Abstract
Background:
Adherence to disease-modifying therapies (DMTs) in pediatric multiple sclerosis (MS) is not well understood. We examined the prevalence and risk factors for poor adherence in pediatric MS.
Methods:
This cross-sectional study recruited youth with MS from 12 North American pediatric MS clinics. In addition to pharmacy-refill data, patients and parents completed self-report measures of adherence and quality of life. Additionally, patients completed measures of self-efficacy and well-being. Factor analysis and linear regression methods were used.
Results:
A total of 66 youth (mean age, 15.7 years) received MS DMTs (33% oral, 66% injectable). Estimates of poor adherence (i.e. missing >20% of doses) varied by source: pharmacy 7%, parent 14%, and patient 41%. Factor analysis yielded two composites: adherence summary and parental involvement in adherence. Regressions revealed that patients with better self-reported physical functioning were more adherent. Parents were more likely to be involved in adherence when their child had worse parent-reported PedsQL School Functioning and lower MS Self-Efficacy Control. Oral DMTs were associated with lesser parental involvement in adherence.
Conclusion:
Rates of non-adherence varied by information source. Better self-reported physical functioning was the strongest predictor of adherence. Parental involvement in adherence was associated with worse PedsQL School Functioning and lower MS Self-Efficacy-measured confidence in controlling MS.
Keywords: Pediatric multiple sclerosis, adherence, parent, psychosocial, quality of life, protective factors
Introduction
Pediatric-onset multiple sclerosis (MS) patients are increasingly prescribed disease-modifying therapies (DMTs) at earlier stages of disease.1 The moderate to high impact of DMT on clinical course reported in clinical trials is mitigated in real life by adherence to medication. In clinic-based adult MS populations, 30%–70% of patients prematurely discontinue DMTs,2 and 25%–59% are consistently non-adherent with their medications.3–7 Some studies have indicated high rates of non-adherence in pediatric MS.1,8 One of these studies (n = 258) revealed that 44% of children do not remain on the first therapy prescribed: one-third discontinue treatment because of poor tolerance or adherence, while the remainder are prescribed an alternative therapy due to breakthrough disease.1 In a study of 30 adolescents with MS, 37% were non-adherent, primarily due to forgetting to take their medication.9 The rate of non-adherence in MS youth may increase with disease duration, as evidenced by an increasing rate of non-adherence over a 5-year period of treatment (non-adherence rates of 18%, 25%, 41%, 50%, and 62% at years 1, 2, 3, 4, and 5, respectively).8 Ultimately, almost half of the patients studied discontinued DMTs altogether.8
Evaluating adherence can be challenging methodologically, as all sources of information about adherence have their own limitations. Patient reports underestimate non-adherence as compared to an electronic monitoring device.10 While parents are the logical source of witnessed adherence reporting, the patient–parent dyad may influence adherence itself. Young children typically receive injections from their parents and are supervised when ingesting oral medications. Adolescents, however, may seek independence and may even resent parental reminders regarding DMT use.
Physicians have been found to miss indicators of poor adherence in patients.11 This may reflect the topic not being broached in clinical encounters. Furthermore, patients—and youth in particular—are not forthcoming about their non-adherence when speaking to their own doctors, or if doing so in the company of their parents.
Objective measures of adherence, while ostensibly more accurate, are also limited depending on the method used. Electronic devices, such as those that record the number of times a pill bottle is opened or the number of needles disposed of in a safety container, accurately record DMT access but do not necessarily capture the actual ingestion or injection frequency. Patient- or parent-recorded logs, while encouraging documentation, suffer from the same issues as well as requiring adherence to documentation as well as therapy administration.
Psychosocial and environmental factors may influence medication adherence in children and youth with MS. Cognitive difficulties, socioeconomic status (e.g. lower education),12 high-risk behaviors (e.g. high levels of alcohol consumption), longer disease duration, and physical disability status are associated with non-adherence in adult MS.6 Psychological morbidity (e.g. mood or anxiety disorders) has also been associated with poor adherence,13 while higher levels of self-efficacy, quality of life, and perceived cognitive functioning have been associated with better adherence.4,14 Whether these or other factors influence adherence in children and youth remains to be determined.
Given the increasing prescribing and range of therapeutic options, increased efficacy, and sizeable cost of DMTs, it is timely to evaluate injectable and oral medication adherence and reasons for non-adherence in youth with MS and to examine risk factors for poor adherence.
Methods
Sample
This multi-site study recruited English-speaking youth with MS, age at enrollment between 10 and 18 years, from 12 pediatric MS clinics in North America from October 2013 to January 2016. Eligible patients had a diagnosis of MS as per the most recent McDonald and International Pediatric MS Study Group criteria15,16 and had been taking an oral or injectable DMT for MS for at least 6 months. Pediatric MS patients receiving intravenous DMT (e.g. natalizumab) were not included. For each eligible participant, at least one parent or guardian was required to be fluent in English and willing to complete the parental questionnaires. Written informed consent was obtained from the parent, and assent was obtained from participants as appropriate. The study was reviewed and approved by the institutional review boards at each site.
Procedure
This study reports baseline data from a randomized trial testing an intervention to improve adherence. We collected demographic information and the Patient-Determined Disease Steps (PDDS)17 to assess perceived MS-specific disability. Recruited and consented participants (patients and parents) were sent an email with a link to a survey using our Health Insurance Portability and Accountability Act (HIPAA)-compliant and secure web-based survey engine (www.surveygizmo.com). The measures included in the web survey are listed below and described in the supplementary text.
Since several of the measures used in this study had been heretofore used primarily with adults, we pre-tested all the measures with 10 youth (age 10–18 years) seen in clinic prior to initiating data collection for this study. These subjects were also asked to complete the study questionnaires and to provide feedback as to whether the questionnaires were understandable. For this study, we retained only those tools that pre-testers were able to complete and endorsed as understandable.
Measures
The supplementary text provides full detail on the measures used in this study. Briefly, adherence was measured using pharmacy-refill data (past 12 months) collected by each site’s research assistant, the self-and parent-reported Morisky Adherence Measure,18 and Multiple Sclerosis Treatment Adherence Questionnaire (MSTAQ).19 Parental involvement in DMT administration was assessed with three items tracking the proportion of time the parent reported (1) reminding the child to take her or his DMT, (2) being present when the child took her or his DMT, and (3) administering the child’s DMT.
Psychosocial risk factors were measured using the following self-report measures: the patient and informant versions of the PedsQL 4.0,20 the patient-reported Multiple Sclerosis Self-Efficacy Scale (MSSE),21 and three subscales from the patient-reported Ryff Scales of Psychological Well-Being.22 Neurocognitive functioning was assessed using the parent-report version of the Multiple Sclerosis Neuropsychological Screening Assessment Questionnaire (MSNQ).23
Statistical analysis
We examined correlations among the continuous measures of adherence. We defined non-adherence for specific analyses as receiving less than 80% of expected doses. Principal component factor analysis was used to create orthogonal composite scores for different aspects of adherence. Due to missing data on several MSTAQ subscales, we included in the factor analyses only those subscales on which we had complete data: the parent-reported proportion missed doses and parent- and patient-reported barrier scores. Given the relatively small sample size of this study, two separate factor analyses (one for each presumed unidimensional construct) were implemented on item sets that were related on the basis of content (i.e. face validity). Unidimensionality was ascertained on the basis of all items loading higher than 0.40 on the first factor, with an eigenvalue greater than 1.0. Factor scores were created by standardizing measures, then multiplying the factor loading of items loading greater than 0.40 on the factor, and summing those weighted item scores. Alpha reliability coefficients were used to assess internal consistency reliability. Linear regression analyses began with univariable analyses to identify relevant predictors for each factor score. Backward stepwise regression was then implemented with a retention rule of p <= 0.10.
Our study has a sample size that has 80% power to detect medium to large effect sizes,24 depending on the analysis and subgrouping. We report effect sizes when relevant rather than p-values. This approach enables one to interpret the magnitude of the detected effects and use it for planning future studies. We report results using Cohen’s criteria for delineating small, medium, and large effects,24 so that future researchers can plan studies to have the power to show statistical significance (Type I error rate (alpha) of 0.05, power (beta) of 80%) given the same detected effect sizes. All analyses were done using Stata 14.25
Results
Sample characteristics
Table 1 shows the demographic characteristics of the sample (n = 66 youth with MS and 66 parents). Table 2 shows the descriptive statistics for the patient- and parent-reported outcomes. On the basis of these data, the sample has a low level of MS-related disability (mean PDDS = 0.50 out of 8). Compared to published norms from healthy youth,20 the sample’s PedsQL scores were in the normal range for all subscales for both parent and patient reports with the exception of parent-reported school functioning, on which they were lower than healthy norms. Compared to published norms for adults on the MS Self-Efficacy scale,21 the sample had similar mean scores. Compared to published values for adolescents using the Ryff Psychological Well-Being measure,26 the sample showed lower values on all subscales.
Table 1.
Sample characteristics (n = 66).
| n | ||
|---|---|---|
| Age (years), mean (SD) | 15.74 (2.02) | 66 |
| Age at diagnosis (years), | 13.20 (3.91) | 60 |
| mean (SD) | ||
| Disease duration (years), | 2.27 (2.25 | 60 |
| mean (SD) | ||
| Age at menarche (years), | 11.61 (1.13) | 38 |
| mean (SD) | ||
| Gender | ||
| Male (%) | 33 | 22 |
| Female (%) | 67 | 44 |
| Race/ethnicitya | ||
| Hispanic or Latino (%) | 6 | 4 |
| Middle Eastern (%) | 6 | 4 |
| South Asian (%) | 5 | 3 |
| Other Asian (%) | 5 | 3 |
| Black or African | 9 | 6 |
| American (%) | ||
| White (%) | 53 | 35 |
| Don’t know (%) | 2 | 1 |
| Missing (%) | 3 | 2 |
| Mother’s education | ||
| Less than 12 years of | 9 | 6 |
| education (%) | ||
| High school | 26 | 17 |
| diploma/GED (%) | ||
| Associate’s degree (%) | 15 | 10 |
| Technical degree (%) | 8 | 5 |
| Bachelor’s degree (%) | 18 | 12 |
| Post graduate education | 20 | 13 |
| (masters, doctorate; %) | ||
| Missing (%) | 5 | 3 |
| Father’s education | ||
| Less than 12 years of | 11 | 7 |
| education (%) | ||
| High school | 35 | 23 |
| diploma/GED (%) | ||
| Associate’s degree (%) | 8 | 5 |
| Technical degree (%) | 9 | 6 |
| Bachelor’s degree (%) | 17 | 11 |
| Post graduate education | 17 | 11 |
| (masters, doctorate; %) | ||
| Missing (%) | 5 | 3 |
| Medication type | ||
| Injectable | 68% | 45 |
| Avonex or Avonex pre- filled syringe (interferon beta1a—intramuscular) |
11 | |
| Copaxone glatiramer acetate) |
24 | |
| Plegridy (peginterferon beta-1a) |
3 | |
| Rebif (interferon beta1b— subcutaneous) |
7 | |
| Oral | 30% | 20 |
| Gilenya (fingolimod) | 4 | |
| Tecfidera (BG-12 or | 14 | |
| dimethyl fumarate) | ||
| Terifluonomide | 2 | |
| Missing | 2% | 1 |
| Time on DMT (years) | ||
| Mean (SD) | 1.97 (1.86) | 61 |
| Range | 0.35–9.46 | |
| No. of DMTs in past 12 months | ||
| 1 | 66% | 40 |
| 2 | 28% | 17 |
| 3 | 5% | 3 |
| 4 | 2% | 1 |
| Site | ||
| Toronto—The Hospital for Sick Children |
33% | 22 |
| Children’s Hospital of Philadelphia |
5% | 3 |
| Children’s Hospital of Pittsburgh |
9% | 6 |
| Boston Children’s Hospital |
11% | 7 |
| St Louis Children’s Hospital |
2% | 1 |
| University of Alabama at Birmingham |
11% | 7 |
| Mayo Clinic | 2% | 1 |
| University of Colorado Denver |
6% | 4 |
| University of California at San Francisco |
8% | 5 |
| Texas Children’s Hospital | 9% | 6 |
| Cleveland Clinic | 3% | 2 |
| Alberta Children’s Hospital |
3% | 2 |
| Method of survey administration |
||
| Using paper and pencil | 21% | 14 |
| Using a computer | 67% | 44 |
| Missing | 12% | 8 |
| Help with questionnaire | ||
| No help | 76% | 50 |
| Help from a parent | 15% | 10 |
| Help from study personnel | 9% | 6 |
SD: standard deviation; DMT: disease-modifying therapy.
Participants could indicate more than one race/ethnicity or not indicate any. We had data on 58 of the 66 respondents, of whom 2 were coded as “missing.”
Table 2.
Descriptive statistics of person-reported outcomes (n = 66).
| Mean (SD) | n | |
|---|---|---|
| Parent-reported measures | ||
| PDDS | 0.50(0.90) | 61 |
| PedsQL Physical Functioning | 79.20(21.31) | 63 |
| PedsQL Emotional Functioning | 72.22(20.84) | 63 |
| PedsQL Social Functioning | 81.59(19.13) | 63 |
| PedsQL School Functioning | 66.90(18.87) | 63 |
| PedsQL Psychosocial Health Summary Score | 73.57(15.89) | 63 |
| MSNQ | 18.69(13.41) | 61 |
| Patient-reported measures | ||
| PedsQL Physical Functioning | 80.17(18.50) | 66 |
| PedsQL Emotional Functioning | 68.03(23.05) | 66 |
| PedsQL Social Functioning | 83.18(17.22) | 66 |
| PedsQL School Functioning | 63.56(18.50) | 66 |
| PedsQL Psychosocial Health Summary Score | 71.59(16.06) | 66 |
| Ryff Autonomy | 27.86(5.52) | 66 |
| Ryff Self Acceptance | 27.08(3.83) | 65 |
| Ryff Environmental Mastery | 24.47(4.25) | 66 |
| MSSE Control | 690.15 (208.03) | 66 |
| MSSE Function | 811.36 (167.35) | 66 |
SD: standard deviation; PDDS: Patient-Determined Disease Steps; MSNQ: Multiple Sclerosis Neuropsychological Screening Assessment Questionnaire; MSSE: Multiple Sclerosis Self-Efficacy Scale.
Estimates of adherence
Table 3 shows the adherence-related scores for the sample, and Figure 1 illustrates differences in esti-mates of non-adherence as a function of source of information. Based on pharmacy records for 12 months prior to study start, the sample received slightly fewer than the expected number of refills (mean of 0.95 out of 1.0), and 7% of the sample (n = 4) were non-adherent (i.e. received less than 80% of expected refills). On the basis of the parent report, the sample missed an average of 10% of DMT doses over the past 28 days, and 14% (n = 8 patients) were non-adherent. Parents reported reminding their child a median of half the time, being present for the DMT doses a median of 75% of the time, and administering the medication a median of 25% of the time (Table 3). Parents reported an average Morisky score reflecting medium adherence (score of 6 or 7 out of 8), with 28% of the sample scoring in the low-adherence range. In contrast, youth reported an average Morisky score reflecting low adherence, with 41% of the sample scoring in the low-adherence range, suggesting either that parents are not aware of their child’s non-adherence and/or over-report compliance. The mean patient rating of the Barriers subscale was also slightly lower than the parents’ rating of Barriers on the MSTAQ.
Table 3.
Adherence-related variables.
| Measure of adherence | Time frame | n | % | Mean | SD |
|---|---|---|---|---|---|
| Pharmacy records | |||||
| Proportion refills received/expected refills | Past 12 days | 56 | 0.95 | 0.12 | |
| Proportion non-adherent (i.e. <80% received vs expected refills) |
4 | 7 | - | - | |
| Parent-reported | |||||
| Proportion missed dosesa | Past 28 days | 58 | 0.1 | 0.3 | |
| Proportion non-adherent (i.e. <80% received vs expected refills) |
8 | 14 | - | - | |
| Parental involvement in DMT use | Past 2 weeks | ||||
| Remind | |||||
| 0% | 18 | 27 | |||
| 25% | 13 | 20 | |||
| 50% | 7 | 11 | |||
| 75% | 11 | 17 | |||
| 100% | 14 | 21 | |||
| Missing | 3 | 5 | |||
| Present | |||||
| 0% | 9 | 14 | |||
| 25% | 8 | 12 | |||
| 50% | 7 | 11 | |||
| 75% | 15 | 23 | |||
| 100% | 24 | 36 | |||
| Missing | 3 | 5 | |||
| Administer | |||||
| 0% | 31 | 47 | |||
| 25% | 8 | 12 | |||
| 50% | 4 | 6 | |||
| 75% | 7 | 11 | |||
| 100% | 13 | 20 | |||
| Missing | 3 | 5 | |||
| Reported adherence | No time frame | 60 | 6.3 | 1.4 | |
| Proportion low adherence | 18 | 28 | - | - | |
| Contextual factors | |||||
| Behavioral coping strategiesa | Past 4 weeks | 41 | 51.5 | 11.3 | |
| Side effectsa | Past 4 weeks | 43 | 51.3 | 9.9 | |
| Barriersa | No time frame | 57 | 51.3 | 11.0 | |
| Patient-reported | |||||
| Reported adherenceb | No time frame | 66 | 5.8 | 1.8 | |
| Proportion low adherenceb | 27 | 41 | - | - | |
| Contextual factors | |||||
| Barriersa | No time frame | 58 | 49.6 | 9.2 | |
| Factor scores (standardized) | |||||
| Adherence summary | 49 | 0.0 | 1.0 | ||
| Parent involvement in adherence | 57 | 0.0 | 1.0 |
SD: standard deviation; DMT: disease-modifying therapy.
Multiple Sclerosis Treatment Adherence Questionnaire.
Morisky Adherence Measure.
Figure 1.
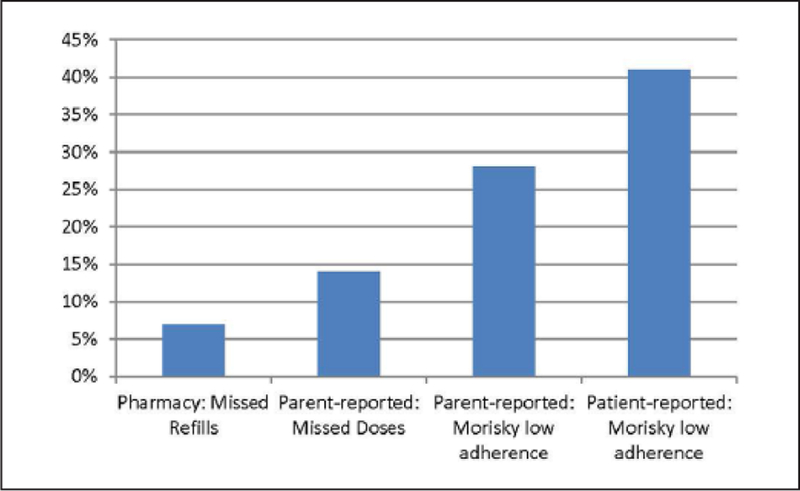
Proportion non-adherent by data source.
Relationships among adherence variables
Table 4 shows the inter-correlations among the adherence measures, with conditional formatting to show small, medium, and large effects using Cohen’s criteria.24 The largest correlations were between the parent being present and administering the child’s DMT, and being present and parent-reported behavioral coping of the child. There was also a large correlation between parent- and patient-reported Morisky adherence scores. There were medium effect-size correlations among pharmacy refills and parent-reported missed doses, and with both sources of Morisky scores (i.e. parent- or patient-reported). Parent-reported missed doses and Morisky scores were moderately correlated, as were parent-reported behavioral coping and side effects. There were small effect-size correlations among the majority of the adherence measures (see Table 4).
Table 4.
Correlation matrix of adherence measures
| Pharmacy proportion refills received/ expected refills |
Proportion missed doses (parent report) |
Parent remind |
Parent present |
Parent administer |
Morisky adherence (parent) |
Behavioral coping (MSTAQ parent) |
Side effects (MSTAQ parent) |
Barriers (MSTAQ parent) |
Morisky adherence (patient) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Pharmacy proportion refills received/expected refills | ||||||||||
| Proportion missed doses (parent report) |
−0.45 | |||||||||
| Parent remind | −0.29 | 0.27 | ||||||||
| Parent present | 0.04 | 0.07 | 0.35 | |||||||
| Parent administer | 0.19 | 0.17 | 0.29 | 0.60 | ||||||
| Morisky adherence (parent) | 0.37 | −0.46 | −0.23 | −0.14 | −0.19 | |||||
| Behavioral coping (MSTAQ parent) |
0.03 | −0.01 | 0.11 | 0.58 | 0.37 | 0.02 | ||||
| Side effects (MSTAQ parent) | 0.05 | 0.12 | 0.03 | 0.16 | 0.23 | −0.14 | 0.43 | |||
| Barriers (MSTAQ parent) | −0.17 | 0.06 | 0.21 | 0.20 | 0.13 | −0.16 | 0.12 | 0.21 | ||
| Morisky adherence (patient) | 0.34 | −0.30 | −0.12 | 0.00 | −0.02 | 0.59 | 0.01 | 0.18 | −0.07 | |
| Barriers (MSTAQ patient) | −0.31 | −0.04 | 0.12 | 0.19 | 0.29 | −0.25 | 0.34 | 0.09 | 0.31 | −0.14 |
MSTAQ: Multiple Sclerosis Treatment Adherence Questionnaire.
Small correlation.
Medium correlation.
Large correlation.
Supplemental Table 1 shows the results of the factor analysis of the adherence variables, which was done for the purpose of data reduction. The first factor score—adherence summary—comprised pharmacy refills, proportion missed doses, and parent- and patient-reported Morisky adherence scores. The second factor score—parental involvement in adherence—comprised the parent reminding, being present, administering the DMT, and parent- and patient-reported barriers. These factors were orthogonal and thus not correlated with each other and had internal consistency reliability of 0.56 and 0.63, respectively, which is on the low end of accepted standards of 0.50–0.70.27
Predictors of adherence
Univariate models predicting adherence summary revealed that higher levels of patient-reported PedsQL Physical Functioning were significantly associated with better adherence; and there were trends suggesting that worse parent-reported cognitive functioning on the MSNQ and better patient-reported PedsQL Emotional Functioning were associated with better adherence (Supplemental Table 2). Univariate models predicting parental involvement in adherence revealed that worse parent-reported PedsQL School Functioning and Psychosocial Health Summary and worse patient-reported Self-Efficacy Function and Control were associated with more parental involvement in adherence, and there were trends suggesting that worse parent-related PedsQL Social Functioning and patient-reported PedsQL School Functioning were associated with more parental involvement in adherence (Supplemental Table 2).
Significant/trend variables from the univariate regression were then entered into the backward step-wise regression models. These models kept only patient-reported PedsQL Physical Functioning in the model predicting adherence summary, suggesting that the patients with better self-reported physical functioning were more adherent, and explaining about 6% of the variance (Table 5). Backward step-wise models predicting parental involvement in adherence kept only parent-reported School Functioning and patient-reported Self-Efficacy Control, suggesting that parents were more likely to be involved in medication administration when their child had worse school functioning and a worse sense of control over their MS (R2 = 0.31; Table 5).
Table 5.
Results of backward stepwise regression models predicting adherence-related factors.
| Coefficient | Standard error | t | p > t | 95% CI | |||
|---|---|---|---|---|---|---|---|
| Adherence summary (n = 48) | |||||||
| PedsQL Physical Function (patient) | 0.01 | 0.01 | 2.01 | 0.05 | 0.00 | 0.03 | |
| Adjusted R2 | 0.06 | ||||||
| Parental involvement in adherence (n = 49) | |||||||
| PedsQL School Function (parent) | −0.01 | 0.01 | −1.84 | 0.072 | −0.02 | 0.00 | |
| MSSE control | 0.00 | 0.00 | −4.15 | 0 | 0.00 | 0.00 | |
| Adjusted R2 | 0.31 |
CI: confidence interval; MSSE: Multiple Sclerosis Self-Efficacy Scale.
We examined initial differences in adherence between injectable and oral DMTs. Figure 2 shows the mean scores on adherence summary and parental involvement in adherence as a function of type of DMT used. While there was no difference between injectable and oral DMTs on adherence summary (Effect Size = −0.06), oral DMTs were associated with lower levels of parental involvement in DMT administration (Effect Size = 0.29). Of note, only 2 of the 20 oral DMT patients were concurrently enrolled in a clinical trial.
Figure 2.
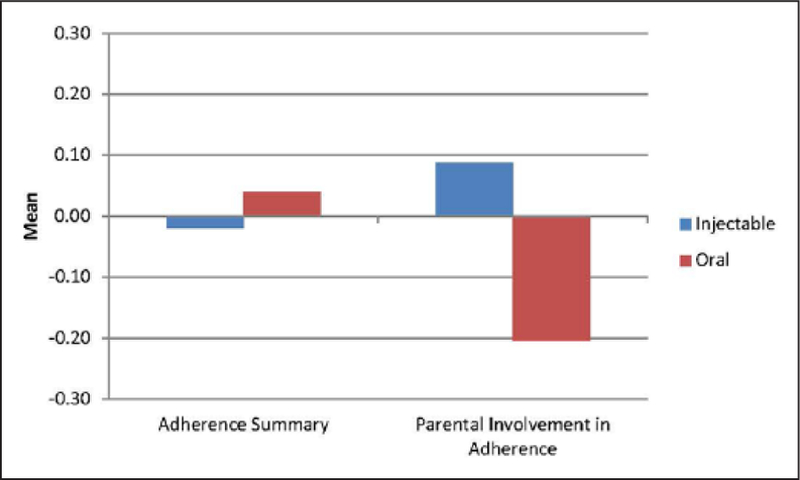
Comparison of injectable versus oral DMT on adherence outcomes.
Discussion
Our study provides an estimate of medication adherence in youth with MS after oral DMTs were introduced to the MS treatment landscape. We found higher levels of medication adherence overall than past research on both youth and adults with MS. We did not find a difference in adherence between oral and injectable DMTs. Worse patient-reported physical functioning was the strongest predictor of lower medication adherence in our study. In contrast to past research on medication adherence which suggests that children over-estimate their levels of adherence,9 our “objective data” (pharmacy-refill data) suggested higher rates of medication adherence than patient self-report.
Other studies have suggested that pharmacy-refill data may comprise a good surrogate measure of medication adherence in some adult populations, but its accuracy in children is unknown. For example, pharmacy-refill data were found to be superior to pill counting in predicting viral load in adult HIV-positive patients on therapy.28 This was not found to be true in pediatric HIV patients; viral load could not be predicted using any single measure (caregiver report, pharmacy refill and appointment maintenance data), but rather use of all three above-mentioned data points were necessary to predict viral load, and, by inference, true medication adherence levels.29 It is possible that multiple measures must be used in our population to establish true medication adherence levels.
Youth with MS reported lower levels of medication adherence than parent reports of their behavior. Further exploration of the discrepancy between parent, child, and pharmacy finding is necessary and will be corroborated in future studies exploring the use of an objective electronic monitoring device in this population. The discrepant reports may be in line with other studies that have found significant discrepancies between parent and child report of other MS symptoms.
We found a relationship between parental involvement in their child’s DMT administration and the child’s DMT adherence. Although there were no differences in adherence as a function of type of DMT, we did find mode-of-administration differences in how much parents were involved in their child’s DMT administration, with less parental involvement with oral than injectable medications. Higher levels of parental involvement in medication adherence were associated with worse reported PedsQL School Functioning and a lower sense of control with regard to MS. These findings suggest that cognitive factors matter in pediatric MS adherence. In this study, the cognitive factors that were found to be important were parent-reported school functioning (i.e. paying attention, forgetting, keeping up with school work, missing school) and cognitive appraisal with regard to self-management (i.e. sense of confidence or self-efficacy in controlling the impact of their MS symptoms on their daily activities). In diabetes, another chronic disease population of adolescents who must also use injectable therapies, self-control in both parents and children has been shown to be associated with higher medication adherence in the adolescents.30 Future research should evaluate the reasons for decreases in quality of life with increasing parental involvement in youth with MS. Based on the aforementioned work, it may relate to adolescent perceptions of self-control. Interventions might focus on these functional and psychosocial aspects of cognition to improve patient self-management and independence in managing their MS treatments.
Despite its strengths of collecting useful and pertinent data on a relatively rare patient population, the study’s limitations should be acknowledged. First, the sample size is limited, which limits the types of analyses that can be done, the statistical power to detect clinically meaningful differences, and the generalizability of the findings. Also, as is typical in pediatric MS populations, patients had very little physical disability and report quality-of-life scores in the same range as healthy youth—challenging our ability to determine clinically meaningful outcomes early in the course of pediatric MS. The cross-sectional design precludes causal inference. Furthermore, the adherence estimates are much higher than documented in previous studies, which may reflect selection biases, changes in the MS population, or advantages of the evolving treatment options for MS. The sample may suffer from selection biases, in part, related to the recruitment sites being pediatric MS centers which may have structures in place that improve adherence and, in part, related to more adherent patients being more interested in a study of adherence or participation in studies in general. Another issue is that pharmacy-refill data are a retrospective review of 12 months of pharmacy-refill data and only provide an estimate of use rather than actual medication administration. Furthermore, our study involved only one of the two possible parents, so we were unable to address the differential effects of the child’s caregivers on patient adherence. Future research might focus on inclusion of both parents in a behavioral intervention study and examine whether parents are influencing the adherence in different ways, and whether the type of parental involvement is associated with differences in a patient’s self-efficacy.
In summary, in our study of 66 youth with MS and their parents, rates of non-adherence were relatively low with discrepancies between sources. Estimates based on pharmacy-refill data would suggest that our sample is remarkably adherent, whereas estimates based on the patients themselves are closer to published adult estimates. This contrast is worth exploring, as it may reflect selection biases or methodological issues in measuring adherence that are important to address. Parental involvement in adherence may be a strong protective factor in pediatric MS, particularly among adolescents struggling in school and with confidence in controlling their MS. These findings have possible implications for intervention research and emphasize the need for focus on parental involvement in future studies. Future longitudinal research will need to confirm our estimates and findings, as well as compare estimates of adherence from pharmacy-refill data as compared to MEMS cap data which capture actual efforts to open medication receptacles (e.g. pill bottles). This will be addressed with prospective exploration of differences between refill data and MEMS cap data. Ongoing efforts by our group will investigate the juxtaposition of parent and patient reports using qualitative interview data.
Supplementary Material
Acknowledgements
The authors are grateful to the youth with MS and their parents for the involvement as well as to the following investigators and their institutions, without whom this study would not have been possible. They are also grateful for the hard work and dedication of the study teams at each site (Supplemental Table 3). C.E.S. and E.A.Y. conceived and designed the study and drafted the manuscript. S.A.G. oversaw patient recruitment and data collection. C.E.S. and E.A.Y. carried out the literature review. C.E.S. and V.E.P. summarized all the findings in tabular and graphic format. C.E.S. and V.E.P. performed the statistical analyses and helped draft the manuscript. All authors read and approved the final manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by a contract from the National Multiple Sclerosis Society (HC 0148).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval and consent to participate
All patients provided informed consent to participate in this research. Because the MS patients were under the age of 18 years, for those patients who did not have the capacity to consent for themselves, their parent provided consent for both the patient and the parent.
Availability of data and supporting materials
Supporting documentation for the study findings is provided in manuscript data and supplementary tables. Scientists wishing to gain access to the study data may contact the last author (E.A.Y.), who will consider such requests on a case-by-case basis, subject to the scientific rigor of the proposed research question.
Contributor Information
Carolyn E Schwartz, DeltaQuest Foundation, Inc., Concord, MA, USA/ Departments of Medicine and Orthopaedic Surgery, School of Medicine, Tufts University, Boston, MA, USA.
Stephanie A Grover, Department of Neuroscience and Mental Health, Research Institute, The Hospital for Sick Children, Toronto, ON, Canada.
Victoria E Powell, DeltaQuest Foundation, Inc., Concord, MA, USA.
Austin Noguera, Hospital for Sick Children, Toronto, ON, Canada/ Division of Neurology and Division of Neuroscience and Mental Health, Department of Pediatrics, Research Institute, Hospital for Sick Children, Toronto, ON, Canada.
Jean K Mah, Division of Neurology, Department of Pediatrics, Cumming School of Medicine, Alberta Children’s Hospital, University of Calgary, Calgary, AB, Canada.
Soe Mar, Departments of Neurology and Pediatrics, St. Louis Children’s Hospital, Washington University School of Medicine in St. Louis, St. Louis, MO, USA.
Lauren Mednick, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA.
Brenda L Banwell, Division of Neurology, Children’s Hospital of Philadelphia, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Gulay Alper, Division of Child Neurology, Department of Pediatrics, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Mary Rensel, Department of Neurology, The Mellen Center, Cleveland Clinic, Cleveland, OH, USA.
Mark Gorman, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA.
Amy Waldman, Division of Neurology, Children’s Hospital of Philadelphia, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Teri Schreiner, Departments of Neurology and Pediatrics, University of Colorado Denver, Denver, CO, USA.
Emmanuelle Waubant, Department of Neurology, University of San Francisco, San Francisco, CA, USA.
E Ann Yeh, Division of Neurology and Department of Neuroscience and Mental Health, Department of Pediatrics, Research Institute, Hospital for Sick Children, Toronto, ON, Canada/Faculty of Medicine, The University of Toronto, Toronto, ON, Canada.
References
- 1.Yeh EA, Waubant E, Krupp LB, et al. Multiple sclerosis therapies in pediatric patients with refractory multiple sclerosis. Arch Neurol 2011; 68(4): 437–444. [DOI] [PubMed] [Google Scholar]
- 2.ong J, Gomes T, Mamdani M, et al. Adherence to multiple sclerosis disease-modifying therapies in Ontario is low. Can J Neurol Sci 2011; 38(3): 429–433. [DOI] [PubMed] [Google Scholar]
- 3.Treadaway K, Cutter G, Salter A, et al. Factors that influence adherence with disease-modifying therapy in MS. J Neurol 2009; 256(4): 568–576. [DOI] [PubMed] [Google Scholar]
- 4.Devonshire V, Lapierre Y, Macdonell R, et al. The Global Adherence Project (GAP): A multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol 2011; 18(1): 69–77. [DOI] [PubMed] [Google Scholar]
- 5.Lafata JE, Cerghet M, Dobie E, et al. Measuring adherence and persistence to disease-modifying agents among patients with relapsing emitting multiple sclerosis. J Am Pharm Assoc 2008; 48(6): 752–757. [DOI] [PubMed] [Google Scholar]
- 6.McKay KA, Tremlett H, Patten SB, et al. Determinants of non-adherence to disease-modifying therapies in multiple sclerosis: A cross-Canada prospective study. Mult Scler J Epub ahead of print 29 June 2016. 10.1177/1352458516657440. [DOI] [PMC free article] [PubMed]
- 7.Menzin J, Caon C, Nichols C, et al. Narrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosis. J Manag Care Pharm 2013; 19(1 Supp A): S24–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thannhauser JE, Mah JK and Metz LM. Adherence of adolescents to multiple sclerosis disease-modifying therapy. Pediatr Neurol 2009; 41(2): 119–123. [DOI] [PubMed] [Google Scholar]
- 9.Lulu S, Julian L, Shapiro E, et al. Treatment adherence and transitioning youth in pediatric multiple sclerosis. Mult Scler Relat Disord 2014; 3(6): 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce JM, Hancock LM and Lynch SG. Objective adherence monitoring in multiple sclerosis: Initial validation and association with self-report. Mult Scler 2010; 16(1): 112–120. [DOI] [PubMed] [Google Scholar]
- 11.Osterberg L and Blaschke T. Adherence to medication. N Engl J Med 2005; 353(5): 487–497. [DOI] [PubMed] [Google Scholar]
- 12.Tremlett H, Van der Mei I, Pittas F, et al. Adherence to the immunomodulatory drugs for multiple sclerosis: Contrasting factors affect stopping drug and missing doses. Pharmacoepidemiol Drug Saf 2008; 17(6): 565–576. [DOI] [PubMed] [Google Scholar]
- 13.Bruce JM, Hancock LM, Arnett P, et al. Treatment adherence in multiple sclerosis: Association with emotional status, personality, and cognition. J Behav Med 2010; 33(3): 219–227. [DOI] [PubMed] [Google Scholar]
- 14.Fraser C, Morgante L, Hadjimichael O, et al. A prospective study of adherence to glatiramer acetate in individuals with multiple sclerosis. J Neurosci Nurs 2004; 36(3): 120–129. [DOI] [PubMed] [Google Scholar]
- 15.Krupp LB, Banwell B and Tenembaum S. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology 2007; 68(16 Suppl. 2): S7–12. [DOI] [PubMed] [Google Scholar]
- 16.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 2011; 69(2): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hohol MJ, Orav EJ and Weiner HL. Disease steps in multiple sclerosis: A simple approach to evaluate disease progression. Neurology 1995; 45: 251–255. [DOI] [PubMed] [Google Scholar]
- 18.Morisky DE, Ang A, Krousel-Wood M, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens 2008; 10(5): 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Wicks P, Massagli M, Kulkarni A, et al. Use of an online community to develop patient-reported outcome instruments: The Multiple Sclerosis Treatment Adherence Questionnaire (MS-TAQ). J Med Internet Res 2011; 13(1): e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varni JW, Seid M and Kurtin PS. PedsQL™ 4.0: Reliability and validity of the Pediatric Quality of Life Inventory™ version 4.0 generic core scales in healthy and patient populations. Med Care 2001; 39(8): 800–812. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz CE, Coulthard-Morris L, Zeng Q, et al. Measuring self-efficacy in people with multiple sclerosis: A validation study. Arch Phys Med Rehabil 1996; 77(4): 394–398. [DOI] [PubMed] [Google Scholar]
- 22.Ryff CD. Happiness is everything, or is it? Explorations on the meaning of psychological well-being. J Person Social Psychol 1989; 57: 1069–1081. [Google Scholar]
- 23.Benedict RH, Cox D, Thompson LL, et al. Reliable screening for neuropsychological impairment in multiple sclerosis. Mult Scler 2004; 10(6): 675–678. [DOI] [PubMed] [Google Scholar]
- 24.Cohen J A power primer. Psychol Bull 1992; 112: 155–159. [DOI] [PubMed] [Google Scholar]
- 25.StataCorp. Stata statistical software: Release 14 College Station, TX: StataCorp, 2016. [Google Scholar]
- 26.Schwartz CE, Keyl P, Bode R, et al. Helping others shows differential benefits on health and well-being for male and female teens. J Happiness Stud 2009; 10(4): 431–448. [Google Scholar]
- 27.Reeve BB, Wyrwich KW, Wu AW, et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res 2013; 22(8): 1889–1905. [DOI] [PubMed] [Google Scholar]
- 28.Sangeda RZ, Mosha F, Prosperi M, et al. Pharmacy refill adherence outperforms self-reported methods in predicting HIV therapy outcome in source-limited settings. BMC Public Health 2014; 14(1): 1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burack G, Gaur S, Marone R, et al. Adherence to antiretroviral therapy in pediatric patients with human immunodeficiency virus (HIV-1). J Pediatr Nurs 2010; 25(6): 500–504. [DOI] [PubMed] [Google Scholar]
- 30.Lansing A, Crochiere R, Cueto C, et al. Mother, father, and adolescent self-control and adherence in adolescents with type 1 diabetes. J Fam Psychol. Epub ahead of print 12 January 2017. 10.1037/fam0000292. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


