Abstract
Background:
Patient perception of colonoscopy varies greatly. Young slender women and patients with irritable bowel syndrome (IBS) appear to be at risk for periprocedural pain. Recent evidence suggests a high prevalence of joint hypermobility related connective tissue disorders in this population. Therefore, we aimed to investigate whether hypermobility spectrum disorder (HSD) is associated with increased pain during colonoscopy.
Methods:
We prospectively included patients undergoing routine colonoscopy. Subjects were assessed for HSD using the 2017 criteria, and IBS and functional dyspepsia using the Rome III criteria. After colonoscopy and recovery from sedation, patients were asked to report pain scores on a 100-mm visual analogue scale (VAS). In addition, caecal intubation time was measured, endoscopists scored the difficulty of the procedure (100-mm VAS) and procedure-related adverse events were registered.
Results:
Of 200 included patients, 22 (11%) met criteria for HSD. A female predominance was observed in patients with HSD (86.4% versus 49.4%, p < 0.001). A crude linear regression model demonstrated that pain scores were 13.30 mm higher in patients with HSD versus non-HSD patients (95% CI 0.07 – 26.53, p = 0.049). When subsequently correcting for possible confounding factors, however, this difference in pain scores could be explained by a confounding effect of female gender. Caecal intubation time, perceived procedural difficulty and complication rate did not differ significantly between groups.
Conclusion:
HSD does not seem to be a predictor of painful colonoscopy, probably due to female gender as a confounding factor. In addition, performing colonoscopy is not more complicated in patients with HSD versus non-HSD patients, nor is it associated with more adverse events.
Keywords: colonoscopy, hypermobility spectrum disorder, visceral hypersensitivity
Introduction
Ehlers–Danlos syndrome (EDS) is a non-inflammatory heritable connective tissue disorder resulting from defects in collagen structure, synthesis or processing. Its manifestations frequently involve the cutaneous and musculoskeletal systems such as skin laxity and joint hypermobility (JHM).1 The combined prevalence of all types of EDS appears to be at least 1 in 5000 individuals worldwide2 but good quality epidemiological data are lacking. The hypermobility type (hEDS) is the most common (80–90%); type 4 (vascular EDS) accounts for 3–6% of all EDS and is considered the most serious because it is associated with vascular rupture. A key defining feature of EDS is JHM, which refers to the characteristic of being able to move joints, actively and/or passively, beyond normal limits. JHM can vary in intensity as well as in location, ranging from localised hyperflexibility (i.e. involving fewer than five joints) to generalised JHM (G-JHM), in which at least five different joints are affected.
As far as gastroenterology practice is concerned, evidence suggests that EDS may pose particular risk for spontaneous or instrumentation-related intestinal rupture.3–8 A recent retrospective study showed, however, that this increased risk was entirely accounted for by patients having vascular EDS but not other EDS subtypes, such as hEDS.9 Another anecdotal but yet scientifically unproven concern is related to colonoscopy being more painful or difficult for the endoscopist in patients with EDS. This is assumed to be the result of the technical difficulty of the colonoscopy by increased tissue laxity, causing the colon to stretch relatively easily upon intubation thus hindering the negotiation of tortuous curves.
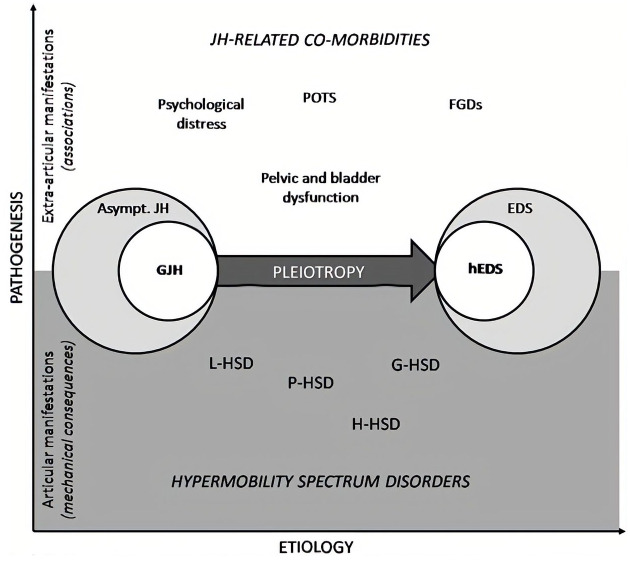
In the current study therefore, we aimed to explore in a pilot study whether hypermobile patients presenting for a colonoscopy had more painful experiences or had an increased risk for adverse events. JHM was assessed according to the novel 2017 criteria for ‘hypermobility spectrum disorder’ (HSD). There is a fairly broad continuum of JHM and related symptoms, ranging between, at one end, asymptomatic G-JHM, i.e. someone who has no symptoms apart from their joints’ capacity to move beyond normal limits, through to hEDS, at the other end. Within this spectrum, the category of HSD represents patients with symptoms related to their JHM but who do not meet the full (new and stricter) criteria for hEDS (see Figure 1). By definition, patients presenting with symptoms and G-JHM would be classified as HSD. HSD will therefore include most people who have been previously diagnosed with joint hypermobility syndrome (JHS) or benign joint hypermobility syndrome, and some people who previously had the diagnosis of EDS type 3. Patients with JHS have previously been shown to have increased risk of chronic pain syndromes, functional gut disorders, for example, functional dyspepsia (FD) and irritable bowel syndrome (IBS), and visceral hypersensitivity.10,11 These factors are known to be associated with increased pain/decreased tolerability during colonoscopy.10,12 We therefore hypothesised that patients with HSD have higher pain scores during colonoscopy than non-HSD patients. In addition, we also assessed the rate of adverse events and other technical details related to colonoscopy to ascertain potential differences related to the patients’ HSD status.
Figure 1.
Visualisation of the spectrum of hypermobility disorders. On the left asymptomatic generalised joint hypermobility, on the right hEDS, with HSD covering the range from right to left. The top of the figure displays extra-articular manifestations.
EDS, Ehlers–Danlos syndrome; hEDS, hypermobility type Ehlers–Danlos syndrome; FGDs, functional gastrointestinal disorders; G-HSD, generalised hypermobility spectrum disorder; GJH, generalised joint hypermobility; H-HSD, historical hypermobility spectrum disorder; JH, joint hypermobility; L-HSD, localised hypermobility spectrum disorder; P-HSD, peripheral hypermobility spectrum disorder; POTS, postural orthostatic tachycardia syndrome. (Reprint from Castori et al.13)
Methods
Study design
This study was performed at the endoscopy department of the Maastricht University Medical Center (Maastricht UMC) in Maastricht, the Netherlands, a secondary/tertiary referral hospital. The study protocol has been approved by the Maastricht UMC Committee of Ethics (IRB identifier 15-4-258) and was executed according to the revised Declaration of Helsinki (64th World Medical Association General Assembly, Fortaleza, Brazil, October 2013).
Participants
All patients aged between 18 years and 75 years undergoing a colonoscopy with regular sedation were considered eligible for inclusion. Patients were excluded when undergoing a colonoscopy in the context of the national screening programme for colorectal cancer (due to age bias, JHM is strongly influenced by age14), a follow-up colonoscopy for inflammatory bowel disease or colorectal cancer, or with a history of previous extended abdominal surgery due to bias towards more painful colonoscopy. Patients with a history of uncomplicated cholecystectomy, appendectomy and/or hysterectomy were considered eligible. Procedures under propofol-based sedation were excluded from the study due to the presumed better tolerability of this method. All participants gave their written informed consent prior to inclusion.
Study procedures
Clinical assessments
All subjects were assessed for HSD using the 2017 criteria by a trained clinical researcher.13 HSD is defined by G-JHM (as assessed using physical examination according to the Beighton score, in addition to the presence of certain clinical features which do not fit the diagnosis of hEDS on the 2017 classification for hEDS, that is, joint trauma, chronic pain, orthopaedic manifestations (pes planus, valgus deformity), etc., as a result of JHM (see https://www.ehlers-danlos.com/heds-diagnostic-checklist/). None of the patients investigated had a diagnosis of HSD, hEDS or JHS prior to inclusion.
In addition, participants were assessed for IBS and FD using the Rome III criteria15 as potential confounders.
Colonoscopy
Colonoscopies were performed either by a consultant gastroenterologist (n = 10) or trainee (n = 21). The endoscopists were blinded to the HSD status of the patient. Pentax HD+ colonoscopes (HOYA Corporation, Tokyo, Japan) with carbon dioxide insufflation were used in all procedures. Furthermore, all colonoscopies were performed under conscious sedation with the use of midazolam, with the addition of an opiate (either pethidine or fentanyl). Standard dosage applied was 2.5 mg for midazolam, 25 µg for fentanyl and 25 mg for pethidine. An additional dose was administered as needed, using a maximum total dosage of 5.0 mg for midazolam, 50 µg for fentanyl and 50 mg for pethidine (considered ‘high dose’). The level of sedation was noted on an ordinal scale from 1 (awake) to 5 (unresponsive) after the endoscopy was completed. There was a minimum of one endoscopist and two assisting nurses present during all procedures. The nurses were present to monitor the patient during the procedure and in addition, all patients were monitored by pulse oximetry and blood pressure measurement every 5 min.
Patient-reported outcomes
After the colonoscopy, when patients had recovered adequately from sedation, they were asked to report periprocedural pain scores on a 100-mm visual analogue scale (VAS) (primary outcome measure). In addition, the patient was asked to score perceived discomfort using the same scale.
Endoscopist-reported outcomes
Endoscopists were requested to report the degree of technical difficulty of the colonoscopy on a 100-mm VAS directly after the colonoscopy. In addition, endoscopists were asked to provide an indication of their experience level (i.e. the number of colonoscopies performed). Experience level was divided into 5 categories, i.e. having performed 0–200 colonoscopies, 200–500 colonoscopies, 500–1500 colonoscopies, 1500–5000 colonoscopies or more than 5000 colonoscopies.
Assisting nurse-reported outcomes
Nurses assisting the colonoscopy were asked to report the level of discomfort of the patient using the Modified Gloucester Discomfort Scale, ranging from 1 (meaning no discomfort) to 5 (meaning severe discomfort).16
Procedure-related outcomes
Sedative and analgesic dose administered before and during endoscopy were registered immediately. During colonoscopy, caecal intubation time (from anal insertion to caecum) was recorded. Patients in whom the caecum was not reached were excluded from the analyses of mean intubation time. Macroscopic diagnoses were registered to correct for possible confounding (e.g. inflammation, diverticulosis). The adequacy of bowel preparation was described using the Boston Bowel Preparation Scale.17 Finally, the occurrence of adverse events was strictly monitored and registered.
Sample size calculation
Sample size calculation was based on the primary outcome measure, patient-reported pain scores during colonoscopy, using a VAS. Mean VAS scores during regular colonoscopy have been reported as 32 mm with a standard deviation (SD) of 21.6 mm.18 A difference in VAS scores of 15 mm was considered clinically relevant, based on earlier studies investigating pain scores during colonoscopy.18,19 To be confident of finding differences in reported discomfort scores of at least 15 mm with a power of 80% and two-sided alpha of 0.05, a sample size of 33 subjects was required in the smallest group. Since we initially expected to find a prevalence of JHM as a trait of about 30%,20 a total sample size of 110 subjects was required. As the prevalence of JHM was found to be substantially less after inclusion of 110 patients, we included additional patients up to a total of 200 patients. This did not, however, result in a change in the prevalence of hypermobility or the diagnosis of HSD. Considering this unaltered prevalence, a sample size of 330 patients would have been necessary to detect a difference of 15 mm in VAS scores. Consequently, we did not have sufficient power to detect a 15 mm difference in VAS scores.
Statistical analysis
Study participants fulfilling 2017 criteria for HSD or not are referred to as ‘HSD’ and ‘non-HSD,’ respectively. Data are presented as mean ± SD for normally distributed continuous variables and proportions for categorical outcomes. Univariate comparison of patient characteristics between HSD and non-HSD was performed using an independent samples t test for continuous variables and χ2 test via cross-tabulation for dichotomous variables. Significance level was corrected for multiple comparisons according to Bonferroni (p < 0.0033 for 15 comparisons).
Subsequently, a crude (unadjusted) linear regression analysis was performed using the primary outcome measure (patient-reported pain scores) as the dependent variable while introducing HSD status as the sole covariate. In addition, multivariable linear regression analysis was performed, adjusting for sex, age, BMI, previous abdominal surgery, FD, IBS, active inflammation, diverticulosis, bowel cleanliness, sedative and analgesic dose, and endoscopist training level. Variance inflation factors revealed no multicollinearity issues. As a result of a suspected non-normal distribution, a sensitivity analysis was performed after a two-step data transformation of patient-reported pain scores.21 After transformation, data were visually confirmed to be normally distributed. As linear analysis with the transformed data yielded similar results, the results of linear regression reported below are based on the original data.
All analyses were performed using IBM SPSS Statistics, version 23 (IBM Statistics for Macintosh, Chicago, IL, USA).
Results
Study participants
During the study period, 348 eligible patients were contacted for participation. A total of 200 patients gave informed consent and were included in the study (mean age 56.4 ± 13.4 years; 52.7% women). A total of 22 patients (11%) met criteria for HSD and were predominantly women (see Table 1). One patient had G-JHM not meeting the criteria for HSD and was therefore included in the control group. The prevalence of G-JHM was therefore 11.5%.
Table 1.
Patient characteristics.
| Non-HSD (n = 178) | HSD (n = 22) | p value | |
|---|---|---|---|
| Age, years (mean ± SD) | 57.1 ± 13.3 | 50.3 ± 13.5 | 0.026 |
| Women, n (%) | 87 (49.4%) | 19 (86.4%) | 0.001 |
| Body mass index (mean ± SD) | 26.7 ± 4.3 | 27.0 ± 4.7 | 0.746 |
| Irritable bowel syndrome, n (%) | 46 (25.8%) | 10 (45.5%) | 0.056 |
| Functional dyspepsia, n (%) | 25 (14.0%) | 11 (50.0%) | <0.001 |
| Macroscopic inflammation, n (%) | 20 (11.2%) | 1 (4.5%) | 0.331 |
| Diverticulosis, (%) | 70 (39.3%) | 8 (36.4%) | 0.773 |
|
Presence of one or
more polyps, n (%) |
73 (41.0%) | 6 (27.3%) | 0.207 |
| Boston Bowel Preparation Scale (mean ± SD) | 8.0 ± 1.2 | 7.9 ± 1.6 | 0.855 |
| High-dose midazolam, n (%) | 43 (24.2%) | 12 (54.5%) | 0.003 |
| High-dose opiate,*n (%) | 116 (65.1%) | 18 (81.8%) | 0.117 |
| Level of sedation, $ n (%) | Awake: 39 (45.3%) Drowsy: 36 (41.9%) Sleeping, responsive to speech: 11 (12.8%) |
Awake: 5 (71.4%) Drowsy: 1 (14.3%) Sleeping, responsive to speech: 1 (14.3%) |
n.a. |
| Adverse events | |||
| Mild desaturation, n (%) | 18 (10.1%) | 3 (13.6%) | 0.618 |
| Vasovagal episode, n (%) | 8 (4.5%) | 0 (0%) | 0.309 |
| Previous uncomplicated abdominal surgery, n (%) | 35 (19.7%) | 7 (31.8%) | 0.187 |
| First-time colonoscopy, n (%) | 93 (52.2%) | 11 (50%) | 0.842 |
| Indication for colonoscopy | 0.494 | ||
| Adenoma follow up | 33 (18.5%) | 3 (13.6%) | |
| Abdominal pain/discomfort | 55 (30.9%) | 9 (40.9%) | |
| Altered stool pattern | 41 (23.0%) | 7 (31.8%) | |
| Rectal blood loss | 42 (23.6%) | 2 (9.1%) | |
| Familial colon cancer | 7 (3.9%) | 1 (4.5%) | |
Either high dose of fentanyl (50 µg) or high dose of pethidine (50 mg).
Incomplete data; level of sedation was not registered for all patients. p values not calculated.
Significance level corrected for multiple comparisons (Bonferroni): p < 0.0033.
HSD, hypermobility spectrum disorder; n.a., not applicable; SD, standard deviation.
FD was more common in HSD compared with non-HSD (50.0% versus 14.0%, p < 0.001). When corrected for age and gender in a logis-tic regression model, this failed to reach the predefined statistical significance level, although a positive trend remained (ORadj 3.62, 95% CI 1.35–9.72, p = 0.01). IBS prevalence rates demonstrated a similar trend, with a higher rate in patients with HSD. No differences were found in macroscopic diagnoses (i.e. diverticulosis, macroscopic inflammation, presence of polyps) (see Table 1).
Patient-reported pain scores
A crude linear regression model demonstrated that pain scores were 13.30 mm higher (95% CI 0.07–26.53, p = 0.049) in patients with HSD than non-HSD patients. When subsequently correcting for possible confounding factors, however, no significant effect on pain scores was found for HSD. The effect of HSD in the crude linear regression appeared to be primarily related to the confounding effect of female gender (see Supplemental data). Indeed, female gender was the strongest predictor of a higher pain score (B: 12.15 mm, 95% CI 2.71–21.59, p = 0.012). In addition, endoscopist training level appeared to have a role in patient pain perception, with lower pain scores when the procedure was performed by a more experienced endoscopist (B: –4.36, 95% CI −7.59 to −0.473, p = 0.027, calculated with the use of experience categories, see Methods section). No other clinically relevant confounders were identified in the current study, including age, BMI, presence of IBS or FD, active inflammation, diverticulosis, bowel cleanliness, and sedative and analgesic dose (see Supplemental data). Less than 20% of variability in pain scores was explained with the use of the complete regression model (R2 = 0.197). In addition to the above, no significant association was found between Beighton score (representing trait JHM) and patient-reported pain scores.
Discomfort scores
Neither patient- nor nurse-reported discomfort scores differed significantly between patients with HSD and non-HSD patients (patient reported: 35.77 mm ± 27.46 versus 42.59 mm ± 29.84 (on a 100-mm VAS), p = 0.278; nurse reported: 2.57 versus 3.00 (on a 5-point Likert scale), p = 0.132. There was a fairly good correlation between patient- and nurse-reported scores: 0.64, p < 0.001.
Procedure-related outcomes
Overall caecal intubation rate was 93.0%; there was no significant difference between failure to reach the caecum (13.6% HSD versus 6.1% non-HSD, p = 0.193). Endoscopist-reported technical difficulty scores were comparable (39.98 mm ± 28.97 versus 40.73 mm ± 30.97, p = 0.910). Similarly, caecal intubation time was comparable between both patient groups: mean caecal intubation time in patients with HSD was 10 min and 34 s (SD 7 min and 36 s) versus 12 min and 14 s (SD 7 min and 27 s) in non-HSD patients, p = 0.358.
A significantly higher proportion of patients with HSD were administered high doses of midazolam (54.5% versus 24.2%, p = 0.003). However, of all patients receiving high-dose midazolam, 83.6% were women, hence gender was likely a confounding factor. Indeed, when corrected for gender, binary logistic regression showed that the odds of high-dose midazolam use were not significantly higher in patients with HSD (ORadj 2.29, 95% CI 0.88–5.98, p = 0.09).
Adverse events
No serious adverse events were reported. Reported adverse events included 21 cases of mild oxygen desaturation (10.5%), all involving only a minor and transient reduction in saturation with a minimum reported oxygen saturation of 80%. In addition, eight vasovagal episodes (4.0%) were reported, which consisted of transient hypotension and/or bradycardia or excessive perspiration. Adverse event rates were similar between groups receiving low- and high-dose midazolam. Similarly, rates did not differ between patient groups (see Table 1). VAS pain scores did not differ significantly between patients who experienced an adverse event and those who did not (data not shown).
Sensitivity analysis
Analyses were rerun when defining the groups as follows: patients with G-JHM (hence including all patients with HSD, n = 23) and patients without G-JHM (n = 177). No differences were detected in any of the outcome parameters when compared with the initial analyses (data not shown).
Discussion
Our results from this exploratory study indicate that HSD, which is a disorder clinically closely related to hEDS, and largely overlapping with the now defunct category of JHS, is not independently associated with higher patient-reported pain scores during colonoscopy. Although an initial crude linear regression model indicated higher pain scores in patients with HSD, subsequent analyses revealed a strong confounding effect of female gender and endoscopist experience. Similarly, there was no association between Beighton score and patient-reported pain scores, indicating that less severe phenotypes of JHM in the general population seem not to be related to periprocedural pain. Furthermore, we found no significant difference in patient- and nurse-reported discomfort scores or the occurance of adverse events between patients with HSD and non-HSD patients. Lastly, endoscopist-reported technical difficulty scores and caecal intubation time did not differ significantly between patients with HSD and non-HSD patients. It therefore appears that colonoscopy in patients with HSD is neither more painful nor less safe than in non-HSD patients. This conclusion might be relevant for both patients and pracitioners when making clinical decisions for colonscopy referral.
Our hypothesis of higher pain scores in patients with HSD was driven by anecdotal evidence in addition to the reported higher prevalence of the female gender and functional gastrointestinal (GI) disorders in patients previously defined as having JHS, according to pre-2017 nosology. Both the high prevalence of functional GI disorders (in particular FD) and female predominance in patients with HSD were corroborated in the current study, albeit the higher prevalence of IBS failed to reach statistical significance. While FD and IBS often coexist as manifestations of visceral hypersensitivity,22 the explanations for why HSD is more strongly linked with FD than IBS remain speculative. It is possible that the upper GI tract is more sensitive to the extracellular matrix alterations seen in HSD.23 Certain extracellular matrix molecules that have been linked to hypermobility are also involved in upper GI sensory function.24 However, more in-depth studies investigating connective tissue alterations in both the upper and lower GI tract, and their correlations with symptomatology, are required to answer this question.
It should be noted that patients with HSD more often needed higher doses of midazolam. One might speculate that the reason patients did not experience more pain was related to the higher level of sedation. However, the level of sedation did not appear to correlate with patient-reported pain scores in the regression model and was not a confounder. In addition, we observed that of all patients receiving high-dose midazolam, 83.6% were women. Therefore, the higher midazolam dosages in the HSD group appeared to be mainly gender related, as also demonstrated by a binary logistic regression analysis.
A strength of the current study is that prospective recruitment took place via the general colonoscopy programme in a secondary/tertiary clinic, without preselection for specific GI diseases. Participants included primary (through open-access for GPs), secondary and tertiary care patients, resulting in a study population that reflects the general hospital population. Furthermore, the evaluation of HSD status was performed by a trained researcher after the colonoscopy in order to reduce bias in the patient-, endoscopist- and nurse-reported outcome measures.
Several limitations of the current exploratory study have to be mentioned. First, the rather small sample size limits the generalisability of the findings. In order to detect a difference in VAS scores of 15 mm, we would have needed to include 330 patients albeit this would probably not have impacted the prevalence and therefore the effect size would not have been influenced either. Therefore, we assume that no clinically relevant effect of HSD on perceived pain during colonoscopy is to be expected in a larger patient sample. Nevertheless, the lack of a statistical association between HSD and pain scores could be due to a type II error. A possible explanation for the lower than expected prevalence of HSD is the relatively high age of the study population, as compared with a previous study where the prevalence of JHS was found to be 33%.20 In addition, the study by Fikree et al. demonstrated that patients with JHS were significantly younger than patients not fulfilling criteria for JHS. A similar trend was observed in the current study, although this age difference was not statistically significant after Bonferroni correction. In fact, it is well-known that JHM decreases by age.25
Second, we identified patients with HSD who need not necessarily fulfil the 2017 criteria for hEDS. Therefore, current results cannot be readily extrapolated to patients with hEDS or any other EDS subtype. On the other hand, we believe the population with HSD represents a clinical relevant patient group to endoscopists (11% of the current overall patient population).
In conclusion, we have not found patients with HSD to perceive colonoscopy as more painful, when correcting for confounders, in particular gender, or for level of sedation. Neither was colonoscopy associated with more adverse events in this patient group. Gastroenterologists therefore do not need to defer from colonoscopy, although findings of this current exploratory study should be corroborated in larger populations.
Supplemental Material
Supplemental material, Supplementary_data for Colonoscopy is safe and not associated with higher pain scores in patients with hypermobility spectrum disorder: results from an exploratory prospective study by Abraham B. Beckers, Lisa Vork, Asma Fikree, Rogier de Ridder, Qasim Aziz, Ad Masclee and Daniel Keszthelyi in Therapeutic Advances in Gastroenterology
Acknowledgments
We acknowledge the assistance of Ms Sanne Krielaart in patient inclusion.
Footnotes
Author contributions: LV and DK were responsible for the study concept and design. LV and ABB were responsible for the acquisition, analysis and interpretation of data. ABB was responsible for drafting the manuscript. LV, RdR, AF, QA, AM and DK were responsible for critical revision of the manuscript for important intellectual content. LV and ABB were responsible for the statistical analysis. All authors approved the final version of the manuscript submitted for publication, and all persons designated as authors qualify for authorship and all those who qualify for authorship are listed.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Data availability: The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Abraham B. Beckers  https://orcid.org/0000-0002-9532-2576
https://orcid.org/0000-0002-9532-2576
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Abraham B. Beckers, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Limburg, The Netherlands
Lisa Vork, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Limburg, The Netherlands.
Asma Fikree, Gastroenterology Department, Royal London Hospital, London, UK.
Rogier de Ridder, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Limburg, The Netherlands.
Qasim Aziz, Wingate Institute of Neurogastroenterology, Queen Mary University of London, London, UK.
Ad Masclee, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Limburg, The Netherlands.
Daniel Keszthelyi, Department of Internal Medicine, Division of Gastroenterology-Hepatology, NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht University Medical Center, PO box 5800, Maastricht, Limburg 6202 AZ, The Netherlands.
References
- 1. Ghali N, Sobey G, Burrows N. Ehlers-Danlos syndromes. BMJ 2019; 366: l4966. [DOI] [PubMed] [Google Scholar]
- 2. Royce PM, Steinmann B. (eds) Connective tissue and its heritable disorders. 2nd ed New York: Wiley-Liss, 2002. [Google Scholar]
- 3. Beighton PH, Murdoch JL, Votteler T. Gastrointestinal complications of the Ehlers-Danlos syndrome. Gut 1969; 10: 1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nardone DA, Reuler JB, Girard DE. Gastrointestinal complications of Ehlers-Danlos syndrome. N Engl J Med 1979; 300: 863–864. [DOI] [PubMed] [Google Scholar]
- 5. Pepin M, Schwarze U, Superti-Furga A, et al. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med 2000; 342: 673–680. [DOI] [PubMed] [Google Scholar]
- 6. Hawk JS, Dellon ES, Martinie JB, et al. Successful ERCP and sphincterotomy in a patient with Ehlers-Danlos syndrome and a history of spontaneous bowel perforation. Gastrointest Endosc 2008; 67: 755–758. [DOI] [PubMed] [Google Scholar]
- 7. Rana M, Aziz O, Purkayastha S, et al. Colonoscopic perforation leading to a diagnosis of Ehlers Danlos syndrome type IV: a case report and review of the literature. J Med Case Rep 2011; 5: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allaparthi S, Verma H, Burns DL, et al. Conservative management of small bowel perforation in Ehlers-Danlos syndrome type IV. World J Gastrointest Endosc 2013; 5: 398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kilaru SM, Mukamal KJ, Nee JW, et al. Safety of endoscopy in heritable connective tissue disorders. Am J Gastroenterol 2019; 114: 1343–1345. [DOI] [PubMed] [Google Scholar]
- 10. Fikree A, Aktar R, Grahame R, et al. Functional gastrointestinal disorders are associated with the joint hypermobility syndrome in secondary care: a case-control study. Neurogastroenterol Motil 2015; 27: 569–579. [DOI] [PubMed] [Google Scholar]
- 11. Beckers AB, Keszthelyi D, Fikree A, et al. Gastrointestinal disorders in joint hypermobility syndrome/Ehlers-Danlos syndrome hypermobility type: a review for the gastroenterologist. Neurogastroenterol Motil. Epub ahead of print 13 January 2017. DOI: 10.1111/nmo.13013 [DOI] [PubMed] [Google Scholar]
- 12. Denters MJ, Schreuder M, Depla AC, et al. Patients’ perception of colonoscopy: patients with inflammatory bowel disease and irritable bowel syndrome experience the largest burden. Eur J Gastroenterol Hepatol 2013; 25: 964–972. [DOI] [PubMed] [Google Scholar]
- 13. Castori M, Tinkle B, Levy H, et al. A framework for the classification of joint hypermobility and related conditions. Am J Med Genet C Semin Med Genet 2017; 175: 148–157. [DOI] [PubMed] [Google Scholar]
- 14. Remvig L, Jensen DV, Ward RC. Epidemiology of general joint hypermobility and basis for the proposed criteria for benign joint hypermobility syndrome: review of the literature. J Rheumatol 2007; 34: 804–809. [PubMed] [Google Scholar]
- 15. Drossman DA, Corazziari E, Delvaux M, et al. Rome III: the functional gastrointestinal disorders. 3rd ed McLean (VA): Degnon Associates, 2006. [Google Scholar]
- 16. Programme NCS. Quality assurance guidelines for colonoscopy, publication no. 6, http://www.cancerscreening.nhs.uk/bowel/publications/nhsbcsp06.pdf (2011, accessed 12 March 2012).
- 17. Lai EJ, Calderwood AH, Doros G, et al. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 2009; 69: 620–625. DOI: 10.1016/j.gie.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ogawa T, Ohda Y, Nagase K, et al. Evaluation of discomfort during colonoscopy with conventional and ultrathin colonoscopes in ulcerative colitis patients. Dig Endosc 2015; 27: 99–105. [DOI] [PubMed] [Google Scholar]
- 19. Lee H, Kim JJ, Min BH, et al. Effectiveness of warm water consumption to reduce patient discomfort during colonoscopy: a randomized controlled trial. Am J Gastroenterol 2009; 104: 2935–2941. [DOI] [PubMed] [Google Scholar]
- 20. Fikree A, Grahame R, Aktar R, et al. A prospective evaluation of undiagnosed joint hypermobility syndrome in patients with gastrointestinal symptoms. Clin Gastroenterol Hepatol 2014; 12: 1680–1687 e1682. [DOI] [PubMed] [Google Scholar]
- 21. Templeton GF. A two-step approach for transforming continuous variables to normal: implications and recommendations for IS research Commun Assoc Inf Syst 2011; 28: 42–58. [Google Scholar]
- 22. Agreus L, Svardsudd K, Nyren O, et al. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology 1995; 109: 671–680. [DOI] [PubMed] [Google Scholar]
- 23. Zoppi N, Chiarelli N, Binetti S, et al. Dermal fibroblast-to-myofibroblast transition sustained by αvß3 integrin-ILK-Snail1/Slug signaling is a common feature for hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders. Biochim Biophys Acta Mol Basis Dis 2018; 1864: 1010–1023. [DOI] [PubMed] [Google Scholar]
- 24. Aktar R, Peiris M, Fikree A, et al. A novel role for the extracellular matrix glycoprotein-Tenascin-X in gastric function. J Physiol 2019; 597: 1503–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Remvig L, Engelbert RH, Berglund B, et al. Need for a consensus on the methods by which to measure joint mobility and the definition of norms for hypermobility that reflect age, gender and ethnic-dependent variation: is revision of criteria for joint hypermobility syndrome and Ehlers-Danlos syndrome hypermobility type indicated? Rheumatology (Oxford) 2011; 50: 1169–1171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_data for Colonoscopy is safe and not associated with higher pain scores in patients with hypermobility spectrum disorder: results from an exploratory prospective study by Abraham B. Beckers, Lisa Vork, Asma Fikree, Rogier de Ridder, Qasim Aziz, Ad Masclee and Daniel Keszthelyi in Therapeutic Advances in Gastroenterology