Gralinski L.E., Eisfeld A.J., Josset L., Bankhead A., Neumann G., Tilton S.C., Schäfer A., Li C., et al. The effect of inhibition of PP1 and TNFα signaling on pathogenesis of SARS coronavirus. BMC Syst. Biol. 2016;10:93. doi: 10.1186/s12918-016-0336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang H., Liu B., Tang Y., Chang P., Yao L., Huang B., Lodato R.F., Liu Z. Corrigendum: Improvement of sepsis prognosis by Ulinastatin: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2020;10:1697. doi: 10.3389/fphar.2019.01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maceyka M., Harikumar K.B., Milstien S., Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walsh K.B., Teijaro J.R., Rosen H., Oldstone M.B.A. Quelling the storm: Utilization of sphingosine-1-phosphate receptor signaling to ameliorate influenza virus-induced cytokine storm. Immunol. Res. 2011;51:15. doi: 10.1007/s12026-011-8240-z. [DOI] [PubMed] [Google Scholar]
- 110.Walsh K.B., Teijaro J.R., Wilker P.R., Jatzek A., Fremgen D.M., Das S.C., Watanabe T., Hatta M., Shinya K., Suresh M., et al. Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus. Proc. Natl. Acad. Sci. USA. 2011;108:12018–12023. doi: 10.1073/pnas.1107024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carbajo-Lozoya J., Müller M.A., Kallies S., Thiel V., Drosten C., von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165:112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Uccelli A., de Rosbo N.K. The immunomodulatory function of mesenchymal stem cells: Mode of action and pathways. Ann. NY Acad. Sci. 2015;1351:114–126. doi: 10.1111/nyas.12815. [DOI] [PubMed] [Google Scholar]
- 113.Ben-Mordechai T., Palevski D., Glucksam-Galnoy Y., Elron-Gross I., Margalit R., Leor J. Targeting Macrophage Subsets for Infarct Repair. J. Cardiovas. Pharmacol. Ther. 2015;20:36–51. doi: 10.1177/1074248414534916. [DOI] [PubMed] [Google Scholar]
- 114.Lee J.W., Fang X., Krasnodembskaya A., Howard J.P., Matthay M.A. Concise Review: Mesenchymal stem cells for acute lung injury: Role of paracrine soluble factors. Stem Cells. 2011;29:913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leuschner F., Courties G., Dutta P., Mortensen L.J., Gorbatov R., Sena B., Novobrantseva T.I., Borodovsky A., Fitzgerald K., Koteliansky V., et al. Silencing of CCR2 in myocarditis. Eur. Heart J. 2015;36:1478–1488. doi: 10.1093/eurheartj/ehu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leuschner F., Dutta P., Gorbatov R., Novobrantseva T.I., Donahoe J.S., Courties G., Lee K.M., Kim J.I., Markmann J.F., Marinelli B., et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Allen C.E., McClain K.L. Pathophysiology and epidemiology of hemophagocytic lymphohistiocytosis. Hematol. Am. Soc. Hematol. Educ. Program. 2015;2015:177–182. doi: 10.1182/asheducation-2015.1.177. [DOI] [PubMed] [Google Scholar]
- 119.Janka G. Familial and Acquired Hemophagocytic Lymphohistiocytosis. Ann. Rev. Med. 2012;63:233–246. doi: 10.1146/annurev-med-041610-134208. [DOI] [PubMed] [Google Scholar]
- 120.Minoia F., Davì S., Horne A., Demirkaya E., Bovis F., Li C., Lehmberg K., Weitzman S., Insalaco A., Wouters C., et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: A multinational, multicenter study of 362 patients. Arthritis Rheumatol. 2014;66:3160–3169. doi: 10.1002/art.38802. [DOI] [PubMed] [Google Scholar]
- 121.Grom A.A., Villanueva J., Lee S., Goldmuntz E.A., Passo M.H., Filipovich A. Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J. Pediatr. 2003;142:292–296. doi: 10.1067/mpd.2003.110. [DOI] [PubMed] [Google Scholar]
- 122.Crayne C.B., Albeituni S., Nichols K.E., Cron R.Q. The Immunology of macrophage activation syndrome. Front. Immunol. 2019;10:119. doi: 10.3389/fimmu.2019.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bracaglia C., Prencipe G., De Benedetti F. Macrophage Activation Syndrome: Different mechanisms leading to a one clinical syndrome. Pediatr. Rheumatol. Online J. 2017;15:5. doi: 10.1186/s12969-016-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Parikh S.A., Kapoor P., Letendre L., Kumar S., Wolanskyj A.P. Prognostic factors and outcomes of adults with hemophagocytic lymphohistiocytosis. Mayo Clin. Proc. 2012;89:484–492. doi: 10.1016/j.mayocp.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 125.Park H.S., Kim D.Y., Lee J.H., Lee J.H., Kim S.D., Park Y.H., Lee J.S., Kim B.Y., Jeon M., Kang Y.A., et al. Clinical features of adult patients with secondary hemophagocytic lymphohistiocytosis from causes other than lymphoma: An analysis of treatment outcome and prognostic factors. Ann. Hematol. 2012;91:897–904. doi: 10.1007/s00277-011-1380-3. [DOI] [PubMed] [Google Scholar]
- 126.Monteagudo L.A., Boothby A., Gertner E. Continuous Intravenous Anakinra Infusion to Calm the Cytokine Storm in Macrophage Activation Syndrome. ACR Open Rheumatol. 2020 doi: 10.1002/acr2.11135. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nicholls J.M., Poon L.L., Lee K.C., Ng W.F., Lai S.T., Leung C.Y., Chu C.M., Hui P.K., Mak K.L., Lim W., et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shakoory B., Carcillo J.A., Chatham W.W., Amdur R.L., Zhao H., Dinarello C.A., Cron R.Q., Opal S.M. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: Reanalysis of a prior phase iii trial. Crit. Care Med. 2016;44:275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., Rawling M., Savory E., Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.03.005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kontzias A., Kotlyar A., Laurence A., Changelian P., O’Shea J.J. Jakinibs: A new class of kinase inhibitors in cancer and autoimmune disease. Curr. Opin. Pharmacol. 2012;12:464–470. doi: 10.1016/j.coph.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ma Y., Wang C., Xue M., Fu F., Zhang X., Li L., Yin L., Xu W., Feng L., Liu P. The coronavirus transmissible gastroenteritis virus evades the type I interferon response through IRE1alpha-mediated manipulation of the microRNA miR-30a-5p/SOCS1/3 axis. J. Virol. 2018;92:e00728-18. doi: 10.1128/JVI.00728-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang C.W., Lee Y.Z., Kang I.J., Barnard D.L., Jan J.T., Lin D., Huang C.W., Yeh T.K., Chao Y.S., Lee S.J. Identification of phenanthroindolizines and phenanthroquinolizidines as novel potent anticoronaviral agents for porcine enteropathogenic coronavirus transmissible gastroenteritis virus and human severe acute respiratory syndrome coronavirus. Antivir. Res. 2010;88:160–168. doi: 10.1016/j.antiviral.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang C.-W., Lee Y.-Z., Hsu H.-Y., Shih C., Chao Y.S., Chang H.Y., Lee S.J. Targeting coronaviral replication and cellular JAK2 mediated dominant NF-κB activation for comprehensive and ultimate inhibition of coronaviral activity. Sci. Rep. 2017;7:4105. doi: 10.1038/s41598-017-04203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 136.Weiskopf D., Weinberger B., Grubeck-Loebenstein B. The aging of the immune system. Transplant. Int. 2009;22:1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- 137.Schett G., Sticherling M., Neurath M.F. COVID-19: Risk for cytokine targeting in chronic inflammatory diseases? Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0312-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]