Abstract
Background: Intrinsic atrophy and debilitating sensory loss are prominent features of severe ulnar neuropathy with limited surgical options to reliably improve recovery. Restoration of sensation is important to provide protection for the vulnerable ulnar border of the hand. Here, we report our experience with side-to-side sensory nerve grafting from the median to ulnar nerve in the palm to enhance ulnar sensory recovery. Methods: A retrospective chart review identified patients with severe ulnar neuropathy who underwent cross-palm nerve grafting. Included patients had objective loss of protective sensation in the ulnar distribution with 2-point discrimination >8 mm, Semmes-Weinstein monofilament testing (SWMT) >4.56, or no sensory response on nerve conduction testing. Cross-palm side-to-side tension-free grafting from median to ulnar sensory components was performed using short-segment allograft or autografts. Analysis included patient etiology, procedures, nerve conduction studies, objective sensory testing, and Disabilities of the Arm, Shoulder, and Hand Disability score. Results: Forty-eight patients with severe ulnar neuropathy underwent cross-palm nerve grafting between 2014 and 2017. Twenty-four patients had adequate follow-up for inclusion. Of the 24 patients, 21 (87%) had return of protective sensation, 16 (66.7%) had return of diminished light touch sensation, and 6 (25%) had return to normal range sensation within 1 year as assessed by SWMT and/or 2-point discrimination. Patients treated with autograft demonstrated referred sensation to the median nerve distribution. Conclusions: Cross-palm nerve grafting may be a useful adjunct to enhance sensory recovery in severe ulnar neuropathy. Further study to quantify differences in sensory recovery between traditional operative techniques and cross-palm nerve grafting is required.
Keywords: nerve reconstruction, nerve graft, side-to-side nerve graft, nerve regeneration, outcomes, research and health outcomes, treatment, surgery, ulnar nerve, nerve injury, nerve compression, cubital tunnel syndrome
Introduction
Cubital tunnel syndrome is the second most common compression neuropathy, with approximately 75 000 cases in the United States per year.1 Pain and paresthesias involving the ring and small finger are the most prominent symptoms, with more severe cases progressing to atrophy and weakness of the ulnar-innervated intrinsic hand muscles.2,3 Ulnar neuropathy less commonly results from laceration of the nerve, brachial plexopathy, and cervical radiculopathy. Regardless of the etiology, severe cases can have a devastating impact on hand function.
Traditional surgical approaches for cubital tunnel syndrome include in situ decompression of the ulnar nerve at the elbow, anterior transposition in its various forms, and medial epicondylectomy.4-6 More complicated clinical scenarios such as severe cubital tunnel syndrome, high ulnar nerve laceration, and brachial plexopathy may require adjunctive reconstructive techniques such as nerve grafting, nerve transfers (eg, anterior interosseous nerve [AIN] to deep motor branch of the ulnar nerve, either end-to-end or supercharge end-to-side), tenodesis, and/or tendon transfers.7-10 Sensory restoration to the ulnar side of the hand is rarely a specific goal of current adjunctive reconstructive techniques.
Sensation to the ulnar side of the hand can be restored using sensory nerve transfers from the third webspace component of the median nerve, the palmar cutaneous of the median, or the lateral antebrachial cutaneous nerve.11,12 These transfers are commonly performed in an end-to-end manner, which leaves a sensory deficit at the donor site.
In clinical scenarios where there is a possibility for recovery from the proximal nerve, a low morbidity procedure to augment sensory function is desirable. Side-to-side nerve grafting involves creating an epineural and perineurial window on the sides of donor and recipient nerves and placement of a bridge graft between the two,13 with a significant differential in between the number of axons in donor and recipient nerves to create a gradient for collateral sprouting. Preoperatively, this can be determined via electrodiagnostic testing to ensure there is a significant sensory nerve action potential (SNAP) gradient between the median and ulnar nerves.
Several animal studies have confirmed the efficacy of side-to-side grafting.13,14 Yüksel et al15 reported a case of high ulnar nerve laceration that was repaired end-to-end and supported distally by side-to-side grafting from the median to ulnar nerves. The patient recovered protective sensation by 9 months after surgery.
Here, we present our experience with side-to-side sensory nerve grafting, termed “cross-palm grafting,” from the sensory component of the median to the sensory ulnar nerve in patients with severe ulnar neuropathy.
Materials and Methods
Institutional review board approval was obtained prior to commencing this study. The study hypothesized that cross-palm grafting would augment sensory recovery in patients with severe ulnar neuropathy; a retrospective review of patients treated surgically for ulnar neuropathy between 2014 and 2016 was performed to identify patients who had undergone cross-palm grafting and had regular clinical follow-up. Preoperative demographics, history, nerve conduction velocity (NCV) reports, physical examination data, Semmes-Weinstein monofilament testing (SWMT) results, and Disabilities of the Arm, Shoulder, and Hand (DASH) scores were reviewed. Intraoperative factors including concomitant procedures and type of graft used were reviewed (Table 1). Patients with preoperatively documented loss of protective sensation and at least 1-year follow-up were included in the study. “Loss of protective sensation” was defined as either SWMT >4.56 or moving 2-point discrimination (2PD) >8 mm in the ulnar-innervated digits.
Table 1.
Patient Characteristics and Causes of Ulnar Neuropathy.
| Total patients | 24 |
| Average age | 51.8 y |
| Female | 8 (33%) |
| Hypertension | 7 (29%) |
| Hypercholesterolemia | 5 (21%) |
| Anxiety | 5 (21%) |
| Depression | 5 (21%) |
| Obesity | 4 (17%) |
| Diabetes | 2 (8%) |
| Hypothyroidism | 1 (4%) |
| Disabilities of the Arm, Shoulder, and Hand score preoperatively (average) | 60.1 |
| Causes of ulnar neuropathy | |
| Cubital tunnel | 14 (58%) |
| Trauma | |
| Laceration | 3 (13%) |
| Other trauma | 7 (29%) |
Postoperative SWMT, physical examination, DASH scores, and complications were reviewed at follow-up intervals up to 1 year. Semmes-Weinstein monofilament testing was completed by hand therapists, independent of follow-up in our clinic. The primary outcome measure determined was return of protective sensation within 1-year time as documented by SWMT.
Surgical Technique
All cases were performed by the senior author under tourniquet control and loupe magnification. Cross-palm grafting was frequently combined with other procedures for the treatment of severe ulnar neuropathy, including ulnar nerve transposition, supercharged end-to-side (SETS) transfer of the pronator quadratus branch of AIN to the ulnar motor nerve, profundus tenodesis, and others.
Access to the median and ulnar nerves for cross-palm grafting was by open release of the carpal tunnel and the Guyon canal via a palmar curved incision as previously described.16 Using 4.0 magnification or greater loupes, or an operating microscope, epi/perineural windows were created in 2 locations along the third webspace fascicle of the median nerve within the carpal tunnel, and 2 reciprocal epi/perineural windows were created in the ulnar nerve sensory fascicles within the Guyon canal (Figure 1). A marking pen was used to “dot” the location of the epi/perineural window and then micro scissors were used to cut the center of the inked dot to create an inked border of epi/perineurium for easier suturing. The exact location on the ulnar sensory fascicles to receive the grafts was fashioned to deliver sensation to the small finger rather than the ring or hypothenar. Two nerve grafts were sewn in a side-to-side fashion between the epi/perineural windows of the median and ulnar nerves using 9-0 nylon suture, fibrin glue, and the operating microscope. A small amount of redundancy was maintained to prevent tension on the coaptations. If autograft was already available from another portion of the surgical procedure (eg, via medial antebrachial cutaneous nerve [MABC] neuromas being treated at the elbow), then this was preferentially used. Otherwise, a 2-3 mm × 50 mm acellular nerve allograft (Avance(R), Axogen, Inc, Alachua, Florida) was utilized, divided sharply into 2 approximately 25 mm segments (surgical video 117). The ends of the graft were trimmed, yielding a typical graft length of 22 mm.
Figure 1.
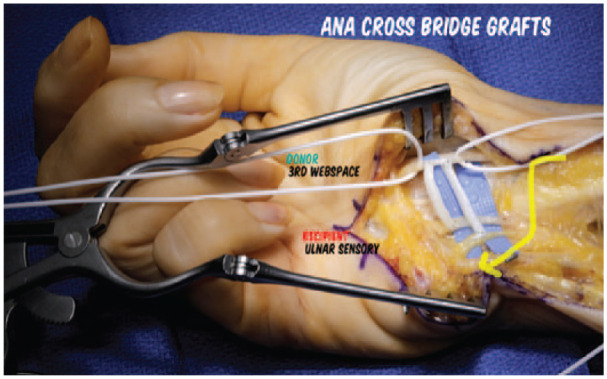
Perineural windows are created in 2 locations along the third webspace fascicle of the median nerve within the carpal tunnel, and 2 reciprocal perineural windows are created in the ulnar nerve sensory fascicles within the Guyon canal.
Note. One graft is anastomosed to the common digital nerve to the fourth webspace and the second to the small finger ulnar digital nerve. Acellular nerve allografts are pictured in this case; when available, autograft is preferentially used.
Results
Forty-eight consecutive patients underwent cross-palm nerve grafting at our institution between 2014 and 2017. Of these, 24 patients had documented loss of ulnar protective sensation (SWMT >4.56) or moving 2PD >8 mm preoperatively and had adequate follow-up for inclusion (Table 1). Preoperative ulnar SNAP amplitude was available for 23 of 24 patients; 16 of 24 had no response, and of the remaining patients, the average recorded was 14.6 µV with a range from 3 to 47 µV. Causes of ulnar neuropathy included cubital tunnel syndrome (58%), ulnar nerve laceration (13%), traction injury (8%), iatrogenic from other surgery (8%), blunt trauma (8%), and multilevel nerve injury (4%) (Table 1).
All patients underwent release of the carpal tunnel and the Guyon canal as a requisite part of the surgical technique. Twenty-one (87.5%) of 24 patients underwent concomitant ulnar nerve transmuscular transposition (UNT). Of these, 10 of 21 were referred for revision UNT, with 4 of 21 being tertiary or quaternary revision cases. Twenty (83%) of 24 patients also had an SETS AIN to ulnar motor nerve transfer.9 Other concomitant procedures are listed in Table 2.
Table 2.
Concomitant Procedures in Patients Undergoing Cross-Palm Sensory Nerve Grafting for Severe Ulnar Neuropathy.
| Procedure | No. (%) |
|---|---|
| UNT | 21 (87.5) |
| Revision UNT | 10 (41.7) |
| Tertiary revision UNT | 3 |
| Quaternary revision UNT | 1 |
| Release of median nerve in forearm | 4 |
| Profundus tenodesisa | 18 |
| SETS AIN to ulnar nerve transfer | 20 |
| MABC neuroma resection | 3 |
| PIN decompression | 2 |
| Radial nerve tendon transfers | 1 |
| Revision carpal tunnel release | 1 |
Note. UNT = ulnar nerve intramuscular transposition; SETS = supercharged end-to-side; AIN = anterior interosseous nerve; MABC = medial antebrachial cutaneous; PIN = posterior interosseous nerve.
Tenodesis of the ring and small finger profundus tendons to the middle and index profundus tendons at the level of the distal forearm.
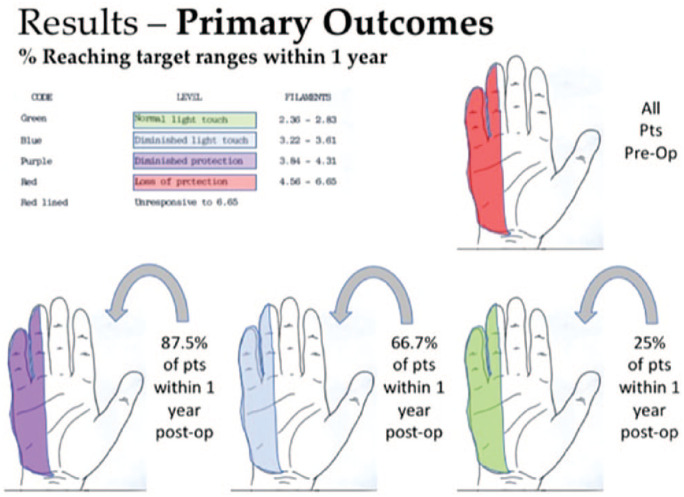
The primary outcome examined was ulnar nerve sensory function in the hand, specifically, return of sensation as measured by SWMT. Twenty-one (87.5%) of 24 patients had return of at least protective sensation within 1 year as assessed by SWMT (<4.31). Sixteen (66.7%) of 24 patients had at least return of diminished light touch sensation (SWMT <3.61). Six (25%) of 24 patients had return of sensation within the normal range (SWMT <2.83). Three (12.5%) of 24 patients did not recover protective sensation within 1 year.
When patients were grouped by SWMT range at final follow-up, 6 (25%) of 24 patients had return of normal light touch sensation at an average of 7 months of follow-up, 10 (41.2%) of 24 patients had return of diminished light touch sensation at an average of 8.1 months of follow-up, 5 (20.8%) of 24 patients had return of diminished protective sensation at an average of 6.8 months of follow-up, and 3 (12.5%) of 24 patients had continued absence of protective sensation at an average of 13.3 months of follow-up (Figure 2). Of the group who regained normal sensation, etiology was cubital tunnel in 4 patients and blunt trauma in 2 patients. Of the group who regained only diminished light touch sensation, etiology was cubital tunnel in 6 patients, brachial plexus traction injury in 2 patients, postoperative from other surgery in 1 patient, and complete transection in 1 patient. Of the group who regained only diminished protective sensation, etiology was cubital tunnel in 2 patients (one of which had superimposed cervical radiculopathy), ulnar nerve transection in 1 patient, postoperative from other surgery in 1 patient, and multilevel injury of the ulnar nerve in 1 patient. Of the group who failed to regain protective sensation, 2 patients had ulnar neuropathy resulting from cubital tunnel syndrome, and 1 had sustained a complete laceration of the ulnar nerve (Table 3).
Figure 2.
Distribution of sensory outcomes among the study group at final follow-up, as measured by Semmes-Weinstein monofilament testing.
Table 3.
Etiology of Ulnar Neuropathy in Patients Grouped by Final Sensory Outcome.
| Final sensory outcome (SWMT) | No. (%) | Average final follow-up, mo | Etiology of ulnar neuropathy |
|---|---|---|---|
| Normal light touch (SWMT: 2.36-2.83) | 6 (25) | 7 | Cubital tunnel: 4 Blunt trauma: 2 |
| Diminished light touch (SWMT: 3.22-3.61) | 10 (41.2) | 8.1 | Cubital tunnel: 6 Traction injury: 2 Other surgery: 1 Ulnar transection: 1 |
| Diminished protective (SWMT: 3.84-4.31) | 5 (20.8%) | 6.8 | Cubital tunnel: 1 Cubital tunnel + cervical radiculopathy: 1 Ulnar transection: 1 Other surgery: 1 Multilevel injury: 1 |
| Loss of protective (SWMT: 4.56-6.65) | 3 (12.5) | 13.3 | Cubital tunnel: 2 Ulnar transection: 1 |
Note. SWMT = Semmes-Weinstein monofilament testing.
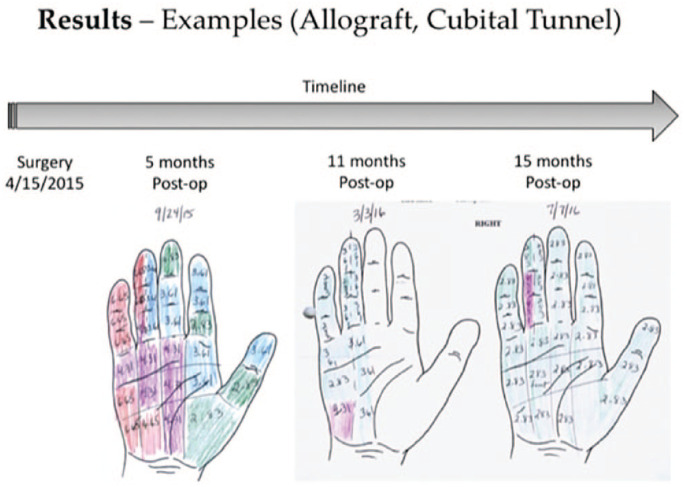
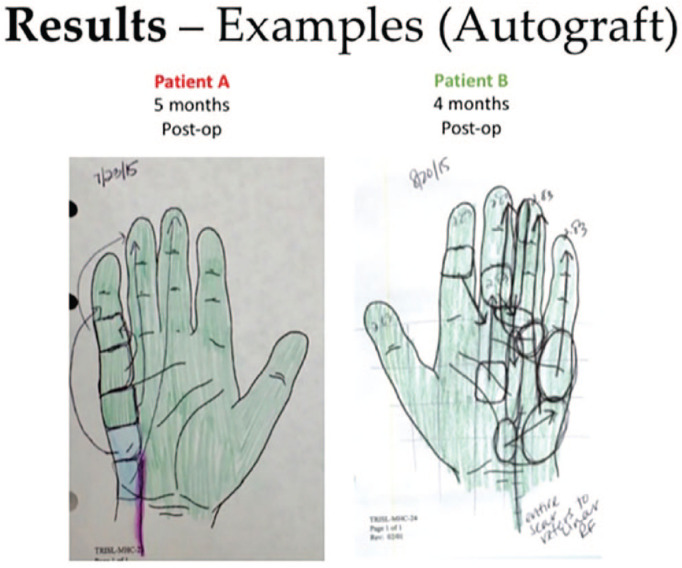
In 2 patients who underwent cross-palm grafting with autograft (derived from the MABC nerve), postoperative SWMT demonstrated evidence of referred sensation to the median-innervated third webspace, in addition to recovery of sensation in ulnar-innervated digits (Figures 3 and 4). No similar pattern of referred sensation was noted among patients who underwent cross-palm grafting with allograft.
Figure 3.
This figure demonstrates postoperative progressive improvement in Semmes-Weinstein monofilament testing during the follow-up period, in a patient with uncomplicated severe cubital tunnel syndrome who underwent cross-cross grafting with acellular allograft along with ulnar nerve transposition.
Figure 4.
This figure demonstrates results from 2 patients who underwent cross-palm grafting with medial antebrachial cutaneous autograft. Semmes-Weinstein monofilament testing results show referred sensation to the (donor) median third webspace distribution.
The DASH disability scores improved from a preoperative average of 60.1 to a postoperative average of 30.1. No surgical complications were observed relating to cross-palm grafting; specifically, no patient was noted to have downgraded median or ulnar sensory function.
Discussion
This report provides an initial description of technique and outcomes for side-to-side nerve grafting in the palm from the median to ulnar nerve in patients with severe ulnar neuropathy of varying etiology. The significance of this preliminary report should be viewed in the context of limited alternative proposed measures to improve sensory outcomes for severe ulnar neuropathy, beyond traditional decompression or transposition procedures. While outcomes of cubital tunnel surgery in general have been extensively reported,1,18-21 many studies report outcomes in grading scales that consider sensory recovery in combination with motor recovery. Relatively few studies specifically examine discrete, objective sensory outcome measures.20-22 Among those that do, there is evidence that long-term sensory recovery in patients undergoing decompression surgeries for severe cubital tunnel syndrome does not uniformly reach the normal range,20,21 suggesting a potential clinical need for adjunctive, sensory-specific reconstruction. Furthermore, surgeons may encounter severe ulnar neuropathy resulting from causes other than cubital tunnel syndrome, where treatment options and outcomes are less standardized, and additional tools to improve sensory recovery may be desirable. In contrast to ulnar intrinsic motor recovery, for which novel procedures have been advanced,23,24 few adjunct procedures are available that may either augment the ultimate quality of sensory recovery or hasten the time to sensory recovery.
Laboratory research supports the clinical premise of side-to-side nerve grafting via several mechanisms. Regenerative and collateral sprouting into nerve grafts attached to the side of a nerve via an epi/perineural window has been extensively confirmed in rodent studies,25-27 where there is suggestion that creation of a epi/perineural window alone is adequate for sensory nerve sprouting, while nerve injury by crush or axotomy may be required for meaningful motor sprouting.25,26,28 It has been shown that creation of a perineural window does not cause nerve fiber damage, with just a temporary break in blood-nerve barrier function of a few days described, and that the larger the window, the greater the sprouting. Hendry et al29 have vividly documented axonal sprouting from one nerve, across side-to-side nerve grafts, into another target denervated nerve using a green fluorescent protein transgenic animal. In this model, the number of crossing axons was greater with 3 side-to-side bridges than with a single bridge. Both Hendry et al and Ladak et al have provided evidence that side-to-side nerve grafts provide “pathway protection” for the chronically denervated distal portions of in-continuity nerves regenerating from a proximal injury. This pathway protection occurs via beneficial effects from both axonal contact (from axons crossing the side-to-side graft into the chronically denervated environment) and dedifferentiation and migration of Schwann cells into the denervated distal nerve, promoting a trophic environment for later regeneration in-continuity.14,29 Prior clinical case reports have also suggested effectiveness of side-to-side nerve grafting using autograft in isolated cases of traumatic reconstruction.15
It is difficult to draw strong conclusions from this study; however, preliminary outcomes are encouraging compared with previously published results of long-term sensory recovery. Matsuzaki et al21 report that 9 (60%) of 15 patients studied continued to have diminished or absent protective sensation postoperatively (SWMT purple or red), despite their overall good functional outcomes, median follow-up of 4.5 years, and less severe preoperative SWMT scores. In contrast, in our group, only 8 (33.3%) of 24 patients retained diminished or absent sensation, and 16 (66.7%) of 24 displayed return of at least diminished light touch sensation (SWMT green or blue) within 1 year from surgery, and no complications or downgrading of function was observed. Other strengths of the study are that outcome measures of sensory function were drawn from SWMT performed by hand therapists outside the study team and provide objective documentation of sensory improvement after cross-palm grafting. Independent SWMT in this study has also provided documentation of referred sensation to the median-innervated third webspace in patients treated with autograft, a novel finding in the literature. Morbidity to the patient is minimized in the presented technique by use of allograft or by redundant nerve autograft in cases where a preexisting injury to the MABC nerve is found. The performance of cross-palm grafting keeps the distance from donor nerve to recipient nerve minimal and also close to sensory end organ, thus improving the likelihood of earlier recovery through collateral sprouting and protecting the most distal and vulnerable portion of the ulnar nerve pathway for an anticipated slow in-continuity recovery.
Limitations of this study include a small, retrospective data set, with a heterogeneous group of patients. In addition, most of the included patients underwent multiple concomitant procedures as part of reconstructive approach to severe ulnar neuropathy; this, along with the lack of a comparison group, creates difficulty in determining the degree to which cross-palm grafting may have contributed to the overall sensory recovery. As with any end-to-side or side-to-side transfer, there is difficulty differentiating between sensory recovery from cross-palm sensory nerve grafting and from proximal ulnar regeneration secondary to decompression. However, our cohort is objectively shown to have significantly improved sensory outcomes when compared with other published series in the literature,21-22 and in a much earlier time frame. Furthermore, nerve autograft patient’s SWMT demonstrated referred sensation from median to ulnar, going some way to solidifying the source of the recovered sensation. Due to the out-of-state and international referral basis of our patients, follow-up was limited and irregular. We acknowledge that had they been followed longer, they may have shown further improvement in their sensory recovery. Finally, this procedure adds significant time and dissection, as well as potentially the expense of a commercial allograft product, when compared with standard ulnar nerve decompression surgeries. Again, it should be emphasized that this procedure is not advocated for simple compression neuropathy, but rather for severe cases, such as those with advanced or long-standing loss of function, secondary or tertiary revision cases, or cases of ulnar nerve injury from various causes.
Future research into this technique may be improved by a prospective study, on a single indication, in demographic-matched patients, with a control group. Regular follow-up intervals with a greater maximum length of follow-up would also improve the quality of data for analysis. Potentially, examining sensation in fully recovered patients after administration of an ulnar nerve block may add information as to the contribution of median nerve sprouting to ultimate sensory recovery. Further comparative research into the outcomes of autograft versus allograft in cross-palm grafting would be useful, given the findings of referred sensation noted in the autograft group in this study.
In summary, cross-palm nerve grafting will not be a useful technique for every patient with ulnar neuropathy. However, given the clear proof-of-concept that has been presented in animal studies and the encouraging early results of this limited case series, further investigation into this technique is warranted. With refined indications and improved outcomes research, cross-palm grafting may offer an adjunctive technique for improved sensory outcomes in difficult cases of severe ulnar neuropathy, where there is so far no other alternative available.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Statement of Informed Consent: Informed consent was obtained from all individual participants included in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Elspeth J. R. Hill  https://orcid.org/0000-0001-9410-6671
https://orcid.org/0000-0001-9410-6671
Hollie A. Power  https://orcid.org/0000-0003-1131-5940
https://orcid.org/0000-0003-1131-5940
Jessica Hasak  https://orcid.org/0000-0001-9541-4625
https://orcid.org/0000-0001-9541-4625
References
- 1. Zlowodzki M, Chan S, Bhandari M, et al. Anterior transposition compared with simple decompression for treatment of cubital tunnel syndrome. J Bone Joint Surg Am. 2007;89(12):2591-2598. doi: 10.2106/JBJS.G.00183. [DOI] [PubMed] [Google Scholar]
- 2. Dellon AL, Mackinnon SE. Human ulnar neuropathy at the elbow: clinical, electrical, and morphometric correlations. J Reconstr Microsurg. 1988;4(3):179-184. doi: 10.1055/s-2007-1006917. [DOI] [PubMed] [Google Scholar]
- 3. Boone S, Gelberman RH, Calfee RP. The management of cubital tunnel syndrome. J Hand Surg Am. 2015;40(9):1897-1904;quiz 1904. doi: 10.1016/j.jhsa.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 4. Caliandro P, La Torre G, Padua R, et al. Treatment for ulnar neuropathy at the elbow. Cochrane Database Syst Rev. 2012;56(7):CD006839. doi: 10.1002/14651858.CD006839.pub3. [DOI] [PubMed] [Google Scholar]
- 5. Soltani AM, Best MJ, Francis CS, et al. Trends in the surgical treatment of cubital tunnel syndrome: an analysis of the national survey of ambulatory surgery database. J Hand Surg Am. 2013;38:1551-1556. doi: 10.1016/j.jhsa.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 6. Mackinnon SE, Novak CB. Compression Neuropathies. In: Wolfe SW, Pederson WC, Hotchkiss RN, SH Kozin, eds. Green’s Operative Hand Surgery. 7th ed. Philadelphia, PA: Elsevier; 2016:921-958. [Google Scholar]
- 7. Tung TH, Mackinnon SE. Nerve transfers: indications, techniques, and outcomes. J Hand Surg Am. 2010;35(2):332-341. doi: 10.1016/j.jhsa.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 8. Jones NF, Machado GR. Tendon transfers for radial, median, and ulnar nerve injuries: current surgical techniques. Clin Plast Surg. 2011;38(4):621-642. doi: 10.1016/j.cps.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 9. Barbour J, Yee A, Kahn LC, et al. Supercharged end-to-side anterior interosseous to ulnar motor nerve transfer for intrinsic musculature reinnervation. J Hand Surg Am. 2012;37(10):2150-2159. doi: 10.1016/j.jhsa.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 10. Xing SG, Tang JB. Entrapment neuropathy of the wrist, forearm, and elbow. Clin Plast Surg. 2014;41(3):561-588. doi: 10.1016/j.cps.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 11. Brown JM, Yee A, Mackinnon SE. Distal median to ulnar nerve transfers to restore ulnar motor and sensory function within the hand: technical nuances. Neurosurgery. 2009;65(5):966-977;discussion 977-978. doi: 10.1227/01.NEU.0000358951.64043.73. [DOI] [PubMed] [Google Scholar]
- 12. Boyd KU, Nimigan AS, Mackinnon SE. Nerve reconstruction in the hand and upper extremity. Clin Plast Surg. 2011;38(4):643-660. doi: 10.1016/j.cps.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 13. Yüksel F, Karacaoğlu E, Güler MM. Nerve regeneration through side-to-side neurorrhaphy sites in a rat model: a new concept in peripheral nerve surgery. Plast Reconstr Surg. 1999;104(7):2092-2099. [DOI] [PubMed] [Google Scholar]
- 14. Ladak A, Schembri P, Olson J, et al. Side-to-side nerve grafts sustain chronically denervated peripheral nerve pathways during axon regeneration and result in improved functional reinnervation. Neurosurgery. 2011;68(6):1654-1666. doi: 10.1227/NEU.0b013e31821246a8. [DOI] [PubMed] [Google Scholar]
- 15. Yüksel F, Peker F, Çeliköz B. Two applications of end-to-side nerve neurorrhaphy in severe upper-extremity nerve injuries. Microsurgery. 2004;24(5):363-368. doi: 10.1002/micr.20058. [DOI] [PubMed] [Google Scholar]
- 16. Mackinnon S, Yee A. Guyon’s Canal Release and Carpal Tunnel Release. https://surgicaleducation.wustl.edu/guyons-canal-release-carpal-tunnel-release. Accessed January 01, 2019.
- 17. Mackinnon S, Yee A. Revision Ulnar Nerve Transposition Following Failed Submuscular Ulnar Nerve Transposition. https://surgicaleducation.wustl.edu/revision-ulnar-nerve-transposition-following-failed-submuscular-ulnar-nerve-transposition. Accessed January 01, 2019.
- 18. Macadam SA, Gandhi R, Bezuhly M, et al. Simple decompression versus anterior subcutaneous and submuscular transposition of the ulnar nerve for cubital tunnel syndrome: a meta-analysis. J Hand Surg Am. 2008;33(8):1314.e1-1314.e12. doi: 10.1016/j.jhsa.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 19. Biggs M, Curtis JA. Randomized, prospective study comparing ulnar neurolysis in situ with submuscular transposition. Neurosurgery. 2006;58(2):296-304. doi: 10.1227/01.NEU.0000194847.04143.A1. [DOI] [PubMed] [Google Scholar]
- 20. Karthik K, Nanda R, Storey S, et al. Severe ulnar nerve entrapment at the elbow: functional outcome after minimally invasive in situ decompression. J Hand Surg Eur Vol. 2017;37(2):115-122. doi: 10.1177/1753193411416426. [DOI] [PubMed] [Google Scholar]
- 21. Matsuzaki H, Yoshizu T, Maki Y, et al. Long-term clinical and neurologic recovery in the hand after surgery for severe cubital tunnel syndrome. J Hand Surg Am. 2004;29(3):373-378. doi: 10.1016/j.jhsa.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 22. Gervasio O, Gambardella G, Zaccone C, et al. Simple decompression versus anterior submuscular transposition of the ulnar nerve in severe cubital tunnel syndrome: a prospective randomized study. Neurosurgery. 2005;56(1):108-117. doi: 10.1227/01.NEU.0000145854.38234.81. [DOI] [PubMed] [Google Scholar]
- 23. Davidge KM, Yee A, Moore AM, et al. The supercharge end-to-side anterior interosseous-to-ulnar motor nerve transfer for restoring intrinsic function. Plast Reconstr Surg. 2015;136(3):344e-352e. doi: 10.1097/PRS.0000000000001514. [DOI] [PubMed] [Google Scholar]
- 24. Farber SJ, Glaus SW, Moore AM, et al. Supercharge nerve transfer to enhance motor recovery: a laboratory study. J Hand Surg Am. 2013;38(3):466-477. doi: 10.1016/j.jhsa.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brenner MJ, Dvali L, Hunter DA, et al. Motor neuron regeneration through end-to-side repairs is a function of donor nerve axotomy. Plast Reconstr Surg. 2007;120(1):215-223. doi: 10.1097/01.prs.0000264094.06272.67. [DOI] [PubMed] [Google Scholar]
- 26. Hayashi A, Pannucci C, Moradzadeh A, et al. Axotomy or compression is required for axonal sprouting following end-to-side neurorrhaphy. Exp Neurol. 2008;211(2):539-550. doi: 10.1016/j.expneurol.2008.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gordon T, Hendry M, Lafontaine CA, et al. Nerve cross-bridging to enhance nerve regeneration in a rat model of delayed nerve repair. PLoS One. 2015;10(5):e0127397. doi: 10.1371/journal.pone.0127397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tarasidis G, Watanabe O, Mackinnon SE, et al. End-to-side neurorraphy: a long-term study of neural regeneration in a rat model. Otolaryngol Head Neck Surg. 1998;119(4):337-341. doi: 10.1016/S0194-5998(98)70074-9. [DOI] [PubMed] [Google Scholar]
- 29. Hendry JM, Alvarez-Veronesi MC, Snyder-Warwick A, et al. Side-to-side nerve bridges support donor axon regeneration into chronically denervated nerves and are associated with characteristic changes in Schwann cell phenotype. Neurosurgery. 2015;77(5):803-813. doi: 10.1227/NEU.0000000000000898. [DOI] [PubMed] [Google Scholar]