Abstract
Introduction:
Resveratrol (RES) exhibits estrogen-like effects and has potential applications to treatment of osteoporosis caused by estrogen deficiency; however, the specific mechanism of action of RES remains unclear. Here, we examined the therapeutic effects of RES on ovariectomized (OVX) rats with osteoporosis and determined the underlying mechanism.
Methods:
We established an OVX rat model to study osteoporosis caused by estrogen deficiency. The treatment groups were given orally with RES (50, 100, and 200 mg/day), the estrogen group received 0.8 mg/kg E2 daily via oral route, and the sham-operated and control groups received an equivalent dose of sodium carboxymethylcellulose orally. After 12 weeks of treatment, we used real-time quantitative polymerase chain reaction (PCR) and Western blot analysis to measure the gene and protein expression of miR-92b-3p, Nox4, NF-κBp65, IκB, BMP2, Smad7, and RUNX-2 in bone tissues. Right femur structural parameters were evaluated by micro-CT. Dual-energy X-ray 4500 W was used to determine systemic bone mineral density (BMD). Enzyme-linked immunosorbent assay (ELISA) kits were used to determine the serum levels of bone alkaline phosphatase (BALP), osteoprotegerin (OPG), anti-tartrate acid phosphatase-5b (PTRA5b), and carboxylated terminal peptide (CTX-I). The rat femoral bone specimens were stained using hematoxylin and eosin for pathological examination
Results:
We observed increased levels of serum estrogen in both ovaries, elevated miR-92b-3p levels in bone tissues, reduced levels of Nox4, NF-κBp65, p-IκB-a, and cathepsin K, and elevated gene and protein expression of BMP2, Smad7, and RUNX-2 in the OVX rat model of osteoporosis after treatment with RES. Elevated levels of BALP, OPG, ALP, and BMD along with reduced levels of TRAP-5b and CTX-I were also observed. The structural model index (SMI) and the trabecular space (Tb. Sp) decreased, while the trabecular thickness (Tb. Th), bone volume fraction (BV/TV), trabecular number (Tb.N), and tissue bone density (Conn.D) increased, thereby improving osteoporosis induced by estrogen deficiency in both ovaries.
Conclusion:
Cathepsin K expression and Nox4/NF-κB signaling pathway were suppressed by the elevated expression of miR-92b-3p. This inhibition was pivotal in the protective effect of RES against osteoporosis induced by estrogen deficiency in both ovaries. Thus, RES efficiently alleviated osteoporosis induced by estrogen deficiency in rats.
Keywords: miR-92b-3p, NF-κBp65, Nox4, osteoporosis, resveratrol
Introduction
Bone remodeling, an important process of bone formation and metabolism, is regulated by a delicate equilibrium between bone synthesis and bone resorption and controlled by osteoblasts and osteoclasts.1 Long-term and excessive bone resorption breaks this balance, leading to increased bone resorption and consequent conditions, such as osteoporosis. Excessive bone resorption by osteoclasts is critical for development of osteoporosis.1 Osteoporosis in ovariectomized rats presents many similar pathophysiological features to postmenopausal osteoporosis in humans; in this regard, ovariectomized rats could be a good model of osteoporosis and have been widely studied.2,3 Reduced bone mineral density (BMD) and damaged bone microstructures after ovariectomy in mice are suitable models for study of post-menopausal osteoporosis.4 Pharmacological therapy for osteoporosis includes drugs that either promote bone synthesis or delay bone resorption; however, some of these drugs, such as bisphosphonates5,6 and estrogens7,8 elicit adverse effects.
Resveratrol (RES, 3, 5, 4′-trihydroxy diphenylene) is a natural polyphenolic compound with a structure similar to estrogen diethylstilbestrol and is widely found in grapes, peanuts, canopy, and so on.9 RES could competitively bind to estrogen receptors in vitro and exert estrogen-like effects; as such, RES is considered a phytoestrogen.9 RES also exhibits several important bioactivities, such as estrogen-like regulatory lipid function, anti-platelet aggregation, anti-oxidation, and anti-tumor.9–12 RES could effectively prevent bone loss, as indicated by the in vitro experiments in rats, which lost bone mass due to lack of physical activity and estrogen deficiency.13 RES inhibits the synthesis of reactive oxygen species, resulting in an increase in the alkaline phosphatase levels and proline hydroxylase activity in MC3 T3-E1 cells and prevention of the differentiation of RAW264.7 cells into osteoclasts.14,15 However, the mechanism of action of RES remains unclear and needs further analysis.
MicroRNAs (miRNAs) are noncoding, endogenous, and single-stranded RNAs (18–25 base) that primarily inhibit their target genes at post-transcriptional levels.16 The results of our previous study on mesenchymal stem cells (MSCs) revealed that miRNAs are important regulators of osteoblast differentiation.16 Induction of osteoblast differentiation in MSCs resulted in downregulated levels of miR-27a, miR-489, and miR-148b.17,18 Inhibition of miR-138 might promote the expression of alkaline phosphatase (ALP) genes in osteoblastic processes in vitro and enhance the osteoblastic ability of MSCs.19 MicroRNA-874 might enhance the osteoblastic ability of MSCs20 by inhibiting the expression of the target gene SUFU.20 Recent studies suggest that miR-92b-3p might promote osteoblastic differentiation in human umbilical cord stem cells (hCMSCs),21 but the underlying mechanism remains unclear.
Nox4 is mainly secreted by osteoclasts, and its activation induces osteoclast differentiation and maturation; thus, promoting bone reabsorption and subsequently leading to osteoporosis.22 A study on Nox4 knockout mice showed elevated bone density and depleted count of osteoclasts and their markers. Osteoclast activity and Nox4 expression increased in the bone of patients with osteoporosis.23 High-fat diet can cause high expression of Nox4 in C57BL/6 J mice, leading to the high expression of NF-κB-p65.24
A previous work found that RES modulated the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1), and NADPH oxidase subunit (Nox4) and reduced endothelial oxidative stress.25 In this experiment, we predicted using www.targetscan.org that Nox4 is a possible regulatory target of miR-92b-3p. Thus, we postulated that miR-92b-3p promoted the development of osteoporosis in an ovariectomized rat model. We also determined whether RES could inhibit the disease by regulating the Nox4/NF-κB signal pathway. Results reveal the mechanism of RES and provide theoretical basis for its use in treatment of osteoporosis induced by estrogen deficiency.
Materials and methods
Animals
Adult Sprague–Dawley (SD) rats (female; age: 6 months (n = 48) and 2 months (n = 8)) were purchased from Kunming Medical University’s Institutional Animal Care and Use Committee. The animals were acclimatized to standard specific pathogen-free laboratory conditions (12 h light–dark cycle; temperature: 23 C ± 2°C) with food/water ad libitum for 7 days. The study followed the recommendations made by the “Institutes of Health Guidelines for the Use of Laboratory Animals.” The animal ethics committee of the Third People’s Hospital of Yunnan Province approved the study protocol (approval number: KYYM-2016-008-F17), which was performed following the institutional ethical guidelines. The animals were treated humanely consistent with the relevant national, international, and/or institutional guidelines and legislations.
Chemicals and reagents
RES (Solarbio Life Sciences, Beijing, China) and 17β estradiol (Shanghai Yuanye Biological Technology Co. Ltd., China) were used for the following experimental procedures.
Isolation of rat bone marrow mesenchymal stem cells (BMSCs)
Femurs were dissected from SD rats (age: 2 months; n = 8) following a previously described protocol.26 The rats were euthanized by injecting pentobarbital sodium (40 mg/kg) interperitoneally. We then collected their bilateral tibia and femur and removed muscle ligaments attached to the femurs and tibias head end. The femur was inserted into the marrow cavity by using a 1 mL syringe. A 5 mL syringe was used to flush out the bone marrow cells by using phosphate-buffered saline (PBS). We isolated mononucleated cells (1.091 g/mL) in lymphocyte cell separation media by using density gradient centrifugation. The isolated cells were cultured in standard low-glucose Dulbecco’s modified Eagles medium (DMEM) containing 15% fetal bovine serum (FBS) at 37°C in 5% CO2 atmosphere until 80% confluency. Fresh culture media were added every 3 days. The confluent cells were trypsinized and re-plated as passage#1. We used passage#3 cells in this study.
Cell culture
The in vitro effects of miR-92b-3p on osteoporosis were evaluated using BMSCs. The harvested BMSCs were cultured at 37°C by using DMEM containing 5% FBS, penicillin–streptomycin (100 U/mL each), and amphotericin B (50 µg/L) in 5% CO2 incubator until they reached subconfluency.
Transfection of miRNA mimics, miRNA inhibitors, and genes
For transfection, serum-free media were used to prepare diluted solutions of miR-92b-3p mimic (miR-92b-3p-M; 50 nM) and miR-92b-3p inhibitor (miR-92b-3p-I; 100 nM). The mimic, inhibitor, and their respective negative controls (NCs) were added individually to Lipofectamine 2000 (50-fold diluted) and then added to 40% confluent BMSCs following a previously described method.17,20 At 24 h post-transfection, we obtained total RNA and proteins, which were stored for qRT-polymerase chain reaction (PCR) and Western blot analyses.
Gene prediction of miR-92b-3p target and dual-luciferase reporter assay
Probable miR-92b-3p targets were determined using miRBase (database) along with Target Scan and PicTar (software). BMSCs (1 × 105) were cultured and transfected with: Nox4-3′UTR-wt, Nox4-3′ UTR-mt, mi-128, or mi-NC. After 24 h, we used the Dual Luciferase Reporter Assay System to measure luciferase activity by using the Renilla luciferase activity as the reference.
MTT assay
BMSCs (2 × 104/mL) were inoculated into 96-well plates, and 200 μL of each well was mixed and incubated for 24 h. We added RES (0, 5, 10, and 20 μL) to the wells and incubated for 72 h. After adding MTT (50 μL×/well), the plate was incubated for 4 h. After adding dimethyl sulfoxide (DMSO) (150 μL), absorbance was recorded at 550 nm. Finally, cellular inhibition rate was calculated using the following formula: (cell inhibition rate (%) = 1—(OD experimental group−OD blank group)/(OD control group−OD blank group) ×100).
In vitro osteoclastogenesis assay
After isolation from the femoral cavity of SD rats,27 bone marrow–derived macrophages (BMMs) were cultured in low-glucose DMEM containing FBS and a mixture of antibiotics. After removing the cell medium containing the non-adherent cells (72 h), we cultured the adherent colonies for 2 weeks and subcultured. The third-generation BMM cultures were added to 48-well plates (2.0 × 104 cells/well) and designated as the control group and RES treatment groups (0, 5, 10, and 20 µg/mL). Macrophage colony-stimulating factor (M-CSF) along with the receptor activator of nuclear factor kappa-B ligand (RANKL, 50 ng/mL each) were added to stimulate RES-treated cells. Five days later, we observed osteoclast activity based on the tartrate-resistant acid phosphatase (TRAP) staining. The cells that showed more than five nuclei were classified as osteoclasts. Similar procedure was repeated with RAW264.7 cells (Chinese Academy of Sciences, China).
Induction of osteoblast differentiation in rat BMSCs
The bone marrow was washed with a culture medium. BMSC suspension was prepared by gentle blowing and cultured in α-medium at 37°C with 10% FBS in a CO2 incubator. The media were replaced every other day, and cells were passaged when 80% confluent. In a six-well plate, P3-generation cells (2 × 105/well) were added, and 90% of the cell length to the bottom of the well was induced by osteogenic inducers (DMEM-10% FBS, 0.05 mmol/L vitamin C, 100 mmol/L dexamethasone, and 10 mmol/L sodium β-glycerophosphate; Sigma-Aldrich, China). Cells not treated with RES but treated with ODM were used as positive controls. BMSCs cultured in media without inducers were treated with RES (0 (control), 5, 10, and 20 µM, Sigma-Aldrich). All experiments were performed three times. The solution was changed every 3 days and induced for 21 days.
ALP enzymatic activity assay
ALP is an osteoblast differentiation marker that is generally expressed during initial stages of development.28 After 7 days, cells treated with ODM/RES/standard culture medium were cultured in six-well plates. At days 4 and 7, the cells were centrifuged at 12,000 r/min for 15 min and 4°C. The cell lysates were collected and detected for ALP activity by LabAssayTM ALP-related test kit. The total protein mass of the cell lysates was measured by bicinchoninic acid assay (BCA) method. The ALP activity of the samples was detected using the ALP-test kit. In brief, 20 μL of the lysates or the cells were mixed with 100 μL of the standard solution and incubated for 15 min at 37°C. Absorbance of the final solution (80 μL) was recorded at 405 nm. The other half of the sample was diluted and mixed with the BCA protein detection working solution and kept at 60°C for 30 min. The same procedure was repeated with the standard. Absorbance at 570 nm was read to obtain total cell protein volume mass. The ALP activity values of the cell lysates were calibrated with the corresponding cell total protein volume mass to obtain the relative ALP activity values of each group. The ALP activity values of the cell lysates were calibrated with the corresponding cell total protein volume mass to obtain the relative ALP activity values of each group.
TRAP staining
We isolated bone marrow mononuclear cells from the femoral cavity of SD rats following a previously described method.26 The cells (4 × 103 cells/well) were cultured in 48-well plates and kept for overnight incubation at 37°C. The cells were induced with 100 ng/L RANKL and RES at 0, 5, 10, and 20 μm once every 2 days and stained with TRAP on day 6. We used 4% paraformaldehyde to fix the cells (20 min) at 37°C for 1 h in the presence of naphthol AS-BI phosphate. The cells were rinsed three times with deionized water. The cells were treated with hematoxylin stain for 3 min, washed three times with PBS, and observed under microscope (100x magnification). Osteoclast cells were counted. TRAP-positive cells were stained with purple, and macrophages with more than three nuclei were mature osteoclast cells.
Animal grouping and ovariectomy
As shown in Table 1, 48 SD rats were randomly assigned to six groups (8 per group) after adaptation for 7 days. Rats in the five groups underwent OVX, and those in the remaining group underwent sham operation. A bilateral ovariectomy was performed via the dorsal approach in the treatment groups under phenobarbital sodium anesthesia (40 mg/kg b.w., ip), except for the sham-operated rats. RES and estradiol (E2) were dissolved in sodium carboxymethyl cellulose (CMC; 0.5%) and cottonseed oil, respectively. Sham-operated group (n = 8): sham-operated rats received 0.5% CMC orally by gavage; OVX group (n = 8): OVX rats received 0.5% CMC orally by gavage); E2 group (n = 8): OVX rats were orally administered with 0.8 mg/kg E2 daily; based on previous references,29–31 low-dose RES group (n = 8): OVX rats received 50 mg/kg RES; medium-dose RES group (n = 8): OVX rats received 100 mg/kg RES, and high-dose RES group (n = 8): OVX rats received 200 mg/kg RES daily orally by gavage. The entire study lasted for 12 weeks. One day after the last treatment, the animals were euthanized through intraperitoneal injection of xylazine (12 mg/kg) and phenobarbital sodium (40 mg/kg) and cervical dislocation. The uterus was excised and weighed. Abdominal aorta puncture was used to collect a blood sample, following centrifugation at 2000×g for 20 min and stored -80°C for further biochemical analysis. Femurs and lumbar vertebrae were excised and stored at -20°C in saline for further bone analyses.
Table 1.
Grouping of 48 rats.
| Group | Number of rats (n) |
|---|---|
| Sham group | 8 |
| OVX group | 8 |
| OVX + E2 group | 8 |
| Low-dose RES group | 8 |
| Medium-dose RES group | 8 |
| High-dose RES group | 8 |
OVX: ovariectomized; RES: resveratrol.
RNA extraction
The right femur bone tissue was taken. The bone tissue was thawed by liquid nitrogen and repeatedly milled to approximately 100 mg. Total RNA was extracted by TRIzol method. Reverse transcriptase as cDNA was applied as per the reverse transcription kit instruction before the real-time PCR reaction. The reaction mixture (20 μL) contained cDNA template (1 μL), ddH2O (8.5 μL), upstream primer (0.25 μL), SYBR Green (10 μL), downstream primer (0.25 μL). miR-92b-3p was identified with qRT-PCR. A looped antisense primer (Table 1) was utilized for reverse transcription. The reverse transcription reaction was diluted 10 times and used as template for qRT-PCR. The reaction conditions were as follows: pre-denaturation at 50°C (2 min), 95°C (10 min), and 95°C (15 s) and 60°C (1 min) for 40 cycles. Each sample was set up with three replicates on a real-time fluorescence quantifier ABI7500, with β-actin as internal reference and snRNA U6 as internal reference for miR-92b-3p. The mRNA levels of NF-κBp65, Nox4, Smad7, cathepsin K, BMP2, miR-92b-3p, and RUNX-2 were analyzed by 2−ΔΔΔCt method. The corresponding primer synthesis was entrusted to Sangon Biotech (Shanghai) Co., Ltd., and the primer sequence is shown in Table 2.
Table 2.
Primers used for qRT-PCR.
| miR-92b-3p | F-5′-UACAGGUGAACCGGUCUCUUU-3′ | 173 bp |
| R-5′-AGAGACGGUUCACGGUGAUU-3′ | ||
| U6 | F-5′-GTGCCGCTTCGGCAGCACATAT-3′ | 168 bp |
| R-5′-AAAAATATGGAACGCTTCACGAA-3′ | ||
| Nox4 | F-5′-TGCCTGCTCATTTGGCTGT-3′ | 180 bp |
| R-5′-CCGGCACATAGGTAAAAGGATG-3′ | ||
| NF-kB | F-5′-GCGAGAGGAGCACAGATACC-3′ | 160 bp |
| R-5′-GCACAGCATTCAGGTCGTAG-3′ | ||
| Smad7 | F-5′-ACGAAGAGAGTCTCGGAGGAA-3′ | 104 bp |
| R-5′-GCTGCTCGCATAAGCTGAC-3′ | ||
| BMP2 | F-5′-CAGCGAGTTTGAGT-TGAGG-3′ | 143 bp |
| R-5′-CGGTACAGGTCGAGCATAT-3′ | ||
| RUNX-2 | F-5′-CCCAACT-TCCTGTGCTCC-3′ | 137 bp |
| 5′-AGTGAAACTCTTGC-CTCGTC-3′ | ||
| Cathepsin K | 5′-GGGAGAAAAACCTGAAGC-3′ | 148 bp |
| 5′-ATTCTGGGGACTCAGAGC-3′ | ||
| IkB | F-5′-TGCTGAGGCACTTCTGAG-3′ | 164 bp |
| R-5′-CTGTATCCGGGTGCTTGG-3′ | ||
| β-actin | F-5′-GATTACTGCTCTGGCTCCTGC-3′ | 174 bp |
| R-5′-GACTCATCGTACTCCTGCTTGC-3′ |
Western blot
We removed the ground bone tissue or the collected BMSCs, added a certain volume of protein lysis solution to fully grind the tissue for 30 min (on ice), centrifuged at 4°C and 12,000×g for 10 min. The BCA protein kit was used to quantify the proteins in the supernatant. The sample (20 μL) was loaded on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). After the electrophoresis was completed, the protein was transferred to a polyvinylidene fluoride membrane (PVDF). After being closed for 2 h with 10% skim milk powder, anti-NF-κBp65 (1:1000; AB21532), Smad7 (1:1000; AN33461), p-IκB-α (1:1000; 33517), BMP2 (1:1000; SM51894), RUNX-2 (1:1500; YK91735), Nox4 (1:1000; 5198463), cathepsin K (1:1000; GT240914), and anti-actin antibody (1:1000; TBI419973) (Abcam, USA) were incubated at 4°C overnight. After washing three times with TBST (15 min each), the corresponding horseradish peroxidase-linked anti-rabbit antibodies (1:4000; NM31583, Molecular Probes, USA) were added and kept for 2 h at room temperature. Post-treatment with enhanced chemiluminescence reagents (GE Healthcare), the membranes were exposed to photographic film. Quantitative analysis was performed using Image J.
Determination of serum BALP, OPG, TRAP-5b, CTX-I, Ca, and P and urinary Ca and P
Serum levels of BALP, OPG, TRAP-5b, and CTX-I were determined strictly according to the enzyme-linked immunosorbent assay (ELISA) kit (Sigma, USA) instructions. After incubation for 2 h, the blood samples were centrifuged at 3000 r/min for 15 min. Next, we collected the supernatant into a 1.5 mL centrifuge tube. The Mindray Medical Automatic Biochemical Analyzer was used to measure the serum concentration of phosphorus (S-P), calcium (S-Ca), along with urine concentrations of phosphorus (U-P), and calcium (U-Ca).
BMD testing
A dual-energy X-ray 4500 W bone densitometer (Norland Corp., USA) was used to measure the right femoral bone BMD in rats anesthetized with 2% pentobarbital sodium. We used the accompanying flexible software for small animals v2.5.0 to perform these scans.32
Micro-computed tomography
We used the eXplore Locus SP micro-CT to scan the fourth lumbar vertebra (L-4) of each group to assess any trabecular microstructural changes. The L-4 was placed in a micro-CT test tube and scanned along the long axis to obtain a continuous micro-CT image using following parameters: scanning accuracy: 8.52 μm, resolution: 17.2 μm after reconstruction, scanning voltage: 80 kV, threshold: 1000, current: 500 μA, interest area: 1600, and width: 2100. After image Gaussian filtering, three-dimensional reconstruction was performed with the fracture site as the center. The Advanced Bone Analysis software was used to analyze the scanned data by bone histochemistry. The main parameters automatically generated by the software include trabecular thickness (Tb. Th), bone mass fraction (BV/TV), trabecular spacing (Tb.Sp), structural model index (SMI), bone surface to bone volume ratio(BS/BV), tissue bone density (Conn.D), and trabecular number (Tb.N) of the sample callus.
Hematoxylin and eosin staining
The femoral bone specimens of the rats, which had been placed in 10% neutral paraformaldehyde solution, were fixed at 25°C for 1 week. The specimens were decalcified in 10% disodium ethylenediamine tetraacetate (EDTANa2, formulated in DEPC water) decalcification solution until tissue softening. After decalcification, the specimens were dehydrated with ethanol gradient, treated transparently with xylene for 2 h, and embedded by paraffin. Then, the specimens were sectioned (5 μm thick) along the long axis of the femoral trunk, and treated with hematoxylin–eosin (H&E) stain. All liquid reagents used must be prepared with DEPC treatment or DEPC water. Each specimen was randomly divided into six tibial slices, and a Nikon Eclipse E800 microscope (Nikon, Japan) was used for bone analysis.
Statistical analyses
The data were analyzed using SPSS v19.0 software, and expressed as mean ± SD, as specified in the figure and table legends. Statistically significant differences between the treatment and sham-operated group or model group were determined using Student’s t-test. Data sets with several comparisons were examined using one-way analysis of variance (ANOVA) with Dunnett’s post-tests. P-value < 0.05 indicated statistically significant data.
Results
Effect of miR-92b-3p on Nox4/NF-kB and BMP2/Smad signaling pathway in BMSCs
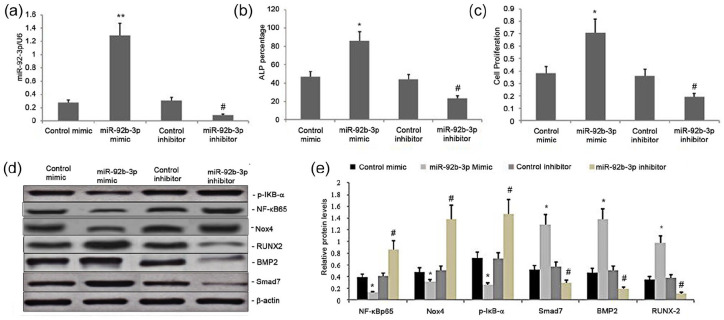
We evaluated the impact of miR-92b-3p on the Nox4/NF-kB and BMP2/Smad signaling pathways by transfecting BMSCs with the mimics and inhibitors of miR-92b-3p, along with their respective NCs. We performed qRT-PCR 24 h post-transfection to measure the levels of miR-92b-3p. Also, Western blot was performed to measure p-IκB-α, NF-κBp65, Smad7, Nox4, RUNX-2, and BMP2 levels. The miR-92b-3p-M significantly upregulated miR-92b-3p levels while the miR-92b-3p-Isignificantly decreased them (Figure 1(a)), which led to increased expression of p-IκB-α, NF-κBp65, and Nox4 and attenuated expression of BMP2, Smad7, and RUNX-2. By contrast, the increase in the miR-128 level significantly decreased the expression of p-IκB-α and NF-κBp65, while increased the expression of Smad7, BMP2, and RUNX-2 (Figure 1(d) and (e)). Thus, miR-92b-3p regulated the Nox4/NF-kB and BMP2/Smad signaling pathways in BMSCs
Figure 1.
Effect of miR-92b-3p on the Nox4 /NF-kB and BMP2/Smad signaling pathway, BMSC proliferation, and osteoblast differentiation in rat BMSCs. (a) miR-92b-3p levels in BMSCs transfected with BMSC mimic, inhibitor, or scrambled sequences. (b) ALP activity levels in the miR-92b-3p inhibitor, miR-92b-3p mimicNC, and miR-92b-3p inhibitor-NC groups. (c) BMSC proliferation in the miR-92b-3p inhibitor, miR-92b-3p mimic-NC, and miR-92b-3p inhibitor-NC groups. (d–e) Representative graphs and statistical analyses of NFκBp65, Nox4, p-IκB-α, Smad7, BMP2, and RUNX-2 by Western blot in BMSCs transfected with miR-92b-3p mimic, inhibitor, or scrambled sequences; Data obtained from quantitative densitometry are presented as mean ± SD of three independent experiments.
*P < 0.05 versus controls and treatment groups. #P < 0.05 versus controls.
Effect of miR-92b-3p on BMSC proliferation and osteoblast differentiation in rat BMSCs
The ALP activity levels and BMSC proliferation in the miR-92b-3p-M group were greater than in the corresponding inhibitor and NC groups (P < 0.01; Figure 1(b)-(c)). However, the ALP activity levels and BMSC proliferation in the miR-92b-3p-I group were lower (P < 0.01) than those in the miR-92b-3p-M-NC and the miR-92b-3p-I-NC groups (Figure 1(b) and (c)). Thus, miR-92b-3p promoted BMSC proliferation and osteoblast differentiation in BMSCs.
Nox4 is a direct miR-92b-3p target
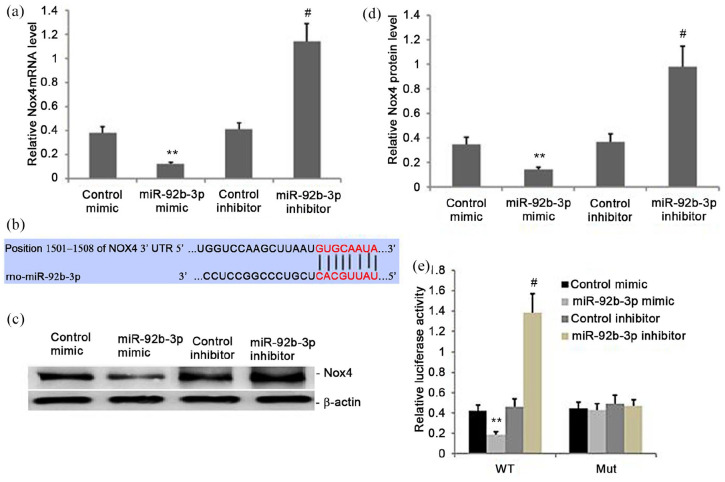
We explored the potential miR-92b-3p targets using Targetscan. Among the candidate targets, Nox4 was selected because of its important roles in bone metabolism disorders, including osteoporosis. Then, we measured the luciferase activity of WT and MUT Nox4 reporter in primary BMSCs. The overexpression of miR-92b-3p had no effect on the MUT Nox4 reporter activity; however, it notably reduced the luciferase activity of the WT Nox4 reporter in BMSCs (Figure 2(b) and (e)). Also, miR-92b-3p overexpression decreased Nox4 levels, both at the mRNA and the protein (Figure 2(a), (c) and (d)) levels in BMSCs, which was reversed by using the miR-92b-3p-I. Hence, Nox4 acted as a miR-92b-3p target.
Figure 2.
Nox4 is a target of miR-92b-3p. (a) Nox4 mRNA levels decreased in miR-92b-3p-overexpressing BMSCs (P < 0.05). (b) miR-92b-3p bound to Nox4-3′UTR-wt, and the binding was blocked by Nox4 -3′UTR-mt. (c-d) Nox4 protein levels decreased in miR-92b-3p-overexpressing cells compared with vector controls (P < 0.05). (e) Dual-Luciferase Reporter assays confirmed that the miR-92b-3p mimic bound to Nox4 -3′UTR-wt but not to the mutated form (P < 0.05).
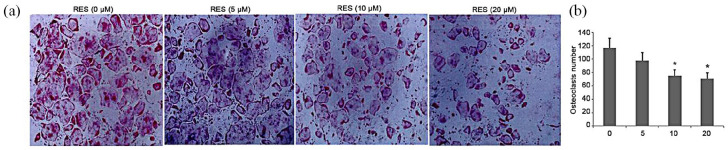
RES inhibited osteoclastogenesis in vitro
An MTT assay was performed before initiating the in vitro study. A standard cellular osteoclastogenesis model involving BMMs was used to explore the effects of RES on osteoclastogenesis. Post-induction with RANKL, the cells were incubated with RES (5, 10, and 20 µg/mL). Seven days later, the TRAP-positive cell count was substantially elevated in the control group, which was suppressed by RES in a dose-dependent way (Figure 3(a) and (b)). However, the 10 and 20 µg/mL RES-treated groups were not statistically different (Figure 3(a) and (b)).
Figure 3.
Effect of treatment with RES on osteoclastogenesis in vitro. All the experiments were performed six times, and the average was taken (TRAP staining, magnification 100×). Formation of TRAP-positive cells from BMMs and quantification of osteoclast. TRAP-positive cells from BMMs was determined; bars indicate mean ± SD.
*P < 0.05, **P < 0.01 versus controls.
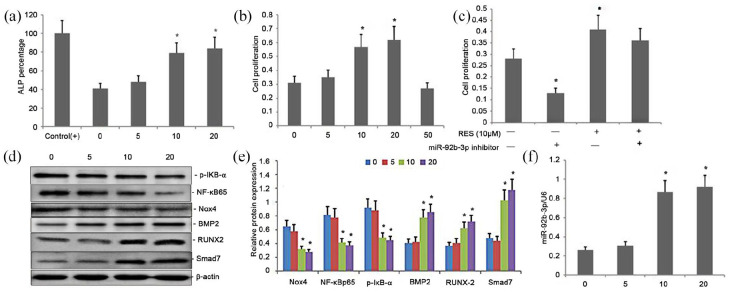
Treatment with RES increased BMSC proliferation and osteoblast differentiation in rat BMSCs though attenuating Nox4 /NF-kB and increasing BMP2/Smad signaling pathway by increasing miR-92b-3p expression
Differentiation of osteoblasts is reflected by the ALP activity. Here, with an increase in the RES concentrations from 1 to 20 μM, a dose-dependent response in the ALP activity in BMSCs was observed (Figure 4(a)). The enzymatic activity of ALP was higher in BMSCs treated with higher concentrations of RES (10 or 20 μM) (P < 0.01) than the BMSCs, which were exposed to neither ODM nor RES (control) (Figure 4(a)). The enzymatic activity of ALP was insignificantly different between BMSCs treated with RES (10 or 20 μM) or ODM (positive control) (Figure 4(a)). Similarly, with an increase in the RES concentrations from 1 to 20 μM, a dose-dependent increase in the proliferation of BMSCs was observed (Figure 4(b)). The BMSCs treated with 5, 10, or 20 μM RES showed increased proliferation compared with the untreated control BMSCs (Figure 4(b)). However, there was an insignificant difference in proliferation of BMSCs among the groups treated with higher concentrations of RES (10 or 20 μM) (Figure 4(b)). The decrease in the expression of Nox4, p-IκB-α, and NF-κBp65 showed an inverse dose-dependency to RES, and the increase in the Smad7, BMP2, and RUNX-2 expression showed a positive dose-dependency to RES (Figure 4(d) and (e)). The expression was also enhanced in response to 20 μM RES. The reducing effects of RES on the expression of Nox4, p-IκB-α, and NF-κBp65 and the enhancing effects on the expression of Smad7, BMP2, and RUNX-2 were regulated by a dose-dependent increase the miR-92b-3p expression (Figure 4(d)–(f)).
Figure 4.
Effect of Treatment with RES on BMSC proliferation and osteoblast differentiation in rat BMSCs though regulating Nox4/NF-kB and BMP2/Smad signaling pathway by attenuating miR-92b-3p expression. (a) ALP activity in BMSCs; (b) MTT assay of BMSC proliferation; (c) Effect of miR-92b-3p on BMSC proliferation; (d–e) Western blots of NF-κBp65, p-IκB-α, Nox4, Smad7, BMP2, and RUNX-2 expression in cells exposed to RES. β-Actin was used as the loading control. (f) RT-PCR of miR-92b-3p expression in cells exposed to RES.
Data indicate mean ± SD. *P < 0.05, **P < 0.01 versus controls.
Treatment with RES increased miR-92b-3p expression in osteoporotic bone tissues
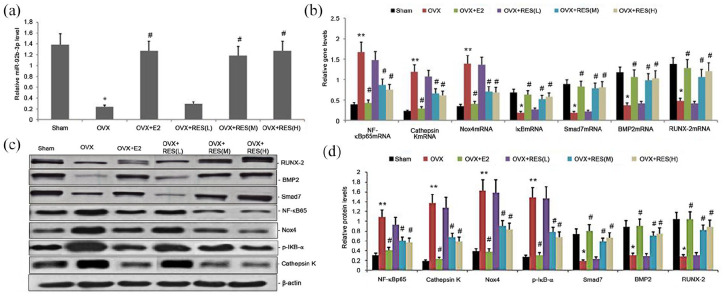
We evaluated the impact of RES on miR-92b-3p levels in osteoporotic bone tissues treated with RES for 12 weeks. qRT-PCR measured miR-92b-3p levels in the bone tissues, which was decreased in estrogen deficiency-induced bone tissues and significantly increased in the RES-treated rats (Figure 5(a)).
Figure 5.
Effect of RES on miR-92b-3p expression and Nox4/NF-kB and BMP2/Smad signaling pathway in estrogen deficiency-induced bone tissues. Rats were treated with RES for 12 weeks. (a) Relative miR-92b3p expression was determined by qRT-PCR; (b) relative mRNA levels of NF-κBp65, Smad7, Nox4, cathepsin K, BMP2, and RUNX-2 were determined by qRT-PCR; (c)–(d) representative Western blots and statistical analyses of NF-κBp65, cathepsin K, Nox4, p-IκB-α, Smad7, BMP2, and RUNX-2 proteins. All expression data were normalized to a housekeeping gene and protein (β-actin). Bars indicate mean ± SD (n = 8). bars indicate mean ± SD.
*P < 0.05, **P < 0.01 versus sham group; #P < 0.05 versus the OVX group.
Treatment with RES increased Nox4/NF-kB and BMP2/Smad signaling pathway in estrogen deficiency-induced bone tissues
Nox4/NF-kB and BMP2/Smad signaling pathway are two important pathways involved in osteoporosis. We analyzed the effect of treatment with RES on Nox4/NF-kB and BMP2/Smad signaling pathway in estrogen deficiency-induced bone tissues. We performed Western blot and qRT-PCR to measure the protein and gene expression, respectively, of cathepsin K, p-IκB-α, NF-κBp65, Smad7, Nox4, BMP2, and RUNX-2. The gene expression levels of NF-κBp65, Nox4, and cathepsin K were substantially elevated (Figure 5(b)). The gene expression of Smad7, BMP2, and RUNX-2 was significantly lower in estrogen deficiency-induced rat bone tissues than the control group (Figures 5(b)). On the contrary, RES treatment considerably decreased the gene expression levels of NF-κBp65, Nox4, and cathepsin K and significantly increased those of Smad7, BMP2, and RUNX-2 (Figure 5(b)). Western blot analysis revealed significantly upregulated protein expression levels of NF-κBp65, p-IκB-α, Nox4, and cathepsin K in estrogen deficiency-induce bone tissues and the significantly low gene expression levels of Smad7, BMP2, and RUNX-2 (Figure 5(c) and (d)). After treatment with RES, we observed a dose-dependent reduction in the protein expression levels of NF-κBp65, p-IκB-α, Nox4, and cathepsin K, and increase in Smad7, BMP2, and RUNX-2 (Figure 5(c) and (d)). Hence, RES regulated osteoporosis by regulating Nox4/NF-kB and BMP2/Smad signaling pathways.
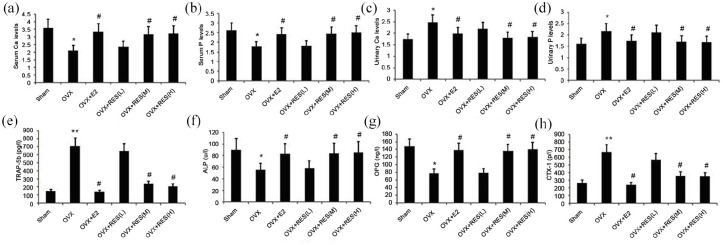
Effect of treatment with RES on serum levels of BALP, OPG, TRAP-5b, CTX-I, Ca, and P, and the urinary levels of Ca and P in estrogen deficiency-induced rats
Serum levels of BALP, OPG, TRAP-5b, and CTX-I were determined using ELISA. Mindray Medical Automatic Biochemical Analyzer was used to measure the serum and urinary levels of Ca and P. As shown in Figure 6, the serum levels of BALP and OPG in estrogen deficiency-induced rats significantly decreased, and those of TRAP-5b and CTX-I substantially increased than the control group and the E2 group (Figure 6(e)–(h)). We found notably elevated serum levels of BALP and OPG in the treatment group, and depleted levels of TRAP-5b and CTX-I than the model group (Figure 6(e)–(h)). The S-Ca and S-P levels evidently decreased, while the U-Ca and U-P levels increased in the model group (Figure 6(a)–(d)), which was alleviated by RES (Figure 6(a)–(d)).
Figure 6.
Effect of treatment with RES on serum levels of BALP, OPG, TRAP-5b, CTX-I, Ca, and P and urinary levels of (a) Ca, and (b) P and urinary (c) Ca and (d) P, and serum levels of (e) TRAP-5b, (f)ALP, (g)OPG, levels of Ca and P in estrogen deficiency-induced rats. Rats were treated with RES for 12 weeks. Serum For Peer Review and (h) CTX-I in estrogen deficiency-induced rats were measured.
Data represent the mean ± SD of 8 rats.
*P < 0.05, **P < 0.01 versus sham group. #P < 0.05 versus OVX group.
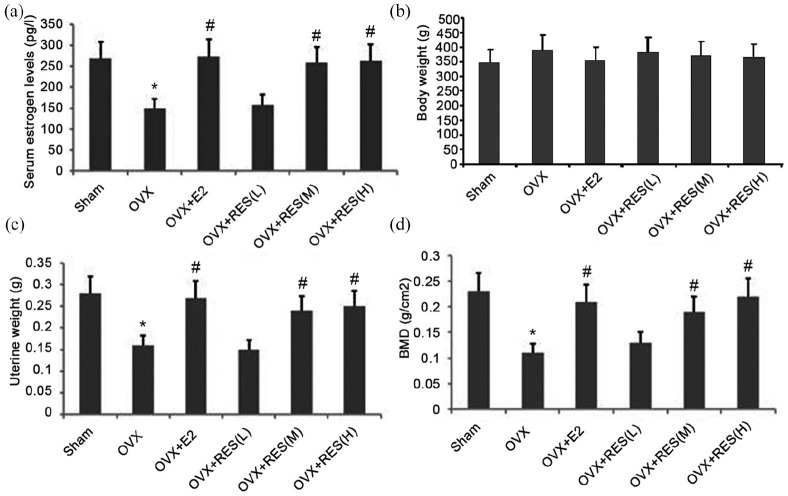
Effect of RES on serum E2, uterine weight, and body weight
After treatment with RES for 12 weeks, serum E2, body weight, and uterine weight were measured. We observed significantly lower serum E2 levels and uterine weight in the OVX rats than in the RES group; however, the reverse was true for the body weight (Figure 7(a)–(c)). RES significantly reversed these changes, however, the change in the weight of the rats was insignificant (Figure 7(a)–(c)). No significant change in the serum E2 levels, body weight and uterine weight between the E2 and sham groups was observed throughout the experiment (Figure 7(a)–(c)). Thus, the increase in E2 induced by RES prevented the loss of uterine weight induced by the bilateral ovariectomy.
Figure 7.
Effect of RES administration on serum E2, body weight, uterine weight, and femoral BMD of OVX rats. (a) Serum E2, (b) body weight, (c) uterine weight, and (d) femoral BMD were measured after RES administration for 12 weeks.
Data represent the mean ± SD of 8 rats.
*P < 0.05 versus sham group. # increase versus OVX group (P < 0.01).
RES increased femoral BMD
The OVX group exhibited a lower mean BMD in the bone than the sham group (Figure 7(d); P < 0.05). The value of mean right BMD was enhanced in the E2, RES 100, and RES200 groups than the OVX group (Figure 7(d)).
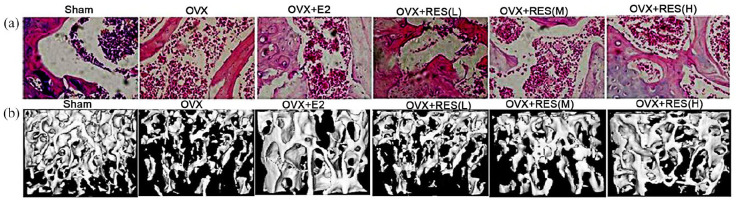
Effect of RES on bone morphology and histology
The parameters related to the fourth lumbar vertebra micro-CT included Tb.Sp, Tb.N, Tb. Sp, BV/TV, SMI, Tb.Th, and Conn.D. In the OVX group, elevated values of SMI and Tb. Sp, and decreased values of BV/TV, Conn.D, Tb.N and Tb.Th confirmed osteoporosis in the OVX rats (Figure 8(b) and Table 2). The increase in Tb.Sp and SMI were diminished, and the decrease in Conn.D, BV/TV, Tb.Th, and Tb.N was considerably restored after RES administration (Figure 8(b) and Table 3). Hence, RES treatment could mitigate osteoporosis. The parameters related to micro-CT significantly improved in the high- and medium-dose RES treatment groups compared with those in the low-dose RES group (Figure 8(b) and Table 3). Based on HE staining (femoral bone), in the OVX rats, we observed a thinning of the bone trabeculae with an increase in the inter-trabecular space and micro-fractures of bone trabeculae (based on the generation of connective tissues in the border area of the fractured trabeculae) (Figure 8(a)). For the RES-treated rats, we found diminished bone trabeculae and intertrabecular space along with minimal micro-fracture of bone trabeculae (Figure 8(a)).
Figure 8.
Change of pathological changes in the femur and representative micro-CT image. (a) Morphology of proximal metaphysis of femoral bone (HE staining, magnification 100×). (b) micro-CT images showing representative trabecular bone microarchitecture of the L-4 vertebral body metaphysis for each treatment group.
Table 3.
Changes of the L-4 vertebral body micro-CT parameters. Rats were treated with RES for 12 weeks. The parameters related to L-4 vertebral body micro-CT parameters included BV/TV, Tb. Sp, Tb. Th, Tb. N, Tb.Sp, SMI, and Conn.D were measured.
| Sham group | OVX | OVX + E2 | OVX + RES(L) | OVX + RES(M) | OVX + RES(H) | |
|---|---|---|---|---|---|---|
| BV/TV | 0.4165 ± 0.053 | 0.2528 ± 0.034* | 0.3498 ± 0.042# | 0.2013 ± 0.074 | 0.3610 ± 0.042# | 0.3629 ± 0.033# |
| Tb.N (mm-1) | 4.52 ± 0.396 | 3.178 ± 0.179* | 3.911 ± 0.278# | 3.446 ± 0.858 | 3.967 ± 0.695# | 4.065 ± 0.715# |
| Tb. Th (μm) | 0.1119 ± 0.043 | 0.0627 ± 0.015* | 0.1058 ± 0.033# | 0.0712 ± 0.027 | 0.0946 ± 0.016# | 0.1072 ± 0.049# |
| Tb. Sp (μm) | 0.1398 ± 0.032 | 0.3691 ± 0.024* | 0.1652 ± 0.025# | 0.3260 ± 0.047 | 0.1641 ± 0.054# | 0.1528 ± 0.075# |
| SMI (%) | 0.5305 ± 0.0713 | 0.953 ± 0.192* | 0.5401 ± 0.045# | 0.885 ± 0.054 | 0.6232 ± 0.034# | 0.5922 ± 0.076# |
| Conn.D(mg/cc) | 47.997 ± 5.123 | 35.159 ± 4.819* | 45.159 ± 7.548# | 38.53 ± 6.717 | 43.27 ± 5.319# | 44.313 ± 5.022# |
OVX: ovariectomized; RES: Resveratrol; SMI: structural model index.
Data represent the mean ± SD of 10 rats.
P < 0.01 versus sham group. #P < 0.05 versus OVX group.
Discussion
Estrogens are the main reproductive hormones in women and are involved in the growth, maturation, and stabilization of bone mass. In cases of pathologic deficiency of estrogen (e.g. ovariectomy, premature ovarian failure, etc.) or a natural deficiency of estrogen (e.g. postmenopausal women), circulating estrogen levels decrease significantly and the body is in a persistent state of estrogen deficiency.33 The reproductive organs and other tissues undergo significant changes, particularly bone loss.33 During bone metabolism, osteoblasts secrete BALP, which regulates the hydrolysis of pyrophospholipids and pyrophosphates to promote bone formation and calcification.33 OPG osteocalcin is an important noncollagen protein in the bone matrix and a specific marker of bone synthesis and is an integral part of the bone remodeling process.34 CTX-I specifically indicates the decomposition of collagen (type I) in bone tissues.33 TRAP-5b, primarily secreted by osteoclasts, accelerates the degradation of calcium and phosphorus mineralization substrates in the bone matrix.35 In the present study, after bilateral removal of the ovaries, the estrogen levels, uterine weight, plasma BALP and OPG levels, and bone metabolic parameters were substantially downregulated. The U-Ca and U-P levels increased. In particular, the Tb.N, BV/TV, Tb.Th, Conn.D, and bone density reduced, whereas the Tb.Sp and SMI increased. After treatment with RES for 12 weeks, the estrogen levels, body weight, uterine weight, plasma levels of BALP and OPG, and S-Ca and S-P levels increased. The U-Ca and U-P levels decreased. Moreover, the Conn.D, BV/TV, Tb. Th, Tb.N, and bone density increased. The Tb.Sp and SMI decreased. Overall, estrogen deficiency-induced osteoporosis was improved. Hence, RES improved estrogen deficiency-induced osteoporosis by increasing the estrogen levels.
BMSCs are one of the most important seed cells in tissue engineering and cell replacement therapy. These cells have the potential for multiple differentiations and can secrete many osteoblasts, osteoblasts, chondrocytes, and adipocytes under certain conditions.36 In this regard, we selected BMSCs to explore the impact of the miR-92b-3p-M on osteoporosis caused by deficiency of estrogen. The miR-92b-3p-M promoted miR-92b-3p expression, enhanced BMSC proliferation and osteoblastic differentiation, and increased the ALP activity. Whereas, the miR-92b-3p-I suppressed its, BMSC proliferation, and osteoblastic differentiation and reduced the ALP activity. The increased miR-92b-3p expression could promote osteoblastic differentiation and exhibits potential for treatment of osteoporosis. Further studies showed that RES showed a dose = dependent increase in the miR-92b-3p expression, ALP activity, BMSC proliferation, and osteoblast differentiation.
The Nox4/ NF-κB/cathepsin K pathway is regulated to improve alveolar bone loss.37 Endogenous miR-92a-3p, miR-92b-3p, miR-99-5p, and miR-100-5p reduced the abundance of NOX4 mRNA in human pulmonary microvascular endothelial cells, reduced H2O2 release, and improved hypertension.38 Here, miR-92b-3p levels declined, Nox4 and NF-κB levels increased in BMSCs, and the proliferation of BMSCs and the differentiation of osteoblasts were inhibited. The depleted miR-92b-3p levels and the elevated Nox4 and NF-κB levels aggravated osteoporosis. Increased miR-92b-3p levels also inhibited the Nox4 and NF-κB expression in BMSCs and enhanced BMSC proliferation and osteoblast differentiation. Nox4 is the regulatory target of miR-128 miR-92b-3p, which regulates BMSC proliferation and osteoblast differentiation through Nox4. RES significantly increased the estrogen levels in estrogen deficiency-induced rats, enhanced the miR-128 miR-92b-3p levels in bone tissues, reduced the levels of Nox4 and NF-κB, and reduced osteoporosis. Cathepsin K is the most important enzyme secreted by osteoclasts to degrade BMPs, especially type I collagen.39 Our study found the increased expression of cathepsin K in estrogen deficiency-induced bone tissues and enhanced osteoporosis. After RES treatment, the cathepsin K expression decreased, osteoclast proliferation was inhibited, and osteoporosis was reduced.
Osteoblast differentiation and extracellular matrix synthesis are associated with the BMP-2/Smad/Runx2 signaling pathways, which regulate bone synthesis.40 Here, the activity of BMP-2/Smad/Runx2 signaling pathway decreased, which aggravated osteoporosis in the bone tissues of OVX rats. Suppression of miR-92b-3p expression enhanced Nox4 and NF-κB levels, decreased the gene and protein expression of Smad7, BMP2, and RUNX-2, and inhibited BMSC proliferation and osteoblast differentiation, leading to aggravated osteoporosis. By contrast, RES and the mimic promoted miR-128miR-92b-3p expression, decreased the levels of Nox4 and NF-κB, increased the gene and protein expression of Smad7, BMP2, and RUNX-2, promoted BMSC proliferation and osteoblast differentiation, resulting in osteoporosis. RES suppressed the activity of the Nox4/NF-κB pathway by increasing miR-128 miR-92b-3p levels and the BMP-2/Smad/Runx2 signaling pathway activity to reduce osteoporosis.
Inflammation is involved in the occurrence and development of osteoporosis induced by estrogen deficiency.41 Serum IL-6 and TNF-a in patients with osteoporosis are elevated. IL-6 and TNF-a can promote osteoclast formation and bone resorption.41 NF-κB is the core of inflammatory transcription.42 Inhibiting the expression of NF-κB in bone tissues may help reduce inflammation and improve osteoporosis induced by estrogen deficiency. In this study, Res reduced the expression of NF-κB in bone tissues and reduced osteoporosis induced by estrogen deficiency, which may be related to the effect of Res on reducing inflammation.
Based on previous references,29–31 clinical studies reported that RES dose of 1000 mg/day for 4 weeks can be tolerated by a patient.28 The low toxicity of RES is partly due to its rapid metabolism and clearance from the body.29 With a different model, the dose of 400 mg RES/kg bwt/day in the young spinal cord of injured rats had an antioxidant effect, increased the serum total antioxidant capacity, and reduced the femoral malondialdehyde concentration compared with those in the absence of RES.31 Therefore, in this experiment, we chose the RES dose range of 50–200 mg/kg. In many studies on RES treatment of tumors, RES inhibits the proliferation of tumor cells and exerts an antitumor effect.43–47 However, in the study of RES treatment of osteoporosis, RES can promote the proliferation of osteoblasts and inhibit the proliferation of osteoclasts48,49 thereby exerting resistance to osteoporosis due to various causes. The results of these studies are consistent with the present work.
Conclusion
Here, we showed that miR-92b-3p was associated with the development of osteoporosis induced by estrogen deficiency in ovariectomized rats. RES enhanced the miR-92b-3p levels, suppressed the Nox4/NF-κB signaling pathway activity, enhanced the BMP-2/Smad/Runx2 signaling pathway activity, inhibited osteoclast proliferation, stimulated BMSC proliferation and osteoblast differentiation, and improved osteoporosis caused by estrogen deficiency. The RES mechanism for treatment of osteoporosis caused by estrogen deficiency provides fundamental theoretical basis. This study also showed that Nox4 is the regulatory target of miR-128 miR-92b-3p.
This study presents some limitations
This study confirmed that RES suppressed the activity of Nox4/NF-κB and cathepsin K levels in bone tissues of OVX rats by upregulating miR-92b-3p expression, promoting osteoblast differentiation, and improving osteoporosis induced by estrogen deficiency. However, this study had few limitations. First, miR-92b-3p regulates osteoblast differentiation and osteoporosis and may involve signaling pathways related to inflammation, apoptosis, and autophagy. Therefore, the mechanism by which miR-92b-3p regulates osteoporosis remains to be further explored. Second, inflammation plays an important role in the development of osteoporosis. However, this experiment did not study the effect of inflammatory cytokines on osteoporosis by regulating the Nox4/NF-κB information pathway. Third, the study could not determine whether RES could regulate osteoclast differentiation by regulating miR-92b-3p and consequently lead to osteoporosis induced by estrogen deficiency. Fourth, this study did not test the toxicity and side effects of different doses of RES on each group of experimental animals and the maximum dosage that the experimental rats could tolerate. In addition, this study did not calculate the half effective dose (ED50), half-inhibited dose (ID50), and minimum inhibitory concentration (MIC) of RES treatment of BMSCs. Thus, the efficacy of RES in osteoporotic rats needs further analysis. In addition, the regulatory mechanism of RES in osteoporosis, involving inflammation, apoptosis, and autophagy, needs to be further elucidated.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received funding via grants Yunnan Applied Basic Research Project-Union Foundation of China (Grant No. 2017FE468(-032), No. 2018FE001(-283)).
Ethical approval: All animal experiments were approved by the animal ethics committee of the First People’s Hospital of Kunming (Approval number: KYYM-2016-008-F17) and performed according to institutional guidelines and ethics.
Animal welfare: The present study involved client-owned animals; it demonstrated a high standard (best practice) of veterinary care and involved informed client consent.
ORCID iDs: Ming-wei Liu  https://orcid.org/0000-0002-3728-2350
https://orcid.org/0000-0002-3728-2350
References
- 1. Chen Y, Guo Y, Zhang X, et al. (2018) Bone susceptibility mapping with MRI is an alternative and reliable biomarker of osteoporosis in postmenopausal women. European Journal of Radiology 28(12): 5027–5034. [DOI] [PubMed] [Google Scholar]
- 2. Henault D, Westley T, Dumitra S, et al. (2018) Divergence from osteoporosis screening guidelines in older breast cancer patients treated with anti-estrogen therapy: A population-based cohort study. Bone 116: 94–102. [DOI] [PubMed] [Google Scholar]
- 3. Macari S, Madeira MFM, Lima ILA, et al. (2018) ST2 regulates bone loss in a site-dependent and estrogen-dependent manner. Journal of Cellular Biochemistry 119(10): 8511–8521. [DOI] [PubMed] [Google Scholar]
- 4. Xu L, Zhang L, Wang Z, et al. (2018) Melatonin suppresses estrogen deficiency-induced osteoporosis and promotes osteoblastogenesis by inactivating the NLRP3 inflammasome. Calcified Tissue International 103(4): 400–410. [DOI] [PubMed] [Google Scholar]
- 5. Orsolini G, Adami G, Rossini M, et al. (2018) Correction to: Is the exposure to bisphosphonates or osteoporosis the predictor of spinal radiographic progression in ankylosing spondylitis? Arthritis Research & Therapy 20(1): 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li LJ, Zhang J, Gao P, et al. (2018) Clinical characteristics and bisphosphonates treatment of rare pregnancy- and lactation-associated osteoporosis. Clinical Rheumatology 37(11): 3141–3150. [DOI] [PubMed] [Google Scholar]
- 7. Gupta T, Das N, Imran S. (2019) The prevention and therapy of osteoporosis: A review on emerging trends from hormonal therapy to synthetic drugs to plant-based bioactives. Journal of Dietary Supplements 16: 699–713. [DOI] [PubMed] [Google Scholar]
- 8. Stern AR, Yao X, Wang Y, et al. (2008) Effect of osteoporosis treatment agents on the cortical bone osteocyte microenvironment in adult estrogen-deficient, osteopenic rats. Bone Reports 8: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khera A, Kanta P, Kalra J, et al. (2019) Resveratrol restores the level of key inflammatory cytokines and RANKL/OPG ratio in the femur of rat osteoporosis model. Journal of Women & Aging 31: 540–552. [DOI] [PubMed] [Google Scholar]
- 10. Jia R, Li Y, Cao L, et al. (2019) Antioxidative, anti-inflammatory and hepatoprotective effects of resveratrol on oxidative stress-induced liver damage in tilapia (Oreochromis niloticus). Comparative Biochemistry and Physiology. Toxicology & Pharmacology 215: 56–66. [DOI] [PubMed] [Google Scholar]
- 11. Li D, Wang G, Jin G, et al. (2019) Resveratrol suppresses colon cancer growth by targeting the AKT/STAT3 signaling pathway. International Journal of Molecular Medicine 43(1): 630–640. [DOI] [PubMed] [Google Scholar]
- 12. Lei J, Chen Q. (2018) Resveratrol attenuates brain damage in permanent focal cerebral ischemia via activation of PI3K/Akt signaling pathway in rats. Neurological Research 40(12): 1014–1020. [DOI] [PubMed] [Google Scholar]
- 13. Feng YL, Jiang XT, Ma FF, et al. (2018) Resveratrol prevents osteoporosis by upregulating FoxO1 transcriptional activity. International Journal of Molecular Medicine 41(1): 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ma J, Wang Z, Zhao J, et al. (2018) Resveratrol attenuates lipopolysaccharides (LPS)-induced inhibition of osteoblast differentiation in MC3T3-E1 Cells. Medical Science Monitor 24: 2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suh KS, Chon S, Choi EM. (2018) Protective effects of piceatannol on methylglyoxal-induced cytotoxicity in MC3T3-E1 osteoblastic cells. Free Radical Research 52(6): 712–723. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Wang K, Hu Z, et al. (2018) MicroRNA-139-3p regulates osteoblast differentiation and apoptosis by targeting ELK1 and interacting with long noncoding RNA ODSM. Cell Death & Disease; 9(11): 1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lü MH, Hu CJ, Chen L, et al. (2013) miR-27b represses migration of mouse MSCs to burned margins and prolongs wound repair through silencing SDF-1a. PLoS ONE 8(7): e68972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sartori EM, Magro-Filho O, Silveira Mendonça DB, et al. (2018) Modulation of Micro RNA Expression and Osteoblast Differentiation by Nanotopography. International Journal of Oral & Maxillofacial Implants 33(2): 269–280. [DOI] [PubMed] [Google Scholar]
- 19. Feng L, Shi L, Lu YF, et al. (2018) Linc-ROR promotes osteogenic differentiation of mesenchymal stem cells by functioning as a competing endogenous RNA for miR-138 and miR-145. Molecular Therapy-nucleic Acids 11: 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin JC, Liu ZG, Yu B, et al. (2018) MicroRNA-874 targeting SUFU involves in osteoblast proliferation and differentiation in osteoporosis rats through the Hedgehog signaling pathway. Biochemical and Biophysical Research Communications 506(1): 194–203. [DOI] [PubMed] [Google Scholar]
- 21. Sun H, Hu S, Zhang Z, et al. (2019) Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. Journal of Cellular Biochemistry 120(1): 171–181. [DOI] [PubMed] [Google Scholar]
- 22. Harrison C. (2013) Bone disorders: Targeting NOX4 knocks down osteoporosis. Nature Reviews Drug Discovery 12(12): 904. [DOI] [PubMed] [Google Scholar]
- 23. Goettsch C, Babelova A, Trummer O, et al. (2013) NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. Journal of Clinical Investigation 123(11): 4731–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watt J, Alund AW, Pulliam CF, et al. (2018) NOX4 deletion in male mice exacerbates the effect of ethanol on trabecular bone and osteoblastogenesis. Journal of Pharmacology and Experimental Therapeutics 366(1): 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spanier G, Xu H, Xia N, et al. (2009) Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). Journal of Physiology and Pharmacology 60(Suppl. 4): 111–116. [PubMed] [Google Scholar]
- 26. Vaez SA, Ebrahimi-Barough S, Soleimani M, et al. (2018) The cardiac niche role in cardiomyocyte differentiation of rat bone marrow-derived stromal cells: Comparison between static and microfluidic cell culture methods. EXCLI Journal 17: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kylmäoja E, Nakamura M, Kokkonen-Puuperä H, et al. (2018) Gap junctional communication is involved in differentiation of osteoclasts from bone marrow and peripheral blood monocytes. Heliyon 4(5): e00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Song H, Wang H, Li X, et al. (2018) Sensitive and selective colorimetric detection of alkaline phosphatase activity based on phosphate anion-quenched oxidase-mimicking activity of Ce(Ⅳ) ions. Analytica Chimica Acta 1044: 154–161. [DOI] [PubMed] [Google Scholar]
- 29. Chow HH, Garland LL, Hsu CH, et al. (2010) Resveratrol modulates drug-and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prevention Research 3(9): 1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cottart CH, Nivet-Antoine V, Beaudeux JL. (2014) Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Molecular Nutrition & Food Research 58(1): 7–21. [DOI] [PubMed] [Google Scholar]
- 31. Wang HD, Shi YM, Li L, et al. (2013) Treatment with resveratrol attenuates sublesional bone loss in spinal cord-injured rats. British Journal of Pharmacology 170(4): 796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raafat SN, Amin RM, Elmazar MM, et al. (2018) The sole and combined effect of simvastatin and platelet rich fibrin as a filling material in induced bone defect in tibia of albino rats. Bone 117: 60–69. [DOI] [PubMed] [Google Scholar]
- 33. Wu J, Henning P, Sjögren K, et al. (2019) The androgen receptor is required for maintenance of bone mass in adult male mice. Molecular and Cellular Endocrinology 479: 159–169. [DOI] [PubMed] [Google Scholar]
- 34. Catalano A, Loddo S, Bellone F, et al. (2018) Pulsed electromagnetic fields modulate bone metabolism via RANKL/OPG and Wnt/β-catenin pathways in women with postmenopausal osteoporosis: A pilot study. Bone 116: 42–46. [DOI] [PubMed] [Google Scholar]
- 35. Liu X, Cai F, Zhang Y, et al. (2016) Celastrol, an NF-κB inhibitor, ameliorates hypercalciuria and articular cartilage lesions in a mouse model of secondary osteoporosis. Journal of Pharmacological Sciences 130(4): 204–211. [DOI] [PubMed] [Google Scholar]
- 36. Shen GS, Zhou HB, Zhang H, et al. (2018) The GDF11-FTO-PPARγ axis controls the shift of osteoporotic MSC fate to adipocyte and inhibits bone formation during osteoporosis. Biochimica et Biophysica Acta-molecular Basis of Disease 1864(12): 3644–3654. [DOI] [PubMed] [Google Scholar]
- 37. Lu SY, Wang CY, Jin Y, et al. (2017) The osteogenesis-promoting effects of alpha-lipoic acid against glucocorticoid-induced osteoporosis through the NOX4, NF-kappaB, JNK and PI3K/AKT pathways. Scientific Reports 7(1): 3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kriegel AJ, Baker MA, Liu Y, et al. (2015) Endogenous microRNAs in human microvascular endothelial cells regulate mRNAs encoded by hypertension-related genes. Hypertension 66(4): 793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Colón-Bernal ID, Duong LT, Pennypacker B, et al. (2018) Cathepsin K inhibition preserves compressive load in lumbar vertebrae of osteoporotic monkeys. Bone Reports 9: 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Segredo -Morales E, García-García P, Reyes R, et al. (2018) Bone regeneration in osteoporosis by delivery BMP-2 and PRGF from tetronic-alginate composite thermogel. International Journal of Pharmaceutics 543(1–2): 160–168. [DOI] [PubMed] [Google Scholar]
- 41. Lacativa PG, Farias ML. (2010) Osteoporosis and inflammation. Arquivos Brasileiros De Endocrinologia E Metabologia 54(2): 123–132. [DOI] [PubMed] [Google Scholar]
- 42. Kudo K, Hagiwara S, Hasegawa A, et al. (2011) Cepharanthine exerts anti-inflammatory effects via NF-κB inhibition in a LPS-induced rat model of systemic inflammation. The Journal of surgical research 171(1): 199–204. [DOI] [PubMed] [Google Scholar]
- 43. Ho Y, Sh Yang YC, Chin YT, et al. (2018) Resveratrol inhibits human leiomyoma cell proliferation via crosstalk between integrin αvβ3 and IGF-1R. Food Chem Toxicol 120: 346–355. [DOI] [PubMed] [Google Scholar]
- 44. Rodríguez-Enríquez S, Pacheco-Velázquez SC, Marín-Hernández Á, et al. (2019) Resveratrol inhibits cancer cell proliferation by impairing oxidative phosphorylation and inducing oxidative stress. Toxicology and Applied Pharmacology 370: 65–77. [DOI] [PubMed] [Google Scholar]
- 45. Flores- Pérez A, Elizondo G. (2018) Apoptosis induction and inhibition of HeLa cell proliferation by alpha-naphthoflavone and resveratrol are aryl hydrocarbon receptor-independent. Chemico-Biological Interactions 281: 98–105. [DOI] [PubMed] [Google Scholar]
- 46. Geng W, Guo X, Zhang L, et al. (2018). Resveratrol inhibits proliferation, migration and invasion of multiple myeloma cells via NEAT1-mediated Wnt/β-catenin signaling pathway. Biomedicine & Pharmacotherapy 107: 484–494. [DOI] [PubMed] [Google Scholar]
- 47. Almeida TC, Guerra CCC, De Assis BLG, et al. (2019) Antiproliferative and toxicogenomic effects of resveratrol in bladder cancer cells with different TP53 status. Environ. Mental and Molecular Mutagenesis 60(8): 740–751. [DOI] [PubMed] [Google Scholar]
- 48. Zhou H, Shang L, Li X, et al. (2009) Resveratrol augments the canonical Wnt signaling pathway in promoting osteoblastic differentiation of multipotent mesenchymal cells. Experimental Cell Research 315(17): 2953–2962. [DOI] [PubMed] [Google Scholar]
- 49. Song LH, Pan W, Yu YH, et al. (2006). Resveratrol prevents CsA inhibition of proliferation and osteoblastic differentiation of mouse bone marrow-derived mesenchymal stem cells through an ER/NO/cGMP pathway. Toxicology in Vitro 20(6): 915–922. [DOI] [PubMed] [Google Scholar]