Abstract
Background
This study provides a detailed imaging assessment in a large series of COVID-19 patients with neurological manifestations.
Purpose
To review the MRI findings associated with acute neurological manifestations in COVID-19 patients.
Methods
This was a cross-sectional study conducted between March 23 and May 7, 2020 at the Pitié-Salpêtrière University Hospital, a reference center for COVID-19 in the Paris area. Inclusion criteria were: adult patients diagnosed with SARS-CoV-2 infection, presenting with acute neurological manifestations and referred for a brain MRI examination. Patients were excluded if they had a previous history of neurological disease. The characteristics and the frequency of different MRI features were investigated. The findings were analyzed separately in patients in intensive care units (ICU) and other departments (non-ICU).
Results
During the inclusion period, 1176 consecutive patients were hospitalized for suspected COVID-19. Out of 308 patients with acute neurological symptoms, 73 patients met the inclusion criteria (23.7%) and were included: 35 ICU patients (47.9%) and 38 non-ICU patients (52.1%). The mean age was 58.5 ± 15.6 years, with a male predominance (65.8% vs. 34.2%). Forty-three patients presented pathological MRI findings 2-4 weeks after symptom onset (58.9%), including 17 with acute ischemic infarct (23.3%), 1 with a deep venous thrombosis (1.4%), 8 with multiple microhemorrhages (11.3%), 22 with perfusion abnormalities (47.7%), 3 with restricted diffusion foci within the corpus callosum consistent with cytotoxic lesions of the corpus callosum (CLOCC, 4.1%). Multifocal white matter enhancing lesions were seen in 4 ICU patients (5%). Basal ganglia abnormalities were seen in 4 other patients (5%). The cerebrospinal fluid (CSF) analysis was negative for SARS-CoV-2 in all tested patients (n=39).
Conclusion
In addition to cerebrovascular lesions, perfusion abnormalities, CLOCC and ICU-related complications, we identified two patterns including white matter enhancing lesions and basal ganglia abnormalities that could be related to SARS-CoV-2 infection.
Keywords: COVID-19, SARS-CoV-2, neurological manifestations, brain MRI, imaging patterns
Summary
MRI abnormalities included cerebrovascular lesions, perfusion abnormalities, cytotoxic lesions of the corpus callosum, ICU-related complications, white matter enhancing lesions and basal ganglia abnormalities.
Key Results
1. Of the 73 patients who presented neurological symptoms, 43 had pathological MRI findings (58.9%), including 17 with acute ischemic infarcts (23.3%), 1 with a deep venous thrombosis (1.4%), 8 with multiple microhemorrhages (11.3%), 22 with perfusion abnormalities (47.7%), 3 with restricted diffusion foci within the corpus callosum consistent with cytotoxic lesions of the corpus callosum (CLOCC, 4.1%).
2. Imaging patterns possibly related to COVID-19 were observed in patients in intensive care and included multifocal white matter enhancing lesions seen (4 patients, 5%) and basal ganglia abnormalities (4 patients, 5%).
Introduction
Since the coronavirus disease 2019 (COVID-19) outbreak in December 2019, there is growing evidence that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has central nervous system (CNS) involvement in addition to the primary respiratory target. In a series of 214 patients infected with SARS-CoV-2, more than a third of patients had neurological manifestations, such as stroke, seizures and anosmia (1). Clinical reports (2–4) and experiments (5,6) from the previous coronavirus epidemics, namely the severe acute respiratory syndrome (SARS-CoV) in 2002 and the Middle East Respiratory Syndrome (MERS-CoV) in 2012, have established that coronaviruses have a neurotropic and neuroinvasive potential. Coronaviruses enter the CNS using a hematogenous pathway or through the olfactory bulb or peripheral nerves (5,6). Using mice models, the infection has been shown to start in the respiratory epithelium, then spread to the brain via the olfactory bulb, and gradually invade the subcortical and cortical regions (5–7). This olfactory involvement could explain the anosmia observed in a large number of COVID-19 patients. Proposed pathophysiological mechanisms include a direct viral replication and an immune-mediated reaction (5,6). It is not yet known whether this knowledge applies to the new SARS-CoV-2. So far, several imaging findings have been described in the setting of COVID-19, but the relationship with SARS-CoV-2 remains unclear (8–14).
This study reports a series of patients with acute neurological manifestations admitted for COVID-19 and referred for a brain MRI in a Parisian reference center for the disease. We describe the MRI findings and their frequency and investigate whether imaging patterns possibly related to COVID-19 could be identified. These patterns were considered as possibly related to COVID-19 when 1) they were found in ≥3 patients, 2) they were similar across patients, and 3) they were not explained by another pathology or condition.
Material and Methods
Study Design and Participants
The study was conducted according to good clinical practice, sponsored by Assistance Publique-Hôpitaux de Paris, and received approval from the local Sorbonne University Hospital Ethics Committee (CER-202028 on 24/04/2020). All patients or their relative signed written informed consent for the use of their medical data in accordance with the French regulation and the European General Data Protection Regulation (GDPR). The study was registered in the clinicaltrials.gov website (NCT04362930). Patients referred for brain MRI in the context of COVID-19 between March 23 and May 7, 2020 were assessed in the Neuroradiology department of the Pitié-Salpêtrière Hospital, a tertiary neurology center and reference center for COVID-19. The inclusion criteria were: i) diagnosis of SARS-CoV-2 infection by detection of RNA in a nasopharyngeal swab, bronchial aspiration or bronchoalveolar lavage using reverse-transcriptase–polymerase-chain-reaction (RT-PCR) or by chest computed tomography (CT) showing results consistent with SARS-CoV-2-associated pneumonia, ii) presence of acute neurological symptoms, iii) age greater than 18 years and iv) availability of a brain MRI. The exclusion criteria were: i) underlying progressive CNS disease excluding stroke, ii) alternative diagnosis assessed during the MRI examination. A case of deep venous thrombosis from the current series has previously been published (15).
Data Collection
Clinical data collected by expert neurologists, electroencephalogram (EEG), cerebrospinal fluid (CSF) and chest CT findings were retrospectively extracted from electronic medical records. Difficult cases were discussed in multidisciplinary meetings.
MRI Acquisition
Patients were scanned using a 3.0 Tesla MRI system (PREMIER, General Electric Healthcare) with a 48-channel receive head coil. The protocol included three-dimensional (3D) T1-weighted (Magnetization-prepared rapid acquisition with gradient-recalled echo, MPRAGE) and 3D Fluid-attenuated inversion recovery (FLAIR) images, susceptibility-weighted imaging (SWI), 3D pseudo-continuous arterial spin labeling (ASL) perfusion imaging, diffusion-weighted imaging (DWI) sequence, 3D post-gadolinium spin echo T1-weighted imaging. In case of suspicion of acute stroke, axial FLAIR sequence, DWI, SWI, ASL and arterial 3D time-of-flight were acquired.
Image Analysis
Two neuroradiologists (L.C., N.P.) independently analyzed all MRI images. In case of disagreement, images were reviewed and a consensus was reached. The following imaging characteristics were evaluated: diffusion abnormalities, white and gray matter FLAIR signal abnormalities, ASL perfusion abnormalities, parenchymal and meningeal enhancement, hemorrhages, vessel permeability, cranial nerve abnormalities including thickening, abnormal T2 hyperintensity and contrast enhancement.
Statistical Analysis
The age was expressed as mean and standard deviation. Categorical variables were expressed as counts and percentages. Participants were divided into an “ICU” group, including patients hospitalized or discharged from an ICU, and a “non-ICU” group, with patients never hospitalized in ICU. The age was compared between groups using Student’s t-test. Proportions for categorical variables were compared using Pearson's Chi-squared test or Fisher's Exact Test. Statistical analyses were performed with R software (version 3.6.1). The significance threshold was set at a p<0.05.
Results
Patient Recruitment
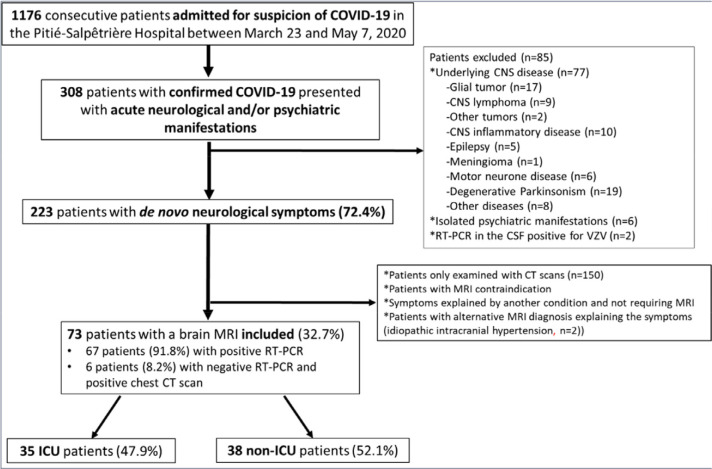
During the inclusion period, 1176 consecutive patients were hospitalized for suspected COVID-19 in the Pitié-Salpêtrière Hospital. Three hundred and eight of these patients (26.2%) presented with neurological and/or psychiatric manifestations, out of whom 223 had de novo acute neurological symptoms (i.e. no previous history of neurological disease) (72.4%). Seventy-three patients met the inclusion criteria (73/223, 32.7%). In these 73 patients, RT-PCR was positive in 67 patients (91.8%). In the 6 remaining patients with negative SARS-CoV-2 RT-PCR (8.2%), chest CT scans were highly suggestive of SARS-CoV-2-associated pneumonia. Thirty-five patients were ICU patients (47.9%) and 38 were non-ICU patients (52.1%). Patient flow chart is detailed in Figure 1.
Figure 1:
Flowchart of patient inclusion. Abbreviations: CNS: central nervous system; COVID-19: coronavirus disease 2019; CSF: cerebrospinal fluid; CT: computed tomography; ICU: intensive care unit; RT-PCR: reverse-transcriptase–polymerase-chain-reaction.
Patient Characteristics
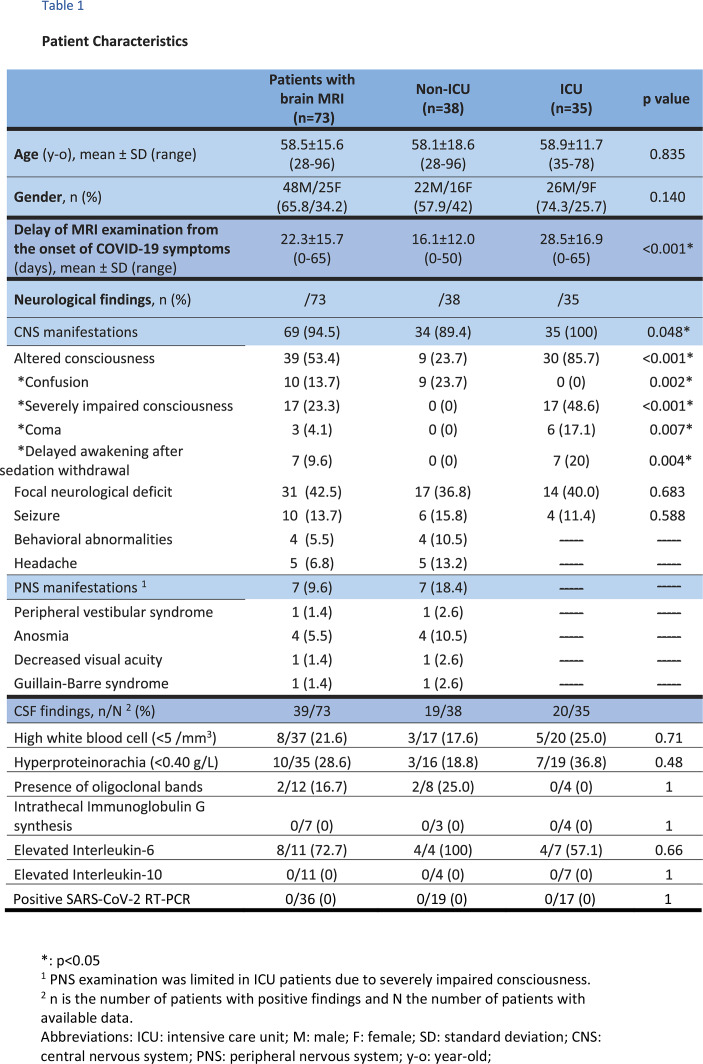
Patient characteristics are summarized in Table 1. The mean age was 58.5±15.6 years (65.8% male), without difference between non-ICU and ICU patients. MRI examinations were performed 22.3±15.7 days (range: 0-65 days, significantly longer in the ICU group, p<0.001) after the onset of COVID-19 symptoms and 5.9±6.7 days (range: 0-30) after from the onset of neurological symptoms. The most frequent neurological manifestations were impaired consciousness not explained by therapy (39/73, 53.4%), focal neurological deficit (31/73, 42.5%) and seizure (10/73, 13.7%). ICU patients had significantly more impaired consciousness than non-ICU patients (85.7% vs. 23.7%, p<0.001), but no difference in the rate of seizures and focal deficits. CSF analysis was available for 39 patients (39/73, 53.4%) showing mild pleiocytosis (8/37, 21.6%) hyperproteinorachia (10/35, 28.6%), oligoclonal bands (2/12, 16.7%) or mild Interleukin 6 increase (8/11, 72.7%). For all patients, RT-PCR for the SARS-CoV-2, herpes simplex viruses 1 and 2, varicella-zoster virus, cytomegalovirus and Epstein-Barr virus as well as the bacterial culture were negative in the CSF. EEG analysis was available for 40 patients (40/73, 54.8%), showing pathological findings related to seizure or encephalopathy in 9 patients (9/40, 22.5%; 2 non-ICU and 7 ICU) and non-specific findings in 24 patients (24/40, 60.0%. 9 non-ICU and 15 ICU). Clinical characteristics of the other non-included patients are provided in Table E1.
Table 1.
Patient Characteristics
MRI Findings
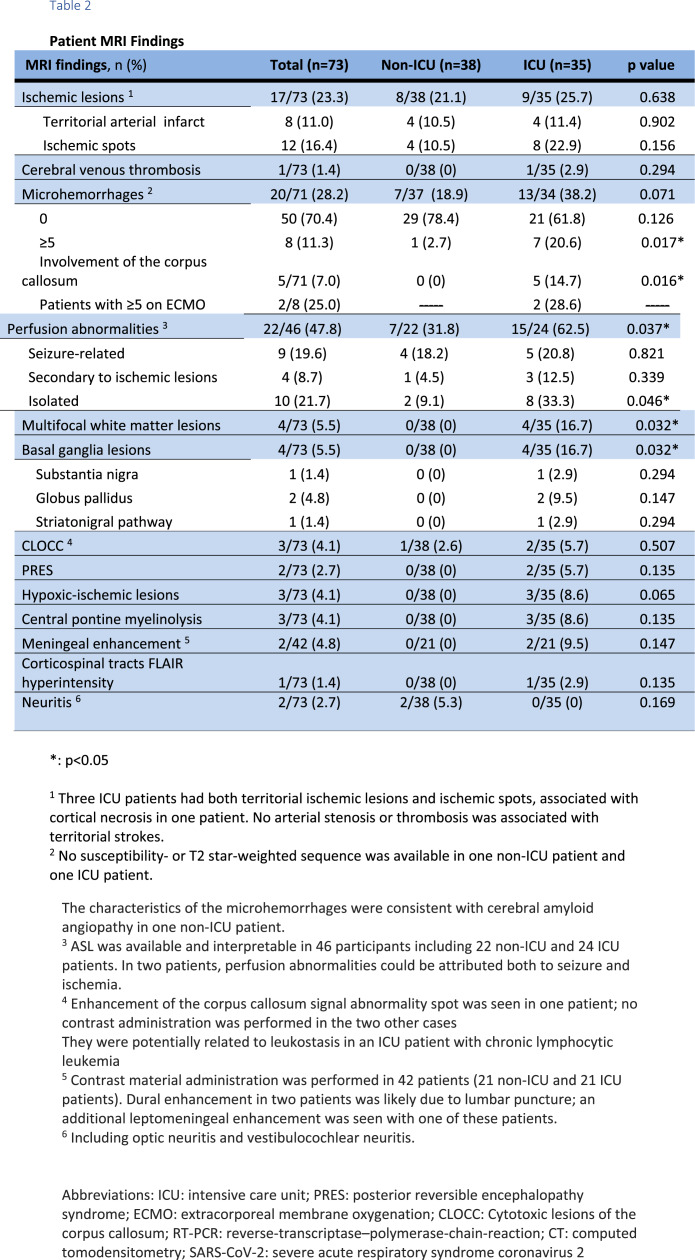
MRI exams revealed no significant abnormalities in 30 patients (22 non-ICU and 8 ICU), apart from changes usually seen in elderly patients, and pathological findings in 43 patients (43/73, 58.9%). Seventeen patients presented with acute ischemic infarct, 1 with a deep venous thrombosis, 8 with multiple microhemorrhages, 9 with seizure-related perfusion abnormalities, 10 with isolated perfusion abnormalities, 4 with multifocal enhancing white matter lesions, 3 with restricted diffusion foci within the splenium of the corpus callosum, 3 with hypoxic-ischemic lesions, 2 with posterior reversible encephalopathy syndrome (PRES), 3 with metabolic abnormalities and 2 with neuritis (Table 2). Clinical details for patients with white matter lesions and basal ganglia abnormalities are presented in Table E2.
Table 2.
Patient MRI Findings
Cerebrovascular complications
Stroke
Twelve of the 17 patients with acute ischemic infarct (70.6%) had multiple ischemic foci while 8 (47.0%) had a territorial infarction (Supplementary Figure E1). Three patients had both types of lesions. There was no significant difference between non-ICU and ICU patients (p=0.638).
Deep cerebral vein thrombosis
Extensive deep cerebral venous thrombosis complicated by hemorrhagic venous infarction was diagnosed in a 72-year-old male ICU patient without known risk factor for thrombosis and with normal baseline coagulation tests (Table E3).
Microhemorrhages
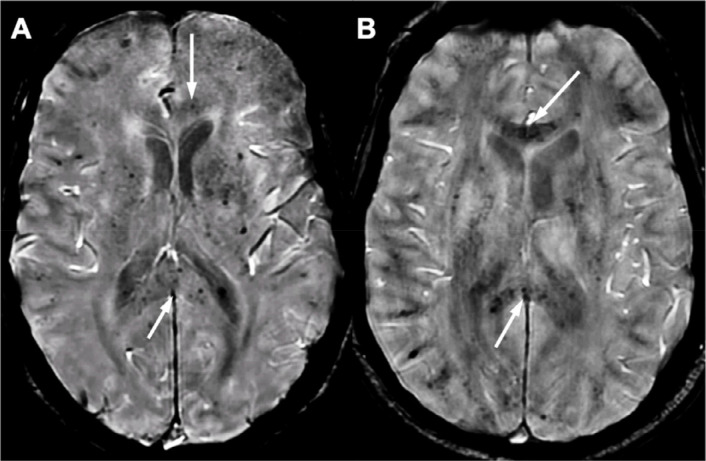
ICU patients had a greater number of multiple microhemorrhages (≥5) than non-ICU patients (20.6% vs. 2.7%, p=0.017), involving the corpus callosum in 5 patients (Figure 2). Patients with microhemorrhages tended to have increased partial thromboplastin time due to preventive anticoagulation therapy (Table E3). Anticoagulation overdose occurred in one ICU patient. Two ICU patients with multiple microhemorrhages (28.6%) had an extracorporeal membrane oxygenation (ECMO).
Figure 2:
Microhemorrhages. Diffuse microhemorrhages involving the corpus callosum in two patients in intensive care on extracorporeal membrane oxygenation (EMCO, A, arrows) and without ECMO (B, arrows) Abbreviations: ECMO: extracorporeal membrane oxygenation.
Perfusion abnormalities
Twenty-two out of 46 patients (47.8%) presented perfusion abnormalities that were related to seizure in nine patients (19.6%), recent or old vascular lesions in four patients (8.7%) and that were unrelated to seizures or ischemia in 10 patients (21.7%). In two patients, perfusion abnormalities were attributed to both seizures and ischemia. ICU patients had more perfusion abnormalities than non-ICU ones (62.5% vs. 31.8%, p=0.037), with a higher rate of isolated perfusion abnormalities (33.3% vs. 9.1%, p=0.046). In particular, marked post-ictal changes associating edema and diffusion restriction within the right frontobasal cortex were observed in a 69-year-old ICU patient who presented with status epilepticus (Supplementary Figure E2).
White matter enhancing lesions
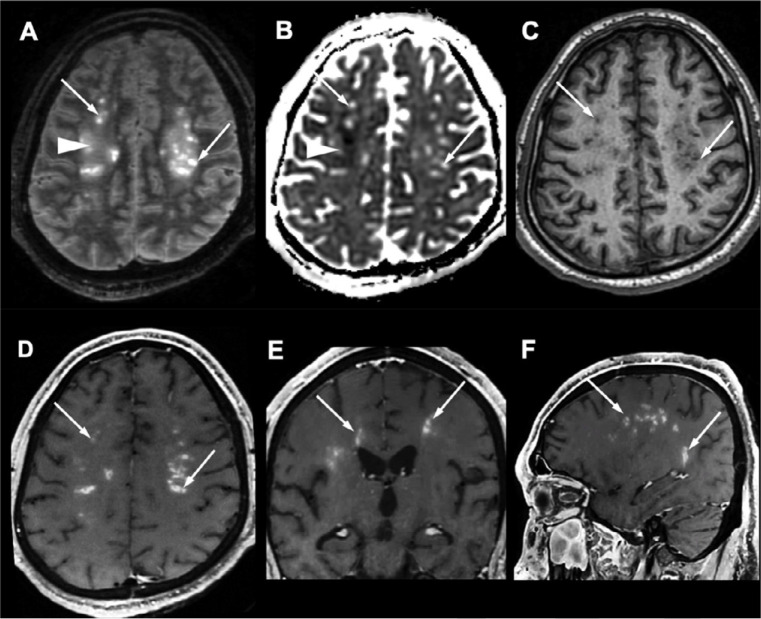
Four ICU patients with late awakening after sedation withdrawal had multifocal bilateral deep and periventricular white matter lesions associated with an enhancement along the perivascular spaces. These lesions had a vacuolated necrotic appearance in one patient (Figures 3 and 4).
Figure 3:
White matter enhancing lesions. A 37-year-old obese male with no medical history, admitted to ICU for SARS-CoV-2-associated pneumonia ten days after the onset of flu-like symptoms, who developed severe acute respiratory syndrome, recurrent venous thrombo-embolism with negative thrombophilia tests, and multiple organ failure. MRI is performed for late awakening following withdrawal of sedation after 38 days of intensive care. The patient did not recover consciousness and died 42 days after the brain MRI. There were symmetrical multifocal periventricular and deep white matter lesions, hyperintense on axial FLAIR images with a vacuolated appearance (A, arrows), without diffusion restriction (B, arrows), hypointense on T1-weighted images (C, arrows), and with a perivascular enhancement on post-contrast T1-weighted images (D E, F, arrows). These lesions were associated with white matter FLAIR hyperintensities (A, arrowhead) with decreased apparent coefficient diffusion (B, arrowhead). Abbreviations: SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
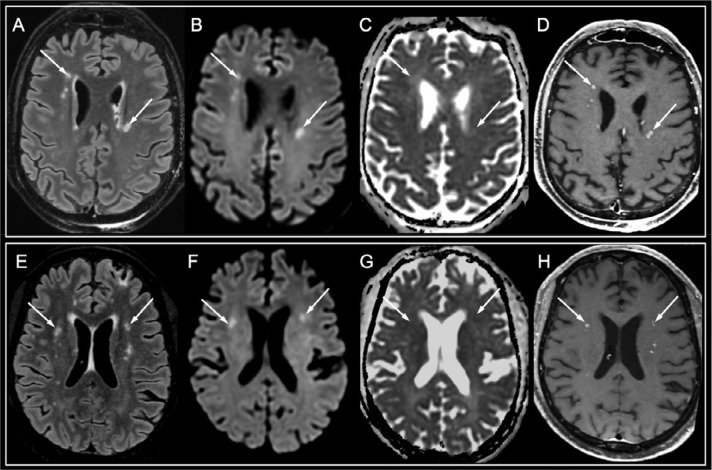
Figure 4:
White matter enhancing lesions. A 50-year-old man with a history of kidney transplantation (A-D) and a 50-year-old ICU man with type 2 diabetes (E-H), who presented with late awakening following sedation withdrawal after 39 and 65 days of intensive care for severe hypoxic SARS-CoV-2-associated pneumonia complicated with severe acute respiratory syndrome, respectively. Both patients progressively recovered consciousness and were able to respond to orders. A similar imaging pattern than that in Figure 4 was seen, but with fewer white matter lesions appearing hyperintense on FLAIR images (A, E, arrows) and diffusion-weighted images (B, F, arrows), without apparent diffusion coefficient decrease (C, G, arrows), with a perivascular enhancement on post-contrast T1-weighted images (D, H, arrows). Abbreviation: SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Basal ganglia abnormalities
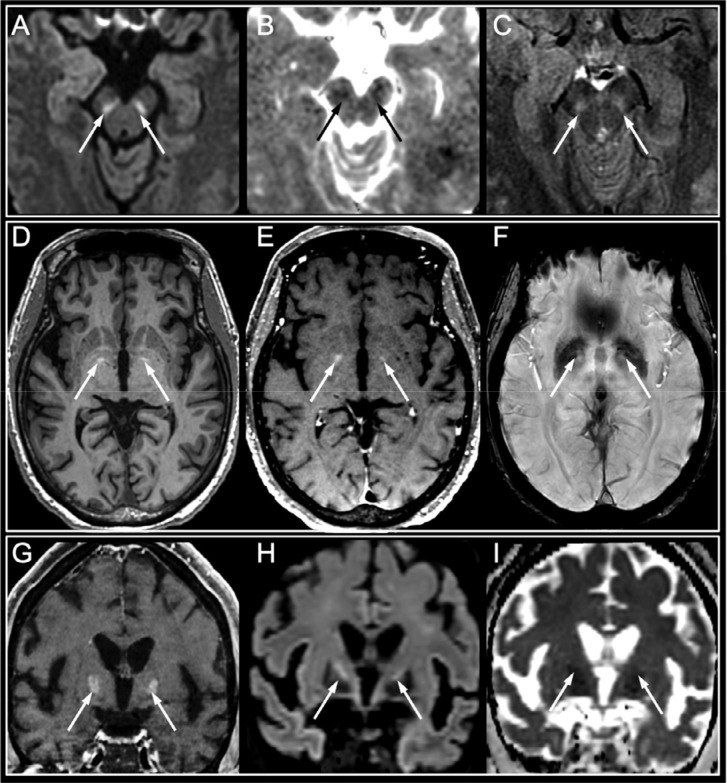
Basal ganglia abnormalities were seen in four other ICU patients who also had lake awakening after sedation withdrawal, including diffusion restriction with FLAIR hyperintensity of the substantia nigra, bilateral enhancement with moderate diffusion restriction in the globus pallidus, and bilateral spontaneous hyperintensity on T1-weighted images in the globus pallidus in one patient and in the upper part of the striatonigral pathways in another patient (Figure 5).
Figure 5:
Basal ganglia abnormalities. Basal ganglia abnormalities in three patients who experienced late awakening after sedation withdrawal in a context of SARS-CoV-2 hypoxic pneumonia with severe acute respiratory syndrome:
1) A 42-year-old male with untreated chronic lymphocytic leukemia who had tetraparesis upon awakening after 47 days of intensive care (A-C). Hyperintensity on DWI within the substantia nigra (A, arrows) with decreased apparent diffusion coefficient (B, arrows) and hyperintensity on axial FLAIR images (C, arrows) were seen. The follow-up neurological examination showed complete consciousness recovery with persistent peripheral motor deficit consistent and mild parkinsonian symptoms.
2) A 62-year-old diabetic female with chronic cardiovascular disease, who suffered cardiorespiratory arrest after 54 days of intensive care (D-F). Hyperintensity on pre-contrast MPRAGE axial T1-weighted images in the globus pallidus (D, arrows), with no enhancement on post-contrast T1-weighted images (E, arrows) or hypointensity on susceptibility-weighted images (F, arrows). The follow-up neurological examination also showed complete consciousness recovery with a persistent motor deficit.
3) A 56-year-old diabetic and obese female who experienced late awakening after 48 days of intensive care (G-I). Bilateral enhancement within the globus pallidus on coronal post-contrast T1-weighted images (G, arrows), with hyperintensity on diffusion-weighted images (G, arrows) and decreased apparent diffusion coefficient (H, arrows). No follow-up was available.
Abbreviation: SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
Corpus callosum abnormalities
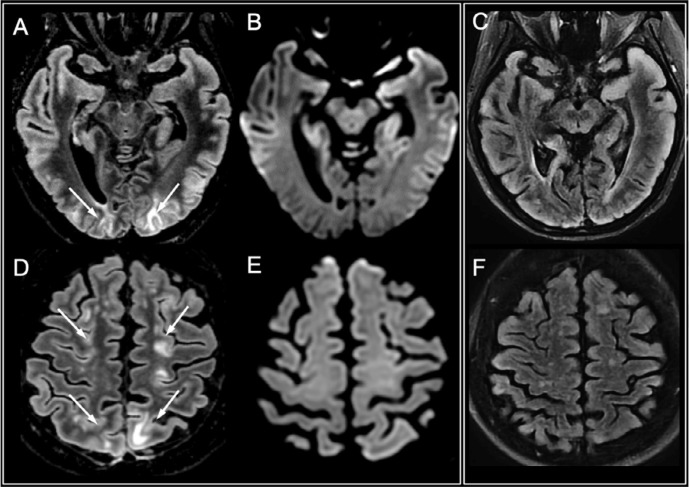
Restricted diffusion foci within the splenium of the corpus callosum were evidenced in three patients. Enhancement was seen in one patient (in the two other patients referred for suspected ischemia, contrast administration was not be performed). In one case, diffusion imaging normalized in the follow-up MRI 25 days later. Follow-up MRI was not performed in the other two patients. This aspect suggested Cytotoxic Lesions of the Corpus Callosum (CLOCC, Supplementary Figure E3).
PRES
Typical findings of PRES with parieto-occipital and superior frontal swelling reversible on the follow-up MRI were detected in one ICU patient. Reversible swelling involving the midbrain and basal ganglia also consistent with PRES was seen in another ICU patient with multiple organ failure (Figure 6).
Figure 6:
Posterior reversible encephalopathy syndrome (PRES). A 66-year-old male infected with SARS-CoV-2 presenting with status epilepticus in a context of pneumonia and severe hyponatremia. There were cortico-subcortical signal abnormalities in the parieto-occipital and superior frontal regions on FLAIR images (A and D, arrows), with no diffusion restriction (B and E). Follow-up MRI performed ten days later showed complete disappearance of the lesions (C, F).
Hypoxic-ischemic lesions
Hypoxic-ischemic lesions were seen in three ICU patients with a history of cardiopulmonary arrest (one case) or severe low flow (two cases).
Metabolic changes
Pontine white matter abnormalities consistent with osmotic demyelination syndrome (central pontine myelinolysis) were detected in three ICU patients with a history of hydrolytic disturbances.
Meningeal enhancement
Meningeal enhancement was detected in only two ICU patients, including dural involvement in two patients who previously had a lumbar puncture, and leptomeningeal involvement in one patient.
Discussion
Neuroimaging examinations at 2-4 weeks after symptom onset in 73 COVID-19 patients with acute de novo neurological manifestations showed ischemic lesions, diffuse microhemorrhages involving the corpus callosum, deep vein thrombosis, perfusion abnormalities, CLOCC and other ICU-related complications, and two other patterns including contrast-enhanced white matter lesions and abnormalities involving the basal ganglia.
The pattern of white matter enhancing lesions observed in four patients differed from the one seen in acute disseminated encephalomyelitis (ADEM) where lesions tend to be asymmetrical, with variable involvement of gray matter and punctate or ring enhancement (16–18). Several features also argued against the diagnosis of subacute embolic infarctions, including i) the periventricular topography of lesions, ii) their morphology associating an oval shape and ill-defined margins, and iii) the perivascular distribution of enhancement. This latter feature has been described in vasculopathies such as PRES and Susac syndrome, and in disorders with angiocentric infiltrates, especially in neurolupus and neurosarcoidosis (19) and in CD8 encephalitis in HIV patients (20). Similarly, our pattern of white matter enhancing lesions could somehow reflect a vasculitis-like phenomenon and/or an inflammatory disorder with angiocentric involvement (19,20). This pattern has also been observed in a recent study (9), reinforcing the hypothesis of a COVID-19-related damage.
Basal ganglia involvement seen in four patients included signal and diffusion abnormalities, with variable contrast enhancement, affecting the substantia nigra, the globus pallidus and the striatonigral pathway in a variable manner. To our knowledge, this pattern has not been previously reported. Patients with hyperglycemia can present spontaneous T1 hyperintensity within the putamen with variable involvement of the caudate and globus pallidus that is usually unilateral (21). Although patients with T1 hyperintensities in our series were diabetic, the topography that we observed was different (symmetrical and involving the globus pallidus and the upper part of the striatonigral pathways). This pattern of brain damage has some similarities to that seen in encephalitis lethargica (EL) also known as Von Economo disease. An epidemic of EL occurred during the 1918 influenza pandemic. In the acute phase, patients with EL had pharyngitis, sleepiness, ocular motility and movement disorders with a fatal presentation in about one third of patients. Delayed parkinsonism and neuropsychiatric signs may occur (22). Neuropathological studies have shown encephalitis of the midbrain and basal ganglia with lymphocytes infiltration (22–24). MRI examinations revealed bilateral edematous changes within the thalami, basal ganglia and midbrain with variable contrast enhancement (22,23). The substantia nigra was involved in 12% of autopsy cases (22) and in one MRI case (25). Although the etiology of EL is still unknown, infectious and environmental causes have been proposed (24). More recently, examination of archived brain material failed to demonstrate Influenza RNA, while the presence of oligoclonal bands in the CSF and the efficacy of corticosteroids suggested that EL might be immune-mediated (23). Follow-up of the patients with basal ganglia abnormalities in our series will help show whether they develop parkinsonism as seen in the chronic phase of EL (24).
The underlying pathophysiology of these two patterns and their association with COVID-19 remain unclear since SARS-CoV-2 RT-PCR in the CSF was negative in all tested patients. A relationship with COVID-19 appears however possible since these lesions were similarly observed in several patients and were not explained by another pathology or condition. To date, SARS-CoV-2 RNA has been reported positive in the CSF in only three previous patients (9,26,27). Several hypotheses could be formulated to explain PCR negativity in the CSF. First, meningeal contrast enhancement was rare in our series, unlike observations from previous studies (8). This difference could be due to the fact that we acquired the FLAIR images before the administration of contrast (the meningeal contrast enhancement in other cases being reported mainly on post-contrast FLAIR images), or to the lack of meningeal involvement in our series, considering that SARS-CoV-2 could have an intra-neuronal localization as reported with SARS-CoV (28). Second, indirect viral pathogenesis through an immune-mediated mechanism and/or a systemic inflammatory response syndrome could be involved. Such an immune process could also explain the occurrence of neuritis or Guillain-Barré syndrome (29). Third, the delay between the RT-PCR and the stage of infection could also explain a negative detection. Brain damage may also result from a first step of CNS viral replication, followed by an immune-mediated phenomenon (5,7) where the virus is no longer detectable, as supported by the relatively long delay between the first respiratory symptoms and the neurological impairment in our study. Post-mortem neuropathological examinations in 18 COVID-19 patients only showed hypoxic changes without sign of encephalitis (30). Viral RNA was detected at low levels, possibly due to in situ viral RNA from the bloodstream (30). Another neuropathological report evidenced vascular and demyelinating changes in one patient, without mention of virus screening (31). The knowledge collected at this stage would favor the hypothesis of a delayed immune-mediated process underlying the physiopathology of CNS damage (14).
Multiple microhemorrhages have been observed in eight patients, with a specific involvement of the corpus callosum, as recently reported (9,11,32). Hemorrhages are known to be a complication of ECMO (33). Microhemorrhages might have been explained by the presence of ECMO in 2 patients and anticoagulation overdose in another patient. In the remaining patients, the physiopathology could involve microvascular damage. A possible implication of SARS-CoV-2 remains unclear.
Ischemic infarcts were diagnosed in 23.3% of patients of our series. It is now recognized that seriously ill COVID-19 patients are at higher risk of venous and arterial thromboembolic events (34–36) despite prophylactic or curative anticoagulation, compared to non-COVID-19 matched patients (35). The higher percentage in our series compared to previous series (14,37) could be explained by the fact that acute ischemic lesions could have been underestimated on CT scans in previous studies while our study only relied on MRI examinations. Coronaviruses use the angiotensin-converting enzyme 2 (ACE2) to enter cells, a receptor which is expressed in the epithelia of the lung and small intestine, and also found in brain endothelial cells (5,6). It has been suggested that recruitment of immune cells by direct viral infection of the endothelium or immune-mediated, with a massive inflammatory cascade, may led to endothelial damage, resulting in stroke, thrombosis and hemorrhage (38).
Perfusion abnormalities, which were related to seizure in 19.6% of patients in our series, were also described in a smaller series (8), although the mechanism was not discussed. Many factors can account for the occurrence of seizures in COVID-19 patients: fever that lowers the seizure threshold, metabolic alterations, iatrogeny and potential changes related to encephalitis.
Lastly, extensive supratentorial white matter FLAIR hyperintensities (9,11,12) and unilateral abnormalities of the medial temporal lobe (9,26) described in previous studies were not seen in our series.
Our study has several limitations. First, clinical examination was limited in this context and CSF data were only available in about half of the participants. Second, most patients had no prior MRI scan. Finally, at this point, information on the outcome of patients was lacking. A cross-analysis correlating clinical, imaging, CSF and pathological data with patient outcome will be the scope of future studies.
Conclusion
This study provides a detailed description of neuroimaging findings in a series of COVID-19 patients. In addition to cerebrovascular thrombotic events, perfusion abnormalities, CLOCC, microhemorrhages involving the splenium of corpus callosum and ICU-related disorders, two MRI patterns were identified including white matter lesions with perivascular enhancement, that may reflect vasculitis lesions and/or inflammatory lesions with angiocentric involvement, and basal ganglia abnormalities including substantia nigra involvement. Future imaging-pathological correlation studies will help determine whether there is a causal relationship between COVID-19 and brain MRI lesions. A longitudinal monitoring will also be necessary to assess the long-term neurological impact of COVID-19.
Acknowledgments
Acknowledgements
The authors gratefully acknowledge the generous support of the Fédération Internationale de l’Automobile (FIA), the FIA Foundation and donors of Paris Brain Institute – ICM. We thank the Cohort COVID-19 Neurosciences (CoCo Neurosciences) study group for its participation to data collection for their contribution to the discussion and the team of Radiology technicians of the Pitié-Salpêtrière Hospital for the data acquisition.
This project was funded by the Paris Brain Institute (ICM). ICM was supported by an unrestricted donation from the Fédération Internationale d’Automobile (https://www.fia.com/fia), a non-profit making association. The research was also supported by funding from the program “Investissements d’avenir” ANR-10- IAIHU-06.
Abbreviations:
- CLOCC
- Cytotoxic lesions of the corpus callosum
- CNS
- central nervous system
- COVID-19
- coronavirus disease 2019
- CT
- computed tomography
- ECMO
- extracorporeal membrane oxygenation
- ICU
- intensive care unit
- PRES
- posterior reversible encephalopathy syndrome
- RT-PCR
- reverse-transcriptase–polymerase-chain-reaction
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
CoCo Neurosciences study group:
Steering committee (Pitié-Salpêtrière Hospital, Paris): Cecile Delorme, Jean-Christophe Corvol, Jean-Yves Delattre, Stephanie Carvalho, Sandrine Sagnes. Scientific committee (Pitié-Salpêtrière Hospital, Paris): Bruno Dubois, Vincent Navarro, Celine Louapre, Tanya Stojkovic, Ahmed Id Baih, Charlotte Rosso, David Grabli, Ana Zenovia Gales, Bruno Millet, Benjamin Rohaut, Eleonore Bayen, Sophie Dupont, Gaelle Bruneteau, Stephane Lehericy, Danielle Seilhean, Alexandra Durr, Foudil Lamari, Marion Houot, Vanessa Batista Brochard. Principal investigators: Pitié-Salpêtrière Hospital (Paris): Sophie Dupont, Catherine Lubetzki, Danielle Seilhean, Pascale Pradat-Diehl, Yves Samson, Khe Hoang-Xuan, Bertrand Fontaine, Lionel Naccache, Philippe Fossati, Isabelle Arnulf, Alexandra Durr, Alexandre Carpentier, Stephane Lehericy, Nadya Pyatigorskaya, Yves Edel,; Rothschild Hospital (Paris): Gilberte Robain, Philippe Thoumie; Hôpital Avicenne (Bobigny): Bertrand Degos; Saint Anne Hospital (Paris): Tarek Sharshar; Tenon Hospital (Paris): Nathalie Dzierzynski; Charles Foix hospital (Ivry): Kiyoka Kinugawa Bourron. Co-investigators: Pitié-Salpêtrière Hospital: Ana Zenovia Gales, Stephanie Bombois, Mehdi Touat, Ahmed Id Baih, Nicolas Villain, David Grabli, Maria del Mar Amador, Gaelle Bruneteau, Louise-Laure Mariani, Nicolas Mezouar, Sara Leder, Graziella Mangone, Aurelie Meneret, Andreas Hartmann, Clement Tarrano, David Bendetowicz, Pierre-François Pradat, Michel Baulac, Sara Sambin, Phintip Pichit, Florence Chochon, Adele Hesters, Bastien Herlin, An Hung Nguyen, Claire Ewenczyk, Giulia Coarelli, Anna Heinzmann, Esteban Munoz Musat, Timothee Lenglet, Lydia Chougar, Nathalia Shor, Sophie Demeret, Benjamin Rouhaut, Tal Seidel Malkinson, Albert Cao, Clemence Marois, Katarzyna Siuda-Krzywicka, Salimata Gassama, Loic Le Guennec, Jean-Yves Rotge, Bertrand Saudreau, Bruno Millet, Philippe Fossati, Victor Pitron, Nassim Sarni, Nathalie Girault, Redwan Maatoug, Ana Zenovia Gales, Smaranda Leu, Eleonore Bayen, Lionel Thivard, Julien Mayaux, Elise Morawiec, Alexandre Demoule; Saint-Antoine Hospital (Paris): Laure Bottin, Marion Yger; Hôpital Rothschild (Paris): Gaelle Ouvrard, Rebecca Haddad. Other contributors: clinical research assistants (ICM, Pitié-Salpêtrière Hospital, Paris): Haysam Salman, Armelle Rametti-Lacroux, Alize Chalançon, Anais Herve, Hugo Royer, Florence Beauzor, Valentine Maheo, Christelle Laganot, Marie Biet, Rania Hilab, Aurore Besnard, Meriem Bouguerra, Gwen Goudard, Saida Houairi, Saba Al-Youssef; data manager(ICM, Paris): Safia Said.
References
- 1.Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020; e201127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau K-K, Yu W-C, Chu C-M, Lau S-T, Sheng B, Yuen K-Y. Possible central nervous system infection by SARS coronavirus. Emerging Infect Dis. 2004;10(2):342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung ECW, Chim SSC, Chan PKS, et al. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. 2003;49(12):2108–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arabi YM, Harthi A, Hussein J, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV). Infection. 2015;43(4):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desforges M, Le Coupanec A, Dubeau P, et al. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses. 2019;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Natoli S, Oliveira V, Calabresi P, Maia LF, Pisani A. Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur J Neurol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desforges M, Le Coupanec A, Brison E, Meessen-Pinard M, Talbot PJ. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol. 2014;807:75–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helms J, Kremer S, Merdji H, et al. Neurologic Features in Severe SARS-CoV-2 Infection. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kremer S, Lersy F, de Sèze J, et al. Brain MRI Findings in Severe COVID-19: A Retrospective Observational Study. Radiology. 2020;202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahammedi A, Saba L, Vagal A, et al. Imaging in Neurological Disease of Hospitalized COVID-19 Patients: An Italian Multicenter Retrospective Observational Study. Radiology. 2020;201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandemirli SG, Dogan L, Sarikaya ZT, et al. Brain MRI Findings in Patients in the Intensive Care Unit with COVID-19 Infection. Radiology. 2020;201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radmanesh A, Derman A, Lui YW, et al. COVID-19 -associated Diffuse Leukoencephalopathy and Microhemorrhages. Radiology. 2020;202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franceschi AM, Arora R, Wilson R, Giliberto L, Libman RB, Castillo M. Neurovascular Complications in COVID-19 Infection: Case Series. AJNR Am J Neuroradiol. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pons-Escoda A, Naval-Baudín P, Majós C, et al. Neurologic Involvement in COVID-19: Cause or Coincidence? A Neuroimaging Perspective. American Journal of Neuroradiology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chougar L, Mathon B, Weiss N, Degos V, Shor N. Atypical Deep Cerebral Vein Thrombosis with Hemorrhagic Venous Infarction in a Patient Positive for COVID-19. American Journal of Neuroradiology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi A. Imaging of acute disseminated encephalomyelitis. Neuroimaging Clin N Am. 2008;18(1):149–161; ix. [DOI] [PubMed] [Google Scholar]
- 17.Tenembaum S, Chitnis T, Ness J, Hahn JS, International Pediatric MS Study Group . Acute disseminated encephalomyelitis. Neurology. 2007;68(16 Suppl 2):S23-36. [DOI] [PubMed] [Google Scholar]
- 18.Menge T, Hemmer B, Nessler S, et al. Acute disseminated encephalomyelitis: an update. Arch Neurol. 2005;62(11):1673–1680. [DOI] [PubMed] [Google Scholar]
- 19.Taieb G, Duran-Peña A, de Chamfleur NM, et al. Punctate and curvilinear gadolinium enhancing lesions in the brain: a practical approach. Neuroradiology. 2016;58(3):221–235. [DOI] [PubMed] [Google Scholar]
- 20.Lescure F-X, Moulignier A, Savatovsky J, et al. CD8 encephalitis in HIV-infected patients receiving cART: a treatable entity. Clin Infect Dis. 2013;57(1):101–108. [DOI] [PubMed] [Google Scholar]
- 21.Lai PH, Tien RD, Chang MH, et al. Chorea-ballismus with nonketotic hyperglycemia in primary diabetes mellitus. AJNR Am J Neuroradiol. 1996;17(6):1057–1064. [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson LL, Vilensky JA, Duvoisin RC. Review: neuropathology of acute phase encephalitis lethargica: a review of cases from the epidemic period. Neuropathol Appl Neurobiol. 2009;35(5):462–472. [DOI] [PubMed] [Google Scholar]
- 23.Dale RC, Church AJ, Surtees RAH, et al. Encephalitis lethargica syndrome: 20 new cases and evidence of basal ganglia autoimmunity. Brain. 2004;127(Pt 1):21–33. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman LA, Vilensky JA. Encephalitis lethargica: 100 years after the epidemic. Brain. 2017;140(8):2246–2251. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Jia G, Li B, Lei G, Sun R. Encephalitis Lethargica With Isolated Substantia Nigra Lesions Followed by a Second Encephalitis in a Child With Humoral Immunodeficiency. Pediatr Neurol. 2015;53(6):519–522. [DOI] [PubMed] [Google Scholar]
- 26.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou L, Zhang M, Wang J, Gao J. Sars-Cov-2: Underestimated damage to nervous system. Travel Med Infect Dis. 2020;101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170(4):1136–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Virani A, Rabold E, Hanson T, et al. Guillain-Barré Syndrome associated with SARS-CoV-2 infection. IDCases. 2020;20:e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological Features of Covid-19. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coolen T, Lolli V, Sadeghi N, et al. Early postmortem brain MRI findings in COVID-19 non-survivors. medRxiv. 2020;2020.05.04.20090316. [DOI] [PubMed] [Google Scholar]
- 33.Xie A, Lo P, Yan TD, Forrest P. Neurologic Complications of Extracorporeal Membrane Oxygenation: A Review. J Cardiothorac Vasc Anesth. 2017;31(5):1836–1846. [DOI] [PubMed] [Google Scholar]
- 34.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients in severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Medicine. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: A systematic review. J Neurol Sci. 2020;413:116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radmanesh A, Raz E, Zan E, Derman A, Kaminetzky M. Brain Imaging Use and Findings in COVID-19: A Single Academic Center Experience in the Epicenter of Disease in the United States. American Journal of Neuroradiology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]