Abstract
The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and Diagnostic Medicine and Therapeutic Pathology. For additional information, and a full list of learning objectives for all three competencies, see http://journals.sagepub.com/doi/10.1177/2374289517715040.1
Keywords: pathology competencies, diagnostic medicine, microbiology, parasitology, babesiosis, malaria, transfusion-transmitted infections
Primary Objective
Objective M6.4: Malaria and Babesiosis. Contrast Plasmodium falciparum with other malaria species and babesiosis on a blood smear and explain the role of thick and thin smears in the diagnosis and management of babesiosis and malaria.
Competency 3: Diagnostic Medicine and Therapeutic Pathology; Topic M: Microbiology; Learning Goal 6: Parasitology.
Secondary Objective
Objective TM1.3: Infectious Risks. Discuss infectious disease risks of transfusion.
Competency 3: Diagnostic Medicine and Therapeutic Pathology; Topic TM: Transfusion Medicine; Learning Goal 1: Concepts of Blood Transfusion.
Patient Presentation
A 68-year-old woman presents to the emergency department on a weekend in July, complaining of 6 days of waxing and waning fever (Tmax 102-104°F), night sweats, and myalgias. She traveled to Haiti this past March and to a Wisconsin farm for 5 days in June. Her other vital signs were within normal limits, and the rest of the physical examination was unremarkable. The patient’s past medical history was notable for hypertension, venous insufficiency, diverticulosis, and a benign heart murmur “for years.”
Diagnostic Findings
Complete blood count (CBC) showed a white blood cell count of 7.4 K/µL (4.2-9.1 K/μL) with 82% neutrophils and 5% bands. Lactate was elevated at 1.3 mmol/L (0.5-1.0 mmol/L). Basic metabolic panel (BMP) and hepatic panel were normal. A computed tomography scan and echocardiogram were unremarkable. A peripheral blood smear was performed (Figure 1). Laboratory serology testing ultimately confirmed the diagnosis from the smear (Table 1).
Figure 1.
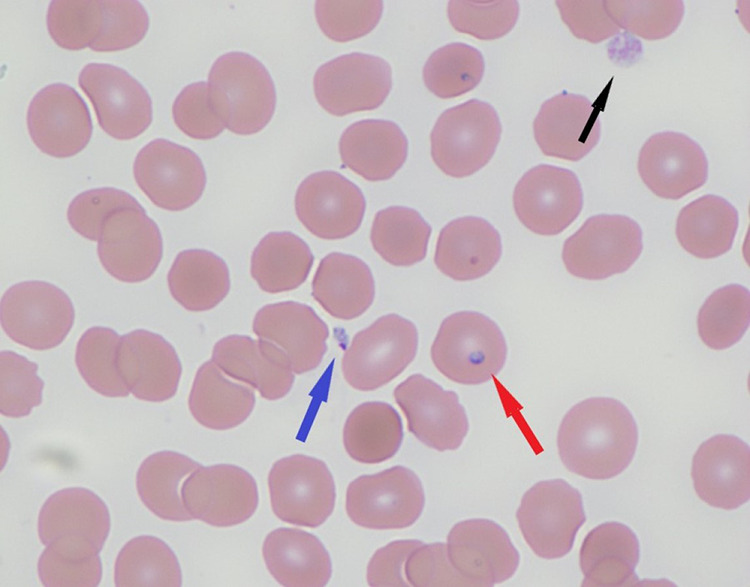
Peripheral blood smear, thin region (Giemsa stain, ×1000). Black arrow, normal platelet. Red arrow, intraerythrocytic trophozoite of Babesia microti. Blue arrow, extraerythrocytic trophozoite of B microti.
Table 1.
Laboratory Results.
| Test | Result |
|---|---|
| Peripheral blood smear | Positive for ring forms (1.1% parasitemia) |
| IFA, malaria | Negative |
| PCR RNA, Babesia microti | Positive |
| Blood culture | No growth |
Abbreviations: IFA, indirect fluorescent antibody; PCR, polymerase chain reaction.
Questions/Discussion Points
Given the Patient’s Presentation, What Is the Differential Diagnosis?
The initial presentation favors an infectious etiology. A waxing and waning fever after recent travel to a tropical area suggests malaria, though other infections may present with a similar clinical picture. Babesia should be considered given the patient’s presenting symptoms, the time spent on a farm in Wisconsin, and the time of year (June). Tuberculosis and HIV are also on the differential diagnosis in addition to potential zoonotic exposures such as Brucella, Francisella, and Erysipelothrix during the visit to the Wisconsin farm. Solid tumors as well as leukemias and lymphomas could present with a fever; however, other symptoms such as weight loss and fatigue are often present. Autoimmune diseases such as lupus erythematosus and other connective tissue disorders could present with fevers, but usually present earlier in life. Additionally, other symptoms, such as joint pain and fatigue, are usually present at diagnosis. Her self-reported chronic medical conditions may contribute to symptoms, but they would not cause a fever.
Which Diagnostic Laboratory Tests Would You Order on Admission?
The patient was medically stable. An initial panel should assess the acuity of her illness and rule out life-threatening infection and malignancy. Tests should include fingerstick glucose, electrocardiogram, CBC w/differential, BMP, lactate (included in venous or arterial blood gases), blood culture, and potentially testing for HIV. Erythrocyte sedimentation rate, liver function tests, and imaging studies may be considered.
Why Was a Peripheral Blood Smear Performed?
In most laboratories, an automated analyzer will perform the CBC. If there is a significant abnormality flagged by the analyzer, a slide is made from the sample and reviewed under the microscope by the laboratory technologist. In this patient, the analyzer flagged a “left shift” indicating that there were a high number of immature granulocytes, such as band neutrophils, metamyelocytes, myelocytes, and promyelocytes. This finding can occur as a response to an inflammatory or infectious process or in certain hematologic malignancies. Depending on the abnormalities on the smear, a pathologist may review the smear for abnormal cell maturation and morphology or microorganisms.
How Would You Describe the Blood Smear? Can an Organism (or Organisms) Be Identified?
Peripheral smears have thick and thin regions, which is inherent to the way the sample is applied to the glass slide. Smears should be read where the red blood cells (RBCs) are evenly distributed and not quite touching each other. Figure 1 is from the thin part with evenly dispersed RBCs. It shows mature erythrocytes. There is one normal platelet present (black arrow). One otherwise normal-appearing red blood cell has a ring-like basophilic structure inside (red arrow). This is a trophozoite or ring form of Babesia. There is also an extracellular structure with a purplish dot and blue cytoplasm present in this smear (blue arrow). This is an extracellular or extraerythrocytic trophozoite. These can sometimes be mistaken for platelets.
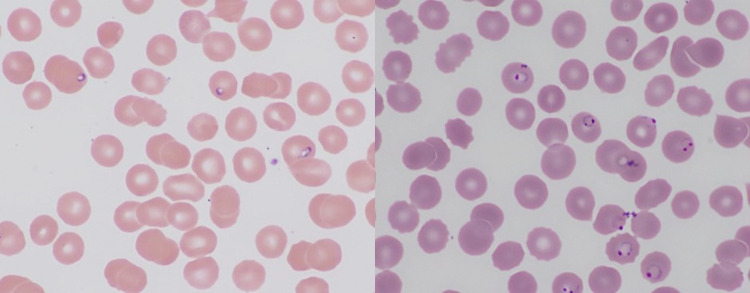
The life cycle of Babesia that is observed in the peripheral blood consists of trophozoites infecting RBCs. These will mature into merozoites. The life cycle of P falciparum present in peripheral blood consists of trophozoites which can mature into either schizonts or gametocytes, although generally only trophozoites are observed. It can be difficult to distinguish the early trophozoite stage of P falciparum from Babesia microti. Other Plasmodium and Babesia species are different sizes and have different morphologies. Only B microti shows tetrad (Maltese cross) forms, which are rarely observed merozoites that form after trophozoites mature. One of the features that distinguishes Babesia from Plasmodium is the presence of extracellular or extraerythrocytic trophozoites in Babesia (Figure 1, blue arrow); malaria parasites are always found inside RBCs. The trophozoites of Babesia are more pleomorphic than P falciparum and can appear vacuolated. Plasmodium falciparum trophozoites are delicate and uniform with occasional double-chromatin dot forms (sometimes called headphone forms) and applique or marginal forms.2,3 The marginal forms are found at the edge of the RBC and may appear to be applied to the periphery of the cell. Figure 2 demonstrates representative peripheral smears with Babesia microti and P falciparum.
Figure 2.
Left: Babesia microti in peripheral blood smear. Several red blood cells (RBCs) with small, vacuolated trophozoites are present. Right: Plasmodium falciparum in peripheral blood smear. Several headphones forms (trophozoites with double chromatin dot) and marginal or applique forms (trophozoites at the periphery of the RBC, sometimes appearing to follow the curvature of the cell) are present.
What Is the Most Likely Diagnosis?
The most likely diagnosis is clinical babesiosis from infection with B microti, a protozoa endemic to the Northeast and upper Midwestern United States. Incubation can take several weeks. Patients may be asymptomatic or manifest severe malaria-like symptoms (fever, chills, myalgias), anemia, and thrombocytopenia, requiring extended hospitalization. Patients with significant comorbidities and immunocompromised patients may experience more severe disease.4-6 Clinicians should not rule out babesiosis even in patients from nonendemic areas.7 Climate change is altering the classic geographic and seasonal distribution of tick-borne diseases. There may be a relevant travel history or history of transfusion with a blood component from a donor in another region, and a thorough travel history should be part of the workup.
What Is the Pathogenesis of Infection?
Babesia species are intraerythrocytic pathogens transmitted to humans by black-legged deer ticks (Ixodes scapularis or Ixodes pacificus in the United States). The deer tick vector may carry other organisms, and coinfections are possible (eg, Lyme disease, ehrlichiosis, human granulocytic anaplasmosis).8
How Would You Confirm This Diagnosis?
Confirmatory tests include serology (immunoglobulin G). Additional tests include enzyme immunoassays (EIA/enzyme-linked immunosorbent assay), nucleic acid amplification tests, and real-time polymerase chain reaction (RT-PCR). Additionally, thick smears should be performed along with the thin smears. In the thick smear, a drop of blood is applied to a slide and spread in a circle over a centimeter of the slide. This results in a thick amount of blood being applied to that area of the slide. The RBCs are lysed and the thickness of the lysed material allows for more of the contents of the RBCs to be examined compared to the thin smear where an even monolayer of RBCs is examined. The thick smear will aid the diagnosis of blood parasites when the level of parasitemia is particularly low, although it does not allow for morphologic speciation. Rapid antigen tests are commercially available for malaria and can be particularly useful for laboratories without expertise in identifying blood parasites on a peripheral smear or during off hours, when a parasitologist may not be available. There are not yet any commercially available rapid tests for the diagnosis of babesiosis. If the hospital does not perform testing in-house, commercial and public health reference laboratories do.9
How Is Babesiosis Treated?
Babesiosis is treated with atovaquone with azithromycin or clindamycin with quinine.2,3 This patient received a 3-day inpatient course of intravenous malarone (atovaquone/proguanil). After discharge, she took daily atovaquone and azithromycin with acetaminophen for her fever. A week later, her CBC and BMP were within normal limits. The patient was monitored as an outpatient with no complications. A year after her illness, the patient notices a blood drive seeking healthy volunteer donors.
Which Aspects of Her Clinical History Might Affect Her Eligibility to Donate Blood?
A history of clinical babesiosis does not exclude the patient from the donor pool, though blood collection centers may defer these patients from donating blood.10 This woman’s recent travel to Haiti, where she may have had been exposed to other infections such as malaria, does exclude her. Donors with a history of malaria are deferred for 3 years following treatment until they are free of symptoms. Those who resided in endemic areas receive 3-year deferrals. Asymptomatic travelers to endemic areas are deferred for 1 year.
How Is the Risk of Infection From Blood Products Reduced? For Which Infections Are Donors Routinely Screened?
In the United States, blood products are routinely screened for HIV, hepatitis B virus, and hepatitis C virus.11 However, there is currently no universal screening for Babesia. There are also other arboviruses (ie, arthropod-borne) including chikungunya, dengue, West Nile, and Zika that can be transmitted via transfusion.11,12 Other strategies for reducing infectious risk include pathogen inactivation and pathogen reduction. Blood products are pretreated using different strategies in order to inactivate or reduce the organisms present. The RBC units can also be leukoreduced to remove most white blood cells from a donated unit, thus reducing the risk of cytomegalovirus infection for the transfusion recipient.
Parasites like Babesia cause a dilemma for blood collection centers. Blood products contaminated with B microti have the potential to case life-threatening infection in critically ill patients.13 Blood collection centers may use screening questionnaires to determine a donor’s risk of Babesia infection. However, donors in endemic areas may not realize that they have been exposed, and some blood collection centers in endemic regions are now routinely screening for Babesia in their products.14 However, screening is expensive and requires specimen pooling, so the risk to the population must be balanced with the cost of this additional testing. There is also no consensus on how to monitor treated patients for reentry into the donor pool as there is for malaria infection or travel to a malaria endemic area.14 The US Food and Drug Administration Blood Products Advisory Committee is considering how to implement screening protocols.
What Are Risk Factors for Transfusion-Transmitted Infections?
Risk factors for transfusion-transmitted infections include age extremes (infancy and over 50 years), malignancy, HIV, chronic immunosuppressant use, hemoglobinopathies, and chronic cardiopulmonary and liver disease. Asplenic patients may show symptoms of clinical babesiosis at very low parasite burdens.15 Babesia microti is the most common transfusion-transmitted pathogen in the United States. It is the leading cause of transfusion-related infectious disease mortality in recipients. There have been at least 160 cases in the United States over the last 40 years, a fifth of them fatal.16-18 It is estimated that 1:300 units in endemic regions contain B microti. However, only 1:18 000 to 1:100 000 people receiving units from endemic regions will contract clinical babesiosis (Table 2).
Table 2.
Risk of Transfusion-Transmitted Infections.11
| Pathogen | Test(s) | Positive donor screen | Transfusion-transmitted infection risk |
|---|---|---|---|
| Trypanosoma cruzi | Ab | 1:15 000 | |
| HBV | HBsAg; anti-HBc; NAT/DNA |
1:12 000 | 1:800 000-1 000 000 |
| HCV | NAT Mini-pool |
1:5000 | 1:1 000 000 |
| HIV ½ | NAT; Ab mini-pool | 1:33 000 (HIV-1; only 5 total HIV-2 units screened since 199211) |
1: 1 000 000 (HIV-1) |
| HTLV I/II | Combined anti-HTLV-I/II Ab; Western blot (confirmatory) | 1:27 000 (HTLV-II slightly more common) | <1: 2 000 000 |
| Treponema pallidum | Ab | No known cases >50 years | |
| Zika virus | NAT (screen); Ab (confirmatory) | To be determined | To be determined |
| West Nile virus | NAT mini-pool | 3500 total (2003-2016) | 14 total from screened blood (low viral loads), or 1:84 million donations |
| Babesia microti | NAT/PCR; Western blot IFA |
1:300 (endemic) | 1:18 000-1:100 000 (endemic) |
| Malaria | <1:1,000 000 |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; IFA, indirect fluorescent antibody; PCR, polymerase chain reaction; HTLV, Human T-Cell Leukemia Virus; Ab, Antibody.
In general, the risk of transfusion-transmitted infection for commonly screened pathogens is low (Table 2). Confirmation of clinical cases requires evidence of transfusion with an infected unit as well as clinical and laboratory results consistent with infection. Epidemiologists take into account the plausibility of infection via transfusion of a contaminated unit versus environmental exposure to the pathogen. In the course of an investigation of a transfusion-transmitted infection, multiple donated units may be infected, requiring investigation into all blood products from the specific donor. Most cases of transfusion-transmitted babesiosis are caused by contaminated red blood cell units as B microti is an intraerythrocytic parasite. However, other components, including platelets, have been linked to adverse events due to babesiosis.19-23
What Qualities Make an Ideal Screening Test for a Transfusion-Transmitted Disease?
Good screening tests are rapid, accessible, and cost-effective. A screening test should have high sensitivity in order to avoid false-negative results. A confirmatory test should be highly specific in order to provide a high rate of true positivity. Babesia antibody screens may yield false positives, either from near ubiquitous exposure in endemic areas or persistent postinfection titers. These assays also do not reliably identify patients in antibody-negative “window periods” and cannot distinguish recent from past infection. Donor units that test positive on sensitive EIA may test negative on more specific PCR tests. The rate of positive screens may be affected by regional incidence and collection day and time.24-26 Commercial tests licensed to diagnose clinical babesiosis are being piloted for use in donor screening.27 Newer tests assess parasite burden, clearance (antibody kinetics), and seroconversion status.28
Teaching Points
Babesia microti is a tick-borne parasite endemic to the Northeast and upper Midwestern United States. Clinical babesiosis is usually asymptomatic but may be life-threatening.
Symptoms of clinical babesiosis may mimic malaria. Symptoms can include malaise, fatigue, chills, fever, headache, myalgias, and arthralgias. Laboratory abnormalities include mild to moderately severe hemolytic anemia, mild neutropenia, and thrombocytopenia.
Laboratory diagnosis of Babesia infection is initially performed by microscopic review of thick and thin smears. The early trophozoite or “ring” form of Babesia microti resembles P falciparum on peripheral smear. Confirmation can be performed by serology and PCR.
Transfusion-transmitted babesiosis is the most common and most fatal infectious disease in the US blood supply, with 1:300 donors screening positive in endemic regions. There is currently no universal screening of blood products for Babesia in the United States.
Transfusion-transmitted infections remain a significant public health burden even after the adoption of universal donor screening, with high morbidity and mortality.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Amy Rapkiewicz  https://orcid.org/0000-0002-3438-022X
https://orcid.org/0000-0002-3438-022X
References
- 1. Knollman-Ritschel BEC, Regula DP, Borowitz MJ, Conran R, Prystowsky MB. Pathology competencies for medical education and educational cases. Acad Pathol. 2017:4 doi:10.1177/2374289517715040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC). Global Health Division of Parasitic Diseases. TickNET. 2007. Accessed April 18, 2019 https://www.cdc.gov/ticknet/index.html
- 3. Centers for Disease Control and Prevention (CDC). Global Health Division of Parasitic Diseases. DPDx—Laboratory Identification of Parasites of Public Health Concern. Babesiosis. 2017. Accessed April 18, 2019 https://www.cdc.gov/dpdx/babesiosis/index.html
- 4. Vannier E, Krause PJ. Human Babesiosis. N Engl J Med. 2012;366:2397–2407. [DOI] [PubMed] [Google Scholar]
- 5. Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am. 2015;29:357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krause PJ, McKay K, Gadbaw J, et al. Increasing health burden of human babesiosis in endemic sites. Am J Trop Med Hyg. 2003;68:431–436. [PubMed] [Google Scholar]
- 7. Ord RL, Lobo CA. Human babesiosis: pathogens, prevalence, diagnosis, and treatment. Curr Clin Micro Rpt. 2015;2:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kowalski TJ, Jobe DA, Dolan EC, Kessler A, Lovrich SD, Callister SM. The emergence of clinically relevant babesiosis in southwestern Wisconsin. WMJ. 2015;114:152–157. [PubMed] [Google Scholar]
- 9. Diuk-Wasser MA, Vannier E, Krause PJ. Coinfection by ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends Parasitol. 2016;32:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Connolly NP, Hinckley AF, Feldman KA, et al. Testing practices and volume of non-Lyme tick borne diseases in the United States. Ticks Tick Borne Dis. 2016;7:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Infectious Disease Testing. American Red Cross. 2020. Accessed April 18, 2019 https://www.redcrossblood.org/biomedical-services/blood-diagnostic-testing/blood-testing.html
- 12. Levin AE, Krause PJ. Transfusion-transmitted babesiosis: is it time to screen the blood supply? Curr Opin Hematol. 2016;23:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Villatoro T, Karp JK. Transfusion-transmitted babesiosis. Arch Pathol Lab Med. 2019;143:130–134. [DOI] [PubMed] [Google Scholar]
- 14. Stramer SL. Current perspectives in transfusion-transmitted infectious diseases: emerging and re-emerging infections. ISBT Sci Ser. 2014;9:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cushing M, Shaz B. Transfusion-transmitted babesiosis: achieving successful mitigation while balancing cost and donor loss. Transfusion. 2012;52:1404–1407. [DOI] [PubMed] [Google Scholar]
- 16. Menis M, Forshee RA, Kumar S, et al. Babesiosis occurrence among the elderly in the United States, as recorded in large medicare databases during 2006–2013. PLoS ONE. 2015;10:e0140332 doi:10.1371/journal.pone.0140332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fang DC, McCullough J. Transfusion-Transmitted Babesia microti . Transfus Med Rev. 2016;30:132–138. [DOI] [PubMed] [Google Scholar]
- 18. Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med. 2011;155:509–519. [DOI] [PubMed] [Google Scholar]
- 19. Lobo CA, Cursino-Santos HR, Alhassan A, Rodrigues M. Babesia: an emerging infectious threat in transfusion medicine. PLoS Pathog. 2013;9:e1003387 doi:10.1371/journal.ppat.1003387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bloch EM, Lee TH, Krause PJ, et al. Development of a real-time polymerase chain reaction assay for sensitive detection and quantitation of Babesia microti infection. Transfusion. 2013;53:2299–2306. [DOI] [PubMed] [Google Scholar]
- 21. Bloch EM, Levin AE, Williamson PC, et al. A prospective evaluation of chronic Babesia microti infection in seroreactive blood donors. Transfusion. 2016;56:1875–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cornillot E, Dassouli A, Pachikara N, et al. A targeted immunomic approach identifies diagnostic antigens in the human pathogen Babesia microti . Transfusion. 2016;56:2085–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levin AE, Williamson PC, Erwin JL, et al. Determination of Babesia microti seroprevalence in blood donor populations using an investigational enzyme immunoassay. Transfusion. 2014;54:2237–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levin AE, Williamson PC, Bloch EM, et al. Serologic screening of United States blood donors for Babesia microti using an investigational enzyme immunoassay. Transfusion. 2016;56:1866–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bish EK, Moritz ED, El-Amine H, Bish DR, Stramer SL. Cost-effectiveness of a Babesia microti blood donation intervention based on real-time prospective screening in endemic areas of the United States. Transfusion. 2016;56:775–777. [DOI] [PubMed] [Google Scholar]
- 26. Bish EK, Moritz ED, El-Amine H, Bish DR, Stramer SL. Cost-effectiveness of Babesia microti antibody and nucleic acid blood donation screening using results from prospective investigational studies. Transfusion. 2015;55:2256–2271. [DOI] [PubMed] [Google Scholar]
- 27. O’Brien SF, Delage G, Scalia V, et al. Seroprevalence of Babesia microti infection in Canadian blood donors. Transfusion. 2016;56:237–243. [DOI] [PubMed] [Google Scholar]
- 28. Teal AE, Habura A, Ennis J, Keithly JS, Madison-Antenucci S. A new real-time PCR assay for improved detection of the parasite Babesia microti. J Clin Microbiol. 2012;50:903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]