Abstract
Objectives:
Nonadherence to medications has been documented, but the combined effect of social risk factors on medication nonadherence has not been investigated.
Methods:
We conducted a cross-sectional analysis of data from the Coronary Artery Risk Development in Young Adults (CARDIA) study, a population-based prospective cohort. The sample (N = 1506) included subjects who at Year 20 (2005–06) were taking prescription medications and completed a 4-item Medication Adherence Scale. Social risk factors were education of high school or less, annual household income <$25,000, high financial strain, high chronic stress, low social support, and high social strain.
Results:
In a fully adjusted logistic regression model, income <$25,000 (OR = 2.37 [95% CI 1.12–4.98], p < .05) and high chronic stress (OR = 2.07 [95% CI 1.09–3.94], p < .05) were significantly associated with medication nonadherence. Individuals with ≥3 social risk factors had >3 times higher odds of nonadherence than counterparts with no social risk factors (OR = 3.26 [95% CI 1.72–6.19], p < .001).
Conclusion:
Low income and chronic stress are associated with medication nonadherence, and the odds of nonadherence increase with the accumulation of social risk factors. Findings may be used to develop risk prediction tools to identify individuals who can benefit from adherence-promoting interventions.
Keywords: medication adherence, social factors, income, chronic stress
Nonadherence to medication has been documented in many chronic health conditions, including pulmonary diseases, hypertension, cardiovascular diseases, arthritis and rheumatic diseases, diabetes, gastrointestinal disorders, HIV, and cancer,1–5 with about 60% of patients failing to take their medications as prescribed.6 Nonadherence reduces the therapeutic effects of medication therapy and can lead to drug resistance and reactions, diminished quality of life, and increased morbidity and mortality.7–9 Medica tion nonadherence also contributes to higher utilization of health services and increased medical costs, adding a burden to both patients and the healthcare system.10–13
Medication nonadherence often has been viewed as an individual choice,14 but this choice cannot be fully understood without taking into account the social context in which it occurs.15,16 Because medication adherence is an interaction between an individual and his or her physical and social environments, the question of nonadherence is essentially a question about the intersection of agency and structure. One theory that explains the dialectic relationship between agency and structure is the health lifestyle theory.17 It posits that health behaviors are shaped by social structures (social class, age, gender, race/ethnicity, collectivities such as religion and ideology, and living conditions) that dictate which behavioral choices are both available and appropriate in one’s social environment. The health choices of individuals (agency) are either constrained or expanded by the available life chances (structure). Although individuals make choices, they choose from a limited menu of available options and according to what is acceptable in their social environment.
The premise of the health lifestyle theory is substantiated by the limited success of adherence-promoting strategies focused on personal intent and motivation.18–20 In the absence of interventional breakthroughs in current adherence research, it is only appropriate to consider the contextual factors that affect a person’s willingness and ability to follow medical advice.21,22 For example, a number of studies report a relationship between socioeconomic factors and adherence in chronic disease. Although the evidence of a direct association of income23–28 and educational achievement29,30 with medication adherence is mixed, cost burden is a widely recognized barrier,31–43 and financial strain has been associated with medication non-adherence among patients with various chronic health conditions.44,45 Conversely, having social support46,47 and being married48 have been associated with higher medication adherence. Various dimensions of stress, including chronic stress burden, also have been noted as obstacles to medication adherence, although few studies have focused specifically on this association.49–52 The simultaneous, combined effect of various social risk factors on medication adherence has not been investigated.
Applying the health lifestyle theory, we conceptualized medication adherence as a health behavior practiced in a social context (Figure 1). Drawing on previous adherence research and using data from the population-based Coronary Artery Risk Development in Young Adults (CARDIA) cohort study, we tested 2 hypotheses: (1) lower education, lower income, higher financial strain, higher chronic stress, less social support, and higher social strain are positively associated with medication nonadherence; and (2) adults with higher number of these social risk factors are more likely to be nonadherent to their prescribed medications.
Figure 1.

Conceptual Framework of the Study
METHODS
Study Design and Population
We performed a cross-sectional analysis of Coronary Artery Risk Development in Young Adults (CARDIA) data collected at the Year 20 (2005–06) exam. The CARDIA study has been described in detail elsewhere.53 In brief, CARDIA is a multi-center, population-based study of young African-American and white adults recruited between June 1985 and May 1986. The cohort has been followed to examine the development of clinical and sub-clinical cardiovascular disease and their risk factors. Recruitment was from 4 urban communities, with participants solicited by random digit dialing in Birmingham (Alabama), Chicago (Illinois), and Minneapolis (Minnesota), with supplemental door-to-door recruitment in Minneapolis ar eas that had low telephone subscription rates. In Oakland (California), participants were selected at random from membership files of the Kaiser Permanente Medical Care Program, which had broad market penetration at the time. Participants with any health condition were eligible to participate, provided their condition would not preclude participation in the study examination itself (eg, being unable to walk on treadmill). The study was approved by each site’s institutional review board, and all subjects signed an informed consent at each examination.
The stratified sampling strategy used at baseline (Year 0) resulted in a cohort of 5115 participants approximately balanced on race (52% African-American, 48% white), sex (55% female), age (45% 18–24 years old, 55% 25–30 years old), and education (high school or less, more than high school). At the Year 20 CARDIA exam (2005–06), the 3547 returning participants also were queried regarding medication use and adherence. Of 1711 persons reporting medication use, 1506 had no missing data on any variable in this analysis. There were no statistically significant differences between participants included in the analysis (N = 1506) and those excluded due to missing data (N = 195, 11.5%).
Measures
Medication adherence was assessed with a validated 4-item scale, the MMAS-4.54 Participants were asked : (1) Do you ever forget to take your medicine? (2) Are you careless at times about taking your medicine? (3) When you feel better do you sometimes stop taking your medicine? and (4) Sometimes if you feel worse when you take the medicine, do you stop taking it? Each item had a Yes/No response option, with 1 point assigned to each “Yes” response and 0 points to each “No” response. A summed score of ≥1 indicated nonadherence, 0 score indicated adherence.
Assessed social factors included educational attainment (high school or less/some college/college degree), annual income (<$25,000/$25,000-$49,999/$50,000-$74,999/≥$75,000), financial strain, chronic stress burden, social support, and social strain.
Financial strain combined responses to 2 questions: How hard is it for you (and your family) to pay for the very basics like food and heating? and How hard is it for you (and your family) to pay for medical care? – each with response options 1 = Very hard, 2 = Hard, 3 = Somewhat hard; 4 = Not very hard. Although there is no common metric for financial strain, the above questions, which are incorporated into several large population-based cohort studies, have been used in previous research.45
Chronic stress burden was assessed with the 8-item Chronic Burden Scale,55 which has also been used in prior studies.56–58 It measures ongoing stress related to health, work, financial, and relationship problems by asking if participants have experienced any of these strains for longer than 6 months: (1) Serious ongoing health problem, with separate response options for Yourself; (2) Your partner; (3) Your parents; (4) Your children; (5) Others close to you; (6) Ongoing difficulties with your job or ability to work; (7) Ongoing financial strain; and (8) Ongoing difficulties in a relationship with someone close to you. Response options were 0 = No, 1 = Yes, but not very stressful, 2 = Yes, moderately stressful, 3 = Yes, very stressful; scores were obtained by summing all 8 items (range 0–24). Scores were categorized into quartiles according to their distribution as has been done previously.59
Social support and social strain from family and friends were assessed with 4 support and 4 strain items on a 4-point Likert scale that have been deployed previously.60,61 For social support, respondents were asked: (1) How much family members or friends care about you? (2) Understand the way you feel? (3) How much you can rely on them? and (4) How much you can open up to them? For social strain, they were asked: (1) How often family members or friends make too many demands on you? (2) Criticize you? (3) Let you down when you are counting on them? and (4) Get on your nerves? Response categories for all items were 1 = Not at all, 2 = A little, 3 = Some, 4 = A lot. Social support and strain scores were obtained by summing the respective support and strain items (range 4–16). Due to the nonlinear distribution of scores, we collapsed them into quartiles as has been done in prior work.61
Social risk factors were defined as low education (high school or less), low income (<$25,000 annually), high financial strain (answering Very hard, Hard, or Somewhat hard to both financial strain questions), high chronic stress (highest quartile of the Chronic Burden Scale), low social support (lowest quartile of social support score), and high social strain (highest quartile of social strain score).
Covariates, defined a priori based on review of the adherence literature, included demographic characteristics (age, race, sex) and 2 known confounders of medication nonadherence: marital status and depressive symptoms assessed by the 20-item Center for Epidemiologic Studies Depression Scale,62 with a score ≥16 indicating depressive symptoms. Analyses additionally controlled for geographic location (Birmingham, Chicago, Minnesota, Oakland) to account for potential regional differences, as well as for self-reported history of heart disease, cancer, diabetes, kidney disease, and lung disease assessed by the question: Has a doctor or nurse ever said that you have […]. Heart disease included heart attack, angina, rheumatic heart disease, mitral valve pro-lapse, and other heart conditions specified by the participant. Cancer included lung, breast, cervical, blood, testes, bone, melanoma, skin, brain, stomach, color, uterine, prostate, and others. Diabetes self-report excluded gestational diabetes. Kidney disease included urine infections from kidney, kidney stones, nephritis, glomerulonephritis, kidney failure, dialysis, and kidney transplant. Lung disease included asthma, emphysema, and chronic obstructive pulmonary disease.
Data Analysis
We calculated participant characteristics by level of medication adherence and compared them using chi-square tests. Next, we used multiple logistic regression models to calculate the odds ratios for medication nonadherence associated with each social risk factor. Model 1 included adjustment for age, sex, race, and center. Model 2 additionally adjusted for marital status, depressive symptoms, and a history of heart disease, cancer, diabetes, lung disease, and kidney disease. The number of social risk factors (0, 1–2, ≥3) by level of medication adherence was calculated, and the statistical significance of the differences was determined with a chi-square test. The association between the number of social risk factors and level of medication adherence was estimated using multiple logistic regression with the same 2 levels of adjustment as described above. Only persons without missing values on all variables were included in the analyses. There were no statistically significant differences between those with missing data (N = 195, 11.5%) and without missing data (N = 1506) on demographic characteristics, social risk factor measures, and covariates. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Study participants (N = 1506), mean ± SD age 45.6 ± 3.6 years, were predominantly female (69.0%), white (55.4%), and married (64.2%) (Table 1). Approximately one-fourth (22.9%) had high school education or less, and 15.5% had annual income less than $25,000. Medication nonadherence was reported by 106 (7.5%) participants. Those individuals were more likely to be African American, unmarried, have depressive symptoms, and a history of diabetes, but less likely to have a history of cancer (Table 1). In terms of social risk factors, compared to participants reporting adherence, those reporting nonadherence had lower education (p < .05), lower income (p < .001), greater financial strain (p < .05), higher chronic stress (p < .001), lower social support (p < .001), and higher social strain (p < .05) (Figure 2).
Table 1.
Characteristics of Participants by Medication Adherence (N = 1506): CARDIA, 2005–06
| Variable | Overall | Medication Adherence | p-value | |
|---|---|---|---|---|
| Adherent N = 1393 (92.5%) | Nonadherent N = 113 (7.5%) | |||
| Age, years | 45.6±3.6 | 45.6±3.5 | 45.2±3.8 | .215 |
| Female, % | 63.1 | 62.6 | 69.0 | .173 |
| African-American, % | 44.6 | 42.9 | 65.5 | < .001 |
| Annual income < $25,000, % | 15.5 | 14.4 | 28.3 | < .001 |
| High school education or less, % | 22.9 | 22.3 | 31.0 | .034 |
| Unmarried, % | 35.8 | 34.9 | 46.9 | .010 |
| Depressive symptoms, % | 19.0 | 17.6 | 36.3 | < .001 |
| Lung disease, % | 19.2 | 19.0 | 22.1 | .410 |
| Heart disease, % | 15.9 | 15.5 | 21.2 | .109 |
| Diabetes, % | 13.6 | 13.1 | 19.5 | .059 |
| Cancer, % | 6.8 | 7.2 | 1.8 | .028 |
| Kidney disease, % | 8.0 | 8.0 | 8.0 | .977 |
| Center | ||||
| Birmingham | 26.7 | 25.9 | 36.3 | .087 |
| Chicago | 19.9 | 19.8 | 20.4 | |
| Minnesota | 24.8 | 25.1 | 20.4 | |
| Oakland | 28.7 | 29.1 | 23.0 | |
Figure 2.
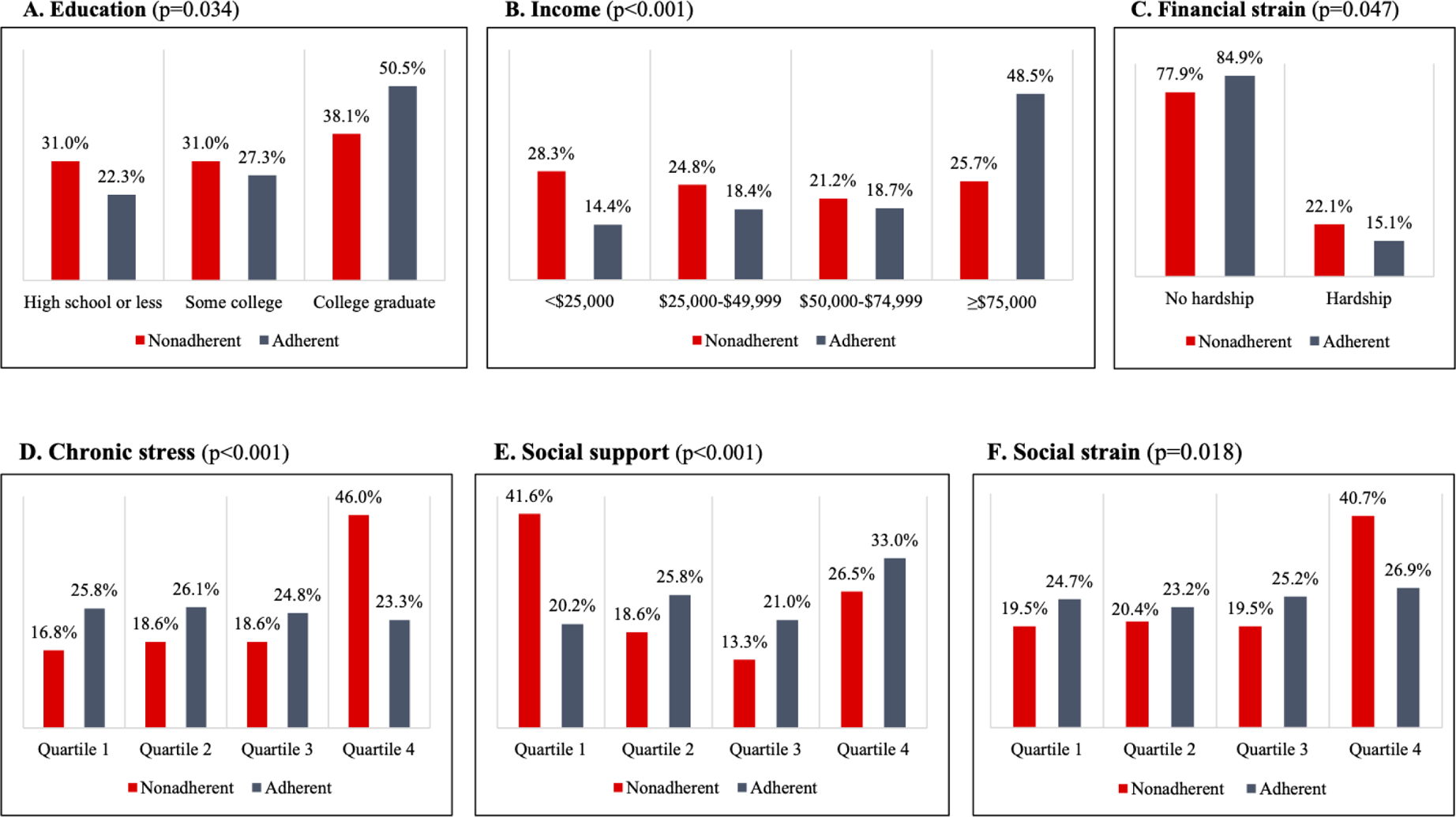
Medication Nonadherence by A) Education; B) Income; C) Financial Strain; D) Chronic Stress; E) Social Support; F) Social Strain
In multiple logistic regression, after adjusting for age, sex, race, and center location (Table 2, Model 1), participants with any annual income less than $75,000 and higher chronic stress were more likely to be nonadherent than their counterparts with annual income ≥$75,000 and low chronic stress. After additionally adjusting for marital status, depressive symptoms, and a history of heart disease, cancer, lung disease, diabetes, and kidney disease (Table 2, Model 2), the odds of nonadherence remained statistically significant for participants with annual income ≤$25,000 (OR = 2.37 [95% CI 1.12–4.98], p < .05) and high chronic stress (OR = 2.07 [95% CI 1.09–3.94], p < .05) compared to those with annual income ≥$75,000 and low chronic stress.
Table 2.
Odds Ratios for Medication Nonadherence (N = 1506): CARDIA 2005–06
| Social risk factors | OR [95% CI] | ||
|---|---|---|---|
| Model 1a | Model 2b | ||
| Education | |||
| College graduate | 1 (ref) | ||
| Some college | 0.95 (0.57 – 1.59) | 0.98 (0.58 – 1.64) | |
| High school or less | 1.05 (0.61 – 1.79) | 0.99 (0.57 – 1.72) | |
| Income | |||
| ≥$75,000 | 1 (ref) | ||
| $50,000–<$75,000 | 1.81 (1.01 – 3.25)* | 1.87 (1.01 – 3.43) | |
| $25,000–<$50,000 | 1.86 (1.01 – 3.41)* | 1.85 (0.96 – 3.59) | |
| <$25,000 | 2.25 (1.18 – 4.31)* | 2.37 (1.12 – 4.98)* | |
| Financial strain | |||
| No | 1 (ref) | 1 (ref) | |
| Yes | 0.71 (0.42 – 1.23) | 0.69 (0.40 – 1.19) | |
| Chronic stress | |||
| Quartile 1 | 1 (ref) | ||
| Quartile 2 | 1.06 (0.55 – 2.44) | 0.99 (0.52 – 1.92) | |
| Quartile 3 | 1.08 (0.56 – 2.11) | 1.01 (0.52 – 1.97) | |
| Quartile 4 | 2.39 (1.29 – 4.43)* | 2.07 (1.09 – 3.94)* | |
| Social support | |||
| Quartile 4 | 1 (ref) | ||
| Quartile 3 | 0.61 (0.32 – 1.19) | 0.64 (0.33 – 1.23) | |
| Quartile 2 | 0.73 (0.40 – 1.34) | 0.77 (0.42 – 1.41) | |
| Quartile 1 | 1.55 (0.88 – 2.75) | 1.53 (0.86 – 2.72) | |
| Social strain | |||
| Quartile 1 | 1 (ref) | ||
| Quartile 2 | 1.09 (0.58 – 2.55) | 1.05 (0.56 – 1.98) | |
| Quartile 3 | 0.88 (0.46 – 1.65) | 0.88 (0.46 – 1.66) | |
| Quartile 4 | 1.08 (0.59 – 1.97) | 1.03 (0.56 – 1.89) | |
p < .05, 2-tailed tests
Note.
Adjusted for age, sex, race, and center
Adjusted for age, sex, race, center, marital status, depressive symptoms, and history of heart disease, cancer, lung disease, diabetes, and kidney disease
Concentration of social risk factors was associated with nonadherence in a gradient pattern (Figure 3). After adjusting for age, sex, race, and center location, participants with ≥3 social risk factors had higher odds of medication nonadherence (OR = 4.16 [95% CI 2.35–7.37], p < .001) compared to counterparts without social risk factors (Table 3, Model 1). Further adjustment for marital status, depressive symptoms, and history of heart disease, cancer, lung disease, diabetes, and kidney disease attenuated the relationship but it remained statistically significant (OR = 3.26 [95% CI 1.72–6.19], p < .001) (Table 3, Model 2).
Figure 3.
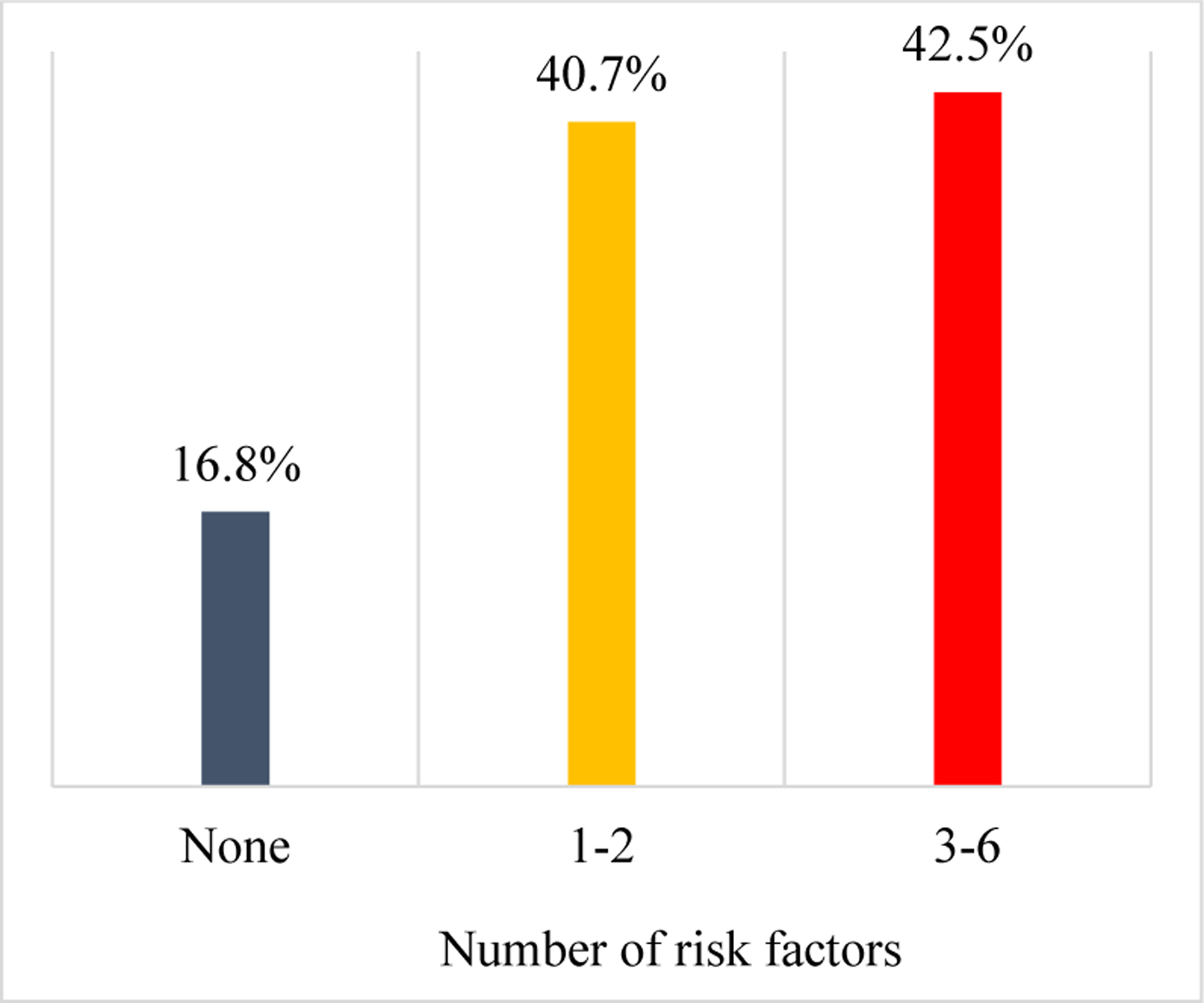
Medication Nonadherence by Number of Social Risk Factors
Table 3.
Odds Ratios for Medication Nonadherence by Number of Social Risk Factors (N = 1506): CARDIA, 2005–06
| Number of social risk factors | OR [95% CI] | |
|---|---|---|
| Model 1a | Model 2b | |
| None | 1 (ref) | |
| 1–2 | 1.73 (0.99 – 3.71) | 1.65 (0.94 – 2.88) |
| 3–6 | 4.16 (2.35 – 7.37)*** | 3.26 (1.72 – 6.19)*** |
p < .001, 2-tailed tests
Note.
Adjusted for age, sex, race, and center
Adjusted for age, sex, race, center, marital status, depressive symptoms, and history of heart disease, cancer, lung disease, diabetes, and kidney disease
DISCUSSION
The negative consequences of medication nonadherence are well described; the reasons for this behavior are less clear. Informed by the health lifestyle theory and using the most recent data from a population-based national cohort, in this study we identified social risk factors for medication nonadherence. In fully adjusted models, nonadherence was associated with having annual income less than $25,000 and reporting high chronic stress. Additionally, individuals with 3–6 social risk factors had 3.3 times higher odds of nonadherence than their counterparts without social risk factors. These findings provide a rationale for targeted interventions aiming to improve medication adherence by mitigating stress exposure.
Few studies have attempted to develop risk stratification tools for medication adherence based on clustering of social and clinical characteristics,63 and even fewer have been successful. For example, a study of antihypertensive medications reported that although certain sociodemographic characteristics and clinical diagnoses were statistically associated with refills of antihypertensive medications, a combination of these factors was not sufficiently accurate to predict adherence.64 Our data show that concentration of social risk factors may be explored as a promising marker of nonadherence. Because medication adherence is not a stable patient characteristic but a dynamic process, efforts would be more fruitful with repeated assessments of adherence, to account for time-varying patterns and identify appropriate time points for targeted interventions.65,66
Our finding that individuals with lower incomes are more likely to be nonadherent to medications reflects results from other studies.67 The association is especially apparent in the lowest income category. Previous evidence suggests that unmet basic needs, typically exacerbated by low income, such as food insecurity, housing insecurity, and access to transportation, are also associated with medication nonadherence.68–71 Our data did not support an association between high financial strain and nonadherence. Because financial strain can occur at all income levels, our results suggest that nonadherence due to financial factors is observed primarily at the lowest end of the income scale.
The highest levels of chronic stress were significantly associated with medication nonadherence in our study. Previous research into chronic stress and medication nonadherence has produced inconsistent results. For example, whereas research into stress and nonadherence in HIV yielded no statistically significant relationship,72 several studies have reported that chronic stress is a predictor for medication nonadherence in diabetes.73,74 In our study, both low income and high chronic stress were associated with medication nonadherence. Therefore, it is important for medical providers to consider the social context when addressing issues related to nonadherence. Connecting patients with resources to help alleviate chronic life stressors may improve adherence rates.
One should note that African-American race, which was not conceptualized as a social risk factor, emerged as a statistically significant correlate of nonadherence in univariate analyses, confirming previously reported racial disparities in medication adherence.75–78 Compared with Caucasians, African Americans are more likely to be nonadherent to statins,79,80 beta blockers,81 and pre-exposure prophylaxis drugs to prevent HIV infection,82 among others. One also should note that racial differences in medication adherence have persisted after adjustment for socioeconomic and health factors. Even among those with close-to-full drug coverage, minorities were 40% less likely to adhere to prescribed treatments compared to Whites, indicating that policies relying on cost reduction alone are unlikely to eliminate racial differences.83 Strategies to address racial/ethnic and gender gaps in medication adherence that take into account social factors beyond cost are needed.
Finally, the proportion of participants reporting nonadherence was only 7.5%. Considering that medication nonadherence typically ranges between 25%4 and 50%,84,85 there likely is a sizeable underestimation of nonadherence in the CARDIA cohort. Self-reported adherence measures the perception of one’s adherence rather than actual adherence.86 Adherence self-reports reflect one’s perceived sense of control, self-esteem, self-efficacy, and knowledge about the prescribed treatment.87–89 It is possible that the demographics of the CARDIA cohort, which was younger and included approximately half of participants with college education and income higher than $75,000, have contributed to the unusually high self-reported adherence.
Our findings expand the literature on the impact of social context beyond its well-documented importance for individual health and well-being, to highlight its role for health-related behaviors such as medication nonadherence. The theoretical premise that medication nonadherence is situated within economic, social, cultural, and family contexts which exert powerful influences on the adherence behavior has profound implications for adherence-promoting interventions. As agency never gets free of structure, meaningful and sustained changes of medication adherence are unlikely by targeting the individual without regard for the circumstances that constrain him or her. Furthermore, decontextualizing medication adherence not only obscures its socioeconomic origin, but also can deteriorate into blaming the victims of socioeconomic inequality for their health-related choices.
Study findings support the notion that efforts to improve medication adherence need to be focused upstream, on expanding access to resources and reducing ongoing stressors. In clinical settings, such an approach may include screening for social adversities, linking patients to resources to address unmet needs, and developing risk stratification tools to aid in this process. However, results also draw attention to the critical role of economic, social, and healthcare policies for creating an environment that is conducive to following medical recommendations.
Limitations
The use of a self-reported adherence scale limits our ability to verify the accuracy of the reported adherence levels. The MMAS-4 is a validated and widely-used scale, but self-reports are known to overestimate adherence.90 Although we dichotomized the scale in the most conservative way, the low proportion of participants reporting nonadherence (7.5%) highlights the unreliable nature of self-reports. Chronic diseases are self-reported as well, and CARDIA did not collect data on disease severity or drug tolerability. We were limited further by our inability to include health insurance status as a covariate due to the high number of missing values. Finally, the cross-sectional study design prevents us from making causal inferences about the reported associations.
Conclusion
Low income and chronic stress are social risk factors for medication nonadherence. Accumulation of social risks is associated with increased medication nonadherence. These findings can be used for the development of risk prediction tools to identify patients who would benefit from targeted interventions. Assessing exposure to social risks and addressing these concerns with the patient may improve medication adherence for the most vulnerable individuals.
Acknowledgements
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I & HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). This manuscript has been reviewed by CARDIA for scientific content. This study was funded by the National Institutes of Health (grant U54MD008176) and the Agency for Healthcare Research and Quality (grant K12HS023009).
Footnotes
Human Subjects Approval Statement
The institutional review board at each center approved the CARDIA study protocol and informed consent was obtained from each participant.
Conflict of Interest Disclosure Statement
The authors declare that they have no conflict of interest.
References
- 1.Thier SL, Yu-Isenberg KS, Leas BF, et al. In chronic disease, nationwide data show poor adherence by patients to medication and by physicians to guidelines. Manag Care. 2008;17(2):48–52, 55–47. [PubMed] [Google Scholar]
- 2.Agh T, Inotai A, Meszaros A. Factors associated with medication adherence in patients with chronic obstructive pulmonary disease. Respiration. 2011;82(4):328–334. [DOI] [PubMed] [Google Scholar]
- 3.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. [DOI] [PubMed] [Google Scholar]
- 4.Khanna R, Pace PF, Mahabaleshwarkar R, et al. Medication adherence among recipients with chronic diseases enrolled in a state Medicaid program. Popul Health Manag. 2012;15(5):253–260. [DOI] [PubMed] [Google Scholar]
- 5.Bugni VM, Ozaki LS, Okamoto KY, et al. Factors associated with adherence to treatment in children and adolescents with chronic rheumatic diseases. J Pediatr (Rio J). 2012;88(6):483–488. [DOI] [PubMed] [Google Scholar]
- 6.Dunbar-Jacob J, Mortimer-Stephens MK. Treatment adherence in chronic disease. J Clin Epidemiol. 2001;54(Suppl 1):S57–S60. [DOI] [PubMed] [Google Scholar]
- 7.Pai AL, Drotar D. Treatment adherence impact: the systematic assessment and quantification of the impact of treatment adherence on pediatric medical and psychological outcomes. J Pediatr Psychol. 2010;35(4):383–393. [DOI] [PubMed] [Google Scholar]
- 8.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. [DOI] [PubMed] [Google Scholar]
- 9.Kronish IM, Ye S. Adherence to cardiovascular medications: lessons learned and future directions. Prog Cardiovasc Dis. 2013;55(6):590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stuart BC, Dai M, Xu J, et al. Does good medication adherence really save payers money? Med Care. 2015;53(6):517–523. [DOI] [PubMed] [Google Scholar]
- 11.Roebuck MC, Kaestner RJ, Dougherty JS. Impact of medication adherence on health services utilization in Medicaid. Med Care. 2018; 56(3):266–273. [DOI] [PubMed] [Google Scholar]
- 12.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. [DOI] [PubMed] [Google Scholar]
- 13.Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawyer SM, Aroni RA. Sticky issue of adherence. J Paediatr Child Health. 2003;39(1):2–5. [DOI] [PubMed] [Google Scholar]
- 15.Butterworth SW. Influencing patient adherence to treatment guidelines. J Manag Care Pharm. 2008;14(6 Suppl B):21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreps GL, Villagran MM, Zhao X, et al. Development and validation of motivational messages to improve prescription medication adherence for patients with chronic health problems. Patient Educ Couns. 2011;83(3):375–381. [DOI] [PubMed] [Google Scholar]
- 17.Cockerham WC. Health lifestyle theory and the convergence of agency and structure. J Health Soc Behav. 2005;46(1):51–67. [DOI] [PubMed] [Google Scholar]
- 18.Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60(7):657–665. [DOI] [PubMed] [Google Scholar]
- 19.Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590–611. [DOI] [PubMed] [Google Scholar]
- 20.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288(22):2868–2879. [DOI] [PubMed] [Google Scholar]
- 21.Mihalko SL, Brenes GA, Farmer DF, et al. Challenges and innovations in enhancing adherence. Control Clin Trials. 2004;25(5):447–457. [DOI] [PubMed] [Google Scholar]
- 22.Russell CL, Ruppar TM, Matteson M. Improving medication adherence: moving from intention and motivation to a personal systems approach. Nurs Clin North Am. 2011;46(3):271–281. [DOI] [PubMed] [Google Scholar]
- 23.Fiese BH, Everhart RS. Medical adherence and childhood chronic illness: family daily management skills and emotional climate as emerging contributors. Curr Opin Pediatr. 2006;18(5):551–557. [DOI] [PubMed] [Google Scholar]
- 24.Rust G, Zhang S, Reynolds J. Inhaled corticosteroid adherence and emergency department utilization among Medicaid-enrolled children with asthma. J Asthma. 2013;50(7):769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hommel KA, Greenley RN, Maddux MH, et al. Self-management in pediatric inflammatory bowel disease: a clinical report of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2013;57(2):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: a meta-analysis. Can J Cardiol. 2012;28(5):574–580. [DOI] [PubMed] [Google Scholar]
- 27.Orzech KM, Vivian J, Huebner Torres C, et al. Diet and exercise adherence and practices among medically under-served patients with chronic disease: variation across four ethnic groups. Health Educ Behav. 2013;40(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collaco JM, Kole AJ, Riekert KA, et al. Respiratory medication adherence in chronic lung disease of prematurity. Pediatr Pulmonol. 2012;47(3):283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalkan K, Bacciogly K, Kalpakliogly AF. Allergic rhinitis: can we identify nonadherence to therapy and its predictors easily in daily practice? J Investig Allergol Clin Immunol. 2013;23(5):315–322 [PubMed] [Google Scholar]
- 30.Singh N, Squier C, Sivek C, et al. Determinants of compliance with antiretroviral therapy in patients with human immunodeficiency virus: prospective assessment with implications for enhancing compliance. AIDS Care. 1996;8(3):261–269. [DOI] [PubMed] [Google Scholar]
- 31.McHorney CA, Spain CV. Frequency of and reasons for medication non-fulfillment and non-persistence among American adults with chronic disease in 2008. Health Expect. 2011;14(3):307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrold LR, Briesacher BA, Peterson D, et al. Cost-related medication nonadherence in older patients with rheumatoid arthritis. J Rheumatol. 2013;40(2):137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785–795. [DOI] [PubMed] [Google Scholar]
- 34.Mackey K, Parchman ML, Leykum LK, et al. Impact of the chronic care model on medication adherence when patients perceive cost as a barrier. Prim Care Diabetes. 2012;6(2):137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole JA, Norman H, Weatherby LB, Walker AM. Drug copayment and adherence in chronic heart failure: effect on cost and outcomes. Pharmacotherapy. 2006;26(8):1157–1164. [DOI] [PubMed] [Google Scholar]
- 36.Piette JD, Wagner TH, Potter MB, Schillinger D. Health insurance status, cost-related medication underuse, and outcomes among diabetes patients in three systems of care. Med Care. 2004;42(2):102–109. [DOI] [PubMed] [Google Scholar]
- 37.Piette JD, Heisler M, Wagner TH. Cost-related medication underuse among chronically ill adults: the treatments people forgo, how often, and who is at risk. Am J Public Health. 2004;94(10):1782–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piette JD, Heisler M, Wagner TH. Cost-related medication underuse: do patients with chronic illnesses tell their doctors? Arch Intern Med. 2004;164(16):1749–1755. [DOI] [PubMed] [Google Scholar]
- 39.Briesacher BA, Gurwitz JH, Soumerai SB. Patients at-risk for cost-related medication nonadherence: a review of the literature. J Gen Intern Med. 2007;22(6):864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chernew ME, Shah MR, Wegh A, et al. Impact of decreasing copayments on medication adherence within a disease management environment. Health Aff (Millwood). 2008;27(1):103–112. [DOI] [PubMed] [Google Scholar]
- 41.Zivin K, Ratliff S, Heisler MM, et al. Factors influencing cost-related nonadherence to medication in older adults: a conceptually based approach. Value Health. 2010;13(4):338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang H, Lobo JM, Kim S, Sohn MW. Cost-related medication non-adherence among U.S. adults with diabetes. Diabetes Res Clin Pract. 2018;143:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaul S, Avila JC, Mehta HB, et al. Cost-related medication nonadherence among adolescent and young adult cancer survivors. Cancer. 2017;123(14):2726–2734. [DOI] [PubMed] [Google Scholar]
- 44.Osborn CY, Kripalani S, Goggins KM, Wallston KA. Financial strain is associated with medication nonadherence and worse self-rated health among cardiovascular patients. J Health Care Poor Underserved. 2017;28(1):499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyles CR, Seligman HK, Parker MM, et al. Financial strain and medication adherence among diabetes patients in an integrated health care delivery system: the Diabetes Study of Northern California (Distance). Health Serv Res. 2016;51(2):610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sherbourne CD, Hays RD, Ordway L, et al. Antecedents of adherence to medical recommendations: results from the Medical Outcomes Study. J Behav Med. 1992;15(5):447–468. [DOI] [PubMed] [Google Scholar]
- 47.Gerlach LB, Kavanagh J, Watkins D, et al. With a little help from my friends?: racial and gender differences in the role of social support in later-life depression medication adherence. Int Psychogeriatr. 2017;29(9):1485–1493. [DOI] [PubMed] [Google Scholar]
- 48.Trivedi RB, Bryson CL, Udris E, Au DH. The influence of informal caregivers on adherence in COPD patients. Ann Behav Med. 2012;44(1):66–72. [DOI] [PubMed] [Google Scholar]
- 49.Rod NH, Gronbaek M, Schnohr P, et al. Perceived stress as a risk factor for changes in health behaviour and cardiac risk profile: a longitudinal study. J Intern Med. 2009;266(5):467–475. [DOI] [PubMed] [Google Scholar]
- 50.Bottonari KA, Safren SA, McQuaid JR, et al. A longitudinal investigation of the impact of life stress on HIV treatment adherence. J Behav Med. 2010;33(6):486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson JR, Novak JR, Johnson MD, et al. A dyadic multiple mediation model of patient and spouse stressors predicting patient dietary and exercise adherence via depression symptoms and diabetes self-efficacy. J Behav Med. 2016;39(6):1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mentz RJ, Greiner MA, Muntner P, et al. Intentional and unintentional medication non-adherence in African Americans: insights from the Jackson Heart Study. Am Heart J. 2018;200:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. [DOI] [PubMed] [Google Scholar]
- 54.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. [DOI] [PubMed] [Google Scholar]
- 55.Bromberger JT, Matthews KA. A longitudinal study of the effects of pessimism, trait anxiety, and life stress on depressive symptoms in middle-aged women. Psychol Aging. 1996;11(2):207–213. [DOI] [PubMed] [Google Scholar]
- 56.Gallo LC, Jimenez JA, Shivpuri S, et al. Domains of chronic stress, lifestyle factors, and allostatic load in middle-aged Mexican-American women. Ann Behav Med. 2011;41(1):21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shivpuri S, Gallo LC, Crouse JR, Allison MA. The association between chronic stress type and C-reactive protein in the Multi-Ethnic Study of Atherosclerosis: does gender make a difference? J Behav Med. 2012;35(1):74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallo LC, Roesch SC, Fortmann AL, et al. Associations of chronic stress burden, perceived stress, and traumatic stress with cardiovascular disease prevalence and risk factors in the Hispanic Community Health Study/Study of Latinos Sociocultural Ancillary Study. Psychosom Med. 2014;76(6):468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogilvie RP, Everson-Rose SA, Longstreth WT Jr., et al. Psychosocial factors and risk of incident heart failure: the Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2016;9(1):e002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang YC, Schorpp K, Harris KM. Social support, social strain, and inflammation: evidence from a national longitudinal study of U.S. adults. Soc Sci Med. 2014;107:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seeman TE, Gruenewald TL, Cohen S, et al. Social relationships and their biological correlates: Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychoneuroendocrinology. 2014;43:126–138. [DOI] [PubMed] [Google Scholar]
- 62.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychol Measure. 1977;1(3):385–401. [Google Scholar]
- 63.Krousel-Wood M, Joyce C, Holt EW, et al. Development and evaluation of a self-report tool to predict low pharmacy refill adherence in elderly patients with uncontrolled hypertension. Pharmacotherapy. 2013;33(8):798–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steiner JF, Ho PM, Beaty BL, et al. Sociodemographic and clinical characteristics are not clinically useful predictors of refill adherence in patients with hypertension. Circ Cardiovasc Qual Outcomes. 2009;2(5):451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Franklin JM, Shrank WH, Lii J, et al. Observing versus predicting: initial patterns of filling predict long-term adherence more accurately than high-dimensional modeling techniques. Health Serv Res. 2016;51(1):220–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Librero J, Sanfelix-Gimeno G, Peiro S. Medication adherence patterns after hospitalization for coronary heart disease. A population-based study using electronic records and group-based trajectory models. PLoS One. 2016;11(8):e0161381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Celano M, Geller RJ, Phillips KM, Ziman R. Treatment adherence among low-income children with asthma. J Pediatr Psychol. 1998;23(6):345–349. [DOI] [PubMed] [Google Scholar]
- 68.Silverman J, Krieger J, Kiefer M, et al. The relationship between food insecurity and depression, diabetes distress and medication adherence among low-income patients with poorly controlled diabetes. J Gen Intern Med. 2015;30(10):1476–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Surratt HL, O’Grady CL, Levi-Minzi MA, Kurtz SP. Medication adherence challenges among HIV positive substance abusers: the role of food and housing insecurity. AIDS Care. 2015;27(3):307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mehta S, Moore RD, Graham NM. Potential factors affecting adherence with HIV therapy. AIDS. 1997;11(14):1665–1670. [DOI] [PubMed] [Google Scholar]
- 71.Wroth TH, Pathman DE. Primary medication adherence in a rural population: the role of the patient-physician relationship and satisfaction with care. J Am Board Fam Med. 2006;19(5):478–486. [DOI] [PubMed] [Google Scholar]
- 72.Vranceanu AM, Safren SA, Lu M, et al. The relationship of post-traumatic stress disorder and depression to antiretroviral medication adherence in persons with HIV. AIDS Patient Care STDS. 2008;22(4):313–321. [DOI] [PubMed] [Google Scholar]
- 73.Farrell SP, Hains AA, Davies WH, et al. The impact of cognitive distortions, stress, and adherence on metabolic control in youths with Type 1 diabetes. J Adolesc Health. 2004;34(6):461–467. [DOI] [PubMed] [Google Scholar]
- 74.Hains AA, Berlin KS, Davies WH, et al. Attributions of adolescents with Type 1 diabetes in social situations: relationship with expected adherence, diabetes stress, and metabolic control. Diabetes Care. 2006;29(4):818–822. [DOI] [PubMed] [Google Scholar]
- 75.Federspiel JJ, Sueta CA, Kucharska-Newton AM, et al. Antihypertensive adherence and outcomes among community-dwelling Medicare beneficiaries: the Atherosclerosis Risk in Communities study. J Eval Clin Pract. 2018;24:48–55. [DOI] [PubMed] [Google Scholar]
- 76.Arbuckle C, Tomaszewski D, Aronson BD, et al. Evaluating factors impacting medication adherence among rural, urban, and suburban populations. J Rural Health. 2018;34(4)339–346. [DOI] [PubMed] [Google Scholar]
- 77.Rolnick SJ, Pawloski PA, Hedblom BD, et al. Patient characteristics associated with medication adherence. Clin Med Res. 2013;11(2):54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tran HV, Waring ME, McManus DD, et al. Underuse of effective cardiac medications among women, middle-Oates et al aged adults, and racial/ethnic minorities with coronary artery disease (from the National Health and Nutrition Examination Survey 2005 to 2014). Am J Cardiol. 2017;120(8):1223–1229. [DOI] [PubMed] [Google Scholar]
- 79.Albright KC, Zhao H, Blackburn J, et al. Racial differences in statin adherence following hospital discharge for ischemic stroke. Neurology. 2017;88(19):1839–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Go AS, Fan D, Sung SH, et al. Contemporary rates and correlates of statin use and adherence in nondiabetic adults with cardiovascular risk factors: the KP CHAMP study. Am Heart J. 2017;194:25–38. [DOI] [PubMed] [Google Scholar]
- 81.Lauffenburger JC, Robinson JG, Oramasionwu C, Fang G. Racial/ethnic and gender gaps in the use of and adherence to evidence-based preventive therapies among elderly Medicare Part D beneficiaries after acute myocardial infarction. Circulation. 2014;129(7):754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Epps P, Maier M, Lund B, et al. Medication adherence in a nationwide cohort of veterans initiating pre-exposure prophylaxis (Prep) to prevent HIV infection. J Acquir Immune Defic Syndr. 2018;77(3):272–278. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y, Baik SH. Race/ethnicity, disability, and medication adherence among Medicare beneficiaries with heart failure. J Gen Intern Med. 2014;29(4):602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28(4):437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yeaw J, Benner JS, Walt JG, et al. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15(9):728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jerant A, DiMatteo R, Arnsten J, et al. Self-report adherence measures in chronic illness: retest reliability and predictive validity. Med Care. 2008;46(11):1134–1139. [DOI] [PubMed] [Google Scholar]
- 87.Cecere LM, Slatore CG, Uman JE, et al. Adherence to long-acting inhaled therapies among patients with chronic obstructive pulmonary disease (COPD). COPD. 2012;9(3):251–258. [DOI] [PubMed] [Google Scholar]
- 88.Burge S, White D, Bajorek E, et al. Correlates of medication knowledge and adherence: findings from the residency research network of South Texas. Fam Med. 2005;37(10):712–718. [PubMed] [Google Scholar]
- 89.Modi AC, Quittner AL. Barriers to treatment adherence for children with cystic fibrosis and asthma: what gets in the way? J Pediatr Psychol. 2006;31(8):846–858. [DOI] [PubMed] [Google Scholar]
- 90.Voils CI, Hoyle RH, Thorpe CT, et al. Improving the measurement of self-reported medication nonadherence. J Clin Epidemiol. 2011;64(3):250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]


