Abstract
Background: Following availability in the United States in 2011, intravenous acetaminophen (IV APAP) was added to many hospital formularies for multimodal pain control. In 2014, the price of IV APAP increased from $12/g to $33/g and became a top 10 medication expenditure at our institution. Objective: To promote appropriate IV APAP prescribing and reduce costs. Design, Setting, Participants: Quality improvement project at a 562-bed academic medical center involving all inpatient admissions from 2010 to 2017. Interventions: Using Plan-Do-Study-Act (PDSA) methodology, our Pharmacy & Therapeutics (P&T) committee aimed to reduce inappropriate use of IV APAP by refinement of restriction criteria, development of clinical decision support in the electronic medical record, education of clinical staff on appropriate use, and empowerment of hospital pharmacists to enforce restrictions. Measurements: Monthly IV APAP utilization and spending were assessed using statistical process control charts. Balancing measures included monthly usage of IV opioid, IV ketorolac, and oral ibuprofen. Results: Five PDSA cycles were conducted during the study period. Monthly spending on IV APAP decreased from the highest average of $56 038 per month to $5822 per month at study conclusion. Interventions resulted in an 80% annual cost savings, or an approximate savings of $600 000 per year. Usage of IV opioids, IV ketorolac, and oral ibuprofen showed no major changes during the study period. Conclusions: IV APAP can be restricted in a safe and cost effective manner without concomitant increase in IV opioid use.
Keywords: pharmacy & therapeutics, intravenous acetaminophen, quality improvement, opioids, Plan-Do-Study-Act, acetaminophen, multimodal analgesic approach
Introduction
Opioid analgesia is traditionally the mainstay of pain management, especially in the inpatient setting for acute and severe pain.1,2 While effective for pain management, opioids are associated with many costly adverse effects such as ileus, decreased respiratory drive, delirium, pruritus, and potential for addiction.1 Recently, the “multimodal analgesic approach” to pain control through adjunctive use of nonopioid medications (eg, acetaminophen [APAP], nonsteroidal anti-inflammatory (NSAIDs), and gabapentin) has become favored. However, few nonopioid analgesics, with the exception of NSAIDs, were available until recently for patients unable to take oral medications. In the United States, parenteral ketorolac and ibuprofen are available, and several studies have demonstrated that NSAIDs used adjunctively to manage postoperative pain can decrease opioid requirements and adverse events.3,4However, ketorolac and intravenous (IV) ibuprofen use is limited due to risks of gastrointestinal bleeding and renal impairment.5,6 Hence, there is a need for additional IV nonopioid analgesics.
Acetaminophen, or acetyl-para-aminophenol, was brought to market in 1955 for treatment of mild pain and fever and until recently, was limited to oral (PO) and rectal (PR) formulations.7 In 2002, IV APAP was introduced in Europe, providing a new class of parenteral analgesia. IV APAP was subsequently approved for use in the United States in November 2010 and commercially available in January 2011. The introduction of IV APAP provided a convenient parenteral analgesic option for use in acute care or perioperative settings, allowing for early initiation of multimodal analgesia.
IV APAP has been shown to have quicker onset of action and higher peak serum drug levels compared with PO or PR administration.8,9 Peak plasma concentration occurs 15 minutes after administration and approximately 30 minutes faster than PO administration. This is due to the fact that IV infusion does not undergo first-pass hepatic metabolism. When compared, the initial onset of analgesia in adults undergoing teeth extraction was achieved in 3 minutes after a 2-minute IV bolus and 5 minutes after a 15-minute infusion of propacetamol 2 grams IV (equivalent to 1 gram IV APAP), compared with 11 minutes after oral administration of 1 gram APAP.9 Adult studies have suggested that IV APAP may reduce opioid use and length of stay (LOS) in select patient populations, including some postoperative patients (ie, total hip and knee replacement, adult tonsillectomy, endoscopic thyroidectomy).10-13 However, most efficacy studies have had methodologic concerns including retrospective design, single-center, lack of blinding or randomization, and lack of head-to-head comparison with PO/PR routes.
While IV APAP is often prescribed in pediatrics,14 high-quality data are lacking and benefits remain inconclusive. Studies involving noncardiac surgeries showed no differences in postoperative nausea vomiting (PONV), pain scores, or time spent in the recovery unit with use of IV APAP compared with control (both opioid and nonopioid medications).15,16 For tonsillectomy, 1 comparative study reported that patients receiving IV APAP were more likely to require opioids compared with those who received intramuscular meperidine (P < .01).17 However, patients receiving IV APAP were less sedated and ready for discharge from the postoperative recovery unit sooner (P = .045). In a separate study, patients who received 1 dose of IV APAP for tonsillectomy were less likely to receive postoperative opioids than those who did not; however, overall opioid consumption was similar between the groups (0 vs 0.033 μg/kg, P = .61).18
The incorporation of IV APAP into surgical protocols as “fast-track” programs has become increasingly popular to provide adjuvant pain relief and promote early discharge.19,20 These fast-track cardiac protocols have demonstrated decreased opioid consumption, adequate analgesia, decreased side effects, and early extubation.21-26 While data are promising, use of IV APAP was one component of a multicomponent protocol; therefore, benefits were not able to be directly attributed to IV APAP.
Within the first 15 weeks of product launch in the United States, IV APAP was adopted to 675 hospital formularies, including ours, at a cost of $10/g.27 By the end of 2013, it was on formulary in over 2400 hospitals.7 In 2014, the price of IV APAP increased to $33/g, and became one of the top 10 drug expenditures at our institution. This article summarizes the quality improvement (QI) initiatives by our institution’s Pharmacy & Therapeutics (P&T) committee in addressing the high cost of IV APAP and promoting appropriate use.
Methods
Setting and Context
Our setting is a 562-bed academic medical center with a 151 bed children’s hospital with a “hospital-within-hospital” structure, located in Portland, Oregon. Our inpatient pharmacy dispenses approximately 4 million medication doses per year and had an annual pharmaceutical expense budget of $43 million in 2018. Institution-wide medication management decisions are determined by a P&T committee, which is a multidisciplinary, interprofessional committee comprised of physicians, pharmacists, nurses, and hospital administrators.
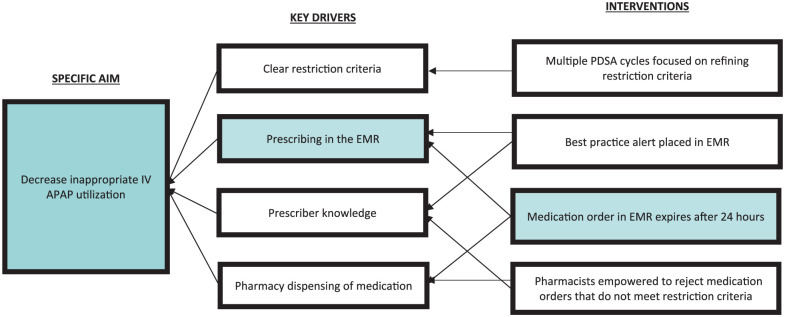
IV APAP was approved by P&T for addition to the hospital formulary in March 2011 with restrictions to adult patients unable to take PO APAP or pediatric patients unable to take PO or PR APAP (Table 1). Use was limited to 24 hours unless approved by the hospital’s pain service team. P&T reexamined cost and utilization data after 3 months and at various intervals over the next 6 years. Using the QI and Plan-Do-Study-Act (PDSA) methodology, multiple changes to restriction criteria were made. Table 1 shows the timeline of events, including PDSA cycles and restriction changes. Figure 1 shows the key driver diagram with the factors and key drivers that directed the work of the P&T committee in crafting restrictions and interventions. All restriction changes were communicated via several mechanisms: clinical decision support (CDS) at point of order entry within the electronic medical record (EMR) and email communication from P&T to its subcommittee members and their constituents.
Table 1.
Timeline of Events Regarding IV APAP.
| Date | Events |
|---|---|
| November 2010 | IV APAP (Ofirmev) approved by the Food and Drug Administration |
| January 2011 | IV APAP commercially available in the United States |
| April 2011 | IV APAP added to institutional formulary with restrictions: Patients unable to take oral APAP, or if a pediatric patient, unable to take oral or per rectal administration of APAP. Use is limited to 24-hour use unless approved by Pain Service. The use will be assessed at 3 months to determine if additional restrictions are necessary. |
| July 2011 | PDSA 1: Reviewed institution usage per restriction criteria and cost (planned medication use evaluation) Revised restrictions to: Restricted to patients who cannot take anything by mouth, including medications, food or water (strictly nil per os), unless approved by the hospital pain service or anesthesia. |
| May 2014 | IV APAP price increased from $12/g to $33/g |
| June 2014 | PDSA 2: Performed MUE and reviewed restriction criteria Surveyed other academic medication centers regarding IV APAP formulary status and restrictions Elected to keep IV APAP on formulary but revised restrictions: Each order is restricted to 24 hours. Order must be reentered every 24 hours. |
| September 2016 | PDSA 3 Revised restriction criteria to: Patients unable to take PO/PFT AND neutropenic (ANC <500) OR Patients unable to take PO/PFT AND also unable to receive per rectum (eg, surgical removal of rectum). |
| January 2017 | PDSA 4 Revised restriction criteria to: Patients unable to take PO/PFT AND neutropenic (ANC <500) OR Patients unable to take PO/PFT AND also unable to receive per rectum (eg, significant rectal pathology, psychosocial concerns, <5 kg) |
| April 2017 | PDSA 5 Revised restriction criteria to: Patients unable to take PO/PFT AND neutropenic (ANC <500) OR Patients unable to take PO/PFT AND also unable to receive per rectum (eg, significant rectal pathology, history of sexual abuse, <5 kg) |
Note. IV = intravenous; APAP = acetaminophen; PDSA = Plan-Do-Study-Act; MUE = medication use evaluation; PO/PFT = oral/per-feeding-tube; ANC = absolute neutrophil count.
Figure 1.
Key drivers diagram.
Note. PDSA = Plan-Do-Study-Act; IV = intravenous; APAP = acetaminophen; EMR = electronic medical record.
Interventions
PDSA 1
After 3 months on formulary, IV APAP spending was projected at $2 million annually, which would make it one of the top medication expenditures at our institution. In response, P&T collaborated with the institution’s pain services to identify opportunities to reduce use. From these discussions, the definition of nil per os (NPO) in the restriction criteria was clarified to only patients who cannot take medications, food, or water by mouth, unless approved by the hospital pain service or anesthesia.
PDSA 2
In May 2014, the price of IV APAP increased from $12/g to $33/g, becoming one of the top 10 medication expenditures within the institution, and prompting P&T to reevaluate usage. A retrospective review was conducted of a randomized sample of 10% of IV APAP orders over the preceding 3 months. This review found that over 44% of orders, accounting for 68% of doses, did not meet restriction criteria, resulting in an estimated $427 000/y spent on inappropriate doses.
Institutional and national attitudes around prescribing of IV APAP were also assessed. First, discussions with key stakeholders occurred during P&T and subcommittee meetings. Second, providers who had ordered IV APAP in the preceding 8 months were sent a web-based survey about prescribing opinions. Third, a separate web-based survey was sent to a university health-system pharmacy consortium listserv about inpatient formulary status. Of the 30 institutions to respond to this survey, 4 had removed IV APAP from formulary, 6 had placed additional restriction criteria, 18 were considering placing additional restriction criteria, and 2 were removing it from formulary.
In July 2014, P&T voted to keep IV APAP on formulary with strict enforcement of the 24-hour stop time by automating the discontinue time in the EMR.
PDSA 3
In January 2016, a 10% assessment of orders from August 2014 to September 2015 continued to show 40% not meeting inclusion criteria, corresponding to $120 000/y of inappropriate expense.
In September 2016, after receiving input from key stakeholders, 41% of P&T members voted to remove IV APAP from formulary, while 59% voted to retain it on formulary with additional restrictions. The new restriction criteria further clarified that NPO status also included patients unable to receive medication per feeding tube (PFT), and that IV APAP was acceptable in neutropenic patients to avoid PR administration (Table 1). With the backing of the institution, clinical pharmacists were also empowered to enforce the restriction criteria at the point of order verification.
PDSA 4
In October 2016, restriction criteria were reevaluated by the pediatric P&T subcommittee given new concerns presented by the neonatal intensive care unit (NICU) and pediatric intensive care unit (PICU) groups. NICU stakeholders felt IV APAP was necessary for premature infants who were NPO given that many patients were too small for rectal APAP administration. As utilization within the NICU was low (estimated $6000/y), stakeholders felt their population should be exempt from the restriction. PICU providers were concerned with pain control in patients following cardiac surgery and in children who could hypothetically receive rectal medication, but in whom doing so would cause considerable distress, such as children following physical or sexual abuse, and adolescents. In response to these concerns, restriction criteria were updated to allow use in premature infants and in patients “unable to receive per rectum (eg, significant rectal pathology, psychosocial concerns, <5 kg).”
PDSA 5
In April 2017, ongoing usage analysis revealed that many patients had been administered IV APAP for “psychosocial concerns” per the EMR, but in whom no history of abuse was present. This terminology was removed and the language was revised to patients with a “history of sexual abuse” to avoid therapeutic creep. As usage maintained acceptable levels, no further P&T discussions occurred during the study period regarding IV APAP.
Measures and Analysis
The main outcomes for this study were monthly consumption (based on grams of IV APAP administered) and cost, which was adjusted for inflation to 2018 USD, from January 2011 to February 2018. Monthly consumption data were also adjusted to account for variance in the hospital census based on 1000-inpatient days. Adult and pediatric data were combined. Consumption of IV opioid (eg, fentanyl, morphine, hydromorphone) and NSAIDs (eg, ibuprofen, ketorolac) from June 2010 to December 2017 were analyzed as balancing measures. IV opioids were converted to milligrams of morphine equivalents (MME) in thousands for comparison. Continuous opioid infusions, intraoperative doses, and patient-controlled analgesics (PCAs) were excluded due to the inability to accurately quantify analgesic doses administered retrospectively from the EMR. In addition, use of IV APAP as adjunctive analgesia would likely not impact patients with severe pain requiring continuous opioids or PCAs. Outpatient doses of analgesics were also excluded because the formulary restriction for IV APAP was limited to the inpatient setting. Microsoft Excel QI Macros was used to create statistical process control (SPC) charts. Centerlines (means) within SPC charts were adjusted according to established SPC rules.
Results
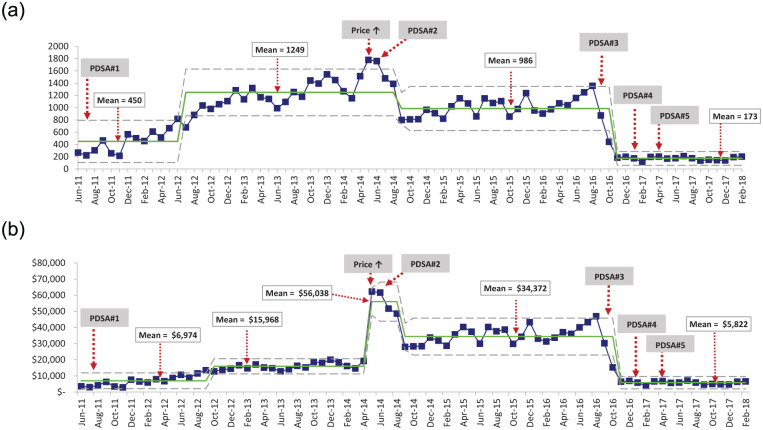
Following formulary addition in April 2011, IV APAP usage increased linearly over time from June 2011 when the first orders were placed until June 2014 (Figure 2a). Mean usage from June 2011 to June 2012 was 450 g/month, or 32 g/month/1000-inpatient days when normalized against the hospital census. The centerline was adjusted upward in July 2012 to a mean of 1249 g/month (92 g/month/1000-inpatient days) and remained at this level until September 2014. Two astronomical points were recorded during May and June of 2014. Following PDSA 2, utilization decreased in September 2014 to a mean of 986 g/month (71 g/month/1000-inpatient days). Following PDSA 3, the centerline shifted down to 173 g/month (13 g/month/1000-inpatient days) and remained at this level for the remainder of the study period. The final IV APAP mean of 173 g/month or 13 g/month/1000-inpatient days represented an 86% reduction in unadjusted and adjusted utilization for the hospital census from the highest average of 1249 g/month (92 g/month/1000-inpatient days).
Figure 2.
(a) IV APAP usage (grams)/month. (b) IV APAP spending by month adjusted for inflation using XmR SPCs where each dot represents 1 month of data.
Note. Center solid line shows average usage (a) and spending (b) for the intervals between major process change. Dotted lines illustrate upper and lower limits. IV = intravenous; APAP = acetaminophen; SPC = statistical process control; PDSA = Plan-Do-Study-Act.
IV APAP expenditure closely mirrored utilization (Figure 2b). The initial spending of $6974/month persisted during the first year, then shifted up to $15 968 in October 2012. As a result of a 3-fold increase in price, monthly spending increased by 350% to $56 038/month in May 2014. Following PDSA 2, spending decreased in September 2014 to $34 372/month. Following PDSA 3, spending decreased to $5822/month and remained constant through the end of the study period. The final monthly expenditure resulted in a savings of $28 550/month (83%) or $50 216/month (90%) when compared with the previous mean or the highest mean, respectively.
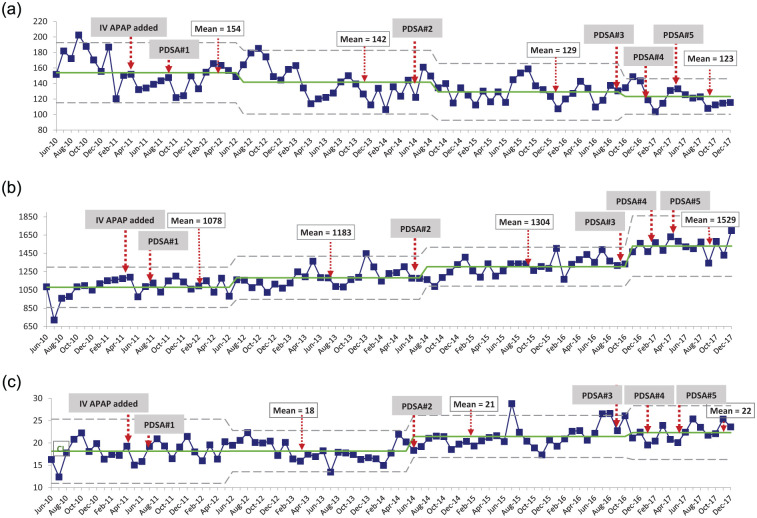
Balancing measures consisted of monthly utilization of IV opioids and NSAIDs from January 2010 to December 2017 (Figure 3). There was a downward trend in IV opioid utilization within the institution over the study period (Figure 3a). The mean MME (in thousands) per month reduced by 20% from 154 MME (June 2010-June 2012) to 123 MME (October 2016-December 2017). Ibuprofen and ketorolac utilization both increased modestly during the study period (Figure 3b and c). Mean ibuprofen use increased by 42% from 1078 grams/month (June 2010-June 2012) to 1529 grams/month (December 2016-December 2017). Mean ketorolac utilization increased by 22% from 18 g/month (June 2010-June 2014) to 22 g/month (November 2016-December 2017). None of these trends seemed associated with IV APAP restriction or PDSA cycles.
Figure 3.
(a) Intravenous opioid, (b) ibuprofen, and (c) ketorolac use by month analyzed by XmR SPCs, where the center solid line shows mean usage and dotted lines show upper and lower limits.
Note. SPC = statistical process control; MME = milligrams of morphine equivalents; IV = intravenous; APAP = acetaminophen; PDSA = Plan-Do-Study-Act.
Discussion
Using an iterative process involving 5 PDSA cycles over 6 years, we were able to significantly and sustainably reduce IV APAP utilization at our institution, with cost savings of approximately $600 000/y. Several mechanisms were vital for achieving lowered usage. First, restriction criteria were continually refined, resulting in greater specificity and clarity for ordering providers. Second, EMR capabilities were utilized in the form of clinical decision support (CDS) such as restriction criteria language at point of physician order entry, order specific questions such as “is the patient NPO?” that require physician answer selection, and an automatic 24-hour order expiration. Third, pharmacists were empowered to act as stewards and gatekeepers of medication delivery. Importantly, opioid use within the institution did not increase as a result of restrictions placed on IV APAP, and instead, showed a gradual reduction over the study period.
This study highlights the difficulty of managing a high-cost, high-demand medication in a setting that involves a diverse group of stakeholders, including pediatric and adult providers. It also illustrates the importance of maintaining an equally diverse group of members on P&T committees to address the various issues that arise for different patient populations in an informed and equitable manner. The fact that the final restriction criteria required 6 years and 5 PDSA cycles to optimize reflects these challenges.
This study also highlights the value and limitations of the EMR in influencing prescriber behavior. Although language around restriction criteria became more specific with each PDSA cycle, we believe the more important driver of decreased utilization related to 2 other interventions: 24-hour order expiration and empowerment of pharmacists as gatekeepers. Use of CDS, such as clarifying questions to prescribers at the point of order entry, and automatic 24-hour order expiration, remain important tools within the EMR for guiding prescribing habits and curtailing use of high-cost medications.28 Empowerment of pharmacists to act in stewardship or gatekeeper roles, as demonstrated in antimicrobial stewardship programs, also represents a powerful tool to reduce waste.29 However, given the culturally ingrained power structures inherent to most institutions, pharmacist stewardship efforts will only be successful if supported by institutional leadership and policies. We were fortunate in this study to have such support.
One of the strengths of our study was the inclusion of monthly IV opioid consumption as a balancing measure for IV APAP restriction which has yet to be reviewed in prior publications. A previously published study on the impact of formulary initiative showed postoperative IV APAP use decreased by 80%; however, balancing measures were other nonopioid analgesics.30 Because decreased IV opioid utilization is a purported benefit of IV APAP, we wanted to make sure IV APAP restriction did not increase IV opioid use. While our study showed no increase in IV opioid use with IV APAP restriction, there is an important caveat to these findings. The study period, 2010 to 2017, corresponded to the rise and recognition of the opioid epidemic.31 Similar to other institutions, our institution incorporated multiple systems changes to reduce opioid prescribing during this time, which limits interpretation of these findings. This factor may also explain the increase in NSAID prescribing (ketorolac, ibuprofen) seen in our study.
This study has several limitations. First, the study was conducted within a single, medium-sized academic medical center, and therefore may not be fully generalizable to other settings. Second, given the retrospective study design and heterogeneous patient population receiving IV APAP, we were unable to assess the effect of IV APAP restriction on LOS. Third, the balancing measures of IV opioid and NSAIDs consumption were confounded by other efforts within the institution that may have directly impacted these measures. The difficulty in finding pure balancing measures, unaffected by other changes in complex healthcare systems, remains a challenging dilemma for QI projects. Similarly to a previous study on formulary management of IV APAP,30 pain scores were not evaluated due the limitations of obtaining reliable and consistent data retrospectively from the EMR. However, use of balancing measures assessing consumption of other analgesics was used to mitigate this limitation. Prospective, randomized controlled trials on specific patient populations with appropriate comparison groups are needed to determine whether IV APAP provides benefit in regard to length of stay, pain reduction, and opioid consumption for specific patient populations.
Conclusion
Through an iterative process, using QI methodology, we demonstrated the ability to restrict IV APAP in a manner that resulted in considerable cost savings to the institution, while at the same time allowing the medication to be used for indicated purposes. Keys to our success included the involvement of a multidisciplinary, interprofessional P&T committee, continuous resource reviews, refinement of restriction criteria via PDSA methodology, enhanced EMR design, and empowerment of pharmacists to be gatekeepers. These results reinforce the importance of expanding the scope of P&T committees to include continuous QI initiatives.
Acknowledgments
The authors acknowledge Yen Pham, RPh, Mike Bonazzollo, MD, FACP, and Rebecca Britton, PharmD, and the members of the Clinical Knowledge and Therapeutics Executive Committee and reporting committees and subcommittees whose hard work and dedication contributed to the success of this project. Acknowledgment to Ken Pickron for assisting with data collection.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Considerations: The study was deemed nonhuman subjects research by the Oregon Health & Science University Institutional Review Board (IRB#00018143).
References
- 1. Argoff CE. Recent management advances in acute postoperative pain. Pain Pract. 2014;14(5):477-487. [DOI] [PubMed] [Google Scholar]
- 2. Brasher C, Gafsous B, Dugue S, et al. Postoperative pain management in children and infants: an update. Paediatr Drugs. 2014;16(2):129-140. [DOI] [PubMed] [Google Scholar]
- 3. Kim SY, Kim EM, Nam KH, Chang DJ, Nam SH, Kim KJ. Postoperative intravenous patient-controlled analgesia in thyroid surgery: comparison of fentanyl and ondansetron regimens with and without the nonsteroidal anti-inflammatory drug ketorolac. Thyroid. 2008;18:1285-1290. [DOI] [PubMed] [Google Scholar]
- 4. Pavy TJ, Paech MJ, Evans SF. The effect of intravenous ketorolac on opioid requirement and pain after cesarean delivery. Anesth Analg. 2001;92:1010-1014. [DOI] [PubMed] [Google Scholar]
- 5. Ketorolac Tromethamine (Toradol) [package insert]. Nutley, NJ: Roche Pharmaceuticals; 2007. [Google Scholar]
- 6. Ibuprofen (Caldolor) Injection [package insert]. Nashville, TN: Cumberland Pharmaceutical; 2016. [Google Scholar]
- 7. Candence Pharmaceuticals. Corporate presentation. https://www.sec.gov/Archives/edgar/data/1333248/000119312511160819/dex991.htm. Published February 2014. Accessed June 5, 2018.
- 8. Singla NK, Parulan C, Samson R, et al. Plasma and cerebrospinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Pract. 2012;12(7):523-532. [DOI] [PubMed] [Google Scholar]
- 9. Moller PL, Sindet-Pedersen S, Petersen CT, Juhl GI, Dillenschneider A, Skoglund LA. Onset of acetaminophen analgesia: comparison of oral and intravenous routes after third molar surgery. Br J Anaesth. 2005;94:642-648. [DOI] [PubMed] [Google Scholar]
- 10. Yang L, Du S, Sun Y. Intravenous acetaminophen as an adjunct to multimodal analgesia after total knee and hip arthroplasty: a systematic review and meta-analysis. Int J Surg. 2017;47:135-146. [DOI] [PubMed] [Google Scholar]
- 11. Liang L, Cai Y, Li A, Ma C. The efficiency of intravenous acetaminophen for pain control following total knee and hip arthroplasty: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(46):e8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Oliveira GS, Jr, Castro-Alves LJ, McCarthy RJ. Single-dose systemic acetaminophen to prevent postoperative pain: a meta-analysis of randomized controlled trials. Clin J Pain. 2015;31(1):86-93. [DOI] [PubMed] [Google Scholar]
- 13. McNicol ED, Ferguson MC, Haroutounian S, Carr DB, Schumann R. Single dose intravenous paracetamol or intravenous propacetamol for postoperative pain. Cochrane Database Syst Rev. 2016;5:CD007126. doi: 10.1002/14651858.CD007126.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shastri N. Intravenous acetaminophen use in pediatrics. Pediatr Emerg Care. 2015;31(6):444-448. [DOI] [PubMed] [Google Scholar]
- 15. Ceelie I, de Wildt SN, van Dijk M, et al. Effect of intravenous paracetamol on postoperative morphine requirements in neonates and infants undergoing major noncardiac surgery: a randomized controlled trial. JAMA. 2013;309:149-154. [DOI] [PubMed] [Google Scholar]
- 16. Kocum AI, Sener M, Caliskan E, et al. Intravenous paracetamol and dipyrone for postoperative analgesia after day-case tonsillectomy in children: a prospective, randomized, double blind, placebo controlled study. Braz J Otorhinolaryngol. 2013;79(1):89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alhashemi JA, Daghistani MF. Effects of intraoperative i.v. acetaminophen vs i.m. meperidine on post-tonsillectomy pain in children. Br J Anaesth. 2006;96(6):790-795. [DOI] [PubMed] [Google Scholar]
- 18. Bowman B, Sanchez L, Sarangarm P. Perioperative intravenous acetaminophen in pediatric tonsillectomies. Hosp Pharm. 2018;53(5):316-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iodice FG, Thomas M, Walker I, Garside V, Elliott MJ. Analgesia in fast-track paediatric cardiac patients. Eur J Cardiothorac Surg. 2011;40(3):610-613. [DOI] [PubMed] [Google Scholar]
- 20. Olbrecht VA, Ding L, Spruance K, Hossain M, Sadhasivam S, Chidambaran V. Intravenous acetaminophen reduces length of stay via mediation of postoperative opioid consumption after posterior spinal fusion in a pediatric cohort. Clin J Pain. 2018;34:593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mamoun NF, Lin P, Zimmerman NM, et al. Intravenous acetaminophen analgesia after cardiac surgery: a randomized, blinded, controlled superiority trial. J Thorac Cardiovasc Surg. 2016;152(3):881-889. [DOI] [PubMed] [Google Scholar]
- 22. White PF, Kehlet H, Neal J, Schricker T, Carr DB, Carli F. The role of the anesthesiologist in fast-track surgery: from multimodal analgesia to perioperative medical care. Anesth Analg. 2007;104(6):1380-1396. [DOI] [PubMed] [Google Scholar]
- 23. Yanatori M, Tomita S, Miura Y, Ueno Y. Feasibility of the fast-track recovery program after cardiac surgery in Japan. Gen Thorac Cardiovasc Surg. 2007;55:445-449. [DOI] [PubMed] [Google Scholar]
- 24. Flynn M, Reddy S, Shepherd W, et al. Fast-tracking revisited: routine cardiac surgical patients need minimal intensive care. Eur J Cardiothorac Surg. 2004;25:116-122. [DOI] [PubMed] [Google Scholar]
- 25. Cattabriga I, Pacini D, Lamazza G, et al. Intravenous paracetamol as adjunctive treatment for postoperative pain after cardiac surgery: a double blind randomized controlled trial. Eur J Cardiothorac Surg. 2007;32:527-531. [DOI] [PubMed] [Google Scholar]
- 26. Akhtar MI, Hamid M, Minai F, et al. Safety profile of fast-track extubation in pediatric congenital heart disease surgery patients in a tertiary care hospital of a developing country: an observational prospective study. J Anaesthesiol Clin Pharmacol. 2013;30(3):355-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cadence Pharmaceuticals. Corporate overview. https://www.sec.gov/Archives/edgar/data/1333248/000119312511160819/dex991.htm. Published June 2011. Accessed June 5, 2018.
- 28. Beeler PE, Bates DW, Hug BL. Clinical decision support systems. Swiss Med Wkly. 2014;144:w14073. [DOI] [PubMed] [Google Scholar]
- 29. Shea KM, Hobbs ALV, Jaso TC, et al. Effect of a health care system respiratory fluoroquinolone restriction program to alter utilization and impact rates of Clostridium difficile infection. Antimicrob Agents Chemother. 2017;61(6):e00125-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vincent WR, III, Huiras P, Empfield J, et al. Controlling postoperative use of I.V. acetaminophen at an academic medical center. Am J Health Syst Pharm. 2018;75(8):548-555. [DOI] [PubMed] [Google Scholar]
- 31. Centers for Disease Control and Prevention. Addressing prescription drug abuse in the United States: current activities and future opportunities. U.S. Department of Health and Human Services. Date unknown. www.cdc.gov/drugoverdose/pdf/hhs_prescription_drug_abuse_report_09.2013.pdf. Accessed October 29, 2018.