Abstract
Food portion size influences energy intake, and sustained high-energy intake often leads to obesity. Virtual portion creation tasks (VPCTs), in which a participant creates portions of food on a computer screen, predict intake in healthy individuals. The objective of this study was to determine whether portions created in VPCTs are stable over time (test-retest reliability) and responsive to factors known to influence food intake, such as eating contexts and food types, and to determine if virtual portions can predict weight loss. Patients with obesity scheduled for bariatric surgery (n=29), and individuals with a normal BMI (18.5–24.9 kg/m2, controls, n=29), were instructed to create virtual portions of eight snack foods, which varied in energy density (low and high) and taste (sweet and salty). Portions were created in response to the following eating situations, or “contexts”: What they would a) eat to stay healthy (healthy), b) typically eat (typical), c) eat to feel comfortably satisfied (satisfied), d) consider the most that they could tolerate eating (maximum), and e) eat if nothing was limiting them (desired). Tasks were completed before and 3 months after surgery in patients, and at two visits, 3 months apart, in controls. Body weight (kg) was recorded at both visits. Virtual portions differed significantly across groups, visits, eating contexts, energy densities (low vs. high), and tastes (sweet vs. salty). Portions created by controls did not change over time while portions created by patients decreased significantly after surgery, for all contexts except healthy. For patients, desired and healthy portions predicted 3-month weight loss. VPCTs are replicable, responsive to foods and eating contexts, and predict surgical weight loss. These tasks could be useful for individual assessment of expectations of amounts that are eaten in health and disease and for prediction of weight loss.
Keywords: Obesity, Bariatric surgery, Portion size, Food choice, Energy density, Sweet, Weight loss
1. Introduction
1.1. Background
Virtual portion creation tasks (VPCTs), in which portion sizes of food are created on a computer screen [1], predict how much individuals plan to eat and will actually eat, and can therefore be considered proxies for intake [2–4]. VPCTs have been primarily used in healthy populations as proxy measures of food intake, mindset [5, 6], and to predict severity of illness in patients with anorexia nervosa (AN) [7, 8]. VPCTs and have been used under a variety of eating contexts (prompts that reflect current or future intentions for which one is eating,) [9].
Because food intake contributes one side of the energy balance equation, VPCTs could also be used in studies of individuals with obesity. In order to demonstrate another clinically relevant application of VPCTs, we adapted and expanded VPCTs to assess ‘normal’ and ‘excessive’ virtual eating behaviors in patients with severe obesity, before and after surgery. Rolls et al suggested that “portion sizes are modifiable and should be further studied in connection with the prevention and treatment of obesity” [4], therefore we posited that virtual portion sizes could be used as proxy measures of a wide variety of contexts and food types that influence food intake.
Bariatric surgery is the leading effective treatment of severe obesity [10], however, after significant weight loss many patients regain weight [11]. Eating behavior likely wields a strong influence on surgically-induced weight loss [12]. Because portion size drives energy intake, which in turn influences body weight [13, 14], it seemed reasonable to also determine whether portion sizes could be used to predict weight loss in patients after bariatric surgery.
This report focuses on the influences to potential food intake from energy density, taste, and eating context, based on the assumption that virtual portion sizes that participants create in these contexts reflect what they would actually eat in these contexts in their everyday lives. This method of portion size assessment could enable investigators to identify abnormalities and mechanisms that control food intake.
1.2. Objective
Actual portion creation during an eating event is influenced by a range of well-established factors (food properties, eating contexts, energy needs, bariatric surgery interventions). Therefore, our overall objective was to examine the influence of these well-established factors on the reliability and responsiveness of VPCTs. Tests of reliability illustrate the stability of response in the instrument over time in similar conditions. Tests of responsiveness demonstrate the ability of the instrument to detect significant differences across factors that the researcher would expect to elicit a difference or change over time [15–18]. Responsiveness is an objective and statistical assessment of differences in portions created and these differences could be deemed “minimal clinically important differences” [19] if correlated with variables of known clinical significance (hormones, activation of areas in the brain, stomach size, weight loss over time, etc.) [17].
1.2.1. Primary aims
The aims of the study were to appraise the: Aim 1) test-retest reliability, by comparison of the same participants at different time points, and Aim 2) responsiveness across factors that influence portion consumption in real eating environments [20] such as BMI status, bariatric surgery interventions, eating contexts, and food properties such as energy density and taste.
1.2.2. Auxiliary aim
An auxiliary aim, to demonstrate potential clinical utility of this tool, was to determine if these VPCTs have any predictive validity, that is, to determine whether portions created in the VPCTs before bariatric surgery predict surgically induced body weight loss in patients.
1.3. Predictions
1.3.1. Predicted outcome addressing Aim 1 (Reliability of responses).
In individuals with normal weight, food intake is stable over time when weight loss or gain is not occurring [21]. Therefore, we predicted that virtual portions created by controls would be stable over time and that baseline portions would be highly correlated with follow-up portions; this stability in portion creation over time would illustrate test-retest reliability of VPCTs.
1.3.2. Predicted outcomes addressing Aim 2 (Responsiveness).
1.3.2.1. Surgery-induced decreases.
Bariatric surgery patients consume less food and lose weight after bariatric surgery [22], therefore, we predicted portions created by patients would significantly decrease after surgery.
1.3.2.2. Group differences.
Because the energy requirements of individuals with obesity is higher than individuals of normal weight [23], we predicted that portions created by patients would be higher than controls at baseline, but similar to controls at follow-up.
1.3.2.3. Eating context differences.
In both groups, we proposed a hierarchy in that contexts promoting higher intakes of food (e.g. select your maximum portion size) would elicit larger portion size responses than those for contexts that encourage lower or “healthier” intakes of food.
1.3.2.4. Differences in portion creation attributable to energy density and taste.
Low energy-dense (LoED) foods are more satiating, but are less palatable, than high energy-dense foods (HiED) [24]. In regard to taste preference, the literature is mixed on whether sweet taste preference supersedes salty taste preference [25]. Therefore, while we expected that portions of HiED snacks (in grams) would be smaller than those for LoED snacks [26], we did not necessarily know if there would be significant differences between sweet and salty portions at either level of energy density.
1.3.2.5. Interactions of energy density and taste.
As there may be differences attributable to energy and/or taste (see 1.3.2.4), we expected an interaction between these two food properties; we expected the difference between sweet LoED and salty LoED would be larger than the difference between sweet HiED and salty HiED portions. This prediction stems from the assumption that while LoED foods may be less palatable than HiED foods [24], adding sweet taste to a LoED food could increase palatability, which could result in higher portion selection [27]. The interaction could also be attributable to greater room for movement in the LoED snacks, which would be expected to generate larger portions, than HiED.
1.3.2.6. Interactions of eating context, energy density, and taste.
Further, we expected these energy density-by-taste interactions (see 1.3.2.5) to be context-specific, in that the magnitude of these differences attributable energy density and taste would be larger in “high intake” contexts than in “low intake” contexts, because we expected that portions of food, regardless of energy density and taste, would be similar at “low intake” contexts.
1.3.3. Predicted outcome addressing auxiliary aim - Weight loss predictions.
Sugarman et al found that “sweets eaters” (i.e. individuals who consume more than 300 kCal of desserts, candy, chocolate, or sweetened beverages more than three times/week), lose significantly less weight than non-sweets eaters do [22]. Therefore, we expected virtual portions of sweet HiED foods would predict surgical weight loss (i.e. if patients continue to eat these foods in high quantities).
2. Materials & Methods
2.1. Participants
Participant data were collected longitudinally between May 25, 2016 - April 4, 2018 at the New York Obesity Nutrition Research Center (NYONRC) at Columbia University. Individuals scheduled to undergo Roux-en-Y gastric bypass or vertical sleeve gastrectomy surgery (patients) were recruited and consented 1–2 weeks before their scheduled surgery at Mt. Sinai – Morningside Hospital (New York, NY). Individuals with normal BMI (18.5 – 24.9 kg/m2) and of similar race, ethnicity, and age to patients (controls), responded to classified ads online (RecruitMe database and Craigslist), or flyers posted around upper Manhattan and the Bronx, NY and were consented at the NYONRC.
Inclusion criteria were: 18–65 years of age; BMI between 35 and 65 kg/m2 (patients) or BMI between 18.5 and 24.9 kg/m2 (controls); blood pressure below 160/100 mmHg (patients). Additionally, during screening, all participants were required to rate liking (at least 110 out of 190mm general labeled visual analog scale [28]) for a commercially-available chocolate-flavored nutritional shake (Ensure™, Abbot Laboratories), to assure they could drink it to capacity [29] in another component of the study to be reported elsewhere. Exclusion criteria were: fasting triglycerides >600 mg/dL (patients), type 2 diabetes, taking any psychotropic medications, being a current smoker, or current pregnancy.
2.2. Study design
Patients were scheduled for a baseline visit 1–2 weeks before their surgery date and a follow-up visit 3 months after surgery, while controls were scheduled for a baseline visit followed by a second visit, 3 months later. We instructed participants to refrain from eating or drinking anything, except water, for 12 hours prior to each study visit. Patients’ body weights were recorded at the surgeon’s office 1 week before surgery, at screening for controls, and at the 3-month follow-up laboratory visit for all participants. VPCTs were completed by all participants at both visits, which started between 10 and 11 AM. VPCTs took 15 minutes to complete and were included as part of a larger study that explores behavioral, motivational, cognitive, and physiological predictors of weight loss and weight recidivism after bariatric surgery.
2.3. Experimental set up
2.3.1. Method of adjustment
The VPCTs used a method of adjustment [30], in which the participant created a virtual portion of food, on a computer monitor, in response to a described context. Participants pressed the arrow keys on a keyboard to decrease or increase the portion size [30]. In each trial, the foods, contexts, and starting picture were shown at random to limit any order effects.
2.3.2. Foods utilized in the virtual portion creation tasks
2.3.2.1. Energy density and taste.
The VPCTs used eight snack foods (Figure 1) chosen to represent HiED (> 2.0 kcal/g) LoED (< 1.7 kcal/g), crossed with two tastes (salty or sweet) (Figure 1), yielding four “food-types” – sweet LoED, sweet HiED, salty LoED, and salty HiED. These two food properties, energy density and taste, were chosen because they are major drivers of food choice [25]. Sweet, salty, and umami tastes are usually well accepted by humans and contribute to the palatability of foods and promote their intake while bitter and sour tastes reduce palatability of foods [25]. Additionally, decreases in sweet and salt sensitivity are reported in the individuals with morbid obesity after gastric bypass surgery [31, 32]. Mixtures of sugar and fat are considered especially palatable by individuals with obesity [33–35].
Figure 1. Pictures of snacks.
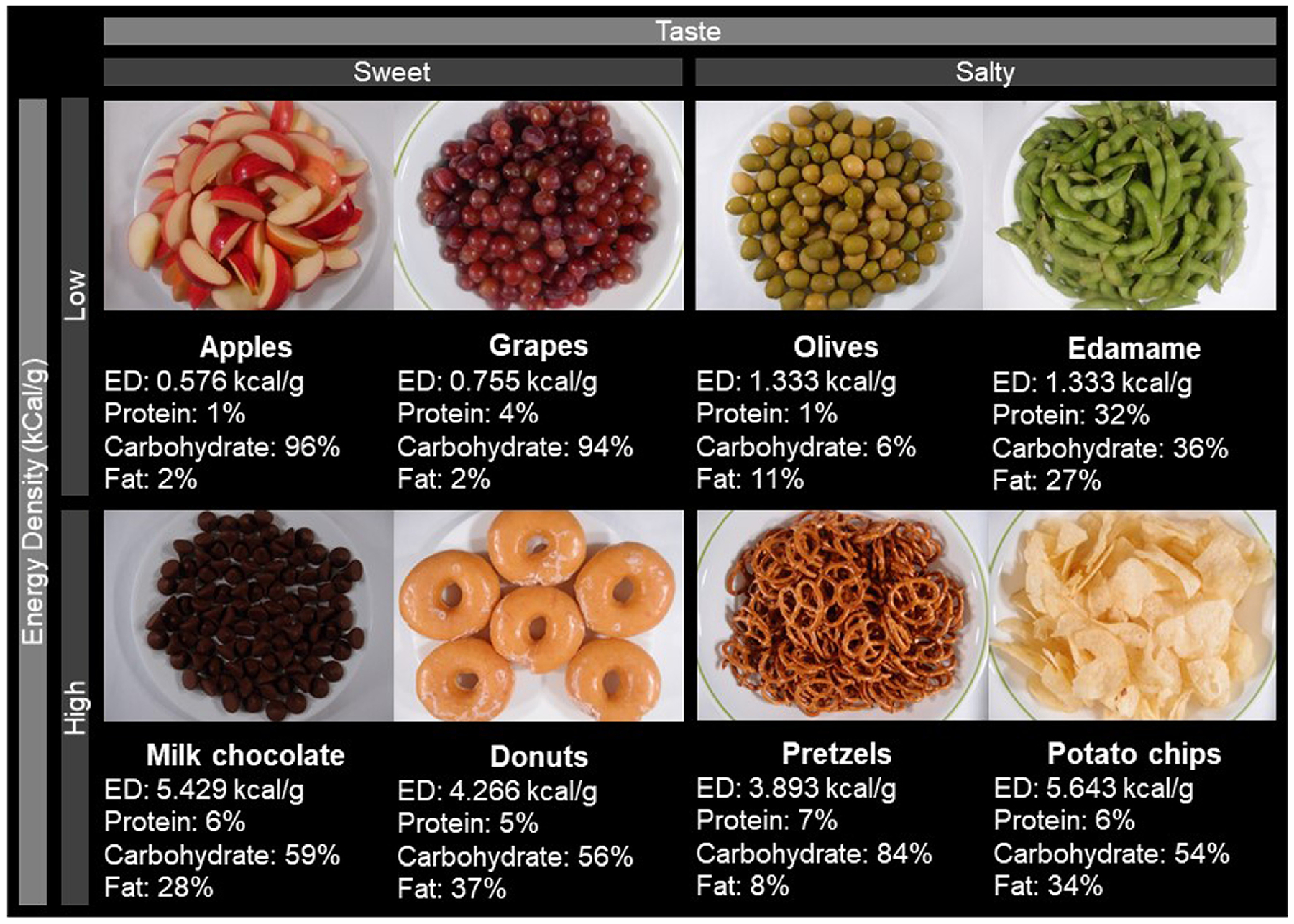
Pictures of the largest portion of the snack portions shown in the virtual portion creation tasks. Arranged by energy density (rows, high or low) and taste (columns, sweet or salty). Energy density (kcal/g) and macronutrient composition (%) are presented for each snack.
The energy density cutoffs were used from those set by Barbara Rolls and colleagues in their diet quality studies [36]. The snacks we utilized in the VPCTs had thresholds that were more widely spaced (HiED, > 3.8 kcal/g, and LoED, < 1.4 kcal/g) to ensure that we could detect larger differences between energy density levels. Snack foods were used because snack-eating and sweet-eating patterns have been associated with different weight loss outcomes after bariatric surgery [37] and only two were used for each food-type to give enough variety for each food-type while also limiting the amount of time spent on this task as there were many other tasks participants completed on the same day. Consequently, the time devoted to this task had to be limited.
2.3.2.2. Choice of snacks.
The snacks that were chosen for the VPCTs have been used in multiple studies and are frequently eaten in the United States. Apples and grapes were chosen because they are sweet, low in energy density, commonly eaten in the United States [38], and have been used in multiple studies [39, 40]. Milk chocolate and donuts were included because they are sweet, high in energy density, frequently purchased from New York City convenience stores and bodegas [41], and have been used in several virtual portion studies, including many conducted by Jeff Brunstrom [7, 8, 20, 42–44]. Similarly, potato chips and pretzels, which are salty and high in energy density, are frequently purchased [41] and used in many studies [20, 42, 43, 45–47]. In contrast, olives and edamame were selected because they are salty, low in energy density, reportedly lower in hedonic ratings [48], consumed infrequently, and perceived to be healthy [41].
2.3.3. Food photography
Snacks were purchased locally, prepared, and digitally photographed by one author (JH). Food photography guidelines developed by Jeff Brunstrom were followed [20]. All foods were photographed on a white 255-mm diameter plate and a lighting apparatus, with two adjustable arms, was used to ensure constant lighting conditions. The camera was mounted at a fixed distance from the plate, which allowed the uploaded photographs to be a real-size replication of the snack when displayed on a 48.26-cm liquid-crystal display monitor. HiED snacks were photographed in 20–40 kcal increments and LoED snacks were photographed in 20 kcal increments, with the highest amount displayed approximately 1000 kcal for LoED snacks and 2000 kcal for HiED snacks. The picture number was recorded when the participant hit ‘enter’, and this number was then converted into a portion response (in grams). Research assistants were available at the beginning of the task to answer any questions, but participants were alone while responding to the VPCTs.
2.4. Eating contexts in the virtual portion creation tasks
2.4.1. Healthy context.
Previous use of VPCTs used method of adjustment, with instruction to create a portion of food that participants “ought to eat” (ideal) [7]. We adapted the ideal context to ask what the participant would eat to “stay healthy” instead of what they think they “ought to eat”. The exact text was “Create the portion you would eat of this food if you were trying to stay healthy.” This healthy context provides a context that bariatric participants may employ when thinking about their weight loss, weight maintenance, and dieting.
2.4.2. Typical context.
Previous VPCTs also had an instruction to create a portion of food that a participant would normally eat [7]. The text we used for this context was “Create the portion you might typically eat of this food.” This context allows for the collection of data on what participants perceive to be their typical portion of these items.
2.4.3. Comfortably satisfied context.
A satisfied context was included to determine if what a participant considered “satisfying” was similar to their typical consumption of the same food. Like the typical context, this context was developed to gauge each participant’s “normal” eating. This context was designed to determine if there are differences between what a participant would call a “typical” portion and one that they would find satisfying; one could pose the question that if satisfied contexts are not redundant to typical contexts, then is this difference indicative of any eating pathology. The same could be asked about typical and maximum (described in the next section).
2.4.4. Maximum context.
In previous studies, participants also indicated if a displayed portion of food would “be too big for [them] to tolerate eating it” (maximum), via method of constant stimuli [7]. In the present study’s VPCTs, the maximum context was extended to a method of adjustment format. The maximum context allows for participants to create portions that may represent perceived physiological limitations and in the future could be correlated with other physiological measures like gastric distension and gastric capacity [49].
2.4.5. Desired context.
A desired context was developed as an extension of maximum, to see what participants would consume without any limits, in a sense, removing the physiological and possibly psychological restriction included in the maximum eating context. This context is novel for virtual paradigm as it allows participants to create portions they would create in an imaginary, unrestrained situation. We note that the term “desire” may hold several meanings; while we have labelled this context as “desire”, the word desire is not used in the prompt.
These five contexts were designed to be somewhat hierarchical, in that desired and maximum are likely to be the highest, followed by typical and satisfied, and then healthy being the lowest. This variety of contexts allow for a spectrum or gradient of responses, which could represent certain patterns of eating behaviors. These patterns, and changes in these patterns, could have clinical significance.
2.5. Ethics and regulatory approval
All participants were provided written informed consent prior to enrollment. These analyses are part of a larger study, Mechanisms Underlying Predictors of Success from Obesity Surgery, which was approved by the Institutional Review Boards of Columbia University and Mount Sinai Morningside Hospital.
2.6. Data analysis and design
SAS9.4 software (SAS Institute, Inc., Cary, NC, USA) was used for all analyses.
2.6.1. Power computation
Power calculation was based on data from a previous study conducted by Jeff Brunstrom and colleagues [20]. In that study, the mean difference between two contexts (maximum and ideal portions) of snacks in individuals with obesity was 18.89 g ± 34.58 SD, and the number of controls and patients needed to show a significant difference, with power of at least 0.8, was n = 29.
2.6.2. Tests of reliability and responsiveness – stability of response in controls over time and differences attributable to group, surgery, context, energy density, and taste
A repeated measures (two time points) design was employed for the two study groups, for portions created of a snack (energy density and taste) for a given context. Descriptive statistics (means ± SEMs) were calculated for demographic variables and portion responses (g) for each context, food-type (mean of snacks of the same energy density and taste), and at each time point for both groups (see 1.3.2). Mean portion response was in grams, not calories, because had we used energy units, the results would be confounded with the differences in energy density across food-types. Note: anyone who wishes to compare the effects with energy density can perform the calculation by means of data in Figure 1, where energy densities are shown. Planned between group tests were conducted to compare the patient and control demographics. Five-way ANOVA was conducted on the design just described, to assess differences between groups, visits, contexts, energy densities, and tastes. The comparisons included estimates for differences between groups, visits, contexts, tastes, and energy densities, with p-values that are Tukey-adjusted.
Planned testing of group x visit, energy x taste, and context x energy x taste interactions were performed. All p-values were held to Bonferroni correction. Interactions measure the influence of one factor on another factor’s influence on an outcome, in this case the outcome being the size of a portion. A significant group x visit interaction occurs when the visit difference of one group is larger in magnitude than the other. A significant energy x taste interaction occurs when the positive difference between sweet LoED and salty LoED snack portions is larger than the difference between sweet HiED and salty HiED snack portions (i.e. how does energy density influence the difference between sweet and salty portions). A significant context x energy x taste interaction occurs when the aforementioned energy x taste interaction is larger at one context compared to another, which would be an illustration of the energy x taste interactions being context-specific.
To assess test-retest reliability under stable conditions (see 1.3.1), Pearson and Spearman correlation tests were conducted on baseline and follow-up responses in controls.
2.6.3. Weight loss analyses
In patients, weight loss (in kg), with baseline weight (kg) as a covariate [50–53], was linearly regressed from pre-operative portions created for a given context and snack type (see 1.3.3). Weight loss is presented as baseline weight subtracted from 3-month weight.
3. Results
3.1. Participants Characteristics
Baseline weight, baseline BMI, 3-month weight, 3-month BMI were higher in patients than in controls (p < 0.05), but age was not different (Table 1). As expected, body weight decreased in patients (p < 0.001) but did not in controls (Table 1). Both groups were primarily female (90% of patients and 83% of controls), black (55% of patients and 59% of controls), and of Hispanic ethnicity (66% of patients and 69% of controls) (Table 1).
TABLE 1.
Demographic data for study participants1
| Patients (n = 29) | Controls (n = 29) | |
|---|---|---|
| Age, y | 36.38 ± 2.25 | 34.45 ± 2.02 |
| Sex, % Female/Male | 90% / 10% | 83% / 17% |
| Race, % Black/White | 55% / 45% | 59% / 41% |
| Ethnicity, % Hispanic/Non-Hispanic | 66% / 34% | 69% / 31% |
| Initial BMI, kg/m2 | 44.67 ± 1.072 | 21.93 ± 0.33 |
| Three-month BMI, kg/m2 | 36.98 ± 0.992 | 22.01 ± 0.37 |
| Baseline Weight, kg | 121.56 ± 3.622 | 59.19 ± 1.28 |
| Three-month Weight, kg | 100.58 ± 3.232 | 59.50 ± 1.34 |
| Three-month Weight Loss, kg | −20.97 ± 1.092, 3 | 0.31 ± 0.38 |
Values are means ± SDs unless otherwise indicated.
Significant group differences (t-test, p < 0.05)
Significant decrease in weight loss (three-month weight – baseline weight) in patients (paired t-test, p < 0.0001).
3.2. Test-retest reliability (controls only)
In controls, mean portion sizes were not significantly different between baseline and 3- month visits (all p’s > 0.05, Supplemental Table 1). Baseline and follow-up portion sizes correlated positively (Spearman R ranging from 0.451 – 0.872, all p’s < 0.001, Supplemental Table 3).
3.3. Responsiveness
3.3.1. Surgery-induced decreases
In patients after surgery, significant decreases in portion size were seen only for sweet LoED snacks. Portions under the typical, satisfied, maximum, and desired contexts decreased (Supplemental Table 2, Figure 2B–2E, all p’s > 0.05), but not under the healthy context (Supplemental Table 2, Figure 2A). No significant decreases were seen for salty HiED, salty LoED, or sweet HiED snacks (Supplemental Table 2, all p’s > 0.05).
Figure 2. Portion means for sweet, low-energy-dense snacks by eating context, group and visit.
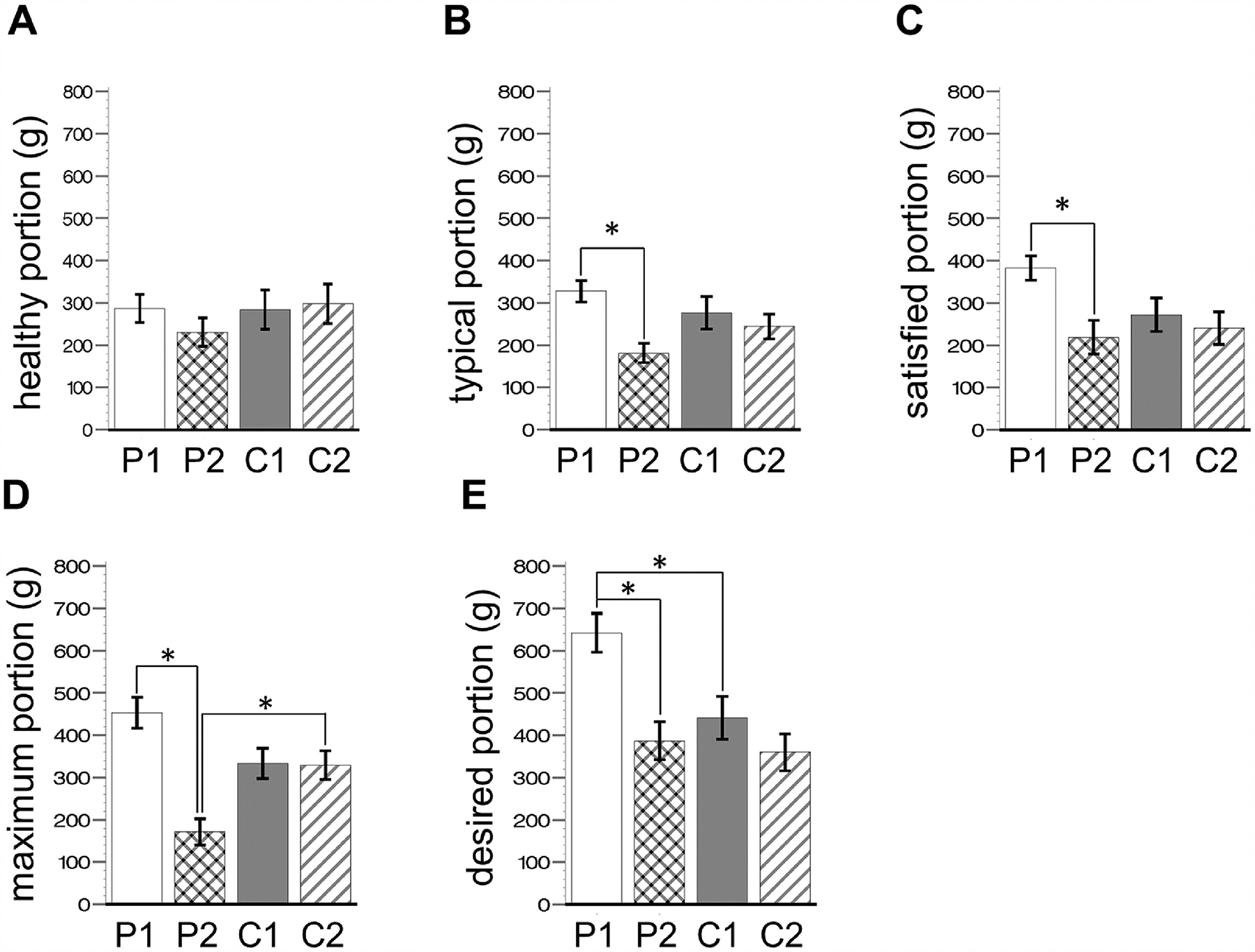
Bar graphs of portion means ± SEM, of sweet, low-energy snacks, for patients (n = 29) and controls (n = 29). All means are reported in Supplemental Table 1 and 2. P, patients; C, controls; 1, at baseline; 2, at follow-up. There were no significant group or visit differences in A. P1 was significantly higher than P2 in B (by 163.6g ± 27.3 SE, t = 5.99, p < 0.0001); C (by 146.1g ± 27.3 SE, t = 5.4, p = 0.0003); D (by 281.9g ± 27.3 SE, t = 10.3, p < 0.0001). P2 was significantly lower than C2 in D (by 157.7g ± 31.1, t = 5.0, p = 0.0013). In E, P1 is significantly higher than P2 (by 255.3g ± 27.3, t = 9.4, p < 0.0001) and C1 (by 200.9g ± 31.3, t = 6.4, p < 0.0001). Testing for group x visit interactions (i.e. visit difference in patients being larger than the visit difference in controls) were significant for D (mean difference: 277.71g ± 61.35 SE, t = 4.53, p < 0.0001) and E (mean difference: 173.92 ± 61.35 SE, t = 2.83, p = 0.005), but not A (mean difference: 69.43g ± 61.35SE, t= 1.13, p = 0.26), B (mean difference: 132.13g ± 61.35 SE, t = 2.15, p = 0.03), or C (mean difference: 113.73g ± 61.35 SE, t = 1.85, p = 0.06).
3.3.2. Group differences
Contrary to prediction (see 1.3.2.2), at baseline, only portions of sweet LoED snacks, in response to the desired context (Figure 2E), were larger in patients than in controls. At follow-up, portions created of sweet LoED snacks were smaller in patients than controls only for the maximum context (Figure 2D). Portions of sweet HiED snacks and all salty snacks were not different for all contexts, at either visit, for both groups (p > 0.05, data not shown). There were significant group x visit interactions for maximum (mean difference: 277.71g ± 61.35 SE, t = 4.53, p < 0.0001), and desired (mean difference: 173.92 ± 61.35 SE, t = 2.83, p = 0.0047) portions of sweet LoED snacks.
3.3.3. Eating context differences
Desired portions of sweet LoED snacks were larger than satisfied and typical portions of the same snacks, for patients and controls (both groups), at baseline and follow-up visits (Figure 3A–D, Table 2). Desired portions of sweet LoED snacks were also larger than healthy portions of the same snacks for both groups at baseline (Figure 3A and C, Table 2), but only for patients at follow-up (Figure 3B, Table 2). Desired portions of sweet LoED snacks were larger than maximum portions of the same snacks, and maximum portions of sweet LoED snacks were larger than typical and healthy portions of the same snacks, but only for patients at baseline (Figure 3A, Table 2). Desired portions of sweet HiED snacks were larger than healthy portions of the same snacks for both groups at baseline, but not at follow-up (Figure 3A–D, Table 2), and maximum portions of sweet HiED snacks were larger than healthy portions of the same snacks for patients at baseline, but not at follow-up (Figure 3A, Table 2).
Figure 3. Portion means of each snack type across eating contexts.
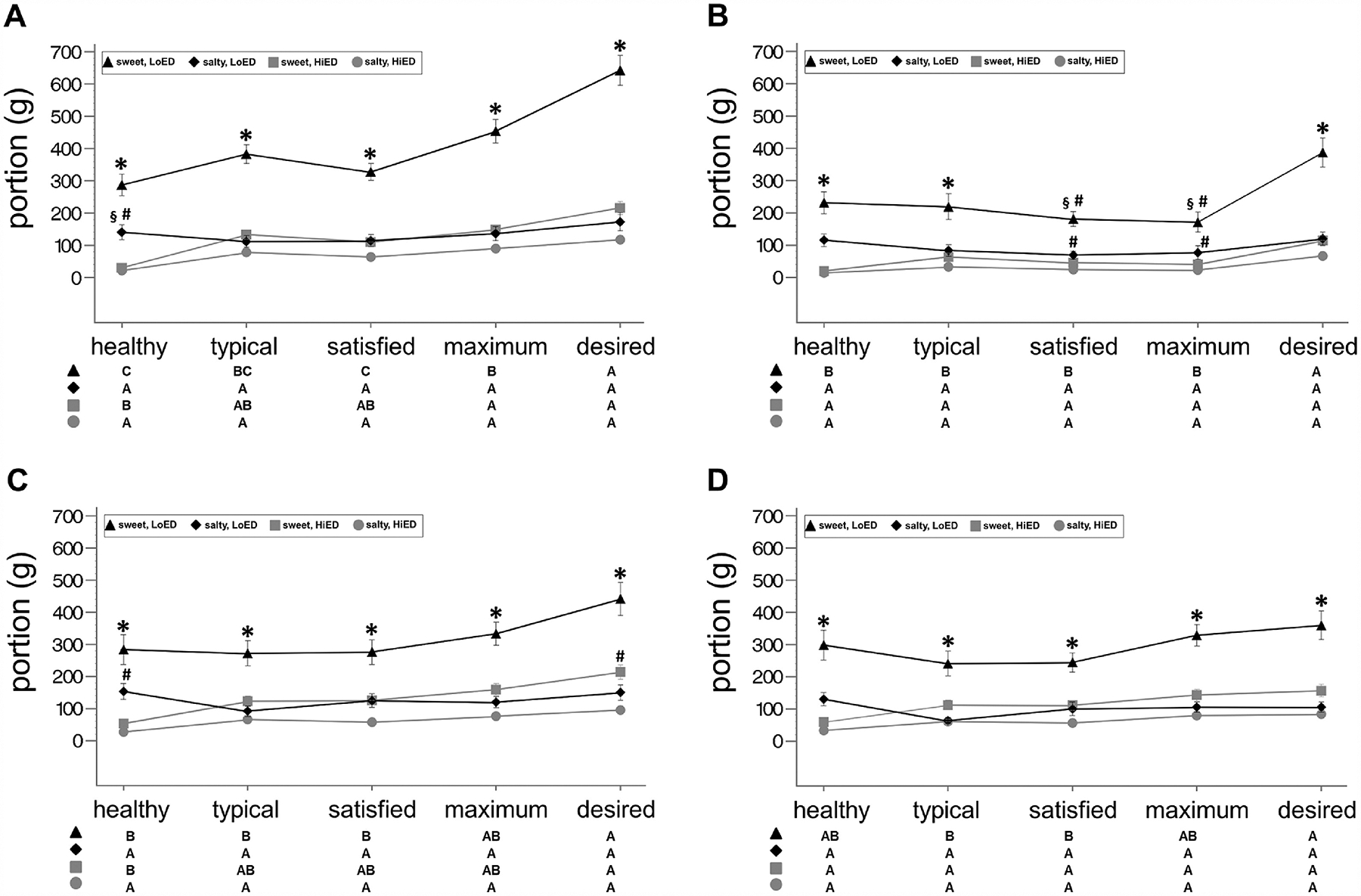
Line graphs of portion means ± SEM across eating contexts (x-axis) for each of the four snack types (lines) in (A) patients (n = 29) at baseline (B) patients at follow-up, (C) controls (n = 29) at baseline, and (D) controls at follow-up. See Supplemental Table 1 and 2 for portion means ± SEM, Supplemental Table 4 for differences between snacks ± SEM, and Table 3 for significant differences between eating contexts (estimated difference ± SEM). For significant differences between snack types, for a given eating context: * signifies that sweet, low energy-dense portions are larger than for all other snacks types; § signifies higher than sweet, high energy-dense snacks; # signifies higher than salty, high energy-dense. See the matrix below the graph for significant differences between eating contexts for a given snack type. In the matrix, letters indicate highest to lowest (A→C) with non-significant differences sharing the same letter.
TABLE 2.
Significant differences between eating contexts1
| Patients (n = 29) | Controls (n = 29) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 1 | Visit 2 | ||||||
| Diff | Mean ± SEM | P | Mean ± SEM | P | Mean ± SEM | P | Mean ± SEM | P | |
| Sweet LoED | D-H | 355.59 ± 27.30 | <0.0001 | 156.02 ± 27.30 | <0.0001 | 157.15 ± 27.30 | <0.0001 | 62.06 ± 27.30 | 0.9995 |
| D-T | 259.61 ± 27.30 | <0.0001 | 167.91 ± 27.30 | <0.0001 | 169.17 ± 27.30 | <0.0001 | 119.26 ± 27.30 | 0.0270 | |
| D-S | 314.86 ± 27.30 | <0.0001 | 205.68 ± 27.30 | <0.0001 | 164.98 ± 27.30 | <0.0001 | 115.99 ± 27.30 | 0.0431 | |
| D-M | 189.09 ± 27.30 | <0.0001 | 215.64 ± 27.30 | <0.0001 | 108.17 ± 27.30 | 0.1178 | 30.93 ± 27.30 | 1.0000 | |
| M-H | 166.5 ± 27.30 | <0.0001 | −59.63 ± 27.30 | 0.9999 | 48.98 ± 27.30 | 1.0000 | 31.14 ± 27.30 | 1.0000 | |
| M-S | 125.78 ± 27.30 | 0.0100 | 9.96 ± 27.30 | 1.0000 | 56.82 ± 27.30 | 1.0000 | 85.06 ± 27.30 | 0.7560 | |
| Sweet HiED | D-H | 185.7 ± 27.30 | <0.0001 | 94.52 ± 27.30 | 0.4426 | 160.16 ± 27.30 | <0.0001 | 98.56 ± 27.30 | 0.3188 |
| M-H | 118.56 ± 27.30 | 0.0299 | 21.01 ± 27.30 | 1.0000 | 106.05 ± 27.30 | 0.1502 | 84.28 ± 27.30 | 0.7788 | |
Values are mean differences ± SEM and p-values are from multiple comparisons (four-way ANOVA with Tukey adjustment). Diff, difference between eating contexts (g); LoED, low energy-dense snacks; HiED, high energy-dense snacks; Eating contexts: H, healthy; T, typical; S, satisfied; M, maximum; D, desired.
3.3.4. Differences attributable to energy density and taste
Portions of sweet LoED snacks were larger than portions created of the other snacks, for all contexts, and for both groups at baseline (Figure 3A and C, Supplemental Table 4). Portions of sweet and salty LoED snacks were no different from each other, for the maximum and satisfied contexts, for patients, but not controls, at follow-up (Figure 3B and D, Supplemental Table 4). Salty LoED portions were larger than salty HiED portions for the healthy context at baseline (Figure 3A and C, Supplemental Table 4), but not for any other context at baseline or follow-up (Figure 3B and D, Supplemental Table 4). Portions of sweet HiED snack were no different from all salty snack portions, for all contexts, for both groups, and at both visits, with an exception for controls at baseline (Figure 3A–D, Supplemental Table 4); portions of sweet HiED snacks were larger than salty HiED snacks for the desired context, for controls at baseline (Figure 3B, Supplemental Table 4).
3.3.5. Interactions of energy density and taste
There were consistent energy x taste interactions for the desired, typical, and healthy contexts for both groups and both visits (Figure 4A–D, Table 3). Additionally, there were energy x taste interactions for the satisfied context for both groups at baseline, and for controls, but not patients, at follow-up (Figure 4A, C, and D, Table 3). There also was an energy x taste interaction for the maximum context for patients at baseline, but not at follow up (Figure 4A, Table 3).
Figure 4. Plots of interactions.
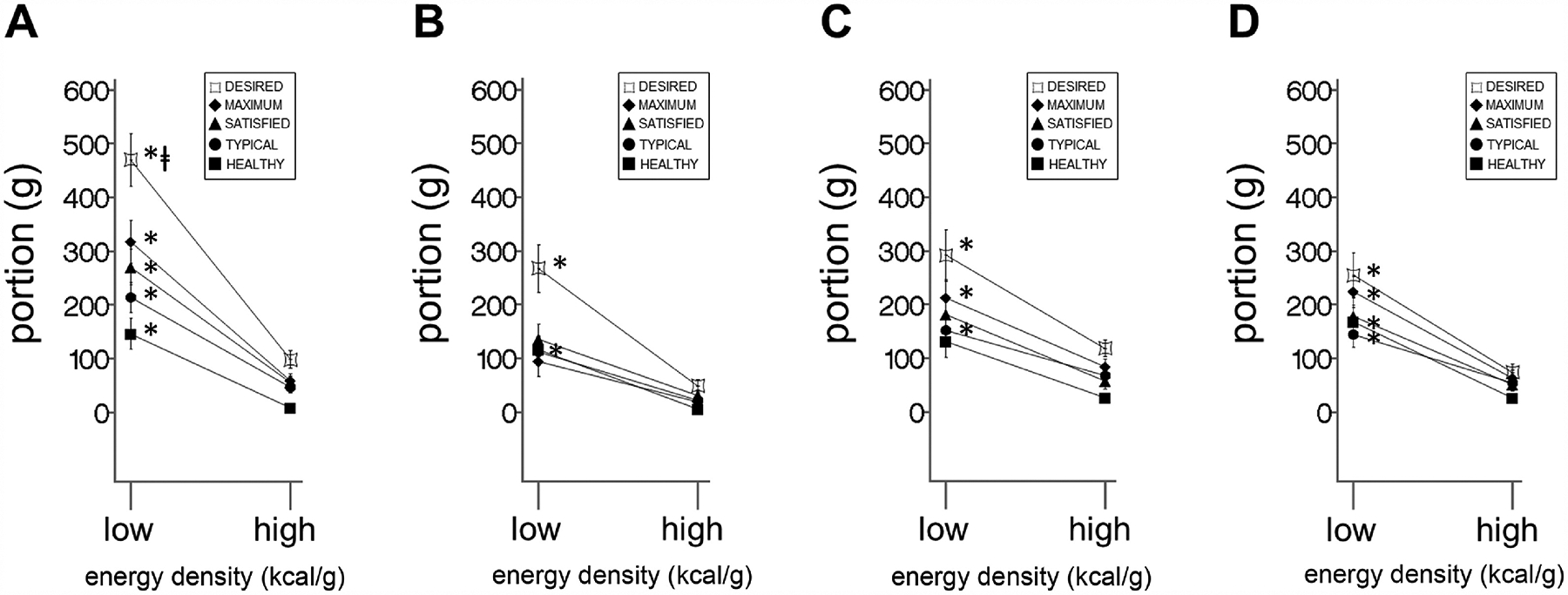
Line graphs of mean difference between sweet and salty portions, ± SEM, across two energy densities (‘high’ and ‘low’, x-axis) for each of the five eating contexts (lines) in (A) patients (n = 29) at baseline (B) patients at follow-up, (C) controls (n = 29) at baseline, and (D) controls at follow-up. See Table 3 for energy x taste interactions for each eating context. See Table 4 for eating context x energy x taste interactions. * signifies significant energy x taste interactions (i.e. the difference between sweet and salty low energy-dense portions being larger than the difference between sweet and salty high energy-dense portions) for a given eating context (see Table 3). ⱡ signifies significant eating context x energy x taste interaction, in this case meaning that in patients at baseline, the energy x taste interaction for the desired eating context is greater than the energy x taste interactions for the satisfied, satisfied, and healthy eating contexts (see Table 4).
TABLE 3.
Energy x taste interaction for each eating context1
| Patients (n = 29) | Controls (n = 29) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 1 | Visit 2 | |||||||||
| Eating context | Mean ± SEM | t | P | Mean ± SEM | t | P | Mean ± SEM | t | P | Mean ± SEM | t | P |
| Healthy | 138.37 ± 38.62 | 3.58 | 0.0003 | 110.71 ± 38.62 | 2.87 | 0.0042 | 104.85 ± 38.62 | 2.72 | 0.0066 | 142.16 ± 38.62 | 3.68 | 0.0002 |
| Typical | 215.46 ± 38.62 | 5.58 | <0.0001 | 104.93 ± 38.62 | 2.72 | 0.0066 | 123.71 ± 38.62 | 3.20 | 0.0014 | 126.31 ± 38.62 | 3.27 | 0.0011 |
| Satisfied | 166.21 ± 38.62 | 4.30 | <0.0001 | 91.11 ± 38.62 | 2.36 | 0.0183 | 83.9 ± 38.62 | 2.17 | 0.0298 | 89.49 ± 38.62 | 2.32 | 0.0205 |
| Maximum | 258.15 ± 38.62 | 6.69 | <0.0001 | 76.27 ± 38.62 | 1.98 | 0.0483 | 129.37 ± 38.62 | 3.35 | 0.0008 | 160.8 ± 38.62 | 4.16 | <0.0001 |
| Desired | 371.39 ± 38.62 | 9.62 | <0.0001 | 219.01 ± 38.62 | 5.67 | <0.0001 | 173.48 ± 38.62 | 4.49 | <0.0001 | 181.5 ± 38.62 | 4.70 | <0.0001 |
Five-way ANOVA with interaction (energy x taste) for each group, visit, and eating context. P-values < 0.005 are significant after Bonferroni-correction (α = 0.005).
3.3.6. Interactions of eating context, energy density, and taste
The energy x taste interaction for desired was larger than for typical, healthy, and satisfied contexts in patients at baseline, but not at follow-up (Figure 4A and B, Table 4), and not in controls at either time point (Figure 4C and D, Table 4).
TABLE 4.
Eating context x energy x taste interaction at each eating context1
| Patients (n = 29) | Controls (n = 29) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 1 | Visit 2 | |||||||||
| Diff | Mean ± SEM | t | P | Mean ± SEM | t | P | Mean ± SEM | t | P | Mean ± SEM | t | P |
| D-M | 113.23 ± 54.61 | 2.07 | 0.0382 | 142.74 ± 54.61 | 261 | 0.0090 | 44.12 ± 54.61 | 0.81 | 0.4192 | 20.69 ± 54.61 | 0.38 | 0.7047 |
| D-S | 205.17 ± 54.61 | 3.76 | 0.0002 | 127.90 ± 54.61 | 2.34 | 0.0192 | 89.59 ± 54.61 | 1.64 | 0.1010 | 92.01 ± 54.61 | 1.68 | 0.0921 |
| D-T | 155.92 ± 54.61 | 2.86 | 0.0043 | 114.0 ± 54.61 | 2.09 | 0.0368 | 49.78 ± 54.61 | 0.91 | 0.3621 | 55.18 ± 54.61 | 1.01 | 0.3123 |
| D-H | 233.02 ± 54.61 | 4.27 | <0.0001 | 108.31 ± 54.61 | 1.98 | 0.0474 | 68.64 ± 54.61 | 1.26 | 0.2089 | 39.33 ± 54.61 | 0.72 | 0.4714 |
| M-S | 91.94 ± 54.61 | 1.68 | 0.0923 | −14.84 ± 54.61 | −0.27 | 0.7858 | 45.47 ± 54.61 | 0.83 | 0.4051 | 71.31 ± 54.61 | 1.31 | 0.1916 |
| M-T | 42.69 ± 54.61 | 0.78 | 0.4344 | −28.66 ± 54.61 | −0.52 | 0.5997 | 5.66 ± 54.61 | 0.10 | 0.9175 | 34.49 ± 54.61 | 0.63 | 0.5277 |
| M-H | 119.79 ± 54.61 | 2.19 | 0.0283 | −34.43 ± 54.61 | −0.63 | 0.5284 | 24.52 ± 54.61 | 0.45 | 0.6535 | 18.64 ± 54.61 | 0.34 | 0.7329 |
| S-T | −49.25 ± 54.61 | −0.9 | 0.3672 | −13.82 ± 54.61 | −0.25 | 0.8002 | −39.81 ± 54.61 | −0.73 | 0.4660 | −36.82 ± 54.61 | −0.67 | 0.5001 |
| S-H | 27.85 ± 54.61 | 0.51 | 0.6101 | −19.59 ± 54.61 | −0.36 | 0.7197 | −20.95 ± 54.61 | −0.38 | 0.7012 | −52.67 ± 54.61 | −0.96 | 0.3348 |
| T-H | 77.10 ± 54.61 | 1.41 | 0.1581 | −5.77 ± 54.61 | −0.11 | 0.9158 | 18.86 ± 54.61 | 0.35 | 0.7298 | −15.85 ± 54.61 | −0.29 | 0.7716 |
Four-way ANOVA with interaction (eating context x energy x taste) for each group and visit. P-values < 0.005 are significant after Bonferroni-correction (α = 0.005); Eating contexts - H, healthy; T, typical; S, satisfied; M, maximum; D, desired.
3.4. Weight loss predictions
Contrary to our weight loss predictions (see 1.3.3), baseline healthy portions of salty LoED snacks inversely predicted 3-month post-operative weight loss (Figure 5A, Table 5 and Supplemental Table 5), that is, the larger the pre-operative portion, the lower the weight loss. However, desired portions of sweet HiED, in addition to sweet LoED, and salty HiED snacks, (Figure 5B–D, Table 5 and Supplemental Table 5) directly predicted weight loss, that is, the larger the pre-operative portion, the higher the weight loss, which was unexpected. The overall regression model was significant as well (Supplemental Table 5).
Figure 5. Weight loss regressions in patients.
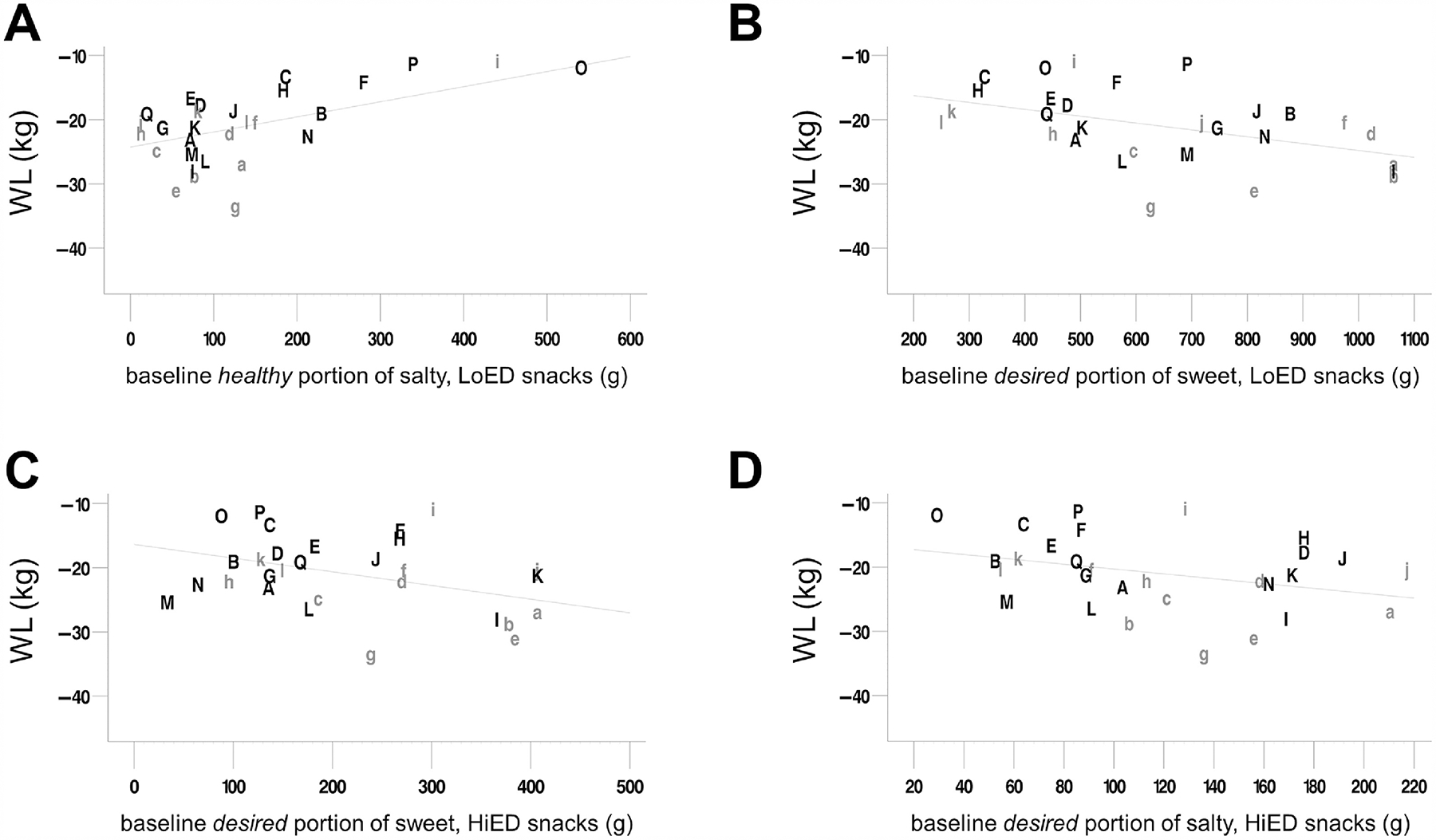
Regressions of body weight loss (kg) at 3 months, in patients (n = 29), from baseline healthy portions of (A) salty, low-energy-dense snacks and desired portions of (B) sweet, high-energy-dense snacks, (C) sweet, low-energy snacks, and (D) salty, high-energy-dense snacks, with baseline weight (kg) as a covariate. Weight loss (kg) is baseline weight subtracted from 3-month weight. The ordinate axis goes from low weight loss (at the top) to highest weight loss (at the bottom). The regression line reflects the relationship between baseline portion and weight loss at mean initial weight (121.59 kg) in all patients. Each letter was assigned to a single participant and remains the same on each plot so that relative positions can be compared between plots. Grey, lowercase letters indicate that the participant underwent Roux-en-Y gastric bypass surgery, while black, uppercase letters indicate that the participant underwent vertical sleeve gastrectomy. See Supplemental Table 5 for regression statistics. WL, weight loss at 3 months; LoED, low energy-dense; HiED, high energy-dense
TABLE 5.
Independent regression models1
| Eating context | Snack type | R2 | t | Slope ± SE | Slope P | Intercept | Intercept P |
|---|---|---|---|---|---|---|---|
| Healthy | Sweet LoED | 0.252 | −0.69 | 0.004 ± 0.006 | 0.502 | 5.307 | 0.458 |
| Healthy | Sweet HiED | 0.238 | 0.23 | 0.001 ± 0.039 | 0.984 | 3.134 | 0.625 |
| Healthy | Salty LoED | 0.449 | −3.21 | 0.024 ± 0.008 | 0.004 | 13.988 | 0.038 |
| Healthy | Salty HiED | 0.246 | −0.64 | 0.034 ± 0.064 | 0.603 | 3.878 | 0.553 |
| Typical | Sweet LoED | 0.289 | 2.61 | −0.009 ± 0.007 | 0.186 | 2.032 | 0.745 |
| Typical | Sweet HiED | 0.264 | 0.69 | −0.012 ± 0.013 | 0.349 | 1.526 | 0.815 |
| Typical | Salty LoED | 0.253 | −1.07 | 0.008 ± 0.011 | 0.483 | 5.659 | 0.439 |
| Typical | Salty HiED | 0.238 | −0.03 | 0.000 ± 0.026 | 0.997 | 3.124 | 0.657 |
| Satisfied | Sweet LoED | 0.241 | 1.53 | −0.002 ± 0.007 | 0.753 | 2.764 | 0.671 |
| Satisfied | Sweet HiED | 0.271 | 0.71 | −0.015 ± 0.014 | 0.287 | 1.519 | 0.813 |
| Satisfied | Salty LoED | 0.239 | −0.30 | 0.002 ± 0.011 | 0.887 | 3.668 | 0.621 |
| Satisfied | Salty HiED | 0.249 | 0.51 | −0.019 ± 0.030 | 0.546 | 2.209 | 0.736 |
| Maximum | Sweet LoED | 0.316 | 2.81 | −0.009 ± 0.005 | 0.097 | 2.976 | 0.624 |
| Maximum | Sweet HiED | 0.253 | 0.75 | −0.010 ± 0.014 | 0.473 | 1.975 | 0.763 |
| Maximum | Salty LoED | 0.240 | −0.51 | −0.002 ± 0.009 | 0.802 | 2.488 | 0.718 |
| Maximum | Salty HiED | 0.274 | 1.38 | −0.033 ± 0.029 | 0.265 | 1.246 | 0.847 |
| Desired | Sweet LoED | 0.441 | 3.33 | −0.011 ± 0.004 | 0.005 | −2.514 | 0.664 |
| Desired | Sweet HiED | 0.403 | 1.30 | −0.021 ± 0.008 | 0.013 | −4.106 | 0.516 |
| Desired | Salty LoED | 0.276 | 0.10 | −0.008 ± 0.007 | 0.253 | 0.209 | 0.975 |
| Desired | Salty HiED | 0.349 | 1.67 | −0.038 ± 0.018 | 0.045 | −1.873 | 0.769 |
Linear regressions of 3-month weight loss (3-month weight – baseline weight), in patients (n = 29), from baseline portion created, with baseline weight as a covariate. Intercept is not the same as regression lines displayed in Figure 3A–D as those intercepts reflect the regression line at the mean of the covariate (baseline weight). Significant slopes and corresponding p-values are bolded.
4. Discussion
4.1. Overview and advantages
We found that responses to the VPCTS are reproducible in a group of normal-weight controls, and that they are influenced by many factors, both food- and non-food related. Responses were group-, visit-, context-, and food-specific, with considerable variability in response to sweet LoED snacks under the desired context. Certain portions also predicted weight loss. We found VPCTs to have reliability, responsiveness, and some predictive validity. Future studies should be conducted to assess additional components of the psychometric quality of this instrument, such as content, construct, and criterion validity.
VPCTs are not cumbersome to use for the researcher or participant and can be administered at a time when bariatric surgery limits the ability to consume solid foods. This instrument allows researchers to collect data on simulated intake of multiple foods in a single assessment [1] that would not be possible if the subject were eating real food. It remains to be determined whether: a) simulations of multiple simultaneous portions would generate the same portions as when they items are presented individually and b) how well virtual portions created with single and multiple foods are related to actual intake with real foods, across different contexts. Different combinations of foods available at the same episode may influence portion creation. We propose that the construct that the VPCTs are measuring is the intention of the individual to eat a portion of a certain size given the food and context, therefore, it is critically important for future studies to determine how well they predict actual intake across contexts and multiple foods. Because of the ease of using this instrument, as well as its stability and responsiveness, this tool could be a valuable dietary assessment tool. In addition, because baseline virtual portions predicted weight loss, these VPCTs hold some predictive validity; however, further study should be conducted to assess clinical prognostic value.
4.2. Overall findings and significance
4.2.1. Reliability of responses.
The prediction that virtual portions created by normal-weight controls will be stable over time, and that their baseline portions will be highly correlated with their follow-up portions was confirmed. What variability, between visits, was seen can be attributable to random fluctuation, since no statistically significant changes were seen after adjustment for multiple comparisons. Future studies should assess reliability in non-surgical controls matched for patient BMI. However, because control data are stable, we suggest these VPCTs are reliable for snack foods.
4.2.2. Surgery-induced decreases.
The prediction that portions created by patients would decrease after surgery was confirmed, but only for one food-type (sweet LoED snacks) for all contexts, except healthy. The failure to see a drop in the healthy context portion sizes may reflect a floor effect. The difference (110g) in portions of sweet LoED between the two “high intake” (desired = 256g; maximum = 282g) and two “low intake” (typical = 164g; satisfied = 146g) contexts may specifically reflect the surgically-induced reduction in gastric capacity, which would affect the propensity to consume larger portions more than it would affect the propensity to consume smaller ones, and would therefore be reflected in the virtual portion size task. However, the fact that difference in desired portion is not affected nearly as much as the other three contexts can be interpreted that desired reflects more of the hedonic or other cognitive influence, whereas the others may reflect more physiologically-related contexts. This hypothesis could be tested by exploration of the effects of similarly worded contexts, in order to separate cognitive from physiologically-driven types of control. It is important to note that the effect of surgery was specific to only one of the four food-types, sweet LoED, which demonstrates that the instrument can detect differences among food-types.
4.2.3. Group differences.
The prediction that portions created by patients will be higher than controls at baseline, but similar to controls at follow-up, was confirmed, but only for sweet LoED snacks, and only for the desired context. While we do not know the reason for the specificity of sweet LoED snacks, the fact that the instrument is selective indicates that it can be useful in discriminating effects between clinical groups, as well as across contexts and food-types. In addition, the restriction of the group differences to one food-type could explain failure of others to see differences between individuals with and without obesity, even in real eating, unless patients were instructed to binge [54]. The literature for portion selection differences under typical eating situations between individuals with obesity and normal-weight controls is also mixed [4, 55, 56]. Perhaps typical portions of foods did not produce differences because the chosen items are not consumed differently or it may be that a typical portion is eaten more frequently in patients than controls [57]. However, when the instruction was changed to “eat as much as you can” [54], which may be similar for a patient with binge eating disorder or bulimia, to the instruction here to “eat without to regard to health” consequences, patients with binge eating disorder or bulimia nervosa ate significantly more than controls.
4.2.4. Eating context differences.
The prediction that, in patients and controls, “high intake” contexts (i.e. maximum and desired) would elicit larger portion size responses than “normal intake” contexts (i.e. satisfied, typical, and healthy) were confirmed in patients for sweet LoED snacks (see 4.2.5 in regard to sweetness and portion size creation). This prediction was also confirmed in controls for all sweet snacks which could be an illustration of another group difference (see 4.2.3) in that controls are context-sensitive to sweet HiED portions while patients are not, and this could contribute to their BMI differences; future studies should explore this hypothesis.
In regard to “normal intake” contexts, when we designed the typical and satisfied contexts, we posited that these contexts might be different in patients, but they were not different from each other at either time point; participants may consider these contexts one in the same. Typical and satisfied portions also became similar to healthy portions after surgery, which illustrates a “normalization” of an excessive-consumption eating style induced by the surgery. The stability of healthy portions in patients could be attributable to food-focused patients, who are dieting, having an idea of what is “healthy” and maintaining this idea, regardless of surgery.
As mentioned (see 4.1.2), desired portions decreased after surgery, but always remained the highest portion out of all contexts; these desired portions may reflect deep-seated neurobehavioral biases that persist after weight loss and could indicate potential failure to maintain weight loss. Future longer-term studies could test the role of desired portion in long-term impaired weight loss and weight recidivism, particularly with a neuroimaging component to determine if changes in reported desire are associated with changes in activation of reward centers of the brain. While individuals with obesity may not always consume larger portions than controls, they may do so for certain foods and under certain contexts. Overall, the differences in portion creation we found between eating contexts suggest that this instrument is context-sensitive and could be useful in assessing eating behavior.
4.2.5. Differences in portion creation attributable to energy density and taste.
The prediction that portions of HiED snacks would be smaller than those for LoED was confirmed in sweet, but not salty. Sweetness may have a greater effect on portion creation than saltiness or energy density in this experimental setting and sample. The creation of diluted diets reduce energy intake [58] may therefore be more effective for sweet tasting than salty tasting items. It is also important to recognize that these differences may be attributable to properties of the food other than energy density and taste, such as familiarity, consumption frequency, availability, cost [59], fat content, palatability and texture [60, 61]. Future studies powered for these additional dimensions of external and internal influences on virtual portion creation should be conducted. Additionally, because fruit, on average, is consumed more than vegetables in the United States [62], additional work is needed to determine whether responses to non-fruit items, that are also sweet and LoED, are similar to those in the current study.
4.2.6. Interactions of energy density and taste.
The prediction that energy density will influence the effects of taste on size of portion created (energy x taste interaction) was confirmed consistently in both groups, at both time points, for the healthy, typical, and desired contexts, which statistically corroborates the differences in food-type that were found and further illustrates the responsiveness of this task to energy density and taste. The prediction that these energy x taste interactions would be larger in “high intake” contexts than in “low intake” contexts was confirmed only for patients at baseline, which further substantiates our discussion about patients’ portion normalization we discussed in Section 4.2.4. From these interactions, we can see that the responsiveness of the VPCTS not only to context, but also to surgery-induced changes. Future studies should explore these interactions with a wider variety of foods in each food type category.
4.2.7. Weight loss predictions.
The prediction that virtual portions of sweet HiED foods would predict surgical weight loss was confirmed in the desired context, however, sweet LoED and salty HiED snacks also directly predicted weight loss, so this prediction was not food-type-specific. Pre-operative healthy portions of salty LoED snacks inversely predicted weight loss as well, which was peculiar; perhaps the variability in portion size can be accounted for by other factors (see 4.2.5). With these weight loss predictions, it is also important to include possible confounders of weight loss, such as physical activity, in future models. While we found some unexpected results, this tool may be useful in future studies delineating the mechanisms in which pre-operative variable work to predict outcome.
4.3. Limitations and future directions
While the present study is limited to eight snack foods, we still found significant differences in portion response. The addition of snacks for each of the food categories and the addition of meal foods is encouraged for future studies.
Social desirability bias during report of dietary intake is common and may influence self-report [63]. To minimize this bias, participants responded to VPCTs in a lab room, alone, and were told that neither their surgeon nor dietitian would see this data. Social factors should be incorporated into these types of studies to determine their influence on portion sizes created and consumed. As in any self-report instrument, there may also be recall bias [64]. Additionally, patients with obesity may have neurocognitive deficits regarding visuospatial memory [65], but most studies examining these deficits use shapes and do not use foods, which are likely more stimulating [66].
Even though VPCTs were given at the same time of day, and under a fasted state, it is not known whether instructions about their state of deprivation (eaten nothing, a small snack, a normal meal, the largest meal they had ever eaten) could influence their portion creation. Future studies should incorporate state of deprivation into VPCTs. Eating and non-eating psychopathology should also be considered in future studies with VPCTs.
We are also limited in interpreting group differences because this particular patient sample was dieting at the time of baseline response. This dieting behavior may have suppressed portions created which could have been larger before pre-surgical nutritional consultation. Future studies should include non-intervention participants with obesity in order to determine if there are differences in portion creation between surgical patients with obesity and non-intervention individuals with obesity; this comparison could help determine the effect of dieting and nutritional counseling on portion creation in individuals with obesity. Despite of this potential limitation, this tool is responsive in that it is able to detect differences due to intervention and BMI (individuals with obesity v. individuals with normal BMI).
Although we know that portions created in response to “typically eaten” contexts accord with amounts actually eaten, and we therefore assume that portions created in other contexts would accord with actual amounts eaten in those contexts, we do not know that for certain, and this assumption remains to be tested. We also do not know the behavioral pathway through which created portions predict weight loss, and assume the pathway is related to food intake and memory for eating episodes. Nevertheless, the robust predictions suggest paths for further investigation.
The present study is also limited by a small, but sufficiently powered, sample that was primarily non-White Hispanic, and included relatively few males. Because weight loss outcomes vary across race, ethnic, socioeconomic, and sex groups [67], this study should be repeated in a larger and more diverse bariatric population. Effect of surgery type on portion size and weight loss should also be explored, as there may be different surgical outcomes, and future studies should explore these effects at long-term post-operative weight loss and weight recidivism.
5. Conclusion
Differences attributable to non-food-related and food-related factors of snack portion creation can be interpreted as an illustration of the ability of this instrument to capture meaningful responses to variability in food-type, eating context, subject characteristics, and weight loss. The stability of the instrument was demonstrated with controls, and the “sensitivity” to surgical manipulation indicates clinical value. We may conclude that VPCTs will become a valuable addition to the ingestive behavior scientist’s tool kit.
Supplementary Material
Highlights.
Virtual portion creation tasks, as proxies for food intake, are stable and reliable
Energy, taste, and eating context influence the size of virtual portions
Pre-operative virtual portion sizes predict weight loss after bariatric surgery
Portion creation tasks can be used in clinical settings and appetite research
Acknowledgements
The authors’ responsibilities were as follows: HRK, JA, AS, JDH, MH designed the research; XP, BL, JA provided clinical oversight; JMB provided materials; JDH, JD wrote the manuscript; JDH, ST, HRK performed the statistical analyses; JDH, JMB, HRK had primary responsibility for the final content of the manuscript. All authors read, edited and approved of the final manuscript and declare no competing or financial interests.
We thank the student volunteers and staff at the New York Obesity Nutrition Research Center at Columbia University Irving Medical Center and the Division of Endocrinology at Mount Sinai - Morningside Hospital in New York, NY for recruitment of patients, data collection, and data entry. These data were presented at the British Feeding and Drinking group congress in Lyon, FR, April 2018, and the New York City Regional Obesity Forum in New York, NY, September 2018.
This study was supported by the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant (R01 DK108643), and the New York Obesity Nutrition Center (DK26687).
Abbreviations
- VPCTs
virtual portion creation tasks
- HiED
high-energy-dense
- LoED
low-energy-dense
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data described in the manuscript, codebook, and analytic code will be made available upon request.
REFERENCES
- 1.Wilkinson LL, et al. , Computer-based assessments of expected satiety predict behavioural measures of portion-size selection and food intake. Appetite, 2012. 59(3): p. 933–938. [DOI] [PubMed] [Google Scholar]
- 2.Diliberti N, et al. , Increased portion size leads to increased energy intake in a restaurant meal. Obesity research, 2004. 12(3): p. 562–568. [DOI] [PubMed] [Google Scholar]
- 3.Fisher JO, et al. , Portion size effects on daily energy intake in low-income Hispanic and African American children and their mothers. American journal of clinical nutrition, 2007. 86(6): p. 1709–1716. [DOI] [PubMed] [Google Scholar]
- 4.Rolls BJ, Morris EL, and Roe LS, Portion size of food affects energy intake in normal-weight and overweight men and women. American journal of clinical nutrition, 2002. 76(6): p. 1207–1213. [DOI] [PubMed] [Google Scholar]
- 5.Hege MA, et al. , Eating less or more–Mindset induced changes in neural correlates of pre-meal planning. Appetite, 2018. 125: p. 492–501. [DOI] [PubMed] [Google Scholar]
- 6.Veit R, et al. , Health, pleasure, and fullness: changing mindset affects brain responses and portion size selection in adults with overweight and obesity. International Journal of Obesity, 2020. 44(2): p. 428–437. [DOI] [PubMed] [Google Scholar]
- 7.Herzog M, et al. , Elasticity in portion selection is predicted by severity of anorexia and food type in adolescents. Appetite, 2016. 103: p. 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herzog M, et al. , Food portion size area mediates energy effects on expected anxiety in anorexia nervosa. Appetite, 2017. 112: p. 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guillocheau E, et al. , Expected satiation alone does not predict actual intake of desserts. Appetite, 2018. 123: p. 183–190. [DOI] [PubMed] [Google Scholar]
- 10.Buchwald H and Williams SE, Bariatric surgery worldwide 2003. J Obesity surgery, 2004. 14(9): p. 1157–1164. [DOI] [PubMed] [Google Scholar]
- 11.Courcoulas AP, et al. , Preoperative factors and 3-year weight change in the Longitudinal Assessment of Bariatric Surgery (LABS) consortium. Surgery for Obesity, 2015. 11(5): p. 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarwer DB, et al. , Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surgery for Obesity, 2008. 4(5): p. 640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Condrasky M, et al. , Chefs’ opinions of restaurant portion sizes. Obesity, 2007. 15(8): p. 2086–2094. [DOI] [PubMed] [Google Scholar]
- 14.Ello-Martin JA, Ledikwe JH, and Rolls BJ, The influence of food portion size and energy density on energy intake: implications for weight management–. American journal of clinical nutrition, 2005. 82(1): p. 236S–241S. [DOI] [PubMed] [Google Scholar]
- 15.Boyce WF, et al. , The Gross Motor Performance Measure: validity and responsiveness of a measure of quality of movement. Physical therapy, 1995. 75(7): p. 603–613. [DOI] [PubMed] [Google Scholar]
- 16.Godfrey J, et al. , Reliability, validity, and responsiveness of the simple shoulder test: psychometric properties by age and injury type. Journal of Shoulder and Elbow Surgery, 2007. 16(3): p. 260–267. [DOI] [PubMed] [Google Scholar]
- 17.Moons P, et al. , Validity, reliability and responsiveness of the” Schedule for the Evaluation of Individual Quality of Life–Direct Weighting”(SEIQoL-DW) in congenital heart disease. Health and Quality of Life Outcomes, 2004. 2(1): p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schatz M, et al. , Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. Journal of Allergy and Clinical Immunology, 2006. 117(3): p. 549–556. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt G, Walter S, and Norman G, Measuring change over time: assessing the usefulness of evaluative instruments. Journal of Clinical Epidemiology, 1987. 40(2): p. 171–178. [DOI] [PubMed] [Google Scholar]
- 20.Brunstrom JM, Shakeshaft NG, and Scott-Samuel NE, Measuring ‘expected satiety’ in a range of common foods using a method of constant stimuli. Appetite, 2008. 51(3): p. 604–614. [DOI] [PubMed] [Google Scholar]
- 21.Hill JO, Wyatt HR, and Peters JC, Energy balance and obesity. Circulation, 2012. 126(1): p. 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz-Tovar J, et al. , Effect of preoperative eating patterns and preoperative weight loss on the short-and mid-term weight loss results of sleeve gastrectomy. Cirugía Española, 2015. 93(4): p. 241–247. [DOI] [PubMed] [Google Scholar]
- 23.Westerterp KR, Control of energy expenditure in humans. European journal of clinical nutrition, 2017. 71(3): p. 340–344. [DOI] [PubMed] [Google Scholar]
- 24.Drewnowski A, Energy Density, Palatability, and Satiety: Implications for Weight Control. Nutrition Reviews, 1998. 56(12): p. 347–353. [DOI] [PubMed] [Google Scholar]
- 25.Nasser J, Taste, food intake and obesity. Obesity Reviews, 2001. 2(4): p. 213–218. [DOI] [PubMed] [Google Scholar]
- 26.Kral TV, Roe LS, and Rolls BJ, Combined effects of energy density and portion size on energy intake in women. American journal of clinical nutrition, 2004. 79(6): p. 962–968. [DOI] [PubMed] [Google Scholar]
- 27.de Castro JM, et al. , Palatability and intake relationships in free-living humans: characterization and independence of influence in North Americans. Physiology & behavior, 2000. 70(3–4): p. 343–350. [DOI] [PubMed] [Google Scholar]
- 28.Hayes JE, Allen AL, and Bennett SM, Direct comparison of the generalized Visual Analog Scale (gVAS) and general Labeled Magnitude Scale (gLMS). Food Qual Prefer, 2013. 28(1): p. 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gras-Miralles B, et al. , Caloric intake capacity as measured by a standard nutrient drink test helps to predict weight loss after bariatric surgery. Obesity surgery, 2014. 24(12): p. 2138–2144. [DOI] [PubMed] [Google Scholar]
- 30.Brunstrom JM, The control of meal size in human subjects: a role for expected satiety, expected satiation and premeal planning. J Proceedings of the Nutrition Society, 2011. 70(2): p. 155–161. [DOI] [PubMed] [Google Scholar]
- 31.Burge JC, et al. , Changes in patients’ taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. Journal of the American Dietetic Association, 1995. 95(6): p. 666–670. [DOI] [PubMed] [Google Scholar]
- 32.Scruggs DM, Buffington C, and Cowan GSM, Taste acuity of the morbidly obese before and after gastric bypass surgery. Obesity surgery, 1994. 4(1): p. 24–28. [DOI] [PubMed] [Google Scholar]
- 33.Drewnowski A, et al. , Food preferences in human obesity: carbohydrates versus fats. 1992. [DOI] [PubMed]
- 34.Gibney M, et al. , Consumption of sugars.[erratum appears in Am J Clin Nutr 1997 May; 65 (5): 1572–4]. American Journal of Clinical Nutrition, 1995. 62(1 Suppl). [DOI] [PubMed] [Google Scholar]
- 35.Mela DJ and Sacchetti DA, Sensory preferences for fats: relationships with diet and body composition. The American journal of clinical nutrition, 1991. 53(4): p. 908–915. [DOI] [PubMed] [Google Scholar]
- 36.Ledikwe JH, et al. , Low-energy-density diets are associated with high diet quality in adults in the United States. Journal of the American Dietetic Association, 2006. 106(8): p. 1172–1180. [DOI] [PubMed] [Google Scholar]
- 37.Faria SL, et al. , Snack-eating patients experience lesser weight loss after Roux-en-Y gastric bypass surgery. Obesity surgery, 2009. 19(9): p. 1293–1296. [DOI] [PubMed] [Google Scholar]
- 38.Foundation, P.f.B.H., State of the Plate, 2015 Study on America’s Consumption of Fruit and Vegetables. 2015.
- 39.Bucher T, Müller B, and Siegrist M, What is healthy food? Objective nutrient profile scores and subjective lay evaluations in comparison. Appetite, 2015. 95: p. 408–414. [DOI] [PubMed] [Google Scholar]
- 40.Cardi V, et al. , The use of a nonimmersive virtual reality programme in anorexia nervosa: A single case-report. European Eating Disorders Review, 2012. 20(3): p. 240–245. [DOI] [PubMed] [Google Scholar]
- 41.Kiszko K, et al. , Corner store purchases in a low-income urban community in NYC. Journal of community health, 2015. 40(6): p. 1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunstrom JM and Rogers PJ, How many calories are on our plate? Expected fullness, not liking, determines meal-size selection. Obesity, 2009. 17(10): p. 1884–1890. [DOI] [PubMed] [Google Scholar]
- 43.Brunstrom JM and Shakeshaft NG, Measuring affective (liking) and non-affective (expected satiety) determinants of portion size and food reward. Appetite, 2009. 52(1): p. 108–114. [DOI] [PubMed] [Google Scholar]
- 44.Ledoux T, et al. , Using virtual reality to study food cravings. Appetite, 2013. 71: p. 396–402. [DOI] [PubMed] [Google Scholar]
- 45.Hogenkamp PS, et al. , Acute sleep deprivation increases portion size and affects food choice in young men. Psychoneuroendocrinology, 2013. 38(9): p. 1668–1674. [DOI] [PubMed] [Google Scholar]
- 46.Brunstrom JM, et al. , Estimating everyday portion size using a ‘method of constant stimuli’: In a student sample, portion size is predicted by gender, dietary behaviour, and hunger, but not BMI. Appetite, 2008. 51(2): p. 296–301. [DOI] [PubMed] [Google Scholar]
- 47.Sakurai S, et al. Affecting our perception of satiety by changing the size of virtual dishes displayed with a tabletop display. Springer. [Google Scholar]
- 48.Wszelaki A, et al. , Consumer liking and descriptive analysis of six varieties of organically grown edamame-type soybean. J Food quality preference, 2005. 16(8): p. 651–658. [Google Scholar]
- 49.Geliebter A, Gastric distension and gastric capacity in relation to food intake in humans. Physiology & behavior, 1988. 44(4–5): p. 665–668. [DOI] [PubMed] [Google Scholar]
- 50.Crager MR, Analysis of covariance in parallel-group clinical trials with pretreatment baselines. Biometrics, 1987. 43(4): p. 895–901. [PubMed] [Google Scholar]
- 51.Fu R, et al. , Handling continuous outcomes in quantitative synthesis, in Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]. 2013, Agency for Healthcare Research and Quality (US). [PubMed] [Google Scholar]
- 52.Senn S, Testing for baseline balance in clinical trials. Stat Med, 1994. 13(17): p. 1715–26. [DOI] [PubMed] [Google Scholar]
- 53.Senn S, Change from baseline and analysis of covariance revisited. Stat Med, 2006. 25(24): p. 4334–44. [DOI] [PubMed] [Google Scholar]
- 54.Guss JL, et al. , Binge eating behavior in patients with eating disorders. Obesity Research, 1994. 2(4): p. 355–363. [DOI] [PubMed] [Google Scholar]
- 55.Kelly MT, et al. , Associations between the portion sizes of food groups consumed and measures of adiposity in the British National Diet and Nutrition Survey. British journal of nutrition, 2009. 101(9): p. 1413–1420. [DOI] [PubMed] [Google Scholar]
- 56.Reily NM, Herman CP, and Vartanian LR, Portion-size preference as a function of individuals’ body mass index. Obesity science & practice, 2016. 2(3): p. 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herman CP, et al. , Are large portions responsible for the obesity epidemic? Physiology & behavior, 2016. 156: p. 177–181. [DOI] [PubMed] [Google Scholar]
- 58.Rolls BJ and Bell EA, Dietary approaches to the treatment of obesity. Medical Clinics of North America, 2000. 84(2): p. 401–418. [DOI] [PubMed] [Google Scholar]
- 59.Glanz K, et al. , Why Americans eat what they do: taste, nutrition, cost, convenience, and weight control concerns as influences on food consumption. Journal of the American Dietetic Association, 1998. 98(10): p. 1118–1126. [DOI] [PubMed] [Google Scholar]
- 60.Flood JE and Rolls BJ, Soup preloads in a variety of forms reduce meal energy intake. Appetite, 2007. 49(3): p. 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Larsen DS, et al. , Increased textural complexity in food enhances satiation. Appetite, 2016. 105: p. 189–194. [DOI] [PubMed] [Google Scholar]
- 62.Lee-Kwan SH, et al. , Disparities in state-specific adult fruit and vegetable consumption—United States, 2015. MMWR. Morbidity and mortality weekly report, 2017. 66(45): p. 1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hebert JR, et al. , Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. International journal of epidemiology, 1995. 24(2): p. 389–398. [DOI] [PubMed] [Google Scholar]
- 64.Poppitt S, et al. , Assessment of selective under-reporting of food intake by both obese and non-obese women in a metabolic facility. International journal of obesity, 1998. 22(4): p. 303–311. [DOI] [PubMed] [Google Scholar]
- 65.Tsai CL, Huang TH, and Tsai MC, Neurocognitive performances of visuospatial attention and the correlations with metabolic and inflammatory biomarkers in adults with obesity. Experimental physiology, 2017. 102(12): p. 1683–1699. [DOI] [PubMed] [Google Scholar]
- 66.Rothemund Y, et al. , Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage, 2007. 37(2): p. 410–421. [DOI] [PubMed] [Google Scholar]
- 67.Khorgami Z, et al. , Effect of ethnicity on weight loss after bariatric surgery. Obesity surgery, 2015. 25(5): p. 769–776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


