Abstract
We report the dispersion of single-walled carbon nanotubes (SWCNTs) using pentiptycene polymers and their use in chemiresistance-based and QCM-D sensors. Poly(p-phenylene ethynylene)s (PPEs) incorporating pentiptycene moieties present a concave surface that promotes π–π interactions and van der Waals interactions with SWCNTs. In contrast to more common polymer-dispersing mechanisms that involve the wrapping of polymers around the SWCNTs, we conclude that the H-shape of pentiptycene groups and the linear rigid-rod structure creates a slot for nanotube binding. UV–vis–NIR, Raman, and fluorescence spectra and TEM images of polymer/SWCNTs support this dispersion model, which shows size selectivity to SWCNTs with diameters of 0.8–0.9 nm. Steric bulk on the channels is problematic, and tert-butylated pentiptycenes do not form stable dispersions with SWCNTs. This result, along with the diameter preference, supports the model in which the SWCNTs are bound to the concave clefts of the pentiptycenes. The binding model suggests that the polymer/SWCNTs complex creates galleries, and we have demonstrated the binding of benzene, toluene, and o-xylene (BTX) vapors as the basis for a robust, sensitive, and selective sensing platform for BTX detection. The utility of our sensors is demonstrated by the detection of benzene at the OSHA short-term exposure limit of 5 ppm in air.
Keywords: carbon nanotubes, pentiptycene polymer, polymer/carbon nanotube composites, benzene sensing, chemiresistive sensing, quartz crystal microbalance with dissipation monitoring
Graphical Abstract
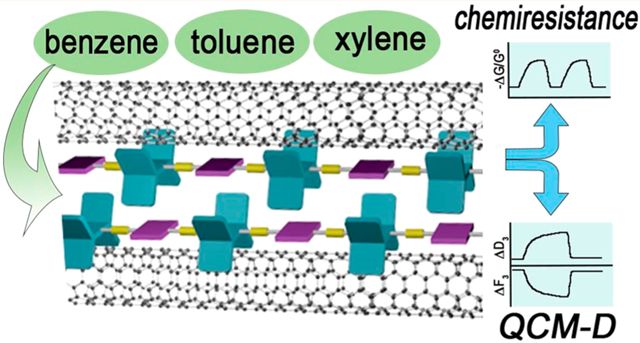
Single-walled carbon nanotubes (SWCNTs) are attractive materials for sensing gaseous analytes as a result of their sensitive resistance changes in response to the binding of molecules.1-5 The sensing performance in these materials is a consequence of a high surface area-to-volume ratio, molecular adsorption onto their electronically active sidewalls, swelling of the matrix, and/or their restricted conduction pathways.5 However, pristine SWCNTs display nonspecific responses to chemical exposures, and covalent or noncovalent functionalization with selectors or receptors is required to produce selectivity to target analytes. Covalent functionalization utilizes reactions that attach chemical groups covalently to the conjugated surfaces or termini of CNTs.6-9 Covalent functionalization has the advantage that it produces stable anchors of functional groups to CNT. However, covalent attachment of groups to the graphene surface transforms sp2 carbons into sites with increased sp3 character that disrupts electronic coherence and decreases carrier mobility. Non-covalent functionalization of SWCNTs is less perturbative to the electronic properties of the nanotubes, and the associated higher carrier mobilities can provide enhanced sensitivity.10-15
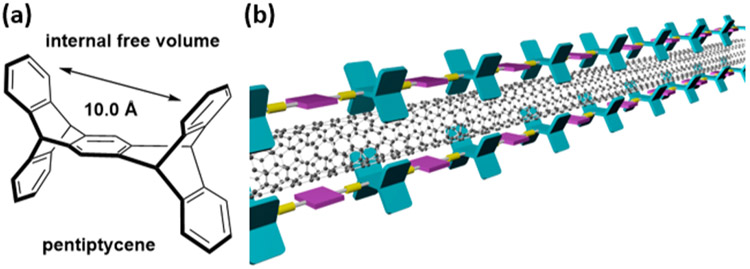
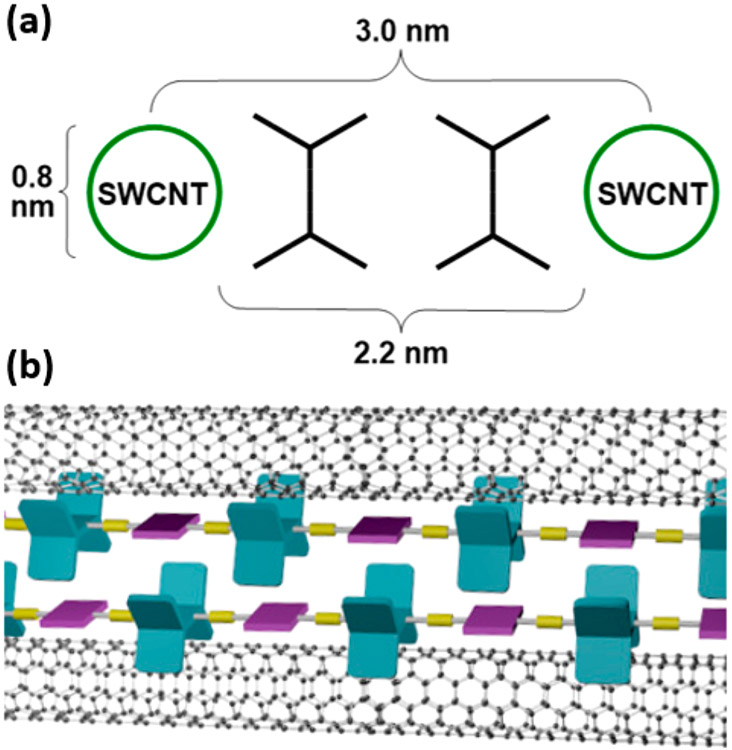
As chemiresistive sensors, SWCNTs have been functionalized noncovalently by physisorption of small aromatic molecules and surfactants through π–π and hydrophobic interactions.16-25 Physisorbed selector molecules or coatings often have limited stability, are prone to environmentally induced changes in their configuration around the CNT, can undergo phase segregation, and can desorb in solution, leading to unstable dispersions. These changes can produce large conductance changes that give rise to drift and degraded performance in chemiresistive sensors. Polymer wrapping or surface-anchored molecular clips produce stable noncovalent functionalized SWCNTs26-34 and have also been widely explored to separate and purify35-47 or orient48,49 these materials. Conjugated polymers with selectors attached can provide selectivity for the detection of specific analytes and even resolve structural isomers of xylenes.13,50-53 Simple poly(phenylene ethynylene)s (PPEs) have been shown to disperse SWCNTs,54-58 and we have been interested to see if PPEs containing pentiptycene, a rigid H-shaped molecule with a cleft of about 10 Å diameter (Figure 1a),59 would have strong interactions with SWCNTs of a complementary diameter. Pentiptycene’s structural properties in conjugated oligomers60,61 and polymers 62,63 prevent intermolecular π–π interactions to maintain high photoluminescence in solid films. The free volume in these structures creates porous structures that have provided size exclusion for the selective detection of small-molecule analytes.64-67
Figure 1.
(a) Structural property of pentiptycene. (b) Schematic drawing of the dispersion mechanism of SWCNTs between pentiptycene polymers.
In this report we propose the dispersion method illustrated in a simplified form in Figure 1b, wherein rather than wrapping around a SWCNT, pentiptycene functions as a linear arrangement of clips that cooperatively bind through van der Waals interactions. Central to this model is that the diameter of the cleft defined by the pentiptycene matches SWCNT with a diameter of about 0.9 nm. Commercially available SWCNTs have a size distribution, and we expect pentiptycene polymers to selectively disperse SWCNTs of a complementary size. To this end we designed five pentiptycene-incorporating polymers, P1–P5 (Chart 1), and investigated their dispersions with SWCNTs. All of the polymers contain long alkyl chains to enhance solubility. P3 and P5 also contain bulky tert-butyl groups on the periphery ring of pentiptycene, which has been shown to increase free volume in polymers.68 The polymer/SWCNT dispersions and thin films were characterized by UV–vis–NIR absorption, photoluminescence, Raman scattering, and TEM imaging. We further demonstrate that the polymer/SWCNT constructions impart enhanced selective sensing responses to vapors of benzene, toluene, and o-xylene (BTX).
Chart 1.
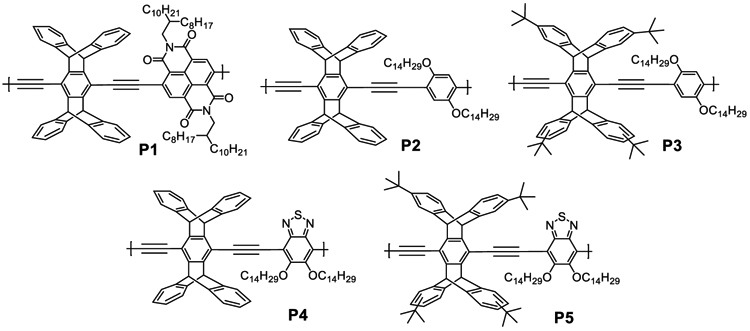
Chemical Structures of P1–P5
RESULTS AND DISCUSSION
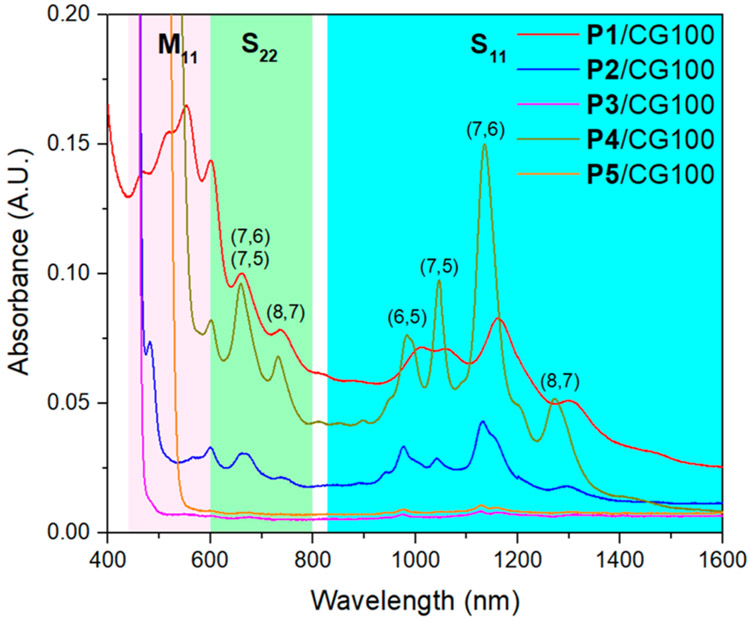
The polymer/SWCNT dispersions were prepared according to the following procedure. The pentiptycene polymer (3 mg) was dissolved in chlorobenzene (4 mL) with 2 mg of CoMoCAT SWCNTs CG100. After 30 min of tip sonication at 63 W, the suspension was centrifuged for 4 h at 30130g. The CG100 SWCNT dispersions with P1, P2, and P4 have high stability and are optically opaque after centrifugation. In contrast, dispersions using tert-butylated pentiptycene polymers, P3 and P5, are unstable and yield clear solutions after centrifugation. The optical absorbance of the supernates obtained directly after the centrifugation was analyzed in a 1 mm short-path quartz cuvette (Figure 2). For P1/CG100, P2/CG100, and P4/CG100, clear interband transitions of the van Hove singularities were observed in the absorption features. Absorption peaks in S11 (830–1600 nm) and S22 (600–800 nm) regions indicate the presence of semiconducting SWCNTs, whereas the broad and featureless absorption background and peaks in the M11 (440–645 nm) region indicate the presence of metallic SWCNTs.69 (6,5), (7,6), (7,5), and (8,7) SWCNTs were found to be the major species in the polymer/SWCNT dispersions.70-72 Notably, P4/CG100 has much sharper absorption peaks than P1/CG100 and P2/CG100, suggesting the superior polymer/SWCNT dispersion quality by P4. The absorption features of P1/CG100 in the S11 (830–1600 nm) region are slightly broader than those of P2/CG100 and P4/CG100, which suggests mild bundling of SWCNTs possibly due to the branched alkyl chains in P1, which could hinder the polymer/SWCNT interaction.44 On the contrary, no clear absorption feature was identified for P3/CG100 and P5/CG100, as they failed to yield stable SWCNT dispersions. The fact that the bulky tert-butylated pentiptycene polymers display poor SWCNT dispersion is also consistent with the binding to the SWCNT being mediated by strong van der Waals interactions between the π-systems.
Figure 2.
UV–vis–NIR absorption spectra of supernates of P1/CG100 (red), P2/CG100 (blue), P3/CG100 (purple), P4/CG100 (green), and P5/CG100 (orange). The pink, green, and cyan boxes indicate the locations of optical transitions of metallic (M11) and semiconducting (S22, S11) SWCNTs, respectively. Characteristic absorption peaks are labeled with the assigned chirality.
To support our proposed noncovalent structural model, we collected Raman spectra of the polymer/SWCNT complexes (Figure S3). Thin film samples were prepared by drop-casting polymer/CG100 supernatant dispersions onto silicon wafers. A pristine CG100 SWCNT thin film was also prepared for reference. The spectra were obtained using a 633 nm excitation wavelength and are normalized to the intensity of the G-band, at 1590 cm−1. The D-band, located at around 1300 cm−1, is indicative of the disruption of the sp2 network in conjugated nanocarbon systems, and the intensity ratio of the D- to G-bands (ID/IG) provides information on the perturbation of the π-system. The ID/IG ratios of polymer/CG100 films were 0.05–0.07, which are close to that of pristine CG100 (0.05) and confirm minimal disruption of the nanotubes’ electronic structure.
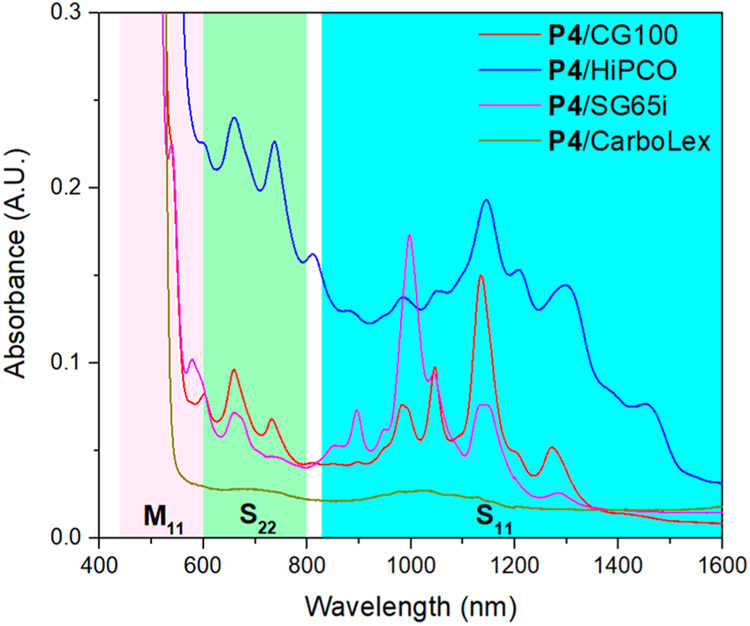
The need to match the SWCNT diameter with the size of the pentiptycene cleft was evaluated in dispersions using P4 and several commercial SWCNTs with different diameter distributions. Besides CoMoCAT SWCNTs CG100 (0.7–1.3 nm diameter), HiPCO SWCNTs (0.8–1.2 nm diameter), (6,5)-enriched CoMoCAT SWCNTs SG65i (0.7–0.9 nm diameter), and arc-discharge SWCNTs CarboLex (1.2–1.5 nm diameter) were investigated. The UV–vis–NIR spectra of the dispersions are shown in Figure 3, and the photographs of the dispersions are shown in Figure 4. Stable SWCNT dispersions were formed in P4/CG100, P4/HiPCO, and P4/SG65i, as well-resolved absorption peaks were observed in S11, S22, and M11 regions. Similarly, the noncovalent binding of P4 to the SWCNTs is confirmed by the minimal change in ID/IG (Figures S6 and S7). For CarboLex, which comprises SWCNTs with diameters larger than the pentiptycene cleft size, the supernatant color is close to that of pure P4, consistent with the UV–vis–NIR spectrum, which indicates that a minimal amount of CarboLex SWCNTs is dispersed.
Figure 3.
UV–vis–NIR absorption spectra of supernates of P4/HiPCO (blue), P4/CG100 (red), P4/SG65i (purple), and P4/CarboLex (green). The pink, green, and cyan boxes indicate the locations of optical transitions of metallic (M11) and semiconducting (S22, S11) SWCNTs, respectively.
Figure 4.
Photographs of supernates of P4/HiPCO, P4/CG100, P4/SG65i, P4/CarboLex, and P4 solution in chlorobenzene (from left to right).
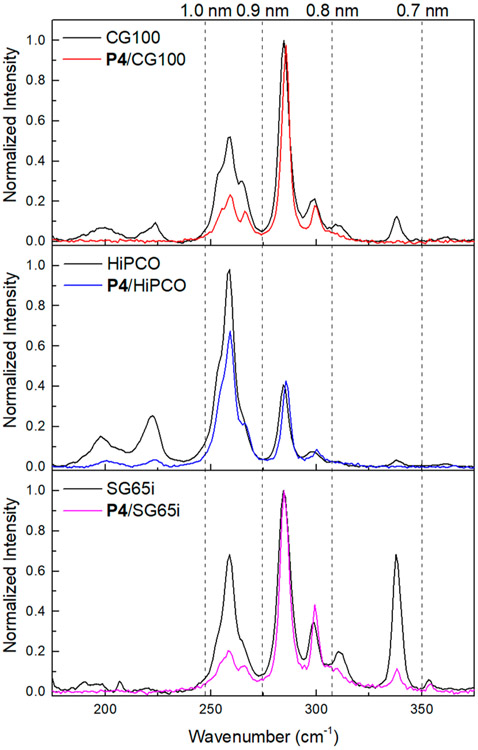
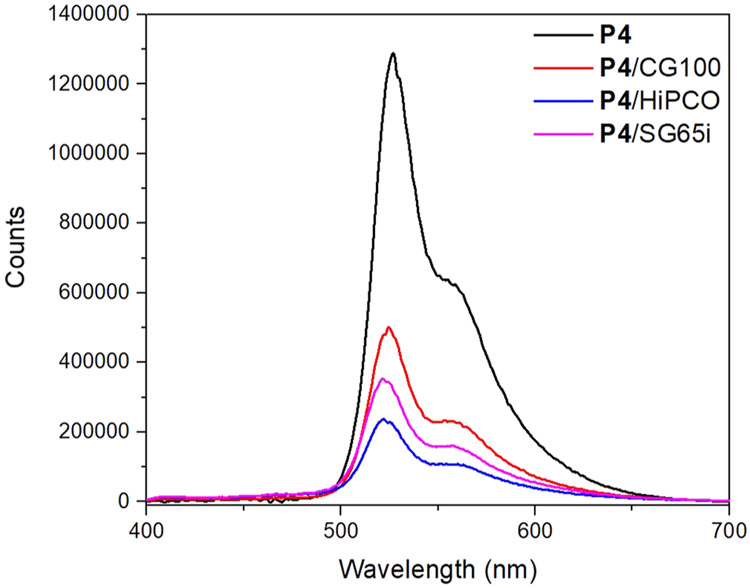
Central to our proposed SWCNT binding model is the diameter selectivity provided by the pentiptycene unit toward a range of SWCNTs. The Raman radial breathing mode (RBM) of the pristine SWCNT and the polymer/SWCNT complexes, which correlates strongly with SWCNT diameter,73-77 is evaluated in Figure 5. Raman intensities are normalized to the peak at 285 cm−1 to illustrate the relative change in the SWCNT diameter distribution. The estimated SWCNT diameters are indicated by the dotted lines following a reported equation.74 As shown in Figure 5, SWCNTs with diameters larger than 1.0 nm or smaller than 0.8 nm are significantly reduced after polymer dispersion. An enrichment of SWCNTs with 0.8–0.9 nm diameters was observed for all dispersions, matching the cleft size of the pentiptycene unit, which facilitates the strong polymer–SWCNT interaction. It should also be noted that the RBM as well as the G-band peaks of the polymer/SWCNT complexes are shifted from their counterparts in pristine SWCNTs (Figures 5 and S8), which suggests effective binding of the polymer on the surface of SWCNTs.73-77 Moreover, the binding of the polymers to the SWCNTs is also apparent from the excited state electron and/or energy transfer that leads to fluorescence quenching. As shown in Figure 6, the emission intensities of P4/SWCNT complexes were found to be attenuated by 60–80% compared to the pure P4 solution at the same concentration. Emission peaks of P4/SWCNT dispersions agree with that of neat P4 solution, indicating no significant polymer scission from the tip sonication procedure, which is also supported by the UV–vis–NIR absorption comparison (Figure S10).
Figure 5.
Radial breathing mode (RBM) region of the Raman spectra of pristine SWCNTs and P4/SWCNT dispersions normalized to the peak at 285 cm−1 (excitation at 633 nm). Dotted lines indicate the estimated SWCNT diameter.
Figure 6.
Emission spectra (λex = 350 nm) of supernates of P4/CG100 (red), P4/HiPCO (blue), P4/SG65i (purple), and P4 solution (black) in chlorobenzene. The polymer concentration is 0.75 mg/mL in all cases.
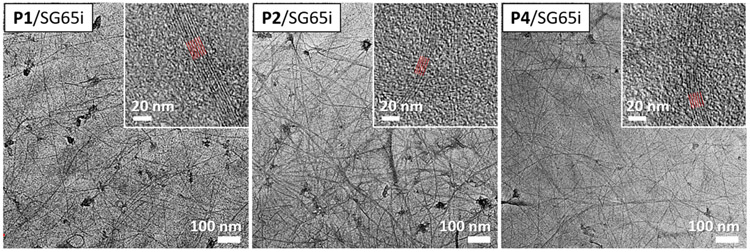
TEM images of drop-cast films of P1/SG65i, P2/SG65i, and P4/SG65i are shown in Figure 7. The linear structures observed are consistent with aligned networks of SWCNT/polymer aggregates. The parallel red lines in the inset highlight the periodic fringes of SWCNTs, which have a center-to-center distance of ~3 nm (Figures S11 and S12). This gives an intertube distance of ~2.2 nm, which is significantly larger than the intertube distance in SWCNT bundles determined previously.78-80 The increased intertube spacing is consistent with the presence of pentiptycene-dispersing polymers between the tubes, as shown schematically in Figure 8. This model is consistent with the 1 nm diameter of pentiptycene clefts and that dispersed SWCNTs will have polymers bound to their surface, consistent with previous reports where PPEs are shown to stack directly on the SWCNT surface by AFM and TEM studies.55,56 Hence, in the film there are on average two polymer chains between each SWCNT, which creates molecularly defined cavities in the films.
Figure 7.
TEM images of drop-cast films of P1/SG65i (left), P2/SG65i (middle), and P4/SG65i (right). Inset figures are at higher magnification and show periodic fringes of SWCNTs.
Figure 8.
Schematic drawing of pentiptycene polymer/SWCNT composition in drop-cast film.
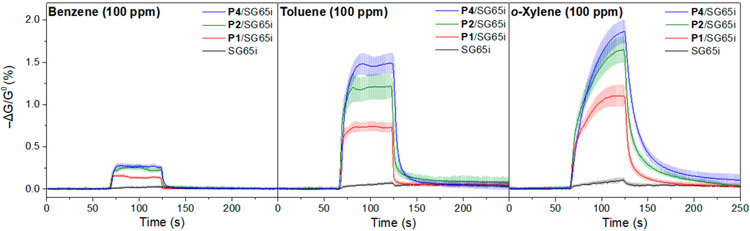
The porous structures with high aspect ratio galleries created by the polymer/SWCNT complexes present an opportunity to detect volatile organic vapors. Considering the planar nature of the comonomers in the pentiptycene polymers, we have evaluated their efficacy for interacting with the planar aromatic organics: benzene, toluene, and o-xylene. Detection of BTX is of interest in environmental health and safety as a result of their toxicity. The parallel bundles of polymer/SWCNT observed in our TEM studies suggest that these composites may create interstitial galleries that can differentiate vapors based on size exclusion. The diffusion of aromatic vapors into these galleries is expected to swell the polymer/SWCNT structures and thereby induce the changes of conductance. (6,5)-Enriched SWCNTs SG65i were used to fabricate the sensors with revised procedures as a result of their high semiconducting content, which has been shown to aid sensing sensitivity.51,81 Devices were prepared by drop-casting polymer/SWCNT supernates (1 μL) on gold electrodes with an interelectrode spacing of 1 mm, followed by drying in vacuo. The change in resistance resulting from the exposure to the analyte vapor was converted to the negative normalized change in conductance, −ΔG/G0, where ΔG and G0 are the change in conductance and baseline conductance, respectively. Figure 9 summarizes the chemiresistive responses (−ΔG/G0) of pristine SG65i, P1/SG65i, P2/SG65i, and P4/SG65i with exposures to benzene, toluene, and o-xylene (100 ppm) for 1 min in air. Minor baseline correction was applied to account for the linear drift of the baseline conductance.
Figure 9.
Chemiresistive responses of pristine SG65i (black), P1/SG65i (red), P2/SG65i (green), and P4/SG65i (blue) in response to benzene (left), toluene (middle), and o-xylene (right) in air. Devices were exposed to analyte at 100 ppm in air for 1 min (N ≥ 6).
As shown in Figure 9, films of pristine SG65i show very weak responses to BTX vapor. For P1/SG65i, the averaged response toward 100 ppm benzene vapor is about 0.15% with an excellent signal-to-background ratio and is approximately 2× more sensitive than our previous BTX chemiresistive sensors.82 For P2/SG65i and P4/SG65i, the responses to benzene vapor (~0.25%) are higher than that of P1/SG65i, while maintaining high signal-to-noise ratios. Exposure to toluene and o-xylene vapors results in much higher signals across all sensors. The averaged sensor responses of P1/SG65i, P2/SG65i, and P4/SG65i to 100 ppm toluene vapor in air are about 0.8%, 1.2%, and 1.5%, respectively. For exposure of 100 ppm o-xylene vapor in air, the averaged sensor responses of P1/SG65i, P2/SG65i, and P4/SG65i are about 1.1%, 1.7%, and 1.9%, respectively. Notably, the sensing responses toward BTX vapors are highly reversible with small variations across different fabricated devices and are equivalent in dry air or nitrogen (Figure S13). The sensing performance is largely maintained at a relatively high humidity of 50–70% (Figure S14). Together, these results confirm the utility and robustness of these sensory materials in BTX detection.
It is worth noting that the resistance of the devices fabricated from similar amounts of different polymer/SWCNT dispersions varies greatly from ~20 kΩ (P1/SG65i) to ~1 MΩ (P4/SG65i). We believe this is also indicative of the quality of the polymer/SWCNT dispersions, and better dispersions will restrict direct SWCNT/SWCNT interactions and thereby lead to high resistivity. The well-resolved absorption spectrum (Figure 3) and the attenuated emission intensity (Figure 6) of P4/SG65i support that SWCNTs are well dispersed, and tight binding to P4 creates molecular insulation on individual tubes. For all sensors, the responses toward benzene and toluene are extremely rapid with a “step-function” type profile that saturates within 10 s and returns to the baseline within 1 min after the exposure. This indicates that the gas molecules diffuse into and out of binding sites quickly. The conductance profile for o-xylene sensing has more sluggish kinetics, which is consistent with its bulkier structure. It should be noted that the BTX molecules have similar electronic and structural properties. The differences in the temporal responses can potentially be used to differentiate between analytes.
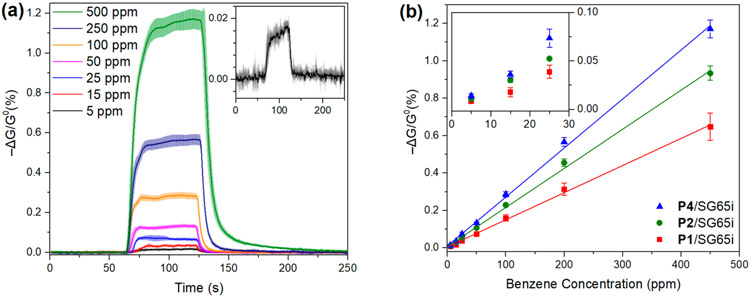
We are particularly interested in detecting benzene, which is a challenging important target in chemical sensing. Figure 10a illustrates the responses of the sensors fabricated from a P4/SG65i dispersion toward benzene vapor at different concentrations in air for 1 min. These results show that we can reproducibly detect benzene down to 5 ppm (Figure 10a, inset), which is at the OSHA short-term exposure limit.83 Similarly, clear signals were observed for all our sensors at 5 ppm of benzene (Figure S15), highlighting the potential utility in real-word benzene monitoring. As summarized in Figure 10b, sensors fabricated from P4/SG65i perform the best with the strongest responses toward benzene at concentrations ranging from 5 to 450 ppm compared to P1/SG65i and P2/SG65i. The change of chemiresistance is proportional to benzene concentration with high linear relationships (R2 > 0.997). The limit of detection (LOD) was calculated to be 4, 3, and 3 ppm for P1/SG65i, P2/SG65i, and P4/SG65i, respectively.
Figure 10.
(a) Chemiresistive responses of P4/SG65i at different concentrations of benzene. The enlarged responses to benzene vapor at 5 ppm are shown in the inset. (b) Summary of chemiresistive responses of P1/SG65i (red), P2/SG65i (green), and P4/SG65i (blue) at different concentrations of benzene. Devices were exposed to benzene in dry air for 1 min (N ≥ 6).
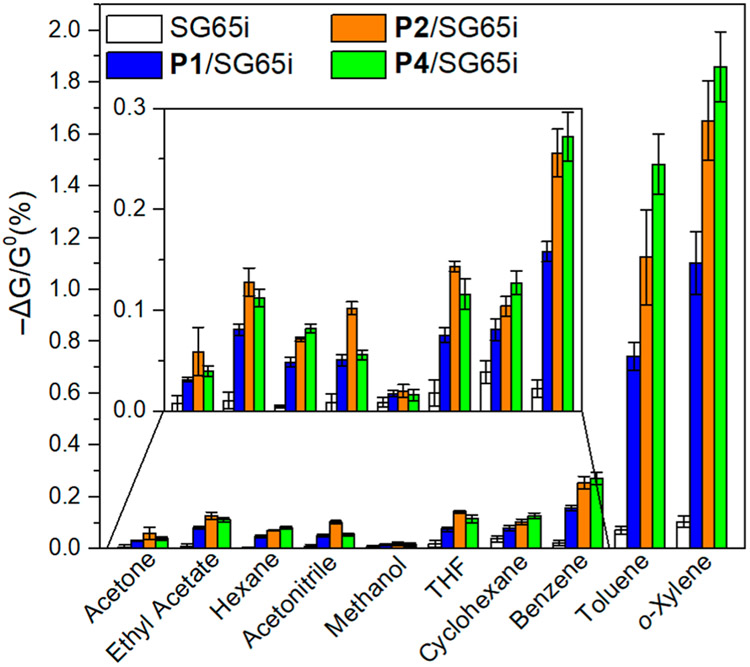
Figure 11 summarizes the selectivity of our sensors when challenged against various volatile organic compounds (VOCs) at 100 ppm in dry air. Overall, the sensors exhibit excellent selectivity toward BTX compared to common VOCs that give 0.15% responses at the same concentrations. Notably, the responses toward vapor of cyclohexane, the alkyl analogue to benzene, are only half of the responses toward benzene vapors. Moreover, the SWCNTs, which are naturally p-doped, generally have higher responses to polar molecules, but in this case the nonpolar BTX analytes have higher responses. These observations confirm the importance of the aromatic pentiptycene polymers for selective BTX sensing via structural recognition and π–π interactions.
Figure 11.
Chemiresistive responses of pristine SG65i (white), P1/SG65i (blue), P2/SG65i (orange), and P4/SG65i (green) in response to different volatile organic vapors. Devices were exposed to analyte at 100 ppm in dry air for 1 min (N ≥ 6).
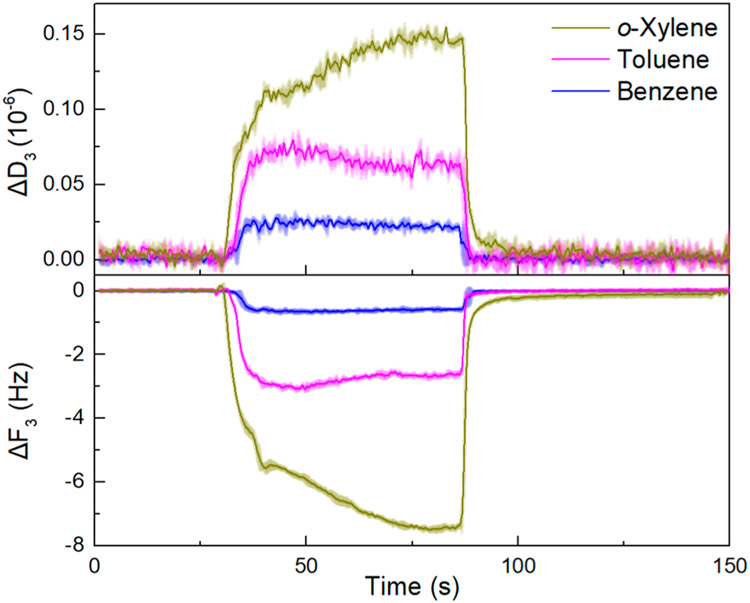
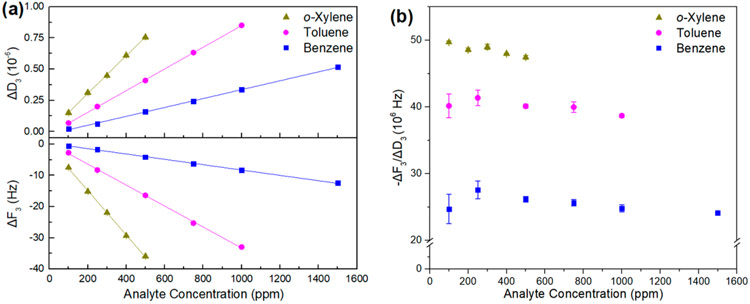
To further access the binding of BTX molecules to the polymer/SWCNT composites, we have performed sensing experiments using a quartz crystal microbalance with dissipation monitoring (QCM-D). Polymer/SWCNT supernates (5 μL) were drop-casted on the gold coating of QCM-D electrodes, followed by drying in vacuo. The sensing responses are represented by the changes in the third overtone of frequency (ΔF3) and the dissipation factor (ΔD3) over three cycles of analyte exposures.82 Representative sensing profiles of P1/SG65i-coated electrodes toward BTX vapors at 100 ppm in air are shown in Figure 12. As expected, the binding of analyte molecules to the electrode surfaces results in an increase in mass and thus a decrease in frequency. The increase in the dissipation factor indicates the change in the viscoelastic properties induced by the binding of guest molecules to the surface of the P1/SG65i-coated electrode. It should be noted that the structural assemblies of the polymer/SWCNT complexes are found to aid BTX binding as the polymer/SWCNT-coated electrodes exhibit significantly higher change in frequency upon the exposure of BTX vapors than pure polymer-coated or uncoated electrodes (Figures S16-S18). The magnitudes of ΔF3 and ΔD3 are in the order of o-xylene > toluene > benzene, which is consistent with the chemiresistance-based sensing results. Moreover, as summarized in Figures 13a, S19a, and S20a, the sensors exhibit a linear response (R2 > 0.993) for BTX vapors within the range of 100–1500, 100–1000, and 100–500 ppm, respectively. These results showcase the feasibility of BTX sensing by the polymer/SWCNT complexes with QCM-D.
Figure 12.
Changes in frequency (ΔF3, 3rd overtone) and dissipation factor (ΔD3, 3rd overtone) of a P1/SG65i-coated electrode upon three cycles of exposure to BTX vapors at 100 ppm for 1 min in air.
Figure 13.
(a) Summary of changes in frequency (ΔF3, 3rd overtone) and dissipation factor (ΔD3, 3rd overtone) of the P1/SG65i-coated electrode upon three cycles of exposure to analyte at different concentrations for 1 min in air. (b) Summary of −ΔF3/ΔD3 of the P1/SG65i-coated electrode upon three cycles of exposure to analyte at different concentrations for 1 min in air.
In order to differentiate the signals between BTX vapors, we herein make use of a characteristic value derived from −ΔF3/ΔD3,84 which has been used to study the contact mechanics of biomolecules,85 microbes,86-88 and nanomaterials89,90 with substrate surfaces. As illustrated in Figure 13a, the absolute magnitudes of ΔF3 and ΔD3 of the P1/SG65i-coated electrode increase with rising analyte concentration. However, the values of −ΔF3/ΔD3 shown in Figure 13b remain nearly constant over the dynamic range of the sensor. The values of −ΔF3/ΔD3 obtained from the BTX exposures are significantly different, with the order of o-xylene > toluene > benzene; they have been found to correlate with the molecular weight of the analytes.91,92 Gratifyingly, we have observed the similar BTX differentiation by the values of ΔF3/ΔD3 in other sensors made from P2/SG65i and P4/SG65i (Figures S19b and S20b). Therefore, with the ability to differentiate BTX molecules, the QCM-D method can complement chemiresistance-based sensing for the sensitive and selective detection of BTX vapors. This illustrates opportunities for the introduction of additional functionality to the pentiptycene polymer/SWCNT complexes in future sensor design to robustly detect and differentiate more complex analytes.
CONCLUSION
We have developed polymer/SWCNT dispersions and thin film compositions that use the clefts of pentiptycene groups to bind the nanotubes. Strong π–π interactions between polymers and SWCNTs are found to require a complementary “fit” between the nanotube and the pentiptycene. TEM results support a structural model wherein SWCNTs bind polymers coincidently with their long axis, which provides for free volume between the tubes. The quality of dispersions is evidenced by UV–vis–NIR absorption spectra, and the minimal perturbation to the SWCNT electronic structure in the dispersion was confirmed by Raman spectroscopy. The polymer construction allows for the robust and selective detection of BTX vapors in air by monitoring the change of conductance, QCM frequency, and the dissipation factor. Notably, our sensors are sensitive enough to detect benzene at the OSHA short-term exposure limit. In total, we have demonstrated that pentiptycene groups can create defined structural assemblies with SWCNTs and will be working to develop functional materials by extending this supramolecular model.
EXPERIMENTAL SECTION
Materials.
Commercial reagents were purchased from Sigma-Aldrich, Alfa Aesar, and TCI and used as received unless otherwise noted. Deuterated solvents were purchased from Cambridge Isotope Laboratories and used as received. CoMoCAT single-walled carbon nanotubes [Signis CG100, lot no.: MKBP3333V; carbon ≥90%; ≥70% carbon as SWNT; 0.7–1.3 nm diameter], (6,5)-enriched single-walled carbon nanotubes [Signis SG65i, lot no.: MKBZ1159V; (6,5) chirality, ≥93% carbon as SWCNT; 0.7–0.9 nm diameter], and arc discharge single-walled carbon nanotubes [CarboLex AP-grade lot no.: 07826BA; 50–70% carbon basis; 1.2–1.5 nm diameter] were purchased from Sigma-Aldrich and used as received. HiPCO single-walled carbon nanotubes [lot no.: P0261, 0.8–1.2 nm diameter] were purchased from Unidym Inc. and used as received.
Instrument.
NMR spectra were recorded using a Bruker Avance 400 MHz NMR or JEOL 500 MHz spectrometer. Tip sonication was performed with a Qsonica Q125 Sonicator. Absorption spectra were obtained using an Agilent Cary 4000 UV–vis–NIR spectrophotometer. Photoluminescence and excitation spectra were acquired on a Horiba Jobin Yvon Fluorolog-3 spectrofluorometer (model FL-321) equipped with a 450 W xenon lamp as the excitation source and an F-3000 fiber optic mount that allows for fluorescence imaging outside of the sample compartment. The F-3000 couples to the T-box; light is focused from the excitation spectrometer onto the fiber-optic bundle and then directed to the sample. Fluorescence emission from the sample is directed back through the bundle and into the front-face collection port in the sample compartment. Polymer/SWCNT supernates after centrifugation were deposited into a short-path-length cell from Starna for the collection of the absorption spectra. Raman spectra were collected using a Horiba Jobin-Yvon LabRam (model HR 800) Raman confocal microscope with a 633 nm laser (1.4 μm spot size). Laser intensity was set to 10% for the 633 nm excitation wavelength. Analyte gases were generated by a FlexStream FlexBase module with precise temperature (±0.01 °C) and gas flow rate control (±1.5% of the reading). Resistance was measured using an Agilent Keysight 34970A potentiostat equipped with a 34901A 20-channel multiplexer (2/4-wire) module. The potentiostat was connected to the sensing laptop using an Agilent 82357B GPIB-USB interface high-speed USB 2.0 serial cable and controlled using BenchLink Data Logger 3 (available free of charge online). The scan rate was set to 1 scan/second. QCM-D experiments were performed using Q-Sense E1 (Q-Sense, Stockholm, Sweden) with gold-coated AT-cut quartz crystal sensors (QSX 301 Gold, Q-Sense) with a 5 MHz fundamental resonance frequency.
Synthesis of Polymer.
2-Octyldodecan-1-amine93 and pentiptycene diacetylene64 were synthesized according to methods previously reported. The syntheses of polymers P2,64 P3,94 P4,95 and P53 were reported by our group. P1 was synthesized through Sonogashira reaction: under a nitrogen atmosphere, pentiptycene diacetylene (1.0 equiv), dibromonaphthalene diimide (1.0 equiv), Pd(PPh3)4 (10 mol %), and CuI (0.5 equiv) were dissolved in a previously degassed mixture of dry toluene and diisopropylamine. The solution was heated at 70 °C for 3 days and then subjected to a CHCl3/H2O workup. The combined organic phase was washed with NH4Cl(aq) and dried by MgSO4. The solvent was removed in vacuo, and the residue was precipitated in methanol three times. GPC (THF vs PS): Mn = 25100, Mw = 42700, PDI = 1.70.
Preparation of Polymer/SWCNT Dispersion.
Polymer (3 mg) was dissolved in chlorobenzene (4 mL), and the solution was sonicated in a water bath for 10 min. To the polymer solution was added 2 mg of SWCNT, and the resulting mixture was chilled with ice and homogenized for 30 min using a Qsonica Q125 sonicator at 63W. Subsequently, the suspension was centrifuged for 4 h at 30130g. For UV–vis–NIR absorption spectroscopy and photoluminescence spectroscopy, the absorption and the emission spectra of the supernatant were directly recorded in a 1 mm short-path quartz cuvette.
Chemiresistive Device Preparation.
Glass slides (VWR microscope slides) were bath sonicated in acetone for 15 min and then dried with a stream of nitrogen. Using an aluminum mask, chromium (15 nm) followed by gold (50 nm) was deposited using a thermal evaporator (Angstrom Engineering), leaving a 1 mm gap between gold electrodes. For pristine SG65i SWCNTs, a stock solution of SG65i SWCNTs (2 mg) was prepared in o-dichlorobenzene (oDCB) (20 mL) by bath sonication at RT for 30 min. A 1 μL amount of the SG65i SWCNT dispersion was drop-casted in between the gold electrodes and dried at RT under house vacuum in a desiccator or vacuum oven. For polymer/SWCNT dispersions, polymer (10 mg) was dissolved in o-dichlorobenzene (oDCB, 10 mL), and the solution was sonicated in a water bath for 10 min. To the polymer solution was added 1 mg of SG65i SWCNT, and the resulting mixture was chilled with ice and homogenized for 20 min using a Qsonica Q125 sonicator at 63W. Subsequently, the suspension was centrifuged for 30 min at 8000g and allowed to stand overnight undisturbed. A 1 μL amount of the polymer/SWCNT supernate was drop-casted in between the gold electrodes and dried at RT under house vacuum in a desiccator or vacuum oven.
TEM Imaging.
The polymer/SWCNT supernate prepared for sensing was diluted by oDCB 10 times. The solution (~10 μL) was then drop-casted onto the TEM grid (lacey C only) and dried to evaporate all oDCB at room temperature.
Analyte Gas Generation.
A gas generator (FlexStream, Kin-Tek) is used to produce gas vapors from liquid sources. A trace amount of analyte is emitted from a permeation tube diluted in air, which is further diluted with air to adjust the concentration (in ppm) of analyte. BTX and VOCs were calibrated by placing 2–3 mL of the liquid in the oven flow and measuring the mass loss after a known length of time at a constant temperature.
Chemiresistive Gas Sensing Measurements.
The chemiresistive device was enclosed in a homemade Teflon gas flow chamber. The resistance of the device was measured over time (1 scan/sec), with typical procedures including 5 min equilibration time (for the baseline resistance to stabilize) followed by 1 min exposure to analyte in air and then 5 min of recovery. All presented data are given as the numeral average (N ≥ 6) accompanied by the standard deviation.
Quartz Crystal Microbalance with Dissipation Monitoring Gas-Sensing Experiments.
The polymer solution or polymer/SWCNT supernatant (5 μL) was drop-casted on the gold coating of QCM-D electrodes, followed by drying in vacuo. The third overtone of frequency (ΔF3) and the dissipation factor (ΔD3) of a film on a QCM sensor were measured by three cycles of exposure of a film to an analyte vapor for 1 min at 23 °C. Typical procedures include 5 min equilibration time followed by 1 min exposure to analyte in air and then 5 min of recovery.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Environmental Health Sciences Superfund Basic Research Program, National Institute of Health, P42 ES027707, and the National Science Foundation, DMR-1809740. We thank Dr. Darryl Fong, Dr. Ruqiang Lu, and Prof. Maggie He for insightful discussions.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnano.0c02570.
Photophysical studies; additional sensing results; NMR spectra of P1 (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Kauffman DR; Star A Carbon Nanotube Gas and Vapor Sensors. Angew. Chem., Int. Ed 2008, 47, 6550–6570. [DOI] [PubMed] [Google Scholar]
- (2).Fennell JF; Liu SF; Azzarelli JM; Weis JG; Rochat S; Mirica KA; Ravnsbæk JB; Swager TM Nanowire Chemical/Biological Sensors: Status and a Roadmap for the Future. Angew. Chem., Int. Ed 2016, 55, 1266–1281. [DOI] [PubMed] [Google Scholar]
- (3).Star A; Joshi V; Skarupo S; Thomas D; Gabriel J-CP Gas Sensor Array Based on Metal-Decorated Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 21014–21020. [DOI] [PubMed] [Google Scholar]
- (4).Meyyappan M Carbon Nanotube-Based Chemical Sensors. Small 2016, 12, 2118–2129. [DOI] [PubMed] [Google Scholar]
- (5).Schroeder V; Savagatrup S; He M; Lin S; Swager TM Carbon Nanotube Chemical Sensors. Chem. Rev 2019, 119, 599–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).He M; Swager TM Covalent Functionalization of Carbon Nanomaterials with Iodonium Salts. Chem. Mater 2016, 28, 8542–8549. [Google Scholar]
- (7).Sakellariou G; Priftis D; Baskaran D Surface-Initiated Polymerization from Carbon Nanotubes: Strategies and Perspectives. Chem. Soc. Rev 2013, 42, 677–704. [DOI] [PubMed] [Google Scholar]
- (8).Schnorr JM; Zwaag D. v. d.; Walish JJ; Weizmann Y; Swager TM Sensory Arrays of Covalently Functionalized Single-Walled Carbon Nanotubes for Explosive Detection. Adv. Funct. Mater 2013, 23, 5285–5291. [Google Scholar]
- (9).Paoletti C; He M; Salvo P; Melai B; Calisi N; Mannini M; Cortigiani B; Bellagambi FG; Swager TM; Di Francesco F; Pucci A Room Temperature Amine Sensors Enabled by Sidewall Functionalization of Single-Walled Carbon Nanotubes. RSC Adv. 2018, 8, 5578–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Fong D; Andrews GM; McNelles SA; Adronov A Decoration of Polyfluorene-Wrapped Carbon Nanotube Thin Films via Strain-Promoted Azide–Alkyne Cycloaddition. Polym. Chem 2018, 9, 4460–4467. [Google Scholar]
- (11).Fong D; Andrews GM; Adronov A Functionalization of Polyfluorene-Wrapped Carbon Nanotubes via Copper-Mediated Azide–Alkyne Cycloaddition. Polym. Chem 2018, 9, 2873–2879. [Google Scholar]
- (12).Zeininger L; He M; Hobson ST; Swager TM Resistive and Capacitive γ-Ray Dosimeters Based On Triggered Depolymerization in Carbon Nanotube Composites. ACS Sens. 2018, 3, 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Fennell JF; Hamaguchi H; Yoon B; Swager TM Chemiresistor Devices for Chemical Warfare Agent Detection Based on Polymer Wrapped Single-Walled Carbon Nanotubes. Sensors 2017, 17, 982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Fong D; Yeung J; McNelles SA; Adronov A Decoration of Polyfluorene-Wrapped Carbon Nanotubes via Strain-Promoted Azide–Alkyne Cycloaddition. Macromolecules 2018, 51, 755–762. [Google Scholar]
- (15).Bilalis P; Katsigiannopoulos D; Avgeropoulos A; Sakellariou G Non-Covalent Functionalization of Carbon Nanotubes with Polymers. RSC Adv. 2014, 4, 2911–2934. [Google Scholar]
- (16).Wei L; Lu D; Wang J; Wei H; Zhao J; Geng H; Zhang Y Highly Sensitive Detection of Trinitrotoluene in Water by Chemiresistive Sensor Based on Noncovalently Amino Functionalized Single-Walled Carbon Nanotube. Sens. Actuators, B 2014, 190, 529–534. [Google Scholar]
- (17).Zhao Y-L; Hu L; Stoddart JF; Grüner G Pyrenecyclodextrin-Decorated Single-Walled Carbon Nanotube Field-Effect Transistors as Chemical Sensors. Adv. Mater 2008, 20, 1910–1915. [Google Scholar]
- (18).Lerner MB; Kybert N; Mendoza R; Villechenon R; Lopez MAB; Johnson ATC Scalable, Non-Invasive Glucose Sensor Based on Boronic Acid Functionalized Carbon Nanotube Transistors. Appl. Phys. Lett 2013, 102, 183113. [Google Scholar]
- (19).Frazier KM; Swager TM Robust Cyclohexanone Selective Chemiresistors Based on Single-Walled Carbon Nanotubes. Anal. Chem 2013, 85, 7154–7158. [DOI] [PubMed] [Google Scholar]
- (20).Zhu R; Azzarelli JM; Swager TM Wireless Hazard Badges to Detect Nerve-Agent Simulants. Angew. Chem., Int. Ed 2016, 55, 9662–9666. [DOI] [PubMed] [Google Scholar]
- (21).Weis JG; Ravnsbæk JB; Mirica KA; Swager TM Employing Halogen Bonding Interactions in Chemiresistive Gas Sensors. ACS Sens. 2016, 1, 115–119. [Google Scholar]
- (22).Liu SF; Petty AR; Sazama GT; Swager TM Single-Walled Carbon Nanotube/Metalloporphyrin Composites for the Chemiresistive Detection of Amines and Meat Spoilage. Angew. Chem., Int. Ed 2015, 54, 6554–6557. [DOI] [PubMed] [Google Scholar]
- (23).Liu SF; Lin S; Swager TM An Organocobalt-Carbon Nanotube Chemiresistive Carbon Monoxide Detector. ACS Sens. 2016, 1, 354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Esser B; Schnorr JM; Swager TM Selective Detection of Ethylene Gas Using Carbon Nanotube-Based Devices: Utility in Determination of Fruit Ripeness. Angew. Chem., Int. Ed 2012, 51, 5752–5756. [DOI] [PubMed] [Google Scholar]
- (25).Chen RJ; Bangsaruntip S; Drouvalakis KA; Wong Shi Kam N; Shim M; Li Y; Kim W; Utz PJ; Dai H Noncovalent Functionalization of Carbon Nanotubes for Highly Specific Electronic Biosensors. Proc. Natl. Acad. Sci U. S. A 2003, 100, 4984–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).O’Connell MJ; Boul P; Ericson LM; Huffman C; Wang Y; Haroz E; Kuper C; Tour J; Ausman KD; Smalley RE Reversible Water-Solubilization of Single-Walled Carbon Nanotubes by Polymer Wrapping. Chem. Phys. Lett 2001, 342, 265–271. [Google Scholar]
- (27).Fong D; Adronov A Recent Developments in the Selective Dispersion of Single-Walled Carbon Nanotubes Using Conjugated Polymers. Chem. Sci 2017, 8, 7292–7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Llanes-Pallas A; Yoosaf K; Traboulsi H; Mohanraj J; Seldrum T; Dumont J; Minoia A; Lazzaroni R; Armaroli N; Bonifazi D Modular Engineering of H-Bonded Supramolecular Polymers for Reversible Functionalization of Carbon Nanotubes. J. Am. Chem. Soc 2011, 133, 15412–15424. [DOI] [PubMed] [Google Scholar]
- (29).Nish A; Hwang J-Y; Doig J; Nicholas RJ Highly Selective Dispersion of Single-Walled Carbon Nanotubes Using Aromatic Polymers. Nat. Nanotechnol 2007, 2, 640–646. [DOI] [PubMed] [Google Scholar]
- (30).Hwang JY; Nish A; Doig J; Douven S; Chen CW; Chen LC; Nicholas RJ Polymer Structure and Solvent Effects on the Selective Dispersion of Single-Walled Carbon Nanotubes. J. Am. Chem. Soc 2008, 130, 3543–3553. [DOI] [PubMed] [Google Scholar]
- (31).Chen RJ; Zhang Y; Wang D; Dai H Noncovalent Sidewall Functionalization of Single-Walled Carbon Nanotubes for Protein Immobilization. J. Am. Chem. Soc 2001, 123, 3838–3839. [DOI] [PubMed] [Google Scholar]
- (32).Zu S-Z; Sun X-X; Liu Y; Han B-H Supramolecular Surface Modification and Solubilization of Single-Walled Carbon Nanotubes with Cyclodextrin Complexation. Chem. - Asian J 2009, 4, 1562–1572. [DOI] [PubMed] [Google Scholar]
- (33).Tromp RM; Afzali A; Freitag M; Mitzi DB; Chen Z Novel Strategy for Diameter-Selective Separation and Functionalization of Single-Wall Carbon Nanotubes. Nano Lett. 2008, 8, 469–472. [DOI] [PubMed] [Google Scholar]
- (34).Hammershøj P; Bomans PHH; Lakshminarayanan R; Fock J; Jensen SH; Jespersen TS; Brock-Nannestad T; Hassenkam T; Nygard J; Sommerdijk NAJM; Kilsa K; Bjørnholm T; Christensen JB A Triptycene-Based Approach to Solubilising Carbon Nanotubes and C60. Chem. - Eur. J 2012, 18, 8716–8723. [DOI] [PubMed] [Google Scholar]
- (35).Zheng M; Jagota A; Semke ED; Diner BA; McLean RS; Lustig SR; Richardson RE; Tassi NG DNA-Assisted Dispersion and Separation of Carbon Nanotubes. Nat. Mater 2003, 2, 338–342. [DOI] [PubMed] [Google Scholar]
- (36).Star A; Joshi V; Han T-R; Altoé MVP; Grüner G; Stoddart JF Electronic Detection of the Enzymatic Degradation of Starch. Org. Lett 2004, 6, 2089–2092. [DOI] [PubMed] [Google Scholar]
- (37).Star A; Steuerman DW; Heath JR; Stoddart JF Starched Carbon Nanotubes. Angew. Chem., Int. Ed 2002, 41, 2508–2512. [DOI] [PubMed] [Google Scholar]
- (38).Cella LN; Chen W; Myung NV; Mulchandani A Single-Walled Carbon Nanotube-Based Chemiresistive Affinity Biosensors for Small Molecules: Ultrasensitive Glucose Detection. J. Am. Chem. Soc 2010, 132, 5024–5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Zhang M; Gorski W Electrochemical Sensing Platform Based on the Carbon Nanotubes/Redox Mediators-Biopolymer System. J. Am. Chem. Soc 2005, 127, 2058–2059. [DOI] [PubMed] [Google Scholar]
- (40).Star A; Liu Y; Grant K; Ridvan L; Stoddart JF; Steuerman DW; Diehl MR; Boukai A; Heath JR Noncovalent Side-Wall Functionalization of Single-Walled Carbon Nanotubes. Macromolecules 2003, 36, 553–560. [Google Scholar]
- (41).Tang BZ; Xu H Preparation, Alignment, and Optical Properties of Soluble Poly(phenylacetylene)-Wrapped Carbon Nanotubes. Macromolecules 1999, 32, 2569–2576. [Google Scholar]
- (42).Steuerman DW; Star A; Narizzano R; Choi H; Ries RS; Nicolini C; Stoddart JF; Heath JR Interactions between Conjugated Polymers and Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2002, 106, 3124–3130. [Google Scholar]
- (43).Bati ASR; Yu L; Batmunkh M; Shapter JG Synthesis, Purification, Properties and Characterization of Sorted Single-Walled Carbon Nanotubes. Nanoscale 2018, 10, 22087–22139. [DOI] [PubMed] [Google Scholar]
- (44).Lei T; Lai Y-C; Hong G; Wang H; Hayoz P; Weitz RT; Chen C; Dai H; Bao Z Diketopyrrolopyrrole (DPP)-Based Donor–Acceptor Polymers for Selective Dispersion of Large-Diameter Semiconducting Carbon Nanotubes. Small 2015, 11, 2946–2954. [DOI] [PubMed] [Google Scholar]
- (45).So H-M; Won K; Kim YH; Kim B-K; Ryu BH; Na PS; Kim H; Lee J-O Single-Walled Carbon Nanotube Biosensors Using Aptamers as Molecular Recognition Elements. J. Am. Chem. Soc 2005, 127, 11906–11907. [DOI] [PubMed] [Google Scholar]
- (46).Chen F; Wang B; Chen Y; Li L-J Toward the Extraction of Single Species of Single-Walled Carbon Nanotubes Using Fluorene-Based Polymers. Nano Lett. 2007, 7, 3013–3017. [DOI] [PubMed] [Google Scholar]
- (47).Lei T; Pochorovski I; Bao Z Separation of Semiconducting Carbon Nanotubes for Flexible and Stretchable Electronics Using Polymer Removable Method. Acc. Chem. Res 2017, 50, 1096–1104. [DOI] [PubMed] [Google Scholar]
- (48).Selmani S; Schipper DJ Orientation Control of Molecularly Functionalized Surfaces Applied to the Simultaneous Alignment and Sorting of Carbon Nanotubes. Angew. Chem., Int. Ed 2018, 57, 2399–2403. [DOI] [PubMed] [Google Scholar]
- (49).Snowdon MR; Selmani S; Schipper DJ Sonication-Enhanced Alignment Relay Technique for the Orientation of Single-Walled Carbon Nanotubes. ACS Appl. Nano Mater 2019, 2, 6637–6645. [Google Scholar]
- (50).Wang F; Gu H; Swager TM Carbon Nanotube/Polythiophene Chemiresistive Sensors for Chemical Warfare Agents. J. Am. Chem. Soc 2008, 130, 5392–5393. [DOI] [PubMed] [Google Scholar]
- (51).Ishihara S; O’Kelly CJ; Tanaka T; Kataura H; Labuta J; Shingaya Y; Nakayama T; Ohsawa T; Nakanishi T; Swager TM Metallic versus Semiconducting SWCNT Chemiresistors: A Case for Separated SWCNTs Wrapped by a Metallosupramolecular Polymer. ACS Appl. Mater. Interfaces 2017, 9, 38062–38067. [DOI] [PubMed] [Google Scholar]
- (52).Ishihara S; Azzarelli JM; Krikorian M; Swager TM Ultratrace Detection of Toxic Chemicals: Triggered Disassembly of Supramolecular Nanotube Wrappers. J. Am. Chem. Soc 2016, 138, 8221–8227. [DOI] [PubMed] [Google Scholar]
- (53).Wang F; Yang Y; Swager TM Molecular Recognition for High Selectivity in Carbon Nanotube/Polythiophene Chemiresistors. Angew. Chem., Int. Ed 2008, 47, 8394–8396. [DOI] [PubMed] [Google Scholar]
- (54).Chen J; Liu H; Weimer WA; Halls MD; Waldeck DH; Walker GC Noncovalent Engineering of Carbon Nanotube Surfaces by Rigid, Functional Conjugated Polymers. J. Am. Chem. Soc 2002, 124, 9034–9035. [DOI] [PubMed] [Google Scholar]
- (55).Rice NA; Soper K; Zhou N; Merschrod E; Zhao Y Dispersing As-Prepared Single-Walled Carbon Nanotube Powders with Linear Conjugated Polymers. Chem. Commun 2006, 4937–4939. [DOI] [PubMed] [Google Scholar]
- (56).Kang YK; Lee O-S; Deria P; Kim SH; Park T-H; Bonnell DA; Saven JG; Therien MJ Helical Wrapping of Single-Walled Carbon Nanotubes by Water Soluble Poly(p-Phenyleneethynylene). Nano Lett. 2009, 9, 1414–1418. [DOI] [PubMed] [Google Scholar]
- (57).Chen Y; Xu Y; Wang Q; Gunasinghe RN; Wang X-Q; Pang Y Highly Selective Dispersion of Carbon Nanotubes by Using Poly(phenyleneethynylene)-Guided Supermolecular Assembly. Small 2013, 9, 870–875. [DOI] [PubMed] [Google Scholar]
- (58).Saem S; Fong D; Adronov A; Moran-Mirabal J Stretchable and Resilient Conductive Films on Polydimethylsiloxane from Reactive Polymer-Single-Walled Carbon Nanotube Complexes for Wearable Electronics. ACS Appl. Nano Mater 2019, 2, 4968–4973. [Google Scholar]
- (59).Yang J-S; Yan J-L Central-Ring Functionalization and Application of the Rigid, Aromatic, and H-Shaped Pentiptycene Scaffold. Chem. Commun 2008, 1501–1512. [DOI] [PubMed] [Google Scholar]
- (60).Lin C-J; Chen C-Y; Kundu SK; Yang J-S Unichromophoric Platinum-Acetylides That Contain Pentiptycene Scaffolds: Torsion-Induced Dual Emission and Steric Shielding of Dynamic Quenching. Inorg. Chem 2014, 53, 737–745. [DOI] [PubMed] [Google Scholar]
- (61).Yang J-S; Yan J-L; Hwang C-Y; Chiou S-Y; Liau K-L; Gavin Tsai H-H; Lee G-H; Peng S-M Probing the Intrachain and Interchain Effects on the Fluorescence Behavior of Pentiptycene-Derived Oligo(p-Phenyleneethynylene)s. J. Am. Chem. Soc 2006, 128, 14109–14119. [DOI] [PubMed] [Google Scholar]
- (62).Zhao X; Cardolaccia T; Farley RT; Abboud KA; Schanze KS A Platinum Acetylide Polymer with Sterically Demanding Substituents: Effect of Aggregation on the Triplet Excited State. Inorg. Chem 2005, 44, 2619–2627. [DOI] [PubMed] [Google Scholar]
- (63).Zhang J; Wu N-W; Xu X-D; Li Q-J; Wang C-H; Tan G; Xu L Branched Platinum–Acetylide Complexes: Synthesis, Properties, and Their Aggregation Behavior. RSC Adv. 2014, 4, 16047–16054. [Google Scholar]
- (64).Yang J-S; Swager TM Fluorescent Porous Polymer Films as TNT Chemosensors: Electronic and Structural Effects. J. Am. Chem. Soc 1998, 120, 11864–11873. [Google Scholar]
- (65).Yang J-S; Swager TM Porous Shape Persistent Fluorescent Polymer Films: An Approach to TNT Sensory Materials. J. Am. Chem. Soc 1998, 120, 5321–5322. [Google Scholar]
- (66).Thomas SW; Joly GD; Swager TM Chemical Sensors Based on Amplifying Fluorescent Conjugated Polymers. Chem. Rev 2007, 107, 1339–1386. [DOI] [PubMed] [Google Scholar]
- (67).Lin C-J; Liu Y-H; Peng S-M; Shinmyozu T; Yang J-S Excimer–Monomer Photoluminescence Mechanochromism and Vapochromism of Pentiptycene-Containing Cyclometalated Platinum(II) Complexes. Inorg. Chem 2017, 56, 4978–4989. [DOI] [PubMed] [Google Scholar]
- (68).Long TM; Swager TM Molecular Design of Free Volume as a Route to Low-κ Dielectric Materials. J. Am. Chem. Soc 2003, 125, 14113–14119. [DOI] [PubMed] [Google Scholar]
- (69).Strano MS; Dyke CA; Usrey ML; Barone PW; Allen MJ; Shan H; Kittrell C; Hauge RH; Tour JM; Smalley RE Electronic Structure Control of Single-Walled Carbon Nanotube Functionalization. Science 2003, 301, 1519–1522. [DOI] [PubMed] [Google Scholar]
- (70).Bachilo SM; Strano MS; Kittrell C; Hauge RH; Smalley RE; Weisman RB Structure-Assigned Optical Spectra of Single-Walled Carbon Nanotubes. Science 2002, 298, 2361–2366. [DOI] [PubMed] [Google Scholar]
- (71).Lolli G; Zhang L; Balzano L; Sakulchaicharoen N; Tan Y; Resasco DE Tailoring (n,m) Structure of Single-Walled Carbon Nanotubes by Modifying Reaction Conditions and the Nature of the Support of CoMo Catalysts. J. Phys. Chem. B 2006, 110, 2108–2115. [DOI] [PubMed] [Google Scholar]
- (72).Ao G; Streit JK; Fagan JA; Zheng M Differentiating Left- and Right-Handed Carbon Nanotubes by DNA. J. Am. Chem. Soc 2016, 138, 16677–16685. [DOI] [PubMed] [Google Scholar]
- (73).Dresselhaus MS; Dresselhaus G; Jorio A; Souza Filho AG; Saito R Raman Spectroscopy on Isolated Single Wall Carbon Nanotubes. Carbon 2002, 40, 2043–2061. [Google Scholar]
- (74).Zheng M; Jagota A; Strano MS; Santos AP; Barone P; Chou SG; Diner BA; Dresselhaus MS; Mclean RS; Onoa GB; Samsonidze GG; Semke ED; Usrey M; Walls DJ Structure-Based Carbon Nanotube Sorting by Sequence-Dependent DNA Assembly. Science 2003, 302, 1545–1548. [DOI] [PubMed] [Google Scholar]
- (75).Chou SG; Ribeiro HB; Barros EB; Santos AP; Nezich D; Samsonidze GG; Fantini C; Pimenta MA; Jorio A; Filho FP; Dresselhaus MS; Dresselhaus G; Saito R; Zheng M; Onoa GB; Semke ED; Swan AK; Ünlü MS; Goldberg BB Optical Characterization of DNA-Wrapped Carbon Nanotube Hybrids. Chem. Phys. Lett 2004, 397, 296–301. [Google Scholar]
- (76).Chen B; Cinke M; Li JZ; Meyyappan M; Chi ZH; Harmon JP; Muisener PAO; Clayton L; D’Angelo J Modifying the Electronic Character of Single-Walled Carbon Nanotubes through Anisotropic Polymer Interaction: A Raman Study. Adv. Funct. Mater 2005, 15, 1183–1187. [Google Scholar]
- (77).Strano MS; Zheng M; Jagota A; Onoa GB; Heller DA; Barone PW; Usrey ML Understanding the Nature of the DNA-Assisted Separation of Single-Walled Carbon Nanotubes Using Fluorescence and Raman Spectroscopy. Nano Lett. 2004, 4, 543–550. [Google Scholar]
- (78).Zhang Y; Ichihashi T; Landree E; Nihey F; Iijima S Heterostructures of Single-Walled Carbon Nanotubes and Carbide Nanorods. Science 1999, 285, 1719. [DOI] [PubMed] [Google Scholar]
- (79).Kim UJ; Gutiérrez HR; Kim JP; Eklund PC Effect of the Tube Diameter Distribution on the High-Temperature Structural Modification of Bundled Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2005, 109, 23358–23365. [DOI] [PubMed] [Google Scholar]
- (80).Ramos-Sanchez G; Chen G; Harutyunyan AR; Balbuena PB Theoretical and Experimental Investigations of the Li Storage Capacity in Single-Walled Carbon Nanotube Bundles. RSC Adv. 2016, 6, 27260–27266. [Google Scholar]
- (81).Fong D; Luo S-X; Andre RS; Swager TM Trace Ethylene Sensing via Wacker Oxidation. ACS Cent. Sci 2020, 6, 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Im J; Sterner SE; Swager MT Integrated Gas Sensing System of SWCNT and Cellulose Polymer Concentrator for Benzene, Toluene, and Xylenes. Sensors 2016, 16, 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Annotated OSHA Z-2 Table. https://www.osha.gov/dsg/annotated-pels/tablez-2.html (accessed March 1, 2020).
- (84).Huang R; Yi P; Tang Y Probing the Interactions of Organic Molecules, Nanomaterials, and Microbes with Solid Surfaces Using Quartz Crystal Microbalances: Methodology, Advantages, and Limitations. Environ. Sci.: Processes Impacts 2017, 19, 793–811. [DOI] [PubMed] [Google Scholar]
- (85).Gutman J; Kaufman Y; Kawahara K; Walker SL; Freger V; Herzberg M Interactions of Glycosphingolipids and Lipopolysaccharides with Silica and Polyamide Surfaces: Adsorption and Viscoelastic Properties. Biomacromolecules 2014, 15, 2128–2137. [DOI] [PubMed] [Google Scholar]
- (86).Olsson ALJ; van der Mei HC; Busscher HJ; Sharma PK Influence of Cell Surface Appendages on the Bacterium–Substratum Interface Measured Real-Time Using QCM-D. Langmuir 2009, 25, 1627–1632. [DOI] [PubMed] [Google Scholar]
- (87).Friedlander RS; Vogel N; Aizenberg J Role of Flagella in Adhesion of Escherichia coli to Abiotic Surfaces. Langmuir 2015, 31, 6137–6144. [DOI] [PubMed] [Google Scholar]
- (88).Molino PJ; Hodson OM; Quinn JF; Wetherbee R The Quartz Crystal Microbalance: A New Tool for the Investigation of the Bioadhesion of Diatoms to Surfaces of Differing Surface Energies. Langmuir 2008, 24, 6730–6737. [DOI] [PubMed] [Google Scholar]
- (89).Chang X; Bouchard DC Multiwalled Carbon Nanotube Deposition on Model Environmental Surfaces. Environ. Sci. Technol 2013, 47, 10372–10380. [DOI] [PubMed] [Google Scholar]
- (90).Li W; Liu D; Wu J; Kim C; Fortner JD Aqueous Aggregation and Surface Deposition Processes of Engineered Superparamagnetic Iron Oxide Nanoparticles for Environmental Applications. Environ. Sci. Technol 2014, 48, 11892–11900. [DOI] [PubMed] [Google Scholar]
- (91).Speller NC; Siraj N; McCarter KS; Vaughan S; Warner IM QCM Virtual Sensor Array: Vapor Identification and Molecular Weight Approximation. Sens. Actuators, B 2017, 246, 952–960. [Google Scholar]
- (92).Regmi BP; Speller NC; Anderson MJ; Brutus JO; Merid Y; Das S; El-Zahab B; Hayes DJ; Murray KK; Warner IM Molecular Weight Sensing Properties of Ionic Liquid-Polymer Composite Films: Theory and Experiment. J. Mater. Chem. C 2014, 2, 4867–4878. [Google Scholar]
- (93).Liu S; Kan Z; Thomas S; Cruciani F; Brédas J-L; Beaujuge PM Thieno[3,4-c]pyrrole-4,6-dione-3,4-difluorothiophene Polymer Acceptors for Efficient All-Polymer Bulk Heterojunction Solar Cells. Angew. Chem., Int. Ed 2016, 55, 12996–13000. [DOI] [PubMed] [Google Scholar]
- (94).Gutierrez GD; Coropceanu I; Bawendi MG; Swager TM A Low Reabsorbing Luminescent Solar Concentrator Employing π-Conjugated Polymers. Adv. Mater 2016, 28, 497–501. [DOI] [PubMed] [Google Scholar]
- (95).Bouffard J; Swager TM Fluorescent Conjugated Polymers That Incorporate Substituted 2,1,3-Benzooxadiazole and 2,1,3-Benzothiadiazole Units. Macromolecules 2008, 41, 5559–5562. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.