Abstract
This review summarizes our contributions during last decade on the synthesis of arylazopyridones that may be used as disperse dyes for hydrophobic fabrics utilizing an environmentally benign high temperature dyeing method. The review also discusses the advantages of select disperse dyes based on pyridone moieties as antioxidant, antimicrobial and anticancer agents.
Keywords: disperse dyes, self-cleaning, ultraviolet protection factor, pyridines, biological activities
1. Introduction
The instincts behind love of color differ from place to place and from one culture to another. Beautiful colors reflect the aesthetic sense of human beings that differs between individuals. Synthetic dyes are a category of high-quality natural materials, basically utilized for dyeing or printing materials that stick to the fabrics by forming a covalent bond during the application procedure. The utilization of natural dyes in textiles has largely been replaced by synthetic dyes because these give a wide assortment of reproducible hues and shades [1]. Heterogeneous amines have been widely used in preparing disperse dyes. These dyes show excellent affinity for polyester fabrics. The disperse dyes are organic colors that are less soluble in water and are widely applied to hydrophobic fibers such as polyester fibers where the dispersed dyes literally dissolve and produce the desired color [2]. The main reason for the development of dispersed dyes is the large increase in the global production of polyester fibers over the past two decades, as a large amount of these dyes is used mainly to dye polyester fabrics [3,4,5]. Some significant heterocyclic diazo components, for example, isothiazoles, thiazoles, thiadiazoles, 4-oxoquinazolines and thiophenes, give generally excellent disperse dyes with good fastness properties. In this review we focus on our contributions to the synthesis and applications of azo disperse dyes based on pyridone derivatives. The target molecules are biologically active and are used as antioxidants and anticancer agents. Environmentally benign uses of these dyes during the last decade are also described.
2. Chemistry of Pyridone Disperse Dyes
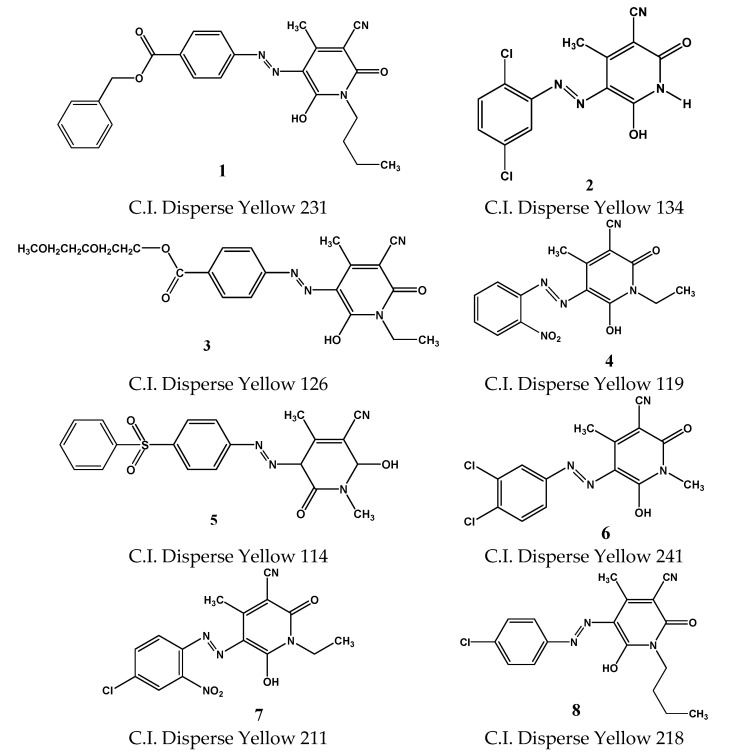
It is of value to mention here that pyridione derivatives have largely supplanted anthraquinones as disperse dyes with desirable properties. Among these dyes compounds 1–8 are commercially accessible (Figure 1) [6,7,8]. Azo pyridone dyes are significant pyridone derivatives that have to a great extent replaced disperse dyes based on pyrazolones due to their excellent hues.
Figure 1.
Some of the commercial dyes.
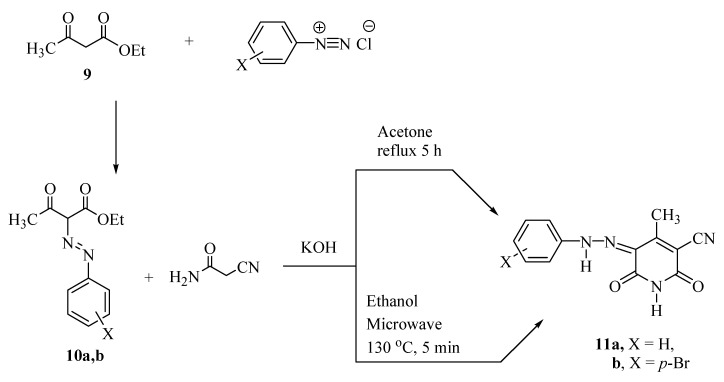
Disperse dyes based on the pyridone moiety can be formed from β-diketones and different diazonium salts, followed by condensation with cyanoacetamide as a first reaction route. Mijin et al. [9,10] reported the synthesis of 5-(substituted phenylazo)-6-hydroxy-4-methyl-3-cyano-2-pyridones, i.e., the known azo pyridone disperse dyes 11a,b via the reaction of ethyl acetoacetate (9) with aryldiazonium salts to give the corresponding ethyl 3-oxo-2-(substituted phenylazo)-butanoates 10 which are then condensed with cyanoacetamide in the presence of potassium hydroxide as a catalyst by either a conventional method with lower reaction yields or by a microwave heating method (Scheme 1).
Scheme 1.
Some of the well-known pyridone dyes.
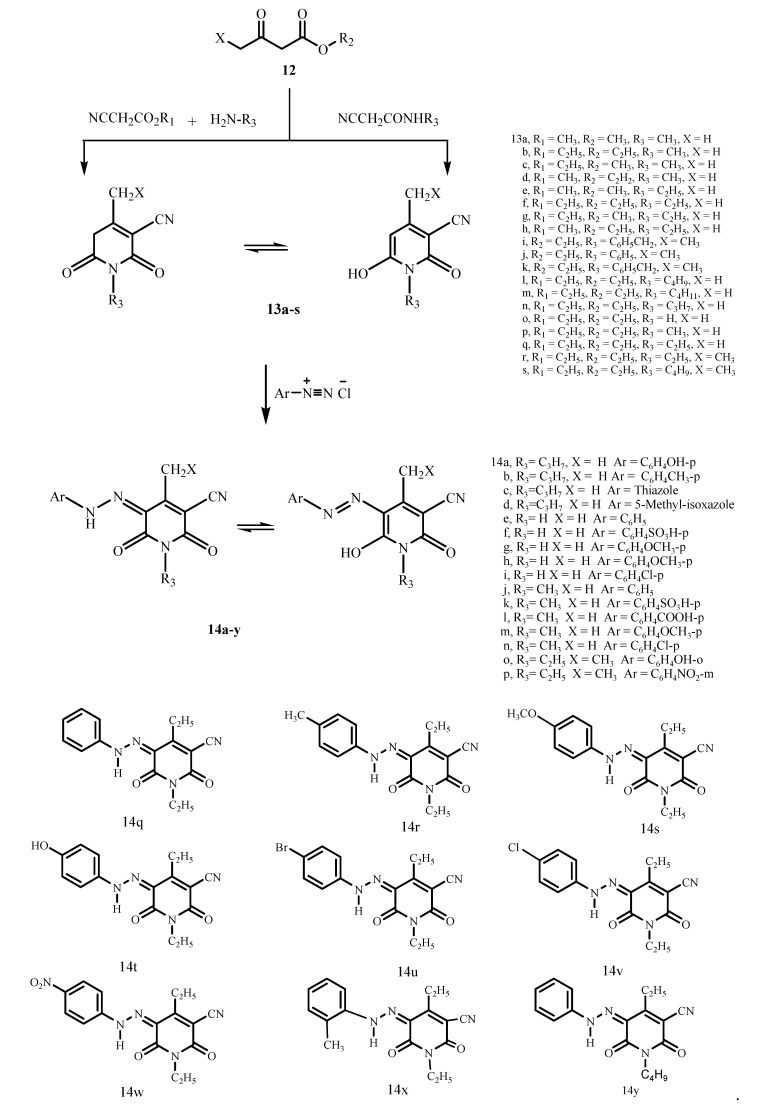
A second reaction route for the synthesis of these azo dyes involves the reaction of pyridone as a coupling component and various diazonium salts. Lately, numerous researchers [10,11] have developed effective syntheses of new substituted aryl and heteroarylazoazines as potential antimicrobial dyes starting from pyridones 13a–s (Scheme 2).
Scheme 2.
Synthesis of some pyridone dyes.
In this manner, a few researchers developed green syntheses of these pyridone derivatives using microwave [10] or ultrasound irradiation [11].
Pyridone derivatives have found wide application in the synthesis of azo dyes, particularly as disperse dyes [12,13,14,15,16,17,18,19,20]. An example of this the work of Balalaie et al. [21,22,23] who have announced an effective three component condensation of alkyl cyanoacetates, primary amines, and β-ketoesters affording more significant yields of 13a–h. These compounds have two tautomeric structures, and in solution there is a quick equilibration between them. Pyridones 13a–s could be promptly coupled with heteroaromatic diazonium salts producing the comparable heteroaromatic azopyridones dyes (Scheme 2).
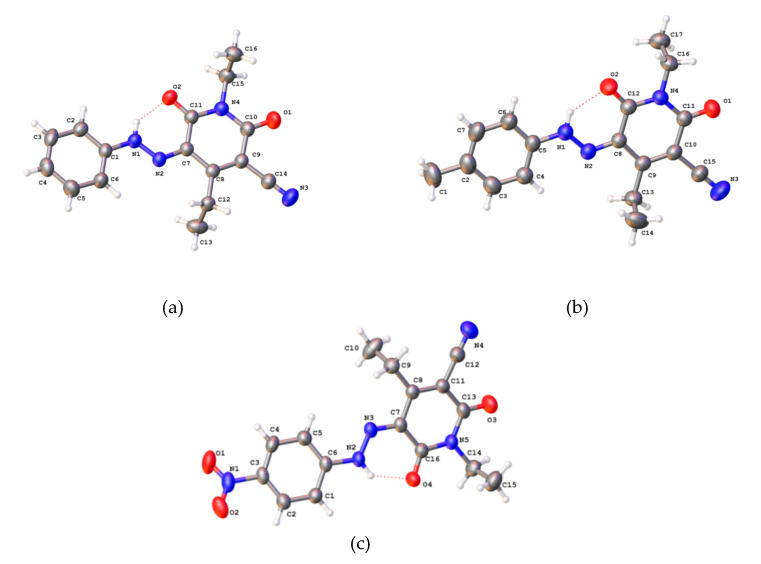
Ashkar et al. [24], Sakoma et al. [25] El-Apasery et al. [26], and Al-Etaibi et al. [27] have evaluated 3-(p-substituted phenylazo)-6-pyridone dyes 14a–d, 14e–n, 14o,p and 14q–w, respectively, as potential disperse dyes that exist in the hydrazone tautomeric form as shown in Figure 2 for the dyes 14q, 14r and 14w and applied them on polyester fabrics, to analyze the impact of substituents on the shade of the synthesized dyes.
Figure 2.
ORTEP structures of (a) dye 14q; (b) dye 14r and (c) dye 14w.
Ashkar et al. [24], Sakoma et al. [25], and El-Apasery et al. [26] reported that the exhaustion of the colors was generally excellent on polyester fabrics, with acceptable wash and light fastness properties. These dyes are essential for their excellent affinity for fabrics. Other remarkable attributes of these dyes are that they give deep and bright hues.
3. Absorption Spectra Characteristics
It should be noted here that the λmax values will be determined in general by the strength of the electronic force in the benzenoid framework. Since the electronic transitions of these prepared dispersed dyes involve a general movement of the electron density from the donor group towards the azo group, the best effect with respect to a longer wavelength is achieved by placing the substituent in a position ortho or para to the azo group for successful conjugation. The maximum absorption of 14q–y colors was estimated in dimethylformamide (DMF) and listed in Table 1, sorted from 410 to 468 nm.
Table 1.
Optical measurements of polyester fabrics.
| Dye No. | Color Shade | Color Strength K/S | Absorption [λmax in DMF (nm)] |
|---|---|---|---|
| 14q | Yellow | 27.39 | 410 |
| 14r | Dark orange | 30.29 | 468 |
| 14s | Orange | 30.28 | 438 |
| 14t | Orange | 8.88 | 464 |
| 14u | Yellow | 28.91 | 417 |
| 14v | Dark yellow | 28.09 | 416 |
| 14w | Very dark yellow | 27.31 | 452 |
| 14x | Deep greenish yellow | 19.38 | 445 |
| 14y | Orange yellow | 12.63 | 435 |
The colors shifts result from changes in substituents on the diazonium part of these dyes. In this way, the introduction of an electron-donating substituent on the arylhydrazono moiety brings about a critical bathochromic shift and the greater the strength of the electron-donating substituent, the more noteworthy the bathochromic move. Along these lines, dyes 14r, 14s, 14t and 14x (λmax 468, 438, 464 and 445 nm) showed critical bathochromic shifts compared to dye 14q (λmax 410 nm) (Δλmax = 58, 28 and 54, 35 nm), attributable to methyl, methoxy, hydroxyl and o-CH3 groups, respectively. The observed bathochromism upon introduction of electron donating methyl, methoxy, and hydroxy groups is in agreement with the theory. The increased electron densities added to the delocalization are because of the electron donating methyl, methoxy, and hydroxy groups are the reason for the observed bathochromic shifts, whereas with the addition of electron-withdrawing groups such as p-Br and p-Cl at the para-position of the arylhydrazono portion, a small bathochromic move could be observed, for example in dyes 14u, and 14v (λmax 417 and 416 nm), can be clearly observed by showing the contrast between their bathochromic shifts compared to dye 14q (Δλmax = 7 and 8) [28].
4. Dyeing Characteristics
Compounds 14q–y were utilized for dyeing polyester textures through a high-pressure high- temperature dyeing technique. After the dyeing process, the dyed fabrics ranged in color from yellow to dark orange. The dyeing properties of polyester fabrics were then evaluated with respect to their fastness properties. The K/S estimates recorded in Table 1 show that the dyes 14q–w showed very high affinity for polyester fabrics and that all K/S values were generally good. The results recorded in Table 1 also show that the introduction of electron withdrawal groups in the benzene ring improved the lightness and brightness; conversely, the incorporating of electron-donating groups reduced the brightness, so the dyes 14u–w were lighter and more brilliant than the dyes 14r–t.
The washing and perspiration fastness evaluation results, listed in Table 2, were generally excellent. When the reduction clearing was overlooked, worse staining was observed. Since these dyes are naturally hydrophobic, high washing and perspiration fastness are expected. The light fastness of the dyes 14q–w on polyester fabrics showed moderate fastness. Light fastness is mainly affected by the nature of the substituents on the diazonium portion. The incorporation of electron-withdrawing substituents (bromine, chlorine, or nitro) improves the light fastness as it reaches values of 3–4, 3–4, and 5, respectively. One feature worth mention here is the light fastness obtained by the dye 14w which contains nitro group in the diazonium part. The nitro group increases the polarity of the dye, which may link it more strongly to the fabric and it opens an additional path of energy dissipation after absorption of light which reduces the chances of photofading [28].
Table 2.
Light fastness of azo dyes.
| Dye No. | Light Fastness |
|---|---|
| 14q | 4 |
| 14r | 2 |
| 14s | 5 |
| 14t | 2 |
| 14u | 3–4 |
| 14v | 3–4 |
| 14w | 5 |
5. Antimicrobial Activities
The inhibition zone diameter data for the arylhydrazopyridone disperse dyes 14q–w against Bacillus subtilis and Staphylococcus aureus (Gram positive bacteria), Escherichia coli and Pseudomonas aeruginosa (Gram negative bacteria) and Candida albicans (yeast) revealed that all tested dyes have potent positive antibacterial activities against four of the five tested microorganisms. Disperse dye 14q indicated a considerable cytolytic impact following five days of incubation [28]. After confirming with certainty that this type of disperse dyes has biological activity and that studies in this field are promising and further investigations are warranted, we synthesized the new arylhydrazono-1.4-diethyl-2,6-dioxo-tetrahydropyridine-3-carbonitrile dye 14x and the aryl- hydrazono-1-butyl-4-ethyl-2,6-dioxotetrahydropyridine-3-carbonitrile dye 14y in an innovative and easy way by reacting pyridones 13r or 13s with aryldiazonium salts. Yields were excellent and these dyes demonstrated acceptable antimicrobial activities [29].
Colors 14x, and 14y were utilized for dyeing polyester fabric with 2% shading, using a commercial carrier HC and Tanavol EP 20017 (supplied by TANATEX Chemicals B.V., Ede, Gelderland, The Netherlands) as an environmentally friendly carrier at dyeing temperatures of 100 °C. The data in Table 3 reveals that achieving the dyeing procedure using the eco-friendly carrier yielded results in a better color depth K/S (4.74 and 3.46) than the non-eco carrier whose results were 4.04 and 3.01 [30].
Table 3.
Optical measurements of polyester fabrics dyed at 100 °C.
| DyeNumber | λmax | K/S |
|---|---|---|
| Eco-friendly carrier | ||
| 14x | 445 | 4.74 |
| 14y | 450 | 3.46 |
| Non-Eco-friendly carrier | ||
| 14x | 445 | 4.04 |
| 14y | 445 | 3.01 |
6. In Vitro Cytotoxicity Screening
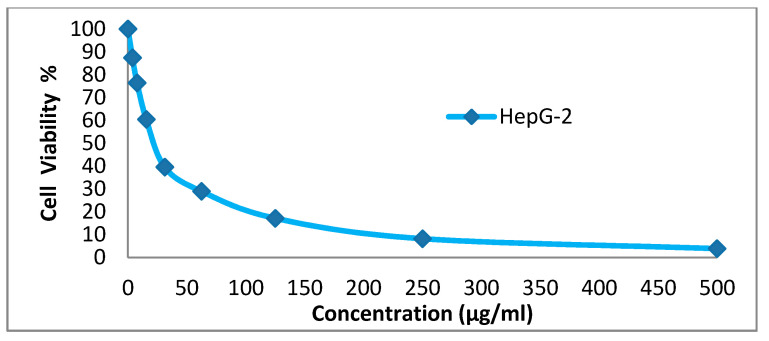
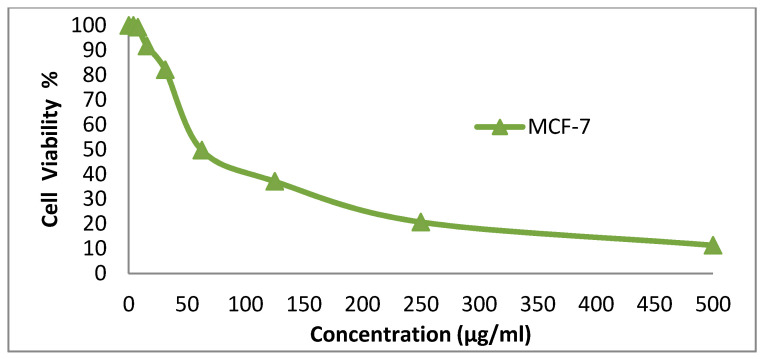
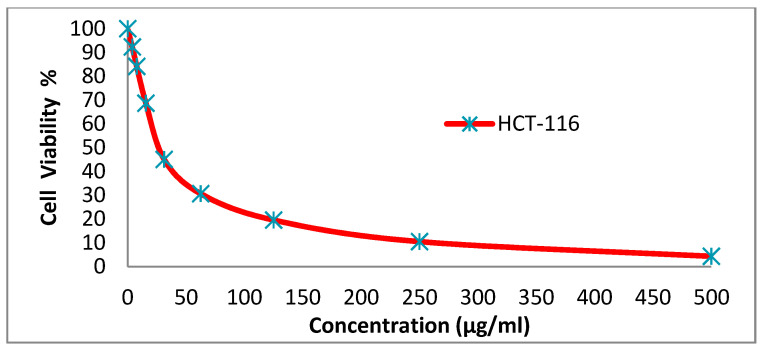
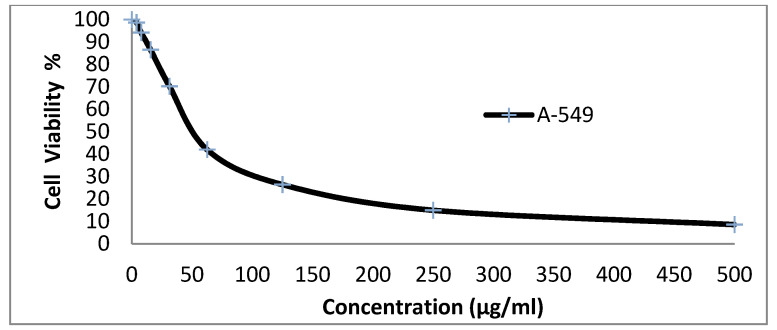
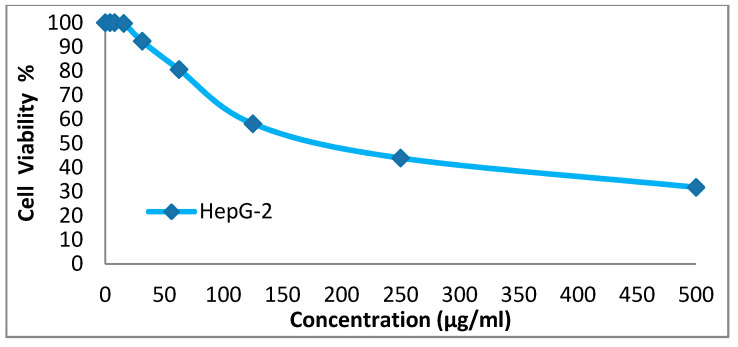
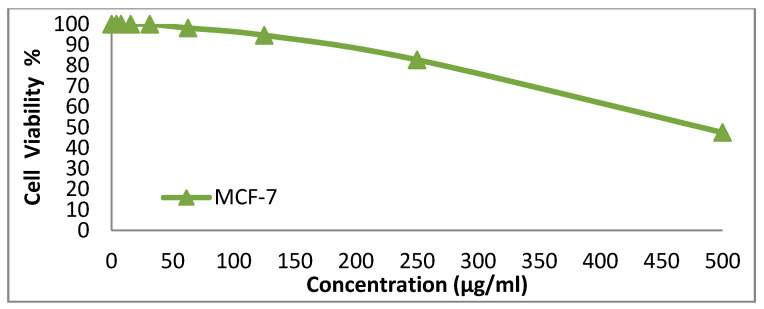
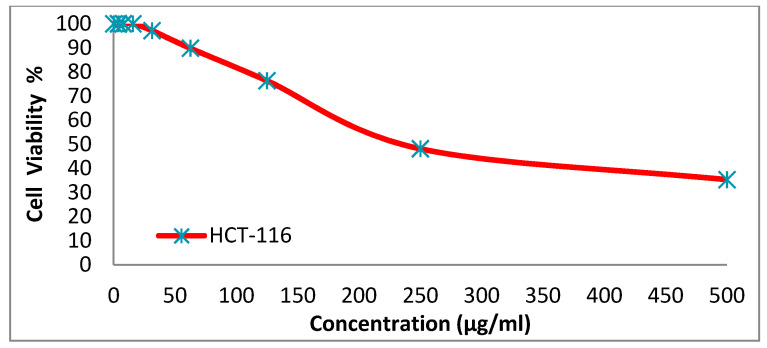
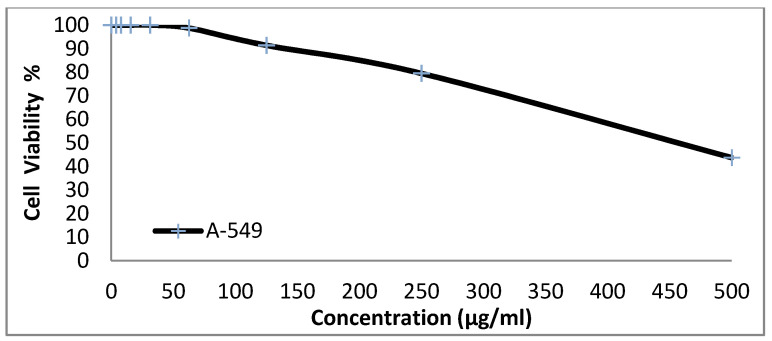
We tested the anticancer activity of the dyes 14x and 14y against the HepG-2, HCT-116, A-549 and MCF-7cell lines. Various concentrations of disperse dye 14x and disperse dye 14y were utilized for evaluating the IC50 (µg/mL). Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10 show that dye 14x had strong activity 23.4 (HepG-2), 62.2 (HCT-116), 28 (A-549) and 53.6 (MCF-7) µg/mL, respectively, while dye 14y revealed weak action 196 (HepG-2), 482 (HCT-116), 242 (A-549) and 456 (MCF-7) µg/mL, respectively [30].
Figure 3.
Evaluation of disperse dye 14x on cell line HepG-2.
Figure 4.
Evaluation of disperse dye 14x on cell line MCF-7.
Figure 5.
Evaluation of disperse dye 14x on cell line HCT-116.
Figure 6.
Evaluation of disperse dye 14x on cell line A-549.
Figure 7.
Evaluation of disperse dye 14y on cell line HepG-2.
Figure 8.
Evaluation of disperse dye 14y on cell line MCF-7.
Figure 9.
Evaluation of disperse dye 14y on cell line HCT-116.
Figure 10.
Evaluation of disperse dye 14y on cell line A-549.
7. Antioxidant Activities
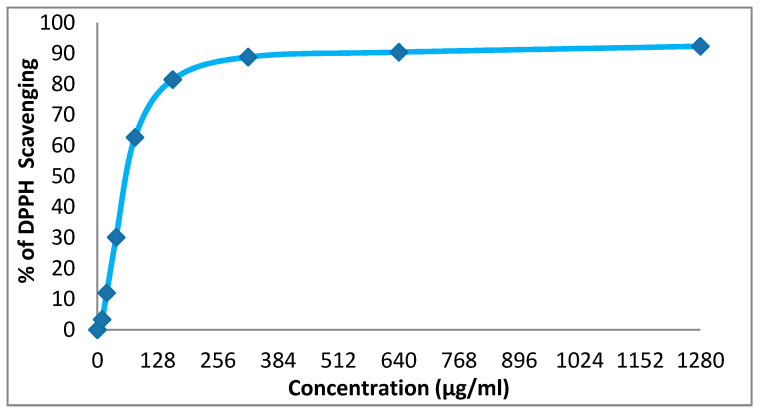
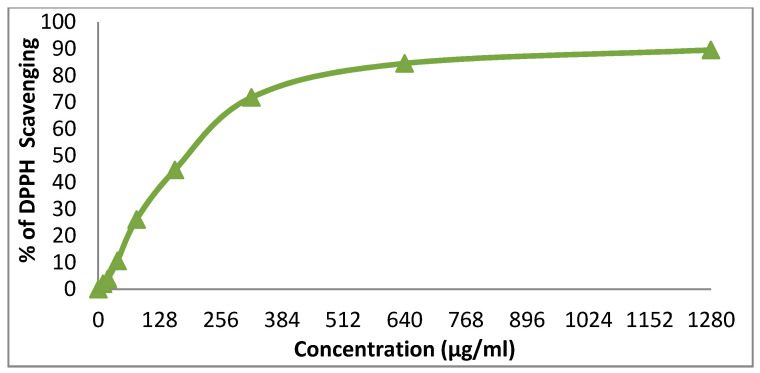
We evaluated the antioxidant properties of the two dispersant dyes prepared by us in an easy and effective way in vitro by their 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals scavenging activity. Figure 11 and Figure 12 demonstrate the good antioxidant activity of the dispersant dye 14x, with IC50 of 64.5 (more than ascorbic acid used as a standard, with an IC50 of 14.2), while the disperse dye 14y had weak antioxidant activity (IC50 191.6) [30].
Figure 11.
Evaluation of the antioxidant effect of disperse dye 14x.
Figure 12.
Evaluation of the antioxidant effect of disperse dye 14y.
8. High Temperature Dyeing as Environmentally Benign Method
During our study, we have taken into account the ability of disperse dyes to preserve the environment by reducing dye residues by coloring polyester fabrics with the dyes 14x and 14y using a high pressure and temperature dyeing strategy. The degree of dye exhaustion in dyebaths has been compared to the low-temperature dyeing technique as dyebath wastes directly and negatively affect the environment. It should be noted that the degree of absorption of the dye by the high-pressure high-temperature (HT) dyeing technique for all polyester fabrics is completely inconsistent with the low-temperature dyeing process, and therefore (HT) expanded and deepened the color strength K/S of the dyes examined, evaluated at 309 and 265% (Table 4). Thus, this gives clear evidence that the measurement of the color present in the dye resulting from the use of the high-pressure high-temperature (HT) dyeing technique is virtually non-existent and ineffective, therefore it is clearly, contaminated, and completely positive for the environment as these remaining colors in the dyebaths are typically dumped into the environment as waste, harming the environment [31].
Table 4.
Color strengths and light fastness of polyester fabrics.
| Dye No. | λmax | K/S | Light Fastness |
|---|---|---|---|
| 14x | 445 | 19.38 | 5–6 |
| 14y | 450 | 12.63 | 5 |
Dyes 14x and 14y were utilized for coloring polyester fabrics by means of the high temperature coloring strategy, Table 4 reveals their excellent perspiration and washing fastness properties and fastness to light which was not as good for the untreated polyester colored fabrics [31]. After we evaluated the pyridone-based disperse dyes and their resistance to bacteria, we also evaluated and tested untreated polyester fabrics dyed with dyes 14x and 14y using the high-temperature and high-pressure dyeing method for their inhibitory effect on the development of six different microorganisms. The antimicrobial screening results showed that untreated fabrics dyed with dye 14x had no antibacterial properties against the tested pathogenic bacterial strains, while untreated polyester fabrics dyed with dye 14y had good antibacterial properties against Staphylococcus sciuri (13 mm), strong antibacterial activity against Pseudomonas aeruginosa (24 mm), and very strong antibacterial properties against Escherichia coli (39 mm) [31].
9. The Multifunctional Performance that nano TiO2 Imparts to Polyester Fabric
In an attempt to acquire fabric dyed by the disperse dyes, the post-treated polyester fabrics dyed with disperse dyes 14x and 14y was subjected to a two-step hot process after they were completely immersed in a solution of TiO2 NPs nanoparticles at 80 °C then we performed a curing process at 140 °C. Here, we can say that the treated fabrics are recognized to provide ideal UV protection factors ranging from 34.9 and 283.6 (Table 5) [31].
Table 5.
UPF values of treated polyester fabrics.
| DyeNo | TiO2 % | Ultraviolet Protection Factor UPF |
|---|---|---|
| Blank | 8.2 | |
| untreated | 236.2 | |
| 1 | 255.3 | |
| 14x | 2 | 201.6 |
| 3 | 283.6 | |
| 4 | 278.7 | |
| 5 | 244.6 | |
| untreated | 25.5 | |
| 1 | 34.3 | |
| 2 | 34.0 | |
| 14y | 3 | 34.9 |
| 4 | 32.6 | |
| 5 | 32.5 |
The dyed fabrics treated with TiO2 NPs additionally exhibited strongly improved light fastness properties, where it became 6 for dye 14x and also 5–6 for dye 14y. Likewise, the possible uses of these fabrics treated with titanium nanoparticles have been studied, for example, anti-fungal and self-cleaning activities. Interestingly, the polyester dyed fabrics with treated TiO2 NPs and disperse dye 14x had strong antifungal properties against Aspergillus flavus and Penicillium chrysogenum. This is predictable in light of published data [32] where the treatment of polyester fabrics with inorganic NPs oxides may confer upon these fabrics an antimicrobial function (Table 6).
Table 6.
Antifungal activity of the treated dyed polyester fabrics.
| Dye No. | Aspergillus Flavus | Penicillium Chrysogenum |
|---|---|---|
| 14x | 21 | 19 |
| Natamycin | 18 | 19 |
10. Conclusions
The large number of uses of disperse dyes have caught the attention of many researchers who have emphasized their importance in the last decade. Our contributions in the field of applied chemistry have been presented through this review article which aims to provide a comprehensive overview of the new disperse dyes that we have synthesized in our laboratories via an environmentally safe route to reduce environmental pollution and demonstrate the characteristics and multiple uses of these new dyes. Since manufacturing of the first disperse dye began in 1923, the prevalence of disperse dyes has made them today the second largest sector in the dye industry. The dye industry faces the challenge of manufacturing high-quality disperse dyes, and this is one of the driving factors for its research and development studies. Nowadays a wide range of dyes is being created on the basis of the potential end-uses such as dyes for sportswear, automotive fabrics and transfer printing. These days efforts are being made to invent dyes for dispersant-free dyeing to reduce the pollution caused by dyeing polyester fabrics with these dyes, and this is what we indicate in this review article about the importance of disperse dyes and their direct correlation with the environment.
Author Contributions
A.M.A.-E. and M.A.E.-A. wrote, reviewed, and edited the manuscript. All authors have read and agree to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Al-Mousawi S.M., EI-Apasery M.A., Elnagdi M.H. Arylazoazines and arylazoazoles as interesting disperse dyes: Recent developments with emphasis on our contribution laboratory outcomes. Eur. J. Chem. 2014;5:990–998. doi: 10.5155/eurjchem.5.1.192-200.883. [DOI] [Google Scholar]
- 2.Gaffer H.E., Shkra S., Abbas D., Allam E.A. Synthesis and antimicrobial activity of some new sulphonamide disperse dyes and their applications to polyesterfibres. J. Appl. Polym. Res. 2013;9:4051–4058. [Google Scholar]
- 3.Elapasery M., Hussein A.M., Eladasy A.A., Saleh M.O., Kamel M. Microwave Assisted Synthesis of Some Azo Disperse Dyes with Antibacterial Activities. Part1. Egypt. J. Chem. 2019;62:853–859. [Google Scholar]
- 4.Hunger K. Industrial Dyes: Chemistry, Properties, Applications. John Wiley & Sons; Hoboken, NJ, USA: 2003. pp. 134–158. [Google Scholar]
- 5.El-Apasery M.A., Yassin F.A., Abd El-Azim M.H.M., Abdellatif M.E.A. Synthesis and Characterization of some Disperse Dyes based on Enaminones. J. Text. Color. Polym. Sci. 2020;17:1–5. doi: 10.21608/jtcps.2020.22547.1034. [DOI] [Google Scholar]
- 6.Mitsuishi M., Naruoka Y., Shimizu M., Hamada K., Ishiwatai T. The effect of disperse dyes on the glass transition temperature of polymers. J. Soc. Dye. Colour. 1996;112:333–335. doi: 10.1111/j.1478-4408.1996.tb01769.x. [DOI] [Google Scholar]
- 7.Huang W., Qian H. Structural characterization of C.I. Disperse Yellow 114. Dyes Pigment. 2008;77:446–450. doi: 10.1016/j.dyepig.2007.07.012. [DOI] [Google Scholar]
- 8.Huang W. Structural and computational studies of azo dyes in the hydrazone form having the same pyridine-2,6-dionecomponent(II):C.I.DisperseYellow119 and C.I.DisperseYellow211. Dyes Pigment. 2008;79:69–75. doi: 10.1016/j.dyepig.2008.01.007. [DOI] [Google Scholar]
- 9.Dostanić J., Valentić N., Ušćumlić G., Mijin D. Synthesis of 5-(substituted phenylazo)-6- hydroxy-4-methyl-3--cyano-2-pyridones from ethyl 3-oxo-2-(substituted phenylazo)butanoates. J. Serb. Chem. Soc. 2011;76:499–504. doi: 10.2298/JSC100618044D. [DOI] [Google Scholar]
- 10.Mijin D.Ž., Baghbanzadeh M., Reidlinger C., Kappe C.O. The microwave-assisted synthesis of 5-arylazo-4,6-disubstituted-3-cyano-2-pyridonedyes. Dyes Pigm. 2010;85:73–78. doi: 10.1016/j.dyepig.2009.10.006. [DOI] [Google Scholar]
- 11.Al-Zayd K.M., Borik R.M., Elnagdi M.H. Studies with arylhydrazonopyridinones: Synthesis of new arylhydrazono thieno[3,4-c]pyridinones as novel D2T2 dye class; classical verse green methodologies. Ultrason. Sonochem. 2009;16:660–668. doi: 10.1016/j.ultsonch.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Helal M.H. Synthesis and characterization of a new series of pyridinone azo dyes for dyeing of synthetic fibers. Pigm. Resin Technol. 2004;33:165–171. doi: 10.1108/03699420410537287. [DOI] [Google Scholar]
- 13.El-Bayouki M., Abdel Hameed K., Asyouni W.M., Mohamed Y.A., Aly M.M., Abbas S.Y. Novel 4(3H)-quinazolinones containing biologically active thiazole, pyridinone and chromene of expected antitumor and antifungal activities. Eur. J. Chem. 2011;2:455–462. doi: 10.5155/eurjchem.2.4.455-462.171. [DOI] [Google Scholar]
- 14.Okada Y., Hihara T., Morita Z. Analysis of the photofading of phenylazo-aniline and phenylazo-pyridone disperse dyes on poly(ethyleneterephthalate) substrate using the semi empirical molecular orbital PM5method. Dye. Pigment. 2008;79:111–125. doi: 10.1016/j.dyepig.2008.01.013. [DOI] [Google Scholar]
- 15.Okada Y., Hihara T., Morita Z. Photofading of phenylazo-aniline,-pyridone and-quinolone disperse dyes on anylon6 substrate. Coloration Technol. 2009;125:86–98. doi: 10.1111/j.1478-4408.2009.00179.x. [DOI] [Google Scholar]
- 16.Ahmed K.A., Elhennawy H.M., Elkashouti M.A. MicrowaveAssiststhe Synthesis of Pyridone azo Dyes and their Application in Polyester Printing. Res. J. Chem. Sci. 2012;2:14–19. [Google Scholar]
- 17.Desai V.R., Patel D., Desai C.M., Joshi H.D. Sulphaphenazolamino-anthraquinoneacid, reactive and substituted pyridine disperse dyes-part-II. J. Inst. Chem. (India) 2001;73:89–91. [Google Scholar]
- 18.Okada Y., Hihara T., Morita Z. Analysis of the catalytic fading of pyridone-azo disperse dyes on polyester using the semi-empirical, molecular orbitalPM5 method. Dye. Pigment. 2008;78:179–198. doi: 10.1016/j.dyepig.2007.12.001. [DOI] [Google Scholar]
- 19.Jang H.K., Doh S.J., Lee J.J. Eco-friendly dyeing of poly(trimethyleneterephthalate) with temporarily solubilized azo disperse dyes based on pyridine derivatives. Fibers Polym. 2009;10:315–319. doi: 10.1007/s12221-009-0315-2. [DOI] [Google Scholar]
- 20.Gadre J.N., Periaswamy R.M.S., Mulay M., Vaze C.S. Synthesis of pyridine based azo disperse dyes. Indian J. Heterocycl. Chem. 2006;16:43–46. [Google Scholar]
- 21.Balalaie S., Kowsari E., Hashtroudi M.S. An Efficient Method for the Synthesis of 3-Cyano-6-hydroxy- 2(1H)-pyridinones under Microwave Irradiation and Solvent-free Conditions. Mon. Chemi. 2003;134:453–456. doi: 10.1007/s00706-002-0551-2. [DOI] [Google Scholar]
- 22.Balalaie S., Nemati N. One-pot preparation of coumarins by Knoevenagel condensation in solvent-free condition under microwave irradiation. Heterocycl. Comm. 2001;7:67. doi: 10.1515/HC.2001.7.1.67. [DOI] [Google Scholar]
- 23.Balalaie S., Sharifi A., Ahangarian B., Kowsari E. Microwave enhanced synthesis of quinazolines in solvent-free condition. Heterocycl. Comm. 2001;7:337. doi: 10.1515/HC.2001.7.4.337. [DOI] [Google Scholar]
- 24.Ashkar S.M., El-Apasery M.A., Touma M.M., Elnagdi M.H. Synthesis of Some Novel Biologically Active Disperse Dyes Derived from 4-Methyl-2,6-dioxo-1-propyl-1,2,5,6-tetrahydropyridine-3-carbonitrile as Coupling Component and Their ColourAssessment on PolyesterFabrics. Molecules. 2012;17:8822–8831. doi: 10.3390/molecules17088822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakoma K.J., Bello K.A., Yakubu M.K. Synthesis of Some Azo Disperse Dyes from1-Substituted 2-Hydroxy-6-pyridone Derivatives and Their Colour Assessment on Polyester Fabric. Open J. App. Sci. 2012;2:54–59. [Google Scholar]
- 26.El-Apasery A.M., Shakra S., Abbas D., Gaffer H., Allam A. Synthesis of some azo disperse dyes based on pyridine moiety and their application on polyester fabrics. Egypt. J. Chem. 2018;60:97–102. [Google Scholar]
- 27.Al-Etaibi A., El-Apasery M.A., Al-Awadi N. The effect of dispersing agent on the dyeing of polyester fabrics with disperse dyes derived from1,4-diethyl-2,6-dioxo-1,2,5,6-tetrahydropyridine-3-carbonitrile. Eur. J. Chem. 2013;4:240–244. doi: 10.5155/eurjchem.4.3.240-244.834. [DOI] [Google Scholar]
- 28.Al-Etaibi A., El-Apasery M.A., Mahmoud H., Al-Awadi N. Synthesis, characterization and antimicrobial activity, and applications of new azo pyridone disperse dyes on polyester fabric. Eur. J. Chem. 2014;5:321–327. doi: 10.5155/eurjchem.5.2.321-327.969. [DOI] [Google Scholar]
- 29.Al-Etaibi A.M. Synthesis andAntimicrobial Activity of some Disperse Dyes derived from Pyridones. Int. J. ChemTech Res. 2019;12:129–133. doi: 10.20902/IJCTR.2019.120515. [DOI] [Google Scholar]
- 30.Al-Etaibi A.M., El-Apasery M.A. Dyeing Performance of Disperse Dyes on Polyester Fabrics Using Eco-Friendly Carrier and Their Antioxidant and Anticancer Activities. Int. J. Environ. Res. Public Health. 2019;16:4603. doi: 10.3390/ijerph16234603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Etaibi A.M., El-Apasery M.A. NanoTiO2 Imparting Multifunctional Performance on Dyed Polyester Fabrics with some Disperse Dyes Using HighTemperature Dyeing as anEnvironmentally Benign Method. Int. J. Environ. Res. Public Health. 2020;17:1377. doi: 10.3390/ijerph17041377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Etaibi A.M., El-Apasery M.A. Dyeing of polyester with disperse dyes: Part3. Characterization of ZnO nanoparticles treated polyester fabrics for antibacterial, self-cleaning and UV protective. Int. J. ChemTech Res. 2016;9:162–169. [Google Scholar]