Abstract
Autophagy is a cellular catabolic process that maintains intracellular homeostasis using lysosomal degradation systems. We demonstrate that inhibiting autophagy by depleting essential autophagy elongation proteins, Atg5 or Atg7, induces ISG15 expression through STING-mediated cytosolic dsDNA response. Genome stability is impaired in ATG5- or ATG7-depleted cells, and thus, double-strand breakages of DNA increase and cytosolic dsDNA accumulates. Accumulated cytosolic dsDNA induces the STING pathway to activate type I IFN signals which induce STAT1 activity and downregulate ATF3. When depletion of ATG5 or ATG7 inhibits autophagy, ATF3 is downregulated and STAT1 is upregulated. Furthermore, inhibiting autophagy induces ISG15 expression through STAT1 activation, which promotes acquisition of tumor-associated phenotypes such as migration, invasion, and proliferation. In conclusion, it appears that via the STING-mediated cytosolic dsDNA response, the STAT1-ISG15 axis mediates the relationship between autophagy and the immune system in relation to tumor progression. Moreover, combined with autophagy control, regulating ISG15 expression could be a novel strategy for cancer immunotherapy.
Subject terms: Cancer, Macroautophagy
Introduction
Autophagy induced by various stress signals is essential for cellular homeostasis to maintain suitable environmental conditions [1, 2]. Under conditions of stress, double-membraned autophagosomes form and fuse with lysosomes where they eliminate and recycle the cargos [3–5]. Every step of autophagy is strictly controlled by diverse mechanisms at transcriptional, translational, or posttranslational levels [6–10]. As reported, autophagy is highly related to the presence of tumors, but the role of autophagy in tumorigenesis remains elusive [11–19]. The role of autophagy in cancer varies with the cellular context or tumor stage and has been linked to both tumor suppression and tumor promotion. Autophagy is also related to immune responses such as inflammation [20–27]. Several cytokines regulate autophagy and control autophagy-related proteins, such as UNC-51-like kinase 1 (ULK1) or Beclin1 [28–30]. Autophagy contributes to the immune response as well, including antiviral responses [21, 26, 27]. Viral pathogen-associated molecular patterns, which are recognized by pattern-recognition receptors, induce autophagy and activate type I interferons [31]. Cytosolic dsDNA-mediated reaction is another well-known example [32, 33].
In response to stressors such as viral infection, cyclic GMP-AMP (cGAMP) synthase (cGAS), a cytosolic dsDNA sensor, recognizes and interacts with cytosolic dsDNA to synthesize cGAMP. cGAMP then activates the stimulator of interferon genes (STING), a pivotal protein of the cytosolic dsDNA response inducing immune pathways by triggering the TANK-binding kinase 1 (TBK1)-interferon regulatory factor 3 (IRF3) axis [34–37]. STING activates TBK1 to phosphorylate IRF3. IRF3 translocates to the nucleus to induce type I interferons (IFN) and other immune-related genes [38]. Type I IFNs, which are multifunctional cytokines, cause activation of the cellular pathways involved in viral infection, such as the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway. After type I IFNs bind to their receptor, STAT1 and STAT2 are phosphorylated by tyrosine kinase 1 and JAK1. The phosphorylated STATs immediately form a dimer to interact with IRF9 and assemble ISGF3, which functions as a transcription factor inducing the expression of IFN-stimulated genes (ISGs) [39, 40].
We demonstrated that STING-meditated cytosolic dsDNA response induces ISG15 expression upon depletion of key autophagy elongation proteins, ATG5 or ATG7. On investigating the interaction between autophagy, immunity, and tumorigenic process, our results provided critical insights into immunotherapy as a cancer treatment strategy.
Results
Genome stability is impaired by defective autophagy
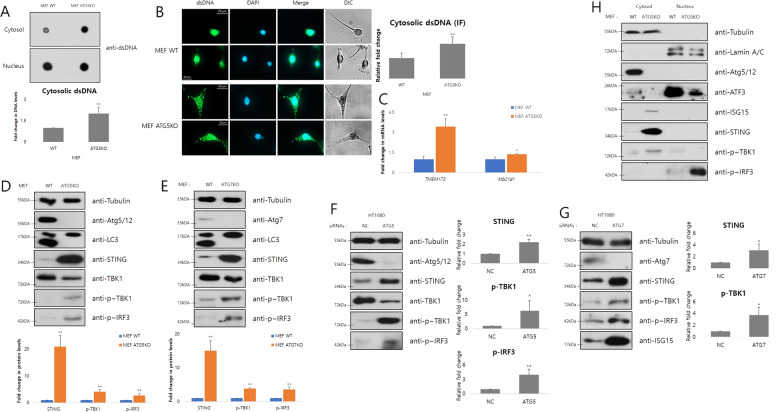
Autophagic proteins contribute to numerous cellular processes such as tumorigenesis, immune response, and cellular signaling pathways [41–43], in addition to their roles in autophagy. We demonstrated a new extra-autophagic function of the autophagy-related proteins, ATG5 and ATG7. Nuclear translocation of Ku70 and Ku80, related to nonhomologous end joining (NHEJ) by directly binding to the breakage site of DNA [44], was decreased in Atg5- or Atg7-depleted mouse embryonic fibroblasts (MEFs) (Figs. 1a and S1A), and in HT1080 or MCF7 cells treated with siRNAs for Atg5 or Atg7 (Fig S1B and C). However, substantial DNA damage rescued the nuclear Ku70 level in Atg5 knockout MEFs (Fig. S1A), indicating that DNA repair processes remains unimpaired, whereas the basal level of nuclear Ku proteins is chronically decreased by defective autophagy. Since NHEJ contributed by Ku proteins plays an important role in genome stability [45], we evaluated levels of rH2AX and phosphorylated ATM, representative molecular markers of genome stability [46]. Consistent with the previous results, rH2AX and phosphorylated ATM were increased after Atg5 or Atg7 depletion (Figs. 1b, S1D, E, and F). It was supported by experimental results with DNA damaging agents, camptothecin or etoposide (Figs. 1c, S1G, D, and H). Nuclear cGAS, an inhibitor of DNA double-strand breakages (DSBs) repair [47, 48], was also increased in Atg5 knockout MEFs (Fig. 1e). Since rH2AX and nuclear cGAS are DSB markers, DSBs level after Atg5 or Atg7 depletion was analyzed using a neutral comet assay. We observed increased comet tail lengths indicating DSBs after depletion of Atg5 or Atg7 (Figs. 1f, S1I, J, and K). These data suggest that inhibition of autophagy elongation by depletion of Atg5 or Atg7 impairs genome stability in various ways, such as increased DNA DSBs.
Fig. 1. Genome stability is impaired by depletion of essential autophagic proteins.
a, e Immunoblot analysis using the indicated antibodies was performed after nuclear fractionation. The density of indicated nuclear proteins was normalized to lamin A/C (n = 4 for Ku70, Ku80 in 1A and n = 3 for cGAS in 1E). *p < 0.05, **p < 0.01 (Student’s t test). b Immunoblot analysis using the indicated antibodies was performed. The density of indicated proteins was normalized to tubulin (n = 3). *p < 0.05, **p < 0.01 (Student’s t test). c After treating the indicated cells with camptothecin (CPT), 1 μM for 2 h or not, immunoblot analysis using the indicated antibodies was performed. The density of indicated proteins was normalized to tubulin (n = 3). *p < 0.05, **p < 0.01 (Student’s t test). d After treating the indicated cells with camptothecin (CPT), 1 μM for 2 h or not and then performing nuclear fractionation, immunoblot analysis using the indicated antibodies was performed. f Neutral comet assay was performed, and cells were visualized using a fluorescence microscope. The data from n > 50 cells were analyzed and digitalized using OpenComet (n = 55 for MEF WT, and n = 52 for MEF ATG5KO). *p < 0.05, **p < 0.01 (Student’s t test).
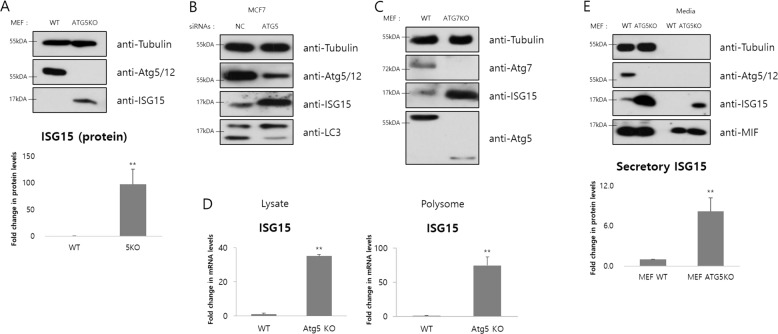
Depletion of autophagic proteins increases ISG15 expression
To identify the effects of impairment of genome stability induced by autophagy inhibition, we performed RNAseq to evaluate changes in gene expression in WT, Atg5 knockout, and Atg7 knockout MEFs. Of genes with more than twofold changes in expression level, 1489 genes overlapped at vs_ATG5KO and vs_ATG7KO (Fig. S2A, left). In addition, of genes filtered with more than twofold, P value under 0.05 and FDR under 10%, 91 genes overlapped at vs_ATG5KO and vs_ATG7KO (Fig. S2A, right). Among the top ten category terms of ontology, 380 genes had more than a twofold decrease and 151 genes had more than a twofold increase in both cells (Fig. S2B). Out of the genes in the top ten category terms of ontology filtered with more than twofold, P value under 0.05 and FDR under 10%, the expression level of eight genes was decreased, and that of 41 genes was increased in the both (Fig. S2C). List of the ten most upregulated or downregulated genes in the common merged area of Fig. S2C are shown in Fig. S2D.
Hence, we focused on immune-related genes classified under the categories of immune system processes, response to virus, defense response to virus and innate immune response. One of these genes, type I IFN induction was confirmed by qRT-PCR at transcriptional level (Fig. S2E), as well as induction of another immune-related genes, for example CXCL10 by defective autophagy (data not shown). We also found that the mRNA levels of IFN-stimulated gene 15(Isg15), which has an antiviral function and is related to the type I IFNs [49, 50], were increased in Atg5- and Atg7-depleted MEFs (Fig. S2D). Similar as the RNAseq data, the ISG15 protein level was increased by depletion of Atg5 or Atg7 (Figs. 2a–c and S2F). We also identified the induction of ISG15 by defective autophagy at transcriptional and translational levels (Fig. 2d). However, the ISGylation was little changed under the same condition (Fig. S2G). The increase in ISG15 remains independent of autophagic degradation induced by bafilomycin A1 (Baf.A1), a late stage inhibitor of autophagic flux inducing accumulation of target proteins in autophagolysosome (Fig. S2H). In other words, the ISG15 induction is not due to posttranslational modifications but due to expressional changes. Since ISG15 had been reported as a secretory protein contributing to immune response [51], we analyzed the ISG15 secretion in Atg5-depleted MEFs compared with WT. As expected, the secreted ISG15 was increased (Fig. 2e), whose band density was normalized to well-known secretory protein MIF. In summary, blocking autophagy by depleting Atg5 or Atg7 induces expression and secretion of ISG15.
Fig. 2. ISG15 expression is increased by depletion of essential autophagic proteins.
a–c Immunoblot analysis using the indicated antibodies was performed. The density of indicated proteins was normalized to tubulin (n = 4). *p < 0.05, **p < 0.01 (Student’s t test). d Relative mRNA levels of the indicated gene in whole cell lysates and translating polysomes were measured by real-time PCR. The mRNA levels were normalized to β-actin (n = 3). *p < 0.05, **p < 0.01 (Student’s t test). e After the concentration of cell growth media without serum for overnight, immunoblot analysis using the indicated antibodies was performed. The density of indicated proteins was normalized to tubulin (lysate) or MIF (media) (n = 3). *p < 0.05, **p < 0.01 (Student’s t test).
STAT1 induction upregulates ISG15 expression
In Fig. 2, we found ISG15 induction after depleting the essential autophagic proteins, ATG5 or ATG7. We then focused on searching for transcription factors related to the expression of ISG15. Among the candidates, STAT protein family, a well-known transcription factor family involved in the type I IFN pathway [40], was found to be upregulated after Atg5 and Atg7 depletion (Fig. S3A and B). Protein levels of total STAT1 were increased in Atg5-depleted cells (Figs. 3a–c and S3C), as well as Atg7-depleted cells (Fig. 3d, e). STAT1 is phosphorylated and then transported to the nucleus to regulate its target gene expression [52]. We observed that the phosphorylated STAT1 level was increased after depleting Atg5 or Atg7 (Figs. 3b–e and S3C). Localization in the nuclear fraction of both total and phosphorylated STAT1 was also increased compared with WT MEFs, indicating the activation of STAT1-mediated gene expression (Figs. 3f, S3D and E). In addition, STAT1 protein level was not changed by blocking autophagic flux with BafA1, suggesting that STAT1 induction is independent of autophagic degradation (Figs. 3g and S3F). To further elucidate the relationship between increased STAT1 and ISG15 expression in Atg5- or Atg7-depleted cells, we transfected the cells with siRNAs for STAT1 and ISG15. In Atg5 knockout MEFs, as expected, Stat1 knockdown blunted the ISG15 protein induction and mRNA expression (Fig. 3h, i). Meanwhile, ISG15 overexpression or knockdown had little effect on STAT1 expression level under the same conditions (Fig. 3j, k). Thus, these data indicate that increased STAT1 positively regulates ISG15 expression by activating its transcription when Atg5 or Atg7 is depleted.
Fig. 3. STAT1 induction positively regulates ISG15 expression after Atg5 or Atg7 depletion.
a–e Immunoblot analysis using the indicated antibodies was performed. The density of indicated proteins was normalized to tubulin (n = 4 for a, b, and d, n = 3 for c and e). *p < 0.05, **p < 0.01 (Student’s t test). f Immunoblot analysis using the indicated antibodies was performed after nuclear fractionation. The density of indicated nuclear proteins was normalized to lamin A/C (n = 3 for STAT1, and n = 4 for phosphorylated STAT1). *p < 0.05, **p < 0.01 (Student’s t test). g After treating indicated cells with bafilomycin A1 (Baf.A1), 1 μM for 2 h or not, immunoblot analysis using the indicated antibodies was performed. h, j After transfection with the indicated siRNAs (50 pmol) for 48 h, immunoblot analysis using the indicated antibodies was performed. i After transfection with the indicated siRNAs (50 pmol) for 48 h, relative mRNA levels of the indicated gene in whole cell lysates were measured by real-time PCR. The mRNA levels were normalized to β-actin (n = 3). *p < 0.05, **p < 0.01 (Student’s t test). k After transient transfecting plasmids pCMV-SPORT6 and pCMV-SPORT6-mISG15 into cells, immunoblot analysis using the indicated antibodies was performed.
Decreased ATF3 after depleting Atg5 or Atg7 regulates STAT1-ISG15
Sood et al. had demonstrated that activating transcription factor 3(ATF3), which belongs to the ATF/cAMP responsive element-binding family, negatively regulates Stat1, Isg15, and Atg5 genes by binding their promoter regions [53]. Accordingly, we evaluated ATF3 expression after depleting autophagic proteins. ATF3 was downregulated in Atg5- or Atg7-depleted cells (Figs. 4a, b, S4A and B), and nuclear ATF3 was decreased in the same cells, indicating that its function as transcription factor is inhibited (Figs. 4c and S4C). Seeing evidence that ATF3 knockdown increases total STAT1, phosphorylated STAT1, and ISG15 levels in WT MEFs (Fig. 4d), we concluded that decrease in ATF3 contributed to the induction of STAT1-ISG15.
Fig. 4. Decreased ATF3 by Atg5 or Atg7 depletion is responsible for the induction of the STAT1-ISG15 axis.
a, b Immunoblot analysis using the indicated antibodies was performed. The density of indicated proteins was normalized to tubulin (n = 4). *p < 0.05, **p < 0.01 (Student’s t test). c Immunoblot analysis using the indicated antibodies was performed after nuclear fractionation. The density of indicated nuclear proteins was normalized to lamin A/C (n = 4). *p < 0.05, **p < 0.01 (Student’s t test). d After transfection with the indicated siRNAs (50 pmol) for 48 h, immunoblot analysis using the indicated antibodies was performed. e After transfection with the indicated siRNAs (50 pmol) for 72 h, cells were pretreated with MG132, 5 μM for 1 h before harvest. Immunoblot analysis using the indicated antibodies was performed. f Relative mRNA levels in whole cell lysates were measured by real-time PCR. The mRNA levels were normalized to β-actin (n = 3). g, h After treating the indicated cells with MG132, 20 μM for 1 h or not (g), or bafilomycin A1 (Baf.A1), 1 μM for 2 h or not (h), immunoblot analysis using the indicated antibodies was performed.
To further confirm this, siRNAs against Atf3 and Stat1 were used in WT and ATG5 knockout MEFs. In Atg5-depleted cells, Atf3 knockdown additionally increased the induction of STAT1-ISG15 (Fig. 4e), whereas ATF3 overexpression negatively regulated the expressions of the two proteins (Fig. S4D). ISG15 overexpression did not affect ATF3, STAT1, or phosphorylated STAT1 levels (Fig. S4E). Meanwhile, Stat1 knockdown rescued ATF3 levels to those of WT MEF in Atg5 knockout MEFs (Fig. S4F). These results suggest that two transcription factors, STAT1 and ATF3, negatively regulate each other’s expression; ISG15 is an outcome of the two regulatings in opposite fashion when elongation step of autophagy is inhibited. Unlike STAT1 and ISG15, Atf3 mRNA levels measured by RNAseq and qRT-PCR were little changed by Atg5 or Atg7 depletion (Figs. 4f and S4G). Reduction of ATF3 protein depends on proteasomal degradation; [54] and inhibition of proteasomal degradation, but not autophagy, blocked the decrease in ATF3 in Atg5-depleted cells (Fig. 4g, h). MDM2, known as an E3 ligase for the ATF3 protein [54], was also increased in Atg5 knockout cells (Fig. S4H), and Mdm2 knockdown rescued ATF3 levels in the same cells (Fig. S4I).
Our results demonstrated that defective autophagy decreases ATF3 levels, which induces the STAT1-ISG15 axis. However, STAT1 negatively regulates ATF3 protein levels simultaneously. This suggests that STAT1 and ATF3 regulate one another in opposite fashion to control the expression of ISG15 when autophagy is inhibited by depletion of Atg5 or Atg7.
STING-mediated response is activated by Atg5 or Atg7 depletion
Thus far, we revealed that Atg5 or Atg7 depletion impairs genome stability and induces the STAT1-ISG15 axis. Our objective was also to locate the link connecting these two phenomena. Several reports had demonstrated that cytosolic dsDNA caused by genome instability induces IFN signaling [29, 30, 34, 37], which regulates STAT1 activation in many different situations. Thus, we hypothesized that the cytosolic dsDNA response was the link between the impairment of genome stability and the induction of STAT1-ISG15 axis. As we hypothesized, cytosolic dsDNA was increased in Atg5- or Atg7-depleted cells (Figs. 5a, b, S5A and B); however apoptotic signals were not induced in the same cells, indicating that increased cytosolic dsDNA is unrelated to damaged mitochondrial DNA (Fig. S5C and D). The increased cytosolic dsDNA level and the induced STAT1-ISG15 axis, in fact, were little changed by EtBr-treated mitochondrial depletion in ATG5KO MEFs (Fig. S5E and F). The mRNA levels of Tmem173(gene encoding STING protein, a central molecule of the cytosolic dsDNA response) were increased and Mb21d1(gene encoding cGAS protein, a cytosolic dsDNA sensor) was also slightly increased (Figs. 5C and S5G), and protein levels of cGAS and STING were also upregulated by defective autophagy (Figs. 5d–f and S5H). When cytosolic dsDNA signals arrive, second messenger 2′3′-cGAMP produced by cGAS activates STING, which then phosphorylates TBK1; in turn phosphorylated TBK1 activates IRF by phosphorylation. Markers of the STING-mediated response, phosphorylated TBK1, and phosphorylated IRF3 [41], were all increased in Atg5- or Atg7-depleted cells as STING was increased (Figs. 5d–f, S5I, J, and O). The binding affinity of phosphorylated IRF3 with STING and TBK1 was also increased (Fig. S5K). However, induction of these proteins was involved only slightly in the inhibition of autophagosome degradation (Fig. S5L and M). Based on the RNAseq data, activation of TBK1 and IRF3 was not due to a change in their mRNA expression levels (Fig. S5G), but due to STING-mediated phosphorylation. In addition, nuclear phosphorylated IRF3 was increased in Atg5-depleted cells, indicating the induction of its transcription factor activities (Figs. 5h, S5N and O). Therefore, increased cytosolic dsDNA by defective autophagy leads to activation of the STING-mediated pathway, including phosphorylation of TBK1 and IRF3. After phosphorylation, IRF3 translocates to the nucleus and functions as a transcription factor to regulate type I IFNs.
Fig. 5. STING-mediated cytosolic dsDNA response is activated by Atg5 or Atg7 depletion.
a Dot blotting analysis using the dsDNA antibody was performed after nuclear fractionation. The density of cytosolic dsDNA was normalized to nuclear dsDNA (n = 3). *p < 0.05, **p < 0.01 (Student’s t test). b Immunofluorescence using the dsDNA antibody and DAPI for nuclear staining was performed and cells were visualized using a fluorescence microscope (n = 115 for MEF WT, and n = 96 for MEF ATG5KO). *p < 0.05, **p < 0.01 (Student’s t test). c Relative mRNA levels of the indicated genes in whole cell lysates were measured by real-time PCR. The mRNA levels were normalized to β-actin (n = 3). *p < 0.05, **p < 0.01 (Student’s t test). d, e Immunoblot analysis using the indicated antibodies was performed. The density of indicated proteins was normalized to tubulin (n = 6 for d, and n = 3 for e). *p < 0.05, **p < 0.01 (Student’s t test). f, g Immunoblot analysis using the indicated antibodies was performed. The density of indicated proteins was normalized to tubulin (n = 3). *p < 0.05, **p < 0.01 (Student’s t test). h Immunoblot analysis using the indicated antibodies was performed after nuclear fractionation.
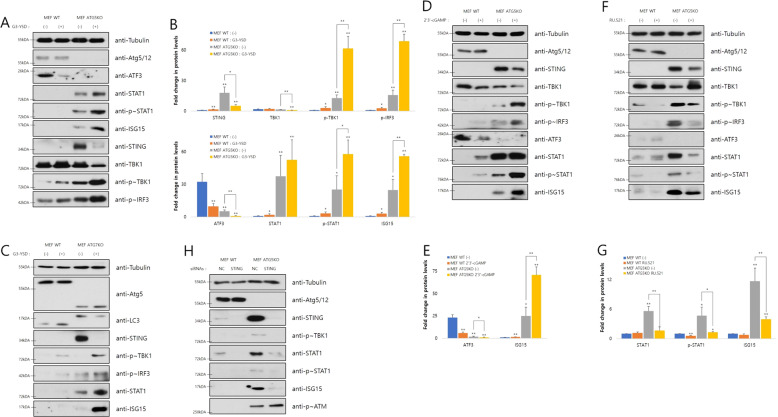
Cytosolic dsDNA pathway regulates the STAT1-ISG15 expression
To verify our hypothesis that activated cytosolic dsDNA response links impairment of genome stability with STAT1-ISG15 induction in Atg5- or Atg7-depleted cells, we performed the following experiments to reveal the relationship between the STING pathway and STAT1-ISG15 regulation. Transfection of G3-YSD, a palindromic DNA sequence stimulating cGAS, activated the STING-mediated pathway, repressed ATF3, and induced the STAT1-ISG15 axis by Atg5 or Atg7 depletion (Figs. 6a–c and S6A). Treating 2′3′-cGAMP showed similar effects of regulating STAT1-ISG15 as G3-YSD transfection (Fig. 6d, e). Meanwhile, treating RU.521, a cGAS inhibitor, repressed STAT1-ISG15 induction in Atg5-depleted cells (Fig. 6f, g), indicating that defective autophagy is an upstream signal of the STING pathway. STING knockdown in Atg5-depleted cells reduced phosphorylated TBK1, phosphorylated STAT1, and ISG15 levels compared to that in control cells (Figs. 6h, S6B and C). ISG15 overexpression or knockdown, however, had little effect on the STING-mediated pathway (Fig. S6D and E), meaning that the STING pathway regulates STAT1-ISG15 induction, while the reverse is impossible.
Fig. 6. Cytosolic dsDNA pathway regulates the expression of STAT1-ISG15.
a After transfecting cells with or without G3-YSD (10 μg/ml) for 24 h, immunoblot analysis using the indicated antibodies was performed. b The density of indicated proteins in a was normalized to tubulin (n = 3). *p < 0.05, **p < 0.01 (Student’s t test). c After treating cells with or without 2′3′-cGAMP (5 μg/ml) for 24 h, immunoblot analysis using the indicated antibodies was performed. d The density of indicated proteins in c was normalized to tubulin (n = 3). *p < 0.05, **p < 0.01 (Student’s t test). e After transfecting cells with or without G3-YSD (10 μg/ml) for 24 h, immunoblot analysis using the indicated antibodies was performed. f After treating cells with or without RU.521 (2 μg/ml) for 24 h, immunoblot analysis using the indicated antibodies was performed. g The density of indicated proteins in f was normalized to tubulin (n = 3). *p < 0.05, **p < 0.01 (Student’s t test). h After transfecting cells with the indicated siRNAs (50 pmol) for 48 h, immunoblot analysis using the indicated antibodies was performed.
Taken together, depletion of autophagy-related proteins involved in the elongation step induces the generation of cytosolic dsDNA caused by the impairment of genome stability. In turn, the increased cytosolic dsDNA activates the STING pathway to stimulate the induction of STAT1-ISG15 axis.
We next tried to determine how inhibiting steps in autophagy other than elongation affects the STAT1-ISG15 axis induction. Unlike the case of elongation proteins, depletion of Beclin1 involved in the nucleation of autophagosome has little effect on the STING pathway (Fig. S7A, B, C and D). However, additive effects on STAT1-ISG15 induction are observed in Atg5-depleted cells when Beclin1 downregulated (Fig. S7E). Treatment with Wortmannin, a Beclin1 complex inhibitor, shows similar effect as Beclin1 knockdown on the STAT1-ISG15 axis induction (Fig. S7F), but also has little effect on the STING pathway in Atg5-depleted cells (Fig. S7G). Interestingly, autophagy inducing signals, rapamycin treatment and starvation, also induce activation of the STAT1-ISG15 axis, without STING induction (Fig. S7H and I), as in the case of Beclin1 inhibition. Since these conditions are all distinct from the Atg5 or Atg7 depletion, further research is needed to comprehend the relationship between autophagy regulation and ISG15 induction.
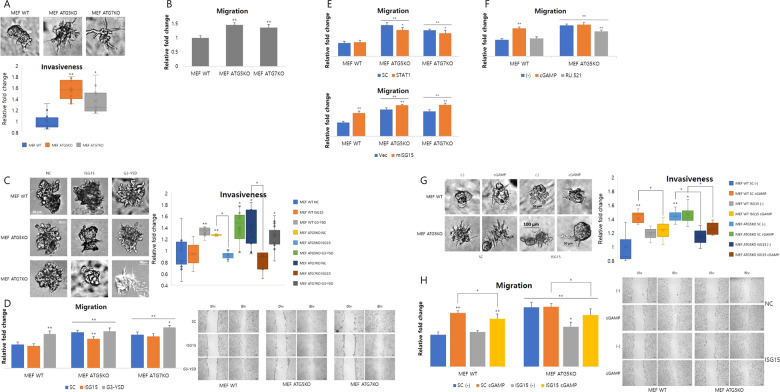
STING pathway induced ISG15 expression in Atg5- or Atg7-depleted cells positively regulate tumor-associated phenotypes
Bakhoum et al. had demonstrated that chromosomal instability is one of the driving forces of metastasis through STING-meditated cytosolic dsDNA response [55]. Moreover, tumor initiation or growth is promoted under defective autophagy conditions, including depletion of several autophagic proteins [11, 12, 16, 24, 56, 57]. Since the STING pathway is activated in Atg5- or Atg7-depleted cells, these cells show tumor-associated phenotypes such as cell migration and invasion. In fact, migration and invasion were increased in Atg5- or Atg7-depleted cells compared with the control (Figs. 7a, b, S8A and B). The induced migration and invasion were further increased after transfection of palindromic DNA, G3-YSD, and they returned to basal levels via Isg15 knockdown (Figs. 7c, d, S8C and D). Thus, the representative tumor-associated phenotypes are regulated by ISG15 involved in the STING-mediated pathway. The multifunctional protein ISG15 has roles in various biological processes such as tumorigenesis that remain elusive [58–64]. It can function as an oncogenic or tumor-suppressive protein depending on tumorigenic stages, circumstance and cancer types. According to our data, ISG15 functions as a oncoprotein to give cells protumorigenic phenotypes in an Atg5- or Atg7-depleted condition, supporting the findings of some previous reports [39, 58–60, 63, 64]. The changes in migration by STAT1 or ISG15 knockdown and ATF3 or ISG15 overexpression demonstrated that the STAT1-ISG15 axis is related to tumor (Figs. 7e, S8C and D). Migration of cells was also regulated by chemical treatments involved in the STING-mediated pathway. 2′3′-cGAMP treatment upregulates migration and RU.521 treatment downregulates it (Figs. 7f and S8D). Furthermore, elevated proliferation in Atg5-depleted cells, which has already been reported, was observed (Fig. S8F and G), which decreased after downregulating ISG15 (Fig. S8F). STING-mediated pathway regulated proliferation similarly (Fig. S8H, I, and J).
Fig. 7. ISG15 induction mediated by the STING pathway positively regulates tumorigenesis in Atg5- or Atg7-depleted cells.
a Invasiveness of indicated cells was identified using a 3D culture assay. Relative fold change of invasiveness is measured by Fiji and normalized to wild type (WT) (n = 3). *p < 0.05, **p < 0.01 (Student’s t test). b Migration of indicated cells was identified using a wound-healing assay. Relative fold change of migration is measured by Fiji and normalized to WT (n = 4). *p < 0.05, **p < 0.01 (Student’s t test). c After transfecting cells with the indicated siRNAs (50 pmol) or G3-YSD (10 μg/ml), invasiveness of the indicated cells was identified using a 3D culture assay. Relative fold change of invasiveness is measured by Fiji and normalized to WT (n = 3). *p < 0.05, **p < 0.01 (Student’s t test). d After transfecting cells with the indicated siRNAs (50 pmol) or G3-YSD (10 μg/ml), migration of the indicated cells was identified using a wound-healing assay. Relative fold change of migration is measured by Fiji and normalized to WT (n = 4). *p < 0.05, **p < 0.01 (Student’s t test). After treating cells with the indicated siRNAs (e), or drugs (f), migration of indicated cells was identified using a wound-healing assay. Relative fold change of migration is measured by Fiji and normalized to WT (n = 4). *p < 0.05, **p < 0.01 (Student’s t test). g After transfecting cells with the indicated siRNAs (50 pmol) followed by 2′3′′-cGAMP treatment, invasiveness of the indicated cells was identified using a 3D culture assay. Relative fold change of invasiveness is measured by Fiji and normalized to WT (n = 3). *p < 0.05, **p < 0.01 (Student’s t test). f After transfecting cells with the indicated siRNAs (50 pmol) followed by 2′3′-cGAMP treatment or not, migration of the indicated cells was identified using a wound-healing assay. Relative fold change of migration was measured by Fiji and normalized to WT (n = 4). *p < 0.05, **p < 0.01 (Student’s t test).
Taken together, properties of tumor such as migration, invasion, and proliferation are all increased by Atg5 or Atg7 depletion through the STAT1-ISG15 axis, induced by the STING-mediated pathway. To further confirm this, we measured migration and invasion after ISG15 knockdown with or without treating 2′3′-cGAMP. 2′3′-GAMP-induced migration and invasion in both control and Atg5-depleted cells were downregulated by ISG15 knockdown (Fig. 7g, h). Proliferation showed a similar pattern under the same conditions (Fig. S8K). As summarized in Fig. 8, the Atg5 or Atg7 depletion promotes tumor-associated phenotypes through STAT1-ISG15 induction. The STAT1-ISG15 axis induction is caused by accumulation of cytosolic dsDNA, which activates the STING pathway. In conclusion, ISG15 induction is responsible for acquisition of tumor-associated phenotypes under Atg5- or Atg7-depleted conditions.
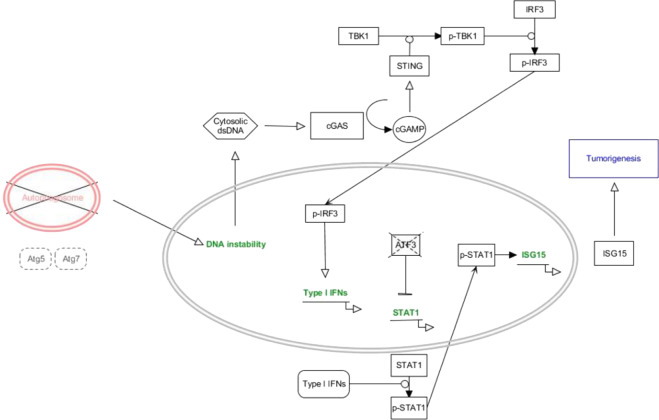
Fig. 8. Model of ISG15 induction involved in acquirement of tumor-associated phenotypes by inhibition of autophagy elongation.
Molecular model for cytosolic dsDNA response-induced STAT1-ISG15 expression in Atg5- or Atg7-depleted cells. The upregulated ISG15 induces acquisition of tumor-associated phenotypes such as migration, invasion, and proliferation.
Discussion
Autophagy inhibition impairs genome stability through various mechanisms [41, 42, 65, 66]. Here, we showed that the absence of Atg5 or Atg7, essential autophagy-related proteins for the membrane elongation, impairs genome stability (Fig. 1) in several different cell lines. The impairment of genome stability gives rise to the accumulation of cytosolic dsDNA activating the cGAS/STING-mediated pathway (Fig. 5). However, more information about the molecular mechanism of this process is needed. Complete understanding of this mechanism can potentially help treat countless diseases caused by impairment of genome stability such as cancer. Additional explanation is also necessary for the reduction of STING under hyperactivation condition with G3-YSD or 2′3′-cGAMP treatment in Atg5-depleted cells (Fig. 5a, c). The recently reported relationship between STING and autophagy such as STING-LC3 interaction or STING-induced autophagy [20, 32] may suggest the reason for STING reduction when cytosolic dsDNA signals constantly arrives at Atg5-depleted cells.
We linked the STING pathway induced by impairment of genome stability in cells with defective autophagy to increased STAT1 and decreased ATF3, which regulate each other in opposite fashion to control ISG15 expression, as transcription factors (Figs. 3 and 4). Mdm2 seems to contribute to their regulation, although the relationship between STAT1 and Mdm2 remains unknown. Since ATG5 and ATG7 are associated with the membrane elongation of autophagosome, inhibition of elongation step could induce the STAT1-ISG15 axis. However, the similar results of ISG15 induction were observed when the Beclin1 complex involved in a nucleation step of autophagy was inhibited. Meanwhile, Beclin1 depletion and Wortmannin treatment cannot induce activation of the STING-mediated pathway, as distinguished from the case of Atg5 or Atg7 depletion (Fig. S7). Likewise, rapamycin treatment and serum deprivation positively regulate STAT1-ISG15 induction without activation of the cGAS/STING pathway although they are regarded as autophagy inducers. In other words, only blockage of the autophagy elongation step activates the cGAS/STING pathway, inducing STAT1-ISG15 axis. Since STING is an ER-associated membrane protein and its activation is involved in translocation from the ER membrane [67, 68], the relationship between autophagy and ER could be considered as a possible explanation in terms of membrane elongation. In order to reveal the detailed process of the STING-mediated ISG15 induction caused by autophagy inhibition and to clarify the autophagy steps involved in tumor progression, and use our findings in cancer therapy, additional studies are necessary. It remains unclear why regulation of other autophagic steps also upregulates ISG15 expression, and further study to elucidate their relationship is required.
Researchers have actively studied the effect of autophagy on tumorigenesis because the regulation of cellular environment is important to antitumor strategies [12, 16, 17, 56]. Though the exact roles of autophagy on tumors are still controversial, several autophagy-related proteins have been considered novel targets of cancer therapy. Immunotherapy using immune system to efficiently treat tumors is an another promising area of cancer treatment [69, 70]. Since autophagy and immunity also affect each other by regulating key proteins of their pathways, regulation of STING known to be controlled by autophagy is a useful target of immunotherapy against tumors by forming negative feedback loop with ULK1 or ATG5/12 to induce autophagic degradation [32, 41]. Another example of their relationship is regulation of the NF-kB pathway by the autophagy receptor SQSTM1, which is accumulated in tumor cells [56, 71, 72]. Here, we pieced several concepts together regarding the relationship between autophagy and immunity in the context of tumor progression, centered on ISG15. According to the RNAseq results described in this paper, induction of various immune-related genes was also observed in Atg5- or Atg7-depleted cells, including Cxcl10. Investigating the expression of immune-related genes other than those of type I IFN system identified by us lead to interesting conclusions under the defective autophagy.
The effect of autophagy on tumors is said to be a “double-edged sword” [63]. According to our results, autophagy has tumor-suppressive roles in the view of STAT1-ISG15 regulation. Invasion, migration and proliferation, representative properties of tumor, are upregulated by ISG15 induction in Atg5- or Atg7-depleted cells (Fig. 7). Although our data suggests that ISG15 plays a role in tumor progression, its contribution to tumorigenesis by regulating the tumor microenvironment is controversial [58, 60–64]. Therefore, further studies elucidating the role of ISG15 in tumor regulation are required, including in vivo studies using a tumor xenograft. Nevertheless, ISG15 downregulation is suggested to be used as an effective auxiliary tool for immunotherapy against tumors to inhibit invasion, migration, proliferation, and epithelial–mesenchymal transition [56].
Materials and methods
Cell culture, antibodies, and reagents
The human fibrosarcoma cell line HT1080, breast cancer cell line MCF7, and human liver cancer cell line HepG2 were obtained from the Korean Cell Line Bank (Seoul, Korea). MEFs were obtained from the Yonsei University. NIH3T3 cells were obtained from the Incheon National University. These cell lines were cultured in DMEM/high glucose medium (Hyclone, SH30243.01) supplemented with 10% fetal bovine serum (FBS, Hyclone) and antibiotic-antimycotics (Gibco, 15240–062). Antibodies against Beclin1, rpS20, lamin A/C, MDM2, Ku70, Ku80, and PARP-1 were purchased from Santa Cruz Biotechnology (USA). Antibodies against ATG5, ATG7, LC3, ISG15, STAT1, phosho-STAT1, ATF3, phosphor-ATM, phposhpor-H2AX (rH2AX), STING, phosphor-TBK1, TBK1, phospho-IRF3, and α-tubulin were obtained from Cell Signaling Technology (USA). Antibodies against dsDNA was obtained from Abcam (UK), and the anti-rpS3 antibody was acquired from HAEL Bio (Seoul, Korea). HRP-conjugated goat anti-rabbit and goat anti-mouse secondary antibodies were purchased from Jackson ImmunoResearch (USA). Ru.521, 2′3′-cGAMP, and G3-YSD were purchased from InvivoGen (USA). Bafilomycin A1 was purchased from AG Scientific and cycloheximide was from Sigma-Aldrich. Chemiluminescence blotting substrate for immunoblot analysis was purchased from Boehringer Mannheim (Germany). Lipofectamine® 2000 and Lipofectamine® RNAiMAX were purchased from Invitrogen (USA).
Transfection of siRNA or plasmid
For siRNA transfection, specific siRNAs for Atg5 (SC-41445), Beclin1 (sc-29798), Atf3 (SC-29785), and Mdm2 (SC-37253) were purchased from Santa Cruz. Stat1 siRNA (EMU071921) was purchased from SIGMA (USA). Negative control (AM4635) and Isg15 (AM16706) siRNAs were purchased from Ambion (USA). The above siRNAs were reverse transfected using Lipofectamine® RNAiMAX according to the manufacturer’s recommendations. For DNA transfection, SPORT6-pCMV-mISG15, SPORT6-pCMV-mATF3, and SPORT6-pCMV-mATG5 were purchased from KHGB (Daejeon, Korea). The Lipofectamine® 2000 reagent (Invitrogen) was used for DNA transfection.
Immunoblot assay
Cells were harvested and lysed with lysis buffer (20 mM Tris-Cl pH 7.5, 150 mM NaCl, 2.5 mM MgCl2, 1% Triton X-100, 0.25% Na-deoxycholate, 2 mM PMSF, 1 µg/ml aprotinin, 1 µg/ml leupeptin) for 30 min on ice. The supernatants were collected by centrifugation at 15,000 g for 10 min at 4 °C, and protein concentrations were determined using the Bradford reagent (Bio-Rad, #500–0006). Whole protein lysates were boiled in 2× SDS-PAGE sample buffer, separated by SDS-PAGE, and transferred to a PVDF membrane for immunoblotting.
Ribosomal pelleting
Cells were harvested after adding cycloheximide (100 µg/ml) to the medium for 30 min, and lysed with 1 ml of lysis buffer (Tris-Cl pH 7.5, 150 mM NaCl, 2.5 mM MgCl2, 1% Triton X-100, 0.25% Na-deoxycholate, 2 mM PMSF, 1 mg/ml aprotinin, 1 mg/ml leupeptin, 1 mM DTT, 100 units/ml RNasin (Promega, N251B), 50 µg/ml cycloheximide). The cell lysates were then applied to a sucrose cushion. The sucrose buffer comprised 20 mM Tris-Cl pH 7.5, 150 mM NaCl, and 2.5 mM MgCl2 with DEPC (Sigma, D5758) DW. The cell lysates ultracentrifuged at 32,000 rpm for 3 h 10 min at 4 °C in a Beckman SW41Ti rotor.
Reverse transcription-polymerase chain reaction (RT-PCR) and real-time PCR
Total cellular RNA was extracted using TRIzol reagent (Ambion, 15596–018). Translating mRNA from the ribosomal fraction was extracted using the TRIZOL LS reagent (Ambion, 10296–028) after ribosomal pelleting. These RNAs were reverse-transcribed using oligo-dT15 primers (Promega) and M-MLV reverse transcriptase (Promega, M170A). Subsequently, gene expression and translation were quantified by real-time PCR of the above-synthesized cDNAs, using a Light Cycler 480 instrument (Roche Molecular Biochemicals). The significance of differences was determined by Student’s t test.
RNAseq
mRNAs in the translating ribosomal pellet of WT MEFs, Atg5 knockout MEFs, and Atg7 knockout MEFs were isolated using the ribosomal pelleting assay. The RNA sequencing was performed by the RNA sequencing platform, nedxseq500, the differentially expressed gene was selected (>twofold, 0.05 > P value, and 10% > FDR) and gene ontology (GO) of each gene was analyzed (DNA LINK, Korea).
Nuclear fractionation
Cells were harvested and lysed with hypotonic lysis buffer (10 mM Tris-Cl pH 7.5, 10 mM NaCl, 2.5 mM MgCl2, 0.75% NP40, 2 mM PMSF, 1 µg/ml aprotinin, 1 µg/ml leupeptin) for 10 min on ice. The cytosolic fraction supernatants were collected by centrifugation at 5000 × g for 4 min at 4 °C, and nuclear fraction pellets were resuspended by lysis buffer (20 mM Tris-Cl pH 7.5, 150 mM NaCl, 2.5 mM MgCl2, 1% Triton X-100, 0.25% Na-deoxycholate, 2 mM PMSF, 1 µg/ml aprotinin, 1 µg/ml leupeptin). Resuspended nuclear pellets were retained by sonication, three times, on ice.
Immunofluorescence
Antibodies against dsDNA obtained from abcam (UK) were used. The staining was carried out according to the manufacturer’s recommendations. Cells were visualized using a fluorescence microscope (Carl Zeiss, Axioscope).
Immunoprecipitation
Cells were harvested and lysed with lysis buffer (20 mM Tris-Cl pH 7.5, 150 mM NaCl, 2.5 mM MgCl2, 1% Triton X-100, 0.25% Na-deoxycholate, 2 mM PMSF, 1 µg/ml aprotinin, 1 µg/ml leupeptin) for 30 min on ice. The supernatants were collected by centrifugation at 15,000 g for 10 min at 4 °C, and protein concentrations were determined using the Bradford reagent (Bio-Rad, #500–0006). One milligram of whole cell lysate was incubated with 1–2 µg of primary antibodies for 24 h at 4 °C, and the immunoprecipitates were collected using protein A- or G-agarose beads. After washing with lysis buffer, the immunoprecipitates were resolved on an SDS-PAGE gel and subjected to immunoblot assay.
Neutral comet assay
CometAssay® Kit (4250–050) was purchased from TREVIGEN (USA). The neutral comet assay was carried out according to manufacturer’s recommendations. Cells were visualized using a fluorescence microscope (Carl Zeiss, Axioscope). The visualized cell images were analyzed by automated tool for comet assay, OpenComet.
Wound-healing assay
Cells were cultured in DMEM/high glucose medium supplemented with 10% FBS until they reached 80–90% confluence and scratched evenly using a 200 μl tip. After 8–24 h, the wounded area was measured to analyze the variation. The variations of wounded area were analyzed by using Fiji, open source image processing software based on imagej.
Colony formation assay
After treatment by drugs or siRNAs, cells were equally plated and cultured in DMEM/high glucose medium supplemented with 10% FBS for 6–8 days. The forming colonies were fixed by methanol for 5 min and stained by 0.4% methylene blue in 20% methanol. The colony numbers were counted by using Fiji, open source image processing software based on imagej.
Three-dimensional (3D) tumor spheroid invasion assay
Using Corning Matrigel, an embedded 3D culture was performed following manufacturer’s instructions. The 3D culture images were analyzed by using Fiji, open source image processing software based on imagej.
Statistical analysis
Statistical significance was determined by Student’s t test. Differences were considered significant if the *p value < 0.05 or **p value < 0.01. Every error bar represents SD.
Supplementary information
Acknowledgements
This work was supported by NRF-2017R1E1A1A01074101, NRF-2019S1A5A2A03050121, and Korea University grant.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by S. Kumar
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41418-020-0519-y) contains supplementary material, which is available to authorized users.
References
- 1.Clarke AJ, Simon AK. Autophagy in the renewal, differentiation and homeostasis of immune cells. Nat Rev Immunol. 2019;19:170–83. doi: 10.1038/s41577-018-0095-2. [DOI] [PubMed] [Google Scholar]
- 2.Lilienbaum A. Relationship between the proteasomal system and autophagy. Int J Biochem Mol Biol. 2013;4:1–26. [PMC free article] [PubMed] [Google Scholar]
- 3.Farre JC, Subramani S. Mechanistic insights into selective autophagy pathways: lessons from yeast. Nat Rev Mol Cell Biol. 2016;17:537–52. doi: 10.1038/nrm.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cebollero E, Reggiori F, Kraft C. Reticulophagy and ribophagy: regulated degradation of protein production factories. Int J Cell Biol. 2012;2012:182834. doi: 10.1155/2012/182834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pietrocola F, Izzo V, Niso-Santano M, Vacchelli E, Galluzzi L, Maiuri MC, et al. Regulation of autophagy by stress-responsive transcription factors. Semin Cancer Biol. 2013;23:310–22. doi: 10.1016/j.semcancer.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Shin HJ, Kim H, Oh S, Lee JG, Kee M, Ko HJ, et al. AMPK-SKP2-CARM1 signalling cascade in transcriptional regulation of autophagy. Nature. 2016;534:553–7. doi: 10.1038/nature18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HD, Kong E, Kim Y, Chang JS, Kim J. RACK1 depletion in the ribosome induces selective translation for non-canonical autophagy. Cell Death Dis. 2017;8:e2800. doi: 10.1038/cddis.2017.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viiri J, Amadio M, Marchesi N, Hyttinen JM, Kivinen N, Sironen R, et al. Autophagy activation clears ELAVL1/HuR-mediated accumulation of SQSTM1/p62 during proteasomal inhibition in human retinal pigment epithelial cells. PLoS ONE. 2013;8:e69563. doi: 10.1371/journal.pone.0069563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubas M, Harder LM, Kumsta C, Tiessen I, Hansen M, Andersen JS, et al. eIF5A is required for autophagy by mediating ATG3 translation. EMBO Rep. 2018;19:e46072. [DOI] [PMC free article] [PubMed]
- 11.Bialik S, Kimchi A. Autophagy and tumor suppression: recent advances in understanding the link between autophagic cell death pathways and tumor development. Adv Exp Med Biol. 2008;615:177–200. doi: 10.1007/978-1-4020-6554-5_9. [DOI] [PubMed] [Google Scholar]
- 12.Poillet-Perez L, Xie X, Zhan L, Yang Y, Sharp DW, Hu ZS, et al. Autophagy maintains tumour growth through circulating arginine. Nature. 2018;563:569–73. doi: 10.1038/s41586-018-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nassour J, Radford R, Correia A, Fuste JM, Schoell B, Jauch A, et al. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature. 2019;565:659–63. doi: 10.1038/s41586-019-0885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dou Z, Xu C, Donahue G, Shimi T, Pan JA, Zhu J, et al. Autophagy mediates degradation of nuclear lamina. Nature. 2015;527:105–9. doi: 10.1038/nature15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenstein M. Molecular biology: remove, reuse, recycle. Nature. 2014;514:S2–4. doi: 10.1038/514S2a. [DOI] [PubMed] [Google Scholar]
- 16.Levine B. Cell biology: autophagy and cancer. Nature. 2007;446:745–7. doi: 10.1038/446745a. [DOI] [PubMed] [Google Scholar]
- 17.Guo JY, Xia B, White E. Autophagy-mediated tumor promotion. Cell. 2013;155:1216–9. doi: 10.1016/j.cell.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Y, Zou Z, Becker N, Anderson M, Sumpter R, Xiao G, et al. EGFR-mediated Beclin 1 phosphorylation in autophagy suppression, tumor progression, and tumor chemoresistance. Cell. 2013;154:1269–84. doi: 10.1016/j.cell.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katheder NS, Khezri R, O’Farrell F, Schultz SW, Jain A, Rahman MM, et al. Microenvironmental autophagy promotes tumour growth. Nature. 2017;541:417–20. doi: 10.1038/nature20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gui X, Yang H, Li T, Tan X, Shi P, Li M, et al. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. 2019;567:262–6. doi: 10.1038/s41586-019-1006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–35. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizzo M, Beffy P, Del Carratore R, Falleni A, Pretini V, D’Aurizio R, et al. Activation of the interferon type I response rather than autophagy contributes to myogenesis inhibition in congenital DM1 myoblasts. Cell Death Dis. 2018;9:1071. doi: 10.1038/s41419-018-1080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Germic N, Frangez Z, Yousefi S, Simon HU. Regulation of the innate immune system by autophagy: neutrophils, eosinophils, mast cells, NK cells. Cell Death Differ. 2019;26:703–14. doi: 10.1038/s41418-019-0295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang GM, Tan Y, Wang H, Peng L, Chen HT, Meng XJ, et al. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol Cancer. 2019;18:17. doi: 10.1186/s12943-019-0944-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin Y, Hong Y, Park CY, Hong Y. Molecular interactions of autophagy with the immune system and cancer. Int J Mol Sci. 2017;18,1694. [DOI] [PMC free article] [PubMed]
- 26.Shibutani ST, Saitoh T, Nowag H, Munz C, Yoshimori T. Autophagy and autophagy-related proteins in the immune system. Nat Immunol. 2015;16:1014–24. doi: 10.1038/ni.3273. [DOI] [PubMed] [Google Scholar]
- 27.Puleston DJ, Simon AK. Autophagy in the immune system. Immunology. 2014;141:1–8. doi: 10.1111/imm.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saleiro D, Kosciuczuk EM, Platanias LC. Beyond autophagy: New roles for ULK1 in immune signaling and interferon responses. Cytokine Growth Factor Rev. 2016;29:17–22. doi: 10.1016/j.cytogfr.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konno H, Konno K, Barber GN. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell. 2013;155:688–98. doi: 10.1016/j.cell.2013.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang Q, Seo GJ, Choi YJ, Kwak MJ, Ge J, Rodgers MA, et al. Crosstalk between the cGAS DNA sensor and Beclin-1 autophagy protein shapes innate antimicrobial immune responses. Cell Host Microbe. 2014;15:228–38. doi: 10.1016/j.chom.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delorme-Axford E, Klionsky DJ. Inflammatory-dependent Sting activation induces antiviral autophagy to limit zika virus in the Drosophila brain. Autophagy. 2019;15:1–3. doi: 10.1080/15548627.2018.1539585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu D, Wu H, Wang C, Li Y, Tian H, Siraj S, et al. STING directly activates autophagy to tune the innate immune response. Cell Death Differ. 2018;26:1735–49. [DOI] [PMC free article] [PubMed]
- 33.Prabakaran T, Bodda C, Krapp C, Zhang BC, Christensen MH, Sun C, et al. Attenuation of cGAS-STING signaling is mediated by a p62/SQSTM1-dependent autophagy pathway activated by TBK1. EMBO J. 2018;37:e97858. [DOI] [PMC free article] [PubMed]
- 34.Ablasser A, Goldeck M, Cavlar T, Deimling T, Witte G, Rohl I, et al. cGAS produces a 2’-5’-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498:380–4. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–6. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motani K, Ito S, Nagata S. DNA-mediated cyclic GMP-AMP synthase-dependent and -independent regulation of innate immune responses. J Immunol. 2015;194:4914–23. doi: 10.4049/jimmunol.1402705. [DOI] [PubMed] [Google Scholar]
- 37.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–91. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oganesyan G, Saha SK, Pietras EM, Guo B, Miyahira AK, Zarnegar B, et al. IRF3-dependent type I interferon response in B cells regulates CpG-mediated antibody production. J Biol Chem. 2008;283:802–8. doi: 10.1074/jbc.M704755200. [DOI] [PubMed] [Google Scholar]
- 39.Forys JT, Kuzmicki CE, Saporita AJ, Winkeler CL, Maggi LB, Jr., Weber JD. ARF and p53 coordinate tumor suppression of an oncogenic IFN-beta-STAT1-ISG15 signaling axis. Cell Rep. 2014;7:514–26. doi: 10.1016/j.celrep.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheon H, Holvey-Bates EG, Schoggins JW, Forster S, Hertzog P, Imanaka N, et al. IFNbeta-dependent increases in STAT1, STAT2, and IRF9 mediate resistance to viruses and DNA damage. EMBO J. 2013;32:2751–63. doi: 10.1038/emboj.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cadwell K, Debnath J. Beyond self-eating: the control of nonautophagic functions and signaling pathways by autophagy-related proteins. J Cell Biol. 2018;217:813–22. doi: 10.1083/jcb.201706157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu F, Fang Y, Yan L, Xu L, Zhang S, Cao Y, et al. Nuclear localization of Beclin 1 promotes radiation-induced DNA damage repair independent of autophagy. Sci Rep. 2017;7:45385. doi: 10.1038/srep45385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park JM, Tougeron D, Huang S, Okamoto K, Sinicrope FA. Beclin 1 and UVRAG confer protection from radiation-induced DNA damage and maintain centrosome stability in colorectal cancer cells. PLoS ONE. 2014;9:e100819. doi: 10.1371/journal.pone.0100819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Vogel H, Holcomb VB, Gu Y, Hasty P. Deletion of Ku70, Ku80, or both causes early aging without substantially increased cancer. Mol Cell Biol. 2007;27:8205–14. doi: 10.1128/MCB.00785-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sishc BJ, Davis AJ. The role of the core non-homologous end joining factors in carcinogenesis and cancer. Cancers. 2017;9:81. [DOI] [PMC free article] [PubMed]
- 46.Kuo LJ, Yang LX. Gamma-H2AX - a novel biomarker for DNA double-strand breaks. Vivo. 2008;22:305–9. [PubMed] [Google Scholar]
- 47.Liu H, Zhang H, Wu X, Ma D, Wu J, Wang L, et al. Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature. 2018;563:131–6. doi: 10.1038/s41586-018-0629-6. [DOI] [PubMed] [Google Scholar]
- 48.Jiang H, Xue X, Panda S, Kawale A, Hooy RM, Liang F, et al. Chromatin-bound cGAS is an inhibitor of DNA repair and hence accelerates genome destabilization and cell death. EMBO J. 2019;38:e102718. [DOI] [PMC free article] [PubMed]
- 49.Farrell PJ, Broeze RJ, Lengyel P. Accumulation of an mRNA and protein in interferon-treated Ehrlich ascites tumour cells. Nature. 1979;279:523–5. doi: 10.1038/279523a0. [DOI] [PubMed] [Google Scholar]
- 50.Perng YC, Lenschow DJ. ISG15 in antiviral immunity and beyond. Nat Rev Microbiol. 2018;16:423–39. doi: 10.1038/s41579-018-0020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Cunha J, Ramanujam S, Wagner RJ, Witt PL, Knight E, Jr., Borden EC. In vitro and in vivo secretion of human ISG15, an IFN-induced immunomodulatory cytokine. J Immunol. 1996;157:4100–8. [PubMed] [Google Scholar]
- 52.Pilz A, Ramsauer K, Heidari H, Leitges M, Kovarik P, Decker T. Phosphorylation of the Stat1 transactivating domain is required for the response to type I interferons. EMBO Rep. 2003;4:368–73. doi: 10.1038/sj.embor.embor802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sood V, Sharma KB, Gupta V, Saha D, Dhapola P, Sharma M, et al. ATF3 negatively regulates cellular antiviral signaling and autophagy in the absence of type I interferons. Sci Rep. 2017;7:8789. doi: 10.1038/s41598-017-08584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mo P, Wang H, Lu H, Boyd DD, Yan C. MDM2 mediates ubiquitination and degradation of activating transcription factor 3. J Biol Chem. 2010;285:26908–15. doi: 10.1074/jbc.M110.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bakhoum SF, Ngo B, Laughney AM, Cavallo JA, Murphy CJ, Ly P, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553:467–72. doi: 10.1038/nature25432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Xiong H, Liu D, Hill C, Ertay A, Li J, et al. Autophagy inhibition specifically promotes epithelial-mesenchymal transition and invasion in RAS-mutated cancer cells. Autophagy. 2019;15:1–14. [DOI] [PMC free article] [PubMed]
- 57.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–6. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 58.Dzimianski JV, Scholte FEM, Bergeron E, Pegan SD. ISG15: it’s complicated. J Mol Biol. 2019;431;21:4203-16. [DOI] [PMC free article] [PubMed]
- 59.Li Y, Bai W, Zhang L. The overexpression of CD80 and ISG15 are associated with the progression and metastasis of breast cancer by a meta-analysis integrating three microarray datasets. Pathol Oncol Res. 2018. 10.1007/s12253-018-0478-5. [DOI] [PubMed]
- 60.Han HG, Moon HW, Jeon YJ. ISG15 in cancer: beyond ubiquitin-like protein. Cancer Lett. 2018;438:52–62. doi: 10.1016/j.canlet.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 61.Tecalco-Cruz AC, Cruz-Ramos E. Protein ISGylation and free ISG15 levels are increased by interferon gamma in breast cancer cells. Biochem Biophys Res Commun. 2018;499:973–8. doi: 10.1016/j.bbrc.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 62.Yoo L, Yoon AR, Yun CO, Chung KC. Covalent ISG15 conjugation to CHIP promotes its ubiquitin E3 ligase activity and inhibits lung cancer cell growth in response to type I interferon. Cell Death Dis. 2018;9:97. doi: 10.1038/s41419-017-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Desai SD. ISG15: a double edged sword in cancer. Oncoimmunology. 2015;4:e1052935. doi: 10.1080/2162402X.2015.1052935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sainz B, Jr., Martin B, Tatari M, Heeschen C, Guerra S. ISG15 is a critical microenvironmental factor for pancreatic cancer stem cells. Cancer Res. 2014;74:7309–20. doi: 10.1158/0008-5472.CAN-14-1354. [DOI] [PubMed] [Google Scholar]
- 65.Eliopoulos AG, Havaki S, Gorgoulis VG. DNA damage response and autophagy: a meaningful partnership. Front Genet. 2016;7:204. doi: 10.3389/fgene.2016.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gomes LR, Menck CFM, Leandro GS. Autophagy roles in the modulation of dna repair pathways. Int J Mol Sci. 2017;18:2351. [DOI] [PMC free article] [PubMed]
- 67.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–8. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dobbs N, Burnaevskiy N, Chen D, Gonugunta VK, Alto NM, Yan N. STING activation by translocation from the ER is associated with infection and autoinflammatory disease. Cell Host Microbe. 2015;18:157–68. doi: 10.1016/j.chom.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt C. The benefits of immunotherapy combinations. Nature. 2017;552:S67–9. doi: 10.1038/d41586-017-08702-7. [DOI] [PubMed] [Google Scholar]
- 71.Wei H, Wang C, Croce CM, Guan JL. p62/SQSTM1 synergizes with autophagy for tumor growth in vivo. Genes Dev. 2014;28:1204–16. doi: 10.1101/gad.237354.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu LZ, Li SS, Zhou W, Kang ZJ, Zhang QX, Kamran M, et al. p62/SQSTM1 enhances breast cancer stem-like properties by stabilizing MYC mRNA. Oncogene. 2017;36:304–17. doi: 10.1038/onc.2016.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.