Abstract
Observed genetic associations with educational attainment may be due to direct or indirect genetic influences. Recent work highlights genetic nurture, the potential effect of parents’ genetics on their child’s educational outcomes via rearing environments. To date, few mediating childhood environments have been tested. We used a large sample of genotyped mother–child dyads (N = 2,077) to investigate whether genetic nurture occurs via the prenatal environment. We found that mothers with more education-related genes are generally healthier and more financially stable during pregnancy. Further, measured prenatal conditions explain up to one third of the associations between maternal genetics and children’s academic and developmental outcomes at the ages of 4 to 7 years. By providing the first evidence of prenatal genetic nurture and showing that genetic nurture is detectable in early childhood, this study broadens our understanding of how parental genetics may influence children and illustrates the challenges of within-person interpretation of existing genetic associations.
Keywords: genetics, childhood development, prenatal
Psychologists have long understood that genetic and environmental influences interact to shape human development and produce individual differences (Turkheimer, 2000). However, as genome-wide association studies over the past decade have created new avenues for the study of genetics at the molecular level (Visscher et al., 2017), the line between genetic and environmental influences has begun to blur. There is increasing appreciation that humans are shaped by both direct genetic effects, the influence of their own genes, and social genetic effects, the indirect influences that other people’s genes have through affecting the shared environment (Domingue & Belsky, 2017).
Social genetic effects represent a novel mechanism through which individual differences may be transmitted from parents to children. Consider recent genetic discoveries for educational attainment (Lee et al., 2018; Okbay et al., 2016), which correlate with numerous related behavioral and social phenotypes: more prestigious occupations and upward social mobility (Belsky et al., 2018; Trejo et al., 2018); intelligence, self-control, and interpersonal skills (Belsky et al., 2016); personality (Mõttus, Realo, Vainik, Allik, & Esko, 2017; Smith-Woolley, Selzam, & Plomin, 2019; Stephan, Sutin, Kornadt, & Terracciano, 2019); brain development (Elliott et al., 2019; Okbay et al., 2016); attention (de Zeeuw et al., 2014); and prosocial behavior (Wertz et al., 2018). Parental genes related to educational attainment may be associated with children’s educational attainment because of the correlation between maternal and child genetics that results from genetic inheritance (Ayorech, Krapohl, Plomin, & von Stumm, 2017; Conley et al., 2015). However, parental genes related to educational attainment may also become associated with children’s educational attainment as a result of an environmentally mediated social genetic effect, whereby parental genes causally influence their children’s educational outcomes via genetically associated parental behaviors or environmental exposures. Recently, nontransmitted parental genes have been used to document such social genetic influences from parents to their children, an effect described as genetic nurture (Kong et al., 2018). Because genome-wide association studies do not discriminate among the various pathways through which genes become associated with outcomes, recent genetic discoveries from such studies capture both direct genetic effects and genetic-nurture effects (Trejo & Domingue, 2019).
Genetic nurture allows genes to be used as a lens for the study of the social processes through which parents influence their children. For example, new research has found that parental genetics for educational attainment are associated with warm, stimulating parenting, which partially explains the association between parental genetics and children’s educational attainment at age 18 (Wertz et al., 2019). However, it is also possible that genetic-nurture processes begin even earlier. In particular, we consider the possibility of such a phenomenon occurring at the earliest stage of development, when the child is still in utero.
The prenatal period is a promising site for genetic nurture for two reasons. First, the prenatal environment is critical for human development, and prenatal adversity (e.g., maternal stress, poverty, and toxicants) is a well-documented developmental risk factor (Piccolo & Noble, 2019). Second, the mother’s womb is the predominant environment for the developing child. External environments may influence the child in utero, but even those are mediated by the mother’s biology. This is not true for the postnatal environment, where many environmental processes that affect a child are independent of the mother.
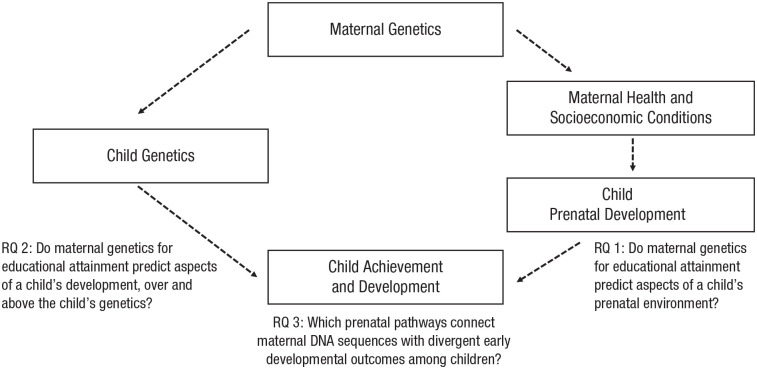
Figure 1 presents a conceptual model for how maternal genetics could influence child development in utero. Maternal genetics are related to both the mother’s behaviors and her environmental exposures while pregnant; these collectively affect the child in utero and impact the child’s downstream outcomes. Identifying the exact causal flows in this graph is challenging, but we note one crucial point. If maternal genetics have a causal effect on child outcomes (independently of genetic transmission), the association between an individual’s prenatal environmental exposures and downstream outcomes will be confounded by both direct genetic and social genetic influences. Put plainly, influences on children’s development that stem from their own genetics, maternal genetics, and independent aspects of the prenatal environment will be challenging to separate.
Fig. 1.
Conceptual model linking maternal genetics with child achievement and development through both a direct pathway (left) and an indirect pathway (child environment; right). Our three primary research questions (RQs) are also shown.
Investigating genetic nurturance during the prenatal period is complicated by the paucity of genetically informed studies that contain both information on a mother’s behaviors and environmental exposures during pregnancy and measures of the subsequent cognitive and social development of her children. The study of genetic nurture has been further constrained by the timing of developmental and cognitive outcome measures. Previous research has focused on offspring outcomes later in life (e.g., at ages 17 and 18; Bates et al., 2018; Wertz et al., 2019), but a complete accounting of genetic nurture would naturally begin earlier in the life course. Can such effects be observed when young children are just entering school?
To investigate (a) whether the prenatal environment is a pathway for genetic nurture and (b) whether these associations are observed early in a child’s life, we asked three main questions. First, do maternal genetics for educational attainment predict aspects of a child’s prenatal environment? Second, do maternal genetics for educational attainment predict aspects of a child’s development in early and middle childhood, over and above the child’s own genetics for educational attainment (i.e., genetic transmission)? Third, which social, behavioral, and psychological prenatal pathways connect maternal genetics with divergent early developmental outcomes among children?
To address these questions, we used rich, prospective data from the Born in Bradford (BiB) birth cohort (Wright et al., 2012). To index maternal genetics, we used a maternal polygenic score for educational attainment (Lee et al., 2018). By controlling for children’s genotype, we isolated genetic nurture from direct genetic influences and clarified the contribution of each. Further, we used a high-quality multi-informant method drawing on parent-reported indexes of prenatal environments, teacher observations of child development, and direct assessments of children’s academic achievement during their first 3 years of schooling (ages 4–7 years). With these robust measures, we offer evidence that maternal genetics are associated with a variety of prenatal exposures and that genetically associated differences in exposure are predictive of downstream differences in educational development early in a child’s life.
Method
Sample
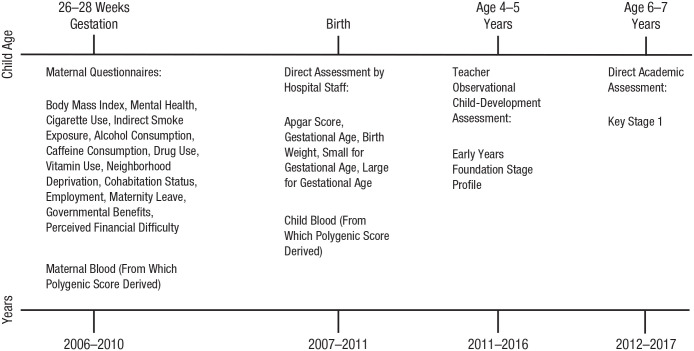
Our analytic sample is drawn from the BiB study, a longitudinal multiethnic birth-cohort study conducted in northern England (Wright et al., 2012). Compared with national averages, the BiB cohort is more ethnically diverse and has higher levels of socioeconomic deprivation; the cohort is broadly characteristic of the city’s maternal population (Wright et al., 2012). BiB enrolled pregnant mothers at 26 to 28 weeks’ gestation and has followed them longitudinally. The full study recruited 12,453 women and 3,353 of their partners across 13,776 pregnancies and 13,858 children from 2007 to 2010. Figure 2 shows a timeline of BiB procedures. After being enrolled during pregnancy, women completed an extensive questionnaire that included information on health behaviors and socioeconomic factors. At the child’s birth, genetic samples were assayed from both mother and child, and measures of neonatal health were taken. Administrative educational records were collected for children, including a structured, teacher-led observational assessment of development at the end of the first year of schooling, when students were 4 to 5 years old (the Early Years Foundation Stage Profile), and an exam-based direct assessment of academic performance at the end of their third year, when students were 6 to 7 years old (Key Stage 1). Genetic data were available for 6,256 mother–child dyads, and valid data for prenatal, academic and developmental measures were available for 6,124 out of the 13,858 children in the BiB cohort.
Fig. 2.
Timeline of procedures for the Born in Bradford cohort.
The cohort was 33.65% White British, 60.35% Pakistani, and 6.00% other ethnicities. Although a strength of the cohort, diversity raises issues in studies of genetic prediction (Martin et al., 2017). Because the polygenic score for educational attainment was derived from genome-wide association studies of 1 million individuals of European ancestry (Lee et al., 2018), we restricted our sample to mother–child dyads in which the mother self-identified as British and was also of European ancestry (N = 2,077, as identified via the first two principal components; see Section 1B in the Supplemental Material available online). We briefly report on preliminary analyses in a Pakistani-ancestry subsample of the BiB cohort (see Section 3 in the Supplemental Material).
Genotyping and polygenic scoring
We used Illumina HumanCore Exome 12 and 24 BeadChip arrays (Version 1/1.1; Illumina, Hayward, CA) to assay common variation in single-nucleotide polymorphism (SNP) in the genomes of our cohort members. As with many traits of interest (Chabris, Lee, Cesarini, Benjamin, & Laibson, 2015), education is highly polygenic. To capture information from across the dispersed loci, we constructed polygenic scores (Dudbridge, 2013) using Plink software (Version 1.9; Chang et al., 2015). We matched mother and child genotypes from the BiB data with the most recent results of genome-wide association studies for educational attainment (Lee et al., 2018; note that the BiB data were not used in this genome-wide association study). We used 216,542 matched SNPs from BiB members to construct polygenic scores. For each genotype, we counted the number of education-associated alleles (0, 1, or 2), multiplied this count by the effect size estimated in the original genome-wide association study, and then summed weighted counts across all genotypes to calculate each BiB participant’s polygenic score. All matched SNPs were used to compute polygenic scores, irrespective of nominal significance for their association with educational attainment. In all analyses, we controlled for maternal age and the first 10 principal components of European-ancestry genotype to account for population stratification and increase the robustness of our findings (Price et al., 2006).
Measures
Additional information for all variables used in the study is available in the Supplemental Material (Section 1).
Prenatal environment
To index salient aspects of the child’s prenatal environment, we measured the mother’s health and socioeconomic status (SES) during pregnancy. Maternal health during pregnancy was indexed via body mass index (BMI; directly assessed by hospital staff), mental health, cigarette use, indirect smoke exposure, alcohol consumption, caffeine consumption, drug use, vitamin use, and sleep problems (via maternal self-report). SES during pregnancy was indexed by maternal education, cohabitation status, employment, maternity leave, governmental benefits, perceived financial difficulty (via maternal self-report), and neighborhood-level socioeconomic neighborhood deprivation (via governmental index). On the basis of the variables separately described for prenatal health and SES, we constructed two composites via principal components analysis. To maximize sample size in downstream analyses, we used an algorithm designed to allow for missing data (Stacklies, Redestig, Scholz, Walther, & Selbig, 2007); additional details on the composites’ construction can be found in Section 1 in the Supplemental Material.
Child outcomes
To index child development, we used children’s scores on the Early Years Foundation Stage Profile, a teacher-led observational assessment with six subscales that indexes physical, personal, social, and emotional development relative to the average child at the end of the first year of schooling (Whitaker, 2014). We created a single composite measure by first standardizing each subscale and then calculating a mean total score (higher scores indicate greater development). To index children’s academic performance, we used their scores on the Key Stage 1, a standardized school-based exam that includes math, reading, and science subscales (Standards and Testing Agency, 2016). We again created a single composite by standardizing each subscale and calculating the total mean (higher scores indicate greater academic performance). Early achievement at the age of 7 years has been shown to have enduring effects on individuals’ downstream educational attainment, SES, and well-being (Ritchie & Bates, 2013).
Analytic sample
Our analytic sample was restricted to mothers and children of European ancestry for whom genetic data and test scores are available (N = 2,077 dyads). Our analytic sample differed from the full BiB sample in several ways (see Section 1C in the Supplemental Material for additional details on sample comparisons); this is to be expected given the diversity of the BiB sample. Given our focus on a genetically homogeneous sample, we concentrated on comparisons within the full set of self-reported White British BiB respondents. Only a small portion of this group is not in the analytic sample (6% of this subsample). Both child and maternal characteristics were largely similar across these two samples (see Table S2A in the Supplemental Material). Within our analytic sample, further data were missing for children’s polygenic scores and developmental and academic outcomes. This is largely due to either children having left the BiB study or students having been too young to be eligible for the Key Stage 1 (see Section 1D in the Supplemental Material for additional details).
Statistical analysis
We conducted linear regressions with standard errors clustered at the mother level (74 mothers had two pregnancies) to test how maternal genetics predict both children’s prenatal conditions and their early academic and developmental outcomes. We conducted a power analysis to probe our ability to detect associations between mothers’ polygenic scores and children’s outcomes (see Section 1E in the Supplemental Material). Given our sample size, our study was well powered to detect association estimates (bs) larger than 0.06; note that previous work has suggested much larger association estimates of around 0.2 (Wertz et al., 2019).
To test possible prenatal pathways through which maternal genetics may be associated with child outcomes, we then considered mediation models—using a recently developed framework (Imai, Keele, & Tingley, 2010)—to test the extent to which maternal-genetics-related differences in early-childhood academic performance and development are explained by prenatal conditions and behaviors. We focused on models in which the composites of prenatal health and SES were separately included as potential mediators linking mothers’ polygenic scores and children’s outcomes (controlling for children’s polygenic scores). We computed confidence intervals on the basis of the bootstrap method. In analyses, all continuous measures were standardized (M = 0, SD = 1).
We refrain from extensive reliance on p values in discussion of our results. However, for the core analyses involving the prenatal composites and the child outcomes, we guard against spurious findings by commenting on p values relative to the recently suggested conservative threshold of p < .005 (Benjamin et al., 2018). Code used for our analysis is publicly available (see Section 4 in the Supplemental Material).
Ethical approvals and data sharing
The research project used only existing, deidentified data; institutional review determined that this project’s study protocol did not meet the definition of human-subjects research. The Bradford Leeds NHS Research Ethics Committee provided ethical approval for the BiB study (15/YH/0455), and adult participants provided written consent before data collection. When participants were children, their parents gave informed consent. Researchers retrieved the sensitive biological, medical, and educational records through a managed-access process approved by the BiB executive board.
Results
Maternal genotypes are associated with maternal health and SES during pregnancy
We first tested whether the mothers’ polygenic score for educational attainment was associated with the mothers’ health composite scores during pregnancy (Table 1). In this model, we did not control for child polygenic score; these are measures derived from data collected before the child’s birth and should thus be largely unaffected by a child’s genetics. We found that, on average, maternal polygenic score was positively associated with greater health (β = 0.089, 95% confidence interval, or CI = [0.046, 0.132], z = 4.077, p < .005). To further investigate this, we also tested each prenatal health factor separately in independent models. We found that a greater maternal polygenic score was associated with lower levels of caffeine consumption, smoking, and indirect smoke exposure and with higher levels of vitamin use (effect sizes ranged from b = 0.07 to b = 0.10). This suggests that a larger polygenic score was generally associated with more optimal health behaviors during pregnancy; however, associations with alcohol consumption were the opposite: mothers with higher polygenic scores were more likely to have drunk alcohol in the last few months than were mothers with lower polygenic scores.
Table 1.
Associations Between Mother’s and Children’s Polygenic Score (PGS) for Educational Attainment and Prenatal Exposures (From Separate Models)
| Outcome | Maternal PGS | Child PGS | n | ||
|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | ||
| Health composite | 0.089 | [0.046, 0.132] | 0.048 | [−0.002, 0.097] | 1,986 |
| Body mass index | −0.030 | [−0.077, 0.017] | −0.021 | [−0.074, 0.032] | 1,903 |
| Mental health | −0.009 | [−0.056, 0.038] | −0.001 | [−0.055, 0.053] | 1,910 |
| Vitamin use | 0.060 | [0.014, 0.107] | 0.092 | [0.042, 0.143] | 1,986 |
| Indirect smoke exposure | −0.076 | [−0.118, −0.034] | −0.050 | [−0.099, −0.002] | 1,984 |
| Smoking | −0.108 | [−0.151, −0.065] | −0.048 | [−0.096, 0.000] | 1,986 |
| Alcohol consumption | 0.067 | [0.021, 0.113] | 0.034 | [−0.017, 0.085] | 1,986 |
| Caffeine use | −0.070 | [−0.118, −0.023] | −0.008 | [−0.056, 0.040] | 1,742 |
| Drug use | −0.013 | [−0.056, 0.031] | 0.014 | [−0.040, 0.069] | 1,923 |
| Sleep problems | −0.005 | [−0.051, 0.041 | 0.001 | [−0.052, 0.053] | 1,907 |
| SES composite | 0.156 | [0.114, 0.198] | 0.096 | [0.048, 0.143] | 1,986 |
| Maternal education | 0.206 | [0.162, 0.250] | 0.070 | [0.019, 0.121] | 1,809 |
| Single | −0.081 | [−0.123, −0.039] | −0.086 | [−0.135, −0.037] | 1,985 |
| Employed | 0.092 | [0.049, 0.136] | 0.036 | [−0.015, 0.086] | 1,986 |
| Maternal leave | −0.019 | [−0.069, 0.031] | −0.026 | [−0.080, 0.029] | 1,554 |
| Neighborhood deprivation | −0.067 | [−0.112, −0.022] | −0.044 | [−0.094, 0.006] | 1,935 |
| Financial difficulty | −0.030 | [−0.076, 0.017] | −0.058 | [−0.112, −0.004] | 1,984 |
| Receipt of governmental benefits | −0.102 | [−0.145, −0.059] | −0.052 | [−0.104, −0.001] | 1,984 |
Note: The rightmost column shows individual ns. For associations with maternal PGS, analyses controlled for age and 10 principal components. For associations with child PGS, analyses controlled for maternal PGS, age, and 10 principal components. CI = confidence interval; SES = socioeconomic status.
We then tested whether the mothers’ polygenic score for educational attainment was associated with the mothers’ SES composite scores during pregnancy. We found that, on average, maternal polygenic score was positively associated with greater SES (β = 0.156, 95% CI = [0.114, 0.198], z = 7.261, p < .005). This association was greater in magnitude than that observed for maternal health. To investigate this further, we also tested each prenatal SES factor separately in independent models. Unsurprisingly, the maternal polygenic score was positively associated with maternal education. Greater maternal polygenic score was associated with lower likelihood of the mother being single, being unemployed, and receiving governmental benefits (effect sizes ranged from 0.08 to 0.1). Echoing previous findings, our results showed that higher polygenic score was also associated with living in a neighborhood with lower levels of deprivation (Belsky et al., 2019; Domingue, Belsky, Conley, Harris, & Boardman, 2015).
As shown in Table 1, these models also revealed that child polygenic score uniquely contributed to aspects of the prenatal environment, even after we controlled for maternal polygenic score. With respect to health, child polygenic score was weakly associated with greater health composite scores, as well as with increased vitamin usage and decreased smoke exposure. With respect to SES, child polygenic score was positively associated with the SES composite and with the mother’s education, and it was negatively associated with the likelihood of the mother being single and experiencing financial difficulty. These results provide further evidence for gene–environment correlation.
Maternal genotypes predict offspring development after analyses control for offspring genes
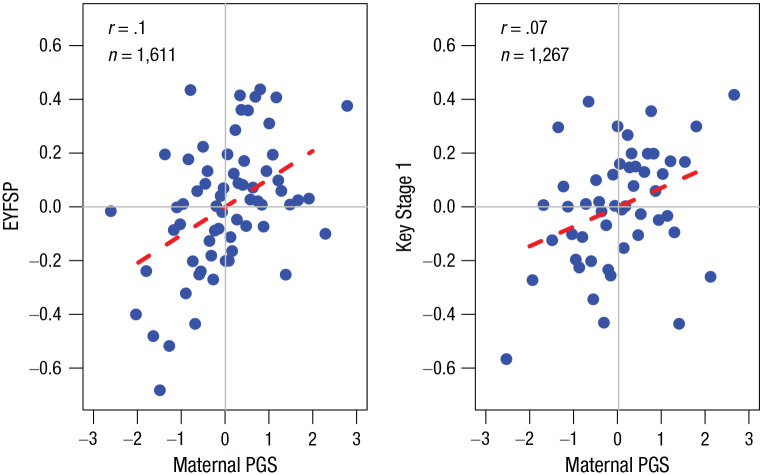
To examine the possibility of genetic nurture among young children, we next tested whether maternal genetics for educational attainment predicted child development (at age 4–5 years) and academic performance (at age 6–7 years) over and above the child’s genetics. As shown in Table 2 and Figure 3, maternal polygenic score was positively associated with offspring development (β = 0.114, 95% CI = [0.058, 0.171], z = 4.002, p < .005) and academic performance (β = 0.087, 95% CI = [0.020, 0.154], z = 2.748, p = 0.006; note that this is marginal in that it is above a conservative threshold of p = .005). In this model, child polygenic score also uniquely predicted greater academic performance (β = 0.083, 95% CI = [0.016, 0.150], z = 2.639, p = .008) and marginally greater child development (β = 0.058, 95% CI = [0.002, 0.133], z = 2.020, p = .043; Table 2).
Table 2.
Results of Regression Analyses Predicting Children’s Development and Academic Performance From Mother’s and Children’s Polygenic Score (PGS)
| Outcome | Child development (n = 1,611, r2 = .044) |
Child academic performance (n = 1,267, r2 = .056) |
||
|---|---|---|---|---|
| Mother PGS | Child PGS | Mother PGS | Child PGS | |
| Standardized | 0.114 [0.058, 0.171] |
0.058 [0.002, 0.113] |
0.087 [0.020, 0.154] |
0.083 [0.016, 0.150] |
| Percentile ranked | 0.034 [0.017, 0.050] |
0.017 [0.001, 0.033] |
0.030 [0.011, 0.049] |
0.025 [0.006, 0.044] |
Note: Standardized coefficients are shown, with robust 95% confidence intervals in brackets. The ns are for mother–child dyads. Child development was indexed by scores on the Early Years Foundation Stage Profile; child academic performance was indexed by scores on the Key Stage 1.
Fig. 3.
Binned scatterplots showing the associations between the Born in Bradford’s mothers’ polygenic score (PGS) and their children’s developmental outcomes (left) and academic outcomes (right). Children’s developmental outcomes are indexed by scores on the Early Years Foundation Stage Profile (EYFSP), and their academic outcomes are indexed by scores on the Key Stage 1. Maternal polygenic score was residualized on child polygenic score, maternal age, and the first 10 principal components of individual genotype. Maternal polygenic score and both developmental outcomes were standardized within sample (M = 0, SD = 1). Each point represents roughly 25 mother–child pairs. The red line represents the best linear fit from a regression on the underlying, unbinned data.
Because the distributions of child outcome variables were highly centralized (see Fig. S2 in the Supplemental Material), we also considered analyses based on outcomes converted to percentiles of their respective distributions. Results were qualitatively similar; a 1-standard-deviation increase in the mother’s polygenic score predicted a gain of around 3 percentile points in the outcome distribution (β = 0.034 for development, β = 0.030 for academic performance). We also tested whether our findings were potentially due to individual differences in child characteristics measured at birth (see Section 2A in the Supplemental Material). Associations between mothers’ polygenic score and children’s gestational age, Apgar score, and birth weight were null.
As other researchers have observed (Bates et al., 2018; Kong et al., 2018; Wertz et al., 2019), these results are consistent with the hypothesis that mothers’ education-associated genetics shape environments that affect offspring outcomes independently of direct mother-child genetic transmission. Our results suggest that such processes are observable during early childhood.
Prenatal environmental exposures mediate associations between maternal genetics and outcomes in early childhood
We next examined whether the observed associations between maternal polygenic score and offspring development and academics were explained by conditions experienced during the prenatal period. To do this, we created mediation models, first with child development as the outcome and next with academic performance as the outcome (see Table 3). Each model separately included the prenatal health and SES composites as mediators (each model additionally controlled for child polygenic score). Note that both the health and SES composites were themselves strongly and positively associated with child development and academic performance (see Section 2B in the Supplemental Material).
Table 3.
Mediation Analysis: Total Effect of Maternal Polygenic Score (PGS) and Proportion of Total Effect Due to Mediator (Controlling for Child PGS and the Alternative Prenatal Composite)
| Outcome and mediator | Total effect (maternal PGS on outcome) | 95% CI | Proportion mediated | 95% CI | n |
|---|---|---|---|---|---|
| Early Years Foundation Stage Profile | |||||
| SES principal component | 0.113 | [0.057, 0.164] | .273 | [.138, .548] | 1,611 |
| Health principal component | 0.115 | [0.058, 0.169] | .112 | [.039, .252] | 1,611 |
| Key Stage 1 | |||||
| SES principal component | 0.087 | [0.025, 0.146] | .321 | [.134, .980] | 1,267 |
| Health principal component | 0.087 | [0.024, 0.150] | .131 | [.038, .484] | 1,267 |
Note: Total effects are given as standardized coefficients. The rightmost column shows individual ns. CI = confidence interval; SES = socioeconomic status.
For child development, maternal SES during pregnancy explained 27.3% (p < .005) of the variance in the association between higher maternal polygenic score and greater child development, and maternal health during pregnancy explained 11.2% (p < .005) of the variance.
For child academic performance, results were similar. Maternal SES during pregnancy explained 32.1% (p < .005) of the variance in the association between higher maternal polygenic score and greater child academic performance, and maternal health during pregnancy explained 13.1% (p < .005) of the variance.
We considered a supplemental analysis wherein each individual prenatal environmental variable was entered as a mediator, instead of the two composites (see Section 2C in the Supplemental Material). Maternal education was an especially salient mediator. One interpretation of these results could be that observed differences were largely mediated by prenatal maternal behaviors that are themselves associated with educational attainment.
Discussion
We investigated whether mothers’ education-associated genetics are associated with offspring’s early development and whether prenatal environmental factors explain variance in these associations. We drew on a large sample of mother–child dyads followed from 28 weeks’ gestation through the first 7 years of life. Our results indicate that mothers with more education-associated alleles tended to be healthier (with the exception of alcohol consumption) and more economically secure during pregnancy. Further, these prenatal factors explained about 30% of the positive association between maternal-education-associated genetics and children’s school readiness and early academic performance, even after analyses accounted for direct genetic transmission. Together, our results suggest that prenatal exposures are salient environmental pathways through which maternal genetics may influence children’s early development and education.
Recent work documents associations between mothers’ genetics and their adolescents’ education (Bates et al., 2018; Kong et al., 2018; Wertz et al., 2019). We showed that maternal genetics are similarly associated with young children’s academic success and broader developmental milestones and that these associations are detectable as early as ages 4 to 5 years. The effect size of the association we observed between maternal polygenic score and child academic achievement was smaller than effect sizes from recent studies of adolescent outcomes (b = 0.12 compared with b = 0.23, as observed by Bates et al., 2018, and Wertz et al., 2019, respectively). Our finding adds to growing evidence that genetic variation linked to educational attainment also predicts a constellation of different behaviors and social circumstances across the life course, and it even spills into the next generation.
This study suggests that genetic nurture may occur during the prenatal period and leave detectable traces earlier in the child’s life than previously observed. Our findings highlight prenatal genetic nurture as a novel pathway through which genetics can confound the observed relationship between prenatal circumstances and child development. For the prenatal environment to be a period of concern for social policymakers, the documented association between prenatal circumstances and life-course development must reflect, at least in part, a causal relationship. However, because a mother’s genetics are both transmitted to her offspring and predict her prenatal circumstances, the degree to which the relation between prenatal circumstances and child development is correlational versus causal is unclear. Most perniciously, such confounding may continue to exist even after analyses control for the genetics a child inherits (Rice et al., 2010; Stein et al., 2014). Researchers interested in exploring the causal chain that connects prenatal circumstances to human development would benefit from controlling for the specific pathways we have identified. In particular, we note the crucial role played by the mother’s social environment during pregnancy. That said, a mother’s SES and other environmental exposures are likely to be relatively unchanging throughout the life course; features of the mother’s pregnancy will become the child’s environmental surroundings in the first few years of life and beyond. This “stickiness” offers further challenges to research connecting prenatal circumstances to later-life outcomes.
Our research expands on the budding phenotypic-annotation literature in which a top-down approach is used for unpacking genetic discoveries (Belsky & Harden, 2019). We showed that this technique can be applied to indirect genetic influences in addition to direct genetic influences. Although findings from genome-wide association studies tend to be a black box, researchers can use data from whole genomes and take a life-course-development approach to explore how genetics for the discovery of specific phenotypes relate to broader nomological networks (Cronbach & Meehl, 1955). Our analyses embody this approach; we utilized a genome-wide polygenic score for educational attainment as a starting point for exploring the broader nomological network of child development that extends beyond purely educational attainment. This is important because children’s socioemotional skills are associated with school readiness and later achievement and well-being (Duncan et al., 2007). Consequently, our results highlight the possibility that maternal genetics may predict a child’s capacity to cope and thrive during the transition into formal education.
We acknowledge limitations of our study. Prenatal environmental measures may be correlated with environmental exposures occurring both before and after pregnancy; for example, the mother’s SES may be relatively stable throughout the life course. Thus, prenatal exposures may also inadvertently capture the effect of, say, persistent exposure to relatively high levels of neighborhood deprivation. Another limitation is that the existing educational-attainment polygenic score contains substantial amounts of measurement error resulting from the finite sample used to obtain the underlying allelic weights. This measurement error attenuates the association between maternal polygenic score and child outcomes, potentially obscuring relevant prenatal pathways. However, this measurement error does not lead to false positives; because mother and child polygenic scores are constructed using the same allelic weights, the child polygenic score indexes the same genetic pathways as the maternal polygenic score, and unmeasured child genetics do not confound the association between maternal polygenic score and child outcomes. In addition, given the complications of interpreting genetic differences across ancestry groups (Martin et al., 2017), our findings only pertain to individuals of White British ancestry. Finally, though we attempted to reduce confounding through the inclusion of control variables, the findings are observational in nature and do not definitively indicate causal pathways.
The present study illustrates ways in which maternal genetics are interwoven in a complex tapestry of health behaviors and social circumstances. Although genes are often used to partition variance in a given outcome into genetic and environmental influences, they also characterize features of the environments that individuals are exposed to. We use genetics to illuminate the important and complex influences on the prenatal environment that in turn shape children’s early development and downstream outcomes. Genetic-nurture influences blur the line between genetic and environmental influences, reminding us that genetic influences are not immutable and environments are rarely exogenous.
Supplemental Material
Supplemental material, Armstrong-Carter_Supplemental_Material_rev for The Earliest Origins of Genetic Nurture: The Prenatal Environment Mediates the Association Between Maternal Genetics and Child Development by Emma Armstrong-Carter, Sam Trejo, Liam Hill, Kirsty Crossley, Dan Mason and Benjamin W. Domingue in Psychological Science
Acknowledgments
We thank Rosie McEachan, Born in Bradford participants, health professionals, researchers, and staff for access to the restricted-use genetic data.
Footnotes
ORCID iDs: Emma Armstrong-Carter  https://orcid.org/0000-0002-5847-9486
https://orcid.org/0000-0002-5847-9486
Sam Trejo  https://orcid.org/0000-0002-9880-5354
https://orcid.org/0000-0002-9880-5354
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797620917209
Transparency
Action Editor: Brent W. Roberts
Editor: D. Stephen Lindsay
Author Contributions
E. Armstrong-Carter and S. Trejo contributed equally to this article. E. Armstrong-Carter, S. Trejo, and B. W. Domingue conducted the analysis and wrote the manuscript. L. J. B. Hill, K. L. Crossley, and D. Mason edited the manuscript and provided support.
Declaration of Conflicting Interests: The author(s) declared that there were no conflicts of interest with respect to the authorship or the publication of this article.
Funding: This work is supported by the Russell Sage Foundation and the Ford Foundation (Grant No. 96-17-04), the National Science Foundation (Grant No. DGE-1656518), the Institute of Education Sciences (Grant No. R305B140009), and a Research Mobility Award grant. Born in Bradford receives funding from the Wellcome Trust (Grant No. WT101597MA), the National Institute for Health Research (NIHR; Grant No. NF-SI-0611-10196), the UK Medical Research Council (Grant No. G0600705), the US National Institutes of Health (Grant No. R01 DK10324), and the European Research Council (Seventh Framework Programme 2007–2013; Grant No. 66954). Opinions are those of the authors alone and not the funders.
Open Practices: Data access can be requested via the Born in Bradford website (https://borninbradford.nhs.uk/). The design and analysis plans for this study were not preregistered.
References
- Ayorech Z., Krapohl E., Plomin R., von Stumm S. (2017). Genetic influence on intergenerational educational attainment. Psychological Science, 28, 1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates T. C., Maher B. S., Medland S. E., McAloney K., Wright M. J., Hansell N. K., . . . Gillespie N. A. (2018). The nature of nurture: Using a virtual-parent design to test parenting effects on children’s educational attainment in genotyped families. Twin Research and Human Genetics, 21(2), 73–83. doi: 10.1017/thg.2018.11 [DOI] [PubMed] [Google Scholar]
- Belsky D. W., Caspi A., Arseneault L., Corcoran D. L., Domingue B. W., Harris K. M., . . . Odgers C. L. (2019). Genetics and the geography of health, behaviour and attainment. Nature Human Behaviour, 3, 576–586. doi: 10.1038/s41562-019-0562-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky D. W., Domingue B. W., Wedow R., Arseneault L., Boardman J. D., Caspi A., . . . Moffitt T. E. (2018). Genetic analysis of social-class mobility in five longitudinal studies. Proceedings of the National Academy of Sciences, USA, 115, E7275–E7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky D. W., Harden K. P. (2019). Phenotypic annotation: Using polygenic scores to translate discoveries from genome-wide association studies from the top down. Current Directions in Psychological Science, 28, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky D. W., Moffitt T. E., Corcoran D. L., Domingue B., Harrington H., Hogan S., . . . Williams B. S. (2016). The genetics of success: How single-nucleotide polymorphisms associated with educational attainment relate to life-course development. Psychological Science, 27, 957–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D. J., Berger J. O., Johannesson M., Nosek B. A., Wagenmakers E.-J., Berk R., . . . Johnson V. E. (2018). Redefine statistical significance. Nature Human Behaviour, 2, 6–10. [DOI] [PubMed] [Google Scholar]
- Chabris C. F., Lee J. J., Cesarini D., Benjamin D. J., Laibson D. I. (2015). The fourth law of behavior genetics. Current Directions in Psychological Science, 24, 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Chow C. C., Tellier L. C., Vattikuti S., Purcell S. M., Lee J. J. (2015). Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience, 4(1), Article 7. doi: 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley D., Domingue B. W., Cesarini D., Dawes C., Rietveld C. A., Boardman J. D. (2015). Is the effect of parental education on offspring biased or moderated by genotype? Sociological Science, 2, 82–105. doi: 10.15195/v2.a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronbach L. J., Meehl P. E. (1955). Construct validity in psychological tests. Psychological Bulletin, 52, 281–302. doi: 10.1037/h0040957 [DOI] [PubMed] [Google Scholar]
- de Zeeuw E. L., van Beijsterveldt C. E., Glasner T. J., Bartels M., Ehli E. A., Davies G. E., . . . Groen-Blokhuis M. M. (2014). Polygenic scores associated with educational attainment in adults predict educational achievement and ADHD symptoms in children. American Journal of Medical Genetics B: Neuropsychiatric Genetics, 165, 510–520. [DOI] [PubMed] [Google Scholar]
- Domingue B. W., Belsky D. W. (2017). The social genome: Current findings and implications for the study of human genetics. PLOS Genetics, 13(3), Article e1006615. doi: 10.1371/journal.pgen.1006615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingue B. W., Belsky D. W., Conley D., Harris K. M., Boardman J. D. (2015). Polygenic influence on educational attainment. AERA Open, 1(3). doi: 10.1177/2332858415599972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudbridge F. (2013). Power and predictive accuracy of polygenic risk scores. PLOS Genetics, 9(3), Article e1003348. doi: 10.1371/journal.pgen.1003348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan G. J., Dowsett C. J., Claessens A., Magnuson K., Huston A. C., Klebanov P., . . . Japel C. (2007). School readiness and later achievement. Developmental Psychology, 43, 1428–1446. doi: 10.1037/0012-1649.43.6.1428 [DOI] [PubMed] [Google Scholar]
- Elliott M. L., Belsky D. W., Anderson K., Corcoran D. L., Ge T., Knodt A., . . . Hariri A. R. (2019). A polygenic score for higher educational attainment is associated with larger brains. Cerebral Cortex, 29, 3496–3504. doi: 10.1093/cercor/bhy219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Keele L., Tingley D. (2010). A general approach to causal mediation analysis. Psychological Methods, 15, 309–334. [DOI] [PubMed] [Google Scholar]
- Kong A., Thorleifsson G., Frigge M. L., Vilhjalmsson B. J., Young A. I., Thorgeirsson T. E., . . . Masson G. (2018). The nature of nurture: Effects of parental genotypes. Science, 359, 424–428. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Wedow R., Okbay A., Kong E., Maghzian O., Zacher M., . . . Cesarini D. (2018). Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nature Genetics, 50, 1112–1121. doi:10.1038/s41588-018-0147-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. R., Gignoux C. R., Walters R. K., Wojcik G. L., Neale B. M., Gravel S., . . . Kenny E. E. (2017). Human demographic history impacts genetic risk prediction across diverse populations. The American Journal of Human Genetics, 100, 635–649. doi: 10.1016/j.ajhg.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mõttus R., Realo A., Vainik U., Allik J., Esko T. (2017). Educational attainment and personality are genetically intertwined. Psychological Science, 28, 1631–1639. [DOI] [PubMed] [Google Scholar]
- Okbay A., Beauchamp J. P., Fontana M. A., Lee J. J., Pers T. H., Rietveld C. A., . . . Benjamin D. J. (2016). Genome-wide association study identifies 74 loci associated with educational attainment. Nature, 533, 539–542. doi: 10.1038/nature17671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo L. R., Noble K. G. (2019). Poverty, early experience, and brain development. In Zeanah C. H., Jr. (Ed.), Handbook of infant mental health (4th ed., pp. 157–171). New York, NY: Guilford Press. [Google Scholar]
- Price A. L., Patterson N. J., Plenge R. M., Weinblatt M. E., Shadick N. A., Reich D. (2006). Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics, 38, 904–909. [DOI] [PubMed] [Google Scholar]
- Rice F., Harold G. T., Boivin J., van den Bree M., Hay D. F., Thapar A. (2010). The links between prenatal stress and offspring development and psychopathology: Disentangling environmental and inherited influences. Psychological Medicine, 40, 335–345. doi: 10.1017/S0033291709005911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie S. J., Bates T. C. (2013). Enduring links from childhood mathematics and reading achievement to adult socioeconomic status. Psychological Science, 24, 1301–1308. [DOI] [PubMed] [Google Scholar]
- Smith-Woolley E., Selzam S., Plomin R. (2019). Polygenic score for educational attainment captures DNA variants shared between personality traits and educational achievement. Journal of Personality and Social Psychology, 117, 1145–1163. doi: 10.1037/pspp0000241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacklies W., Redestig H., Scholz M., Walther D., Selbig J. (2007). pcaMethods – A bioconductor package providing PCA methods for incomplete data. Bioinformatics, 23, 1164–1167. [DOI] [PubMed] [Google Scholar]
- Standards and Testing Agency. (2016). Key Stage 1: 2016 assessment and reporting arrangements (ARA). Retrieved from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/518970/2016_KS1_Assessment_and_reporting_arrangements__ARA__April_PDFA.pdf
- Stein A., Pearson R. M., Goodman S. H., Rapa E., Rahman A., McCallum M., . . . Pariante C. M. (2014). Effects of perinatal mental disorders on the fetus and child. The Lancet, 384, 1800–1819. doi: 10.1016/S0140-6736(14)61277-0 [DOI] [PubMed] [Google Scholar]
- Stephan Y., Sutin A. R., Kornadt A., Terracciano A. (2019). Polygenic scores for education, health, and personality as predictors of subjective age among older individuals of European ancestry: Evidence from the Health and Retirement Study. Psychology and Aging, 34, 139–144. doi: 10.1037/pag0000283 [DOI] [PubMed] [Google Scholar]
- Trejo S., Belsky D. W., Boardman J. D., Freese J., Harris K. M., Herd P., . . . Domingue B. W. (2018). Schools as moderators of genetic associations with life course attainments: Evidence from the WLS and Add Health. Sociological Science, 5, 513–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo S., Domingue B. W. (2019). Genetic nature or genetic nurture? Quantifying bias in analyses using polygenic scores. Biodemography and Social Biology, 64, 187–215. doi: 10.1080/19485565.2019.1681257 [DOI] [PubMed] [Google Scholar]
- Turkheimer E. (2000). Three laws of behavior genetics and what they mean. Current Directions in Psychological Science, 9, 160–164. [Google Scholar]
- Visscher P. M., Wray N. R., Zhang Q., Sklar P., McCarthy M. I., Brown M. A., Yang J. (2017). 10 years of GWAS discovery: Biology, function, and translation. The American Journal of Human Genetics, 101, 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz J., Belsky J., Moffitt T. E., Belsky D. W., Harrington H., Avinun R., . . . Caspi A. (2019). Genetics of nurture: A test of the hypothesis that parents’ genetics predict their observed caregiving. Developmental Psychology, 55, 1461–1472. doi: 10.1037/dev0000709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz J., Caspi A., Belsky D. W., Beckley A. L., Arseneault L., Barnes J. C., . . . Moffit T. E. (2018). Genetics and crime: Integrating new genomic discoveries into psychological research about antisocial behavior. Psychological Science, 29, 791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker A. (2014). Early years foundation stage profile results: 2013 to 2014. Retrieved from https://www.gov.uk/government/statistics/early-years-foundation-stage-profile-results-2013-to-2014
- Wright J., Small N., Raynor P., Tuffnell D., Bhopal R., Cameron N., . . . Petherick E. S. (2012). Cohort profile: The Born in Bradford multi-ethnic family cohort study. International Journal of Epidemiology, 42, 978–991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Armstrong-Carter_Supplemental_Material_rev for The Earliest Origins of Genetic Nurture: The Prenatal Environment Mediates the Association Between Maternal Genetics and Child Development by Emma Armstrong-Carter, Sam Trejo, Liam Hill, Kirsty Crossley, Dan Mason and Benjamin W. Domingue in Psychological Science