Abstract
Pyoderma gangrenosum is a challenging disease to manage, due in part to the lack of approved treatment therapies. Recently, the emergence of biologic agents has expanded treatment options, with tumour necrosis factor alpha inhibitors being the best supported in the literature. In our report, we present a 50-year-old female with pyoderma gangrenosum who was successfully treated with the anti-interleukin-17 biologic agent, secukinumab, after failing other systemic therapies.
Keywords: Pyoderma gangrenosum, secukinumab, biologics, treatment
Introduction
Pyoderma gangrenosum is a rare, poorly understood, neutrophilic dermatosis that presents as a painful inflammatory and ulcerative disorder of the skin. The clinical presentation of pyoderma gangrenosum is variable; however, there is a common clinical course involving the development of a tender papule, vesicle, or pustule that progressively expands and then ulcerates. Pyoderma gangrenosum is often associated with underlying inflammatory or malignant disease, but it can be idiopathic in up to 50% of patients.1,2
Pyoderma gangrenosum is a challenging disease to manage. The rarity of the disease has contributed to the lack of high-quality data on treatment outcomes. No definitive guidelines for the management of pyoderma gangrenosum have been established, but treatment commonly focuses on suppressing the inflammatory process.3 In general, patients are managed with a combination of local wound care management, topical, and/or systemic therapies.1 Factors such as disease severity, presence of underlying systemic disease, side-effect profiles, cost, and patient preference are commonly used to guide treatment decisions.3
Oral corticosteroids and cyclosporine are the most frequently used systemic treatments for pyoderma gangrenosum; however, the emergence of biologic agents has expanded treatment options, with tumour necrosis factor alpha (TNF-α) inhibitors being the best supported in the literature.1,3 In this report, we describe the case of a female patient with pyoderma gangrenosum successfully treated with the anti-interleukin (IL)-17 biologic agent, secukinumab.
Case report
A 50-year-old woman was referred for a progressive, painful ulcerating lesion on her left lower leg. The lesion began as a small blister that ruptured, draining brown fluid. It had recently worsened following physical trauma to the area. The patient had no known history of inflammatory bowel disease or rheumatoid arthritis. Her only medication was desvenlafaxine for depression. She had been evaluated by her family physician and an infectious disease specialist, with a suspected diagnosis of pyoderma gangrenosum. She was initially treated with three courses of antibiotics (clindamycin and trimethoprim/sulfamethoxazole) with no improvement.
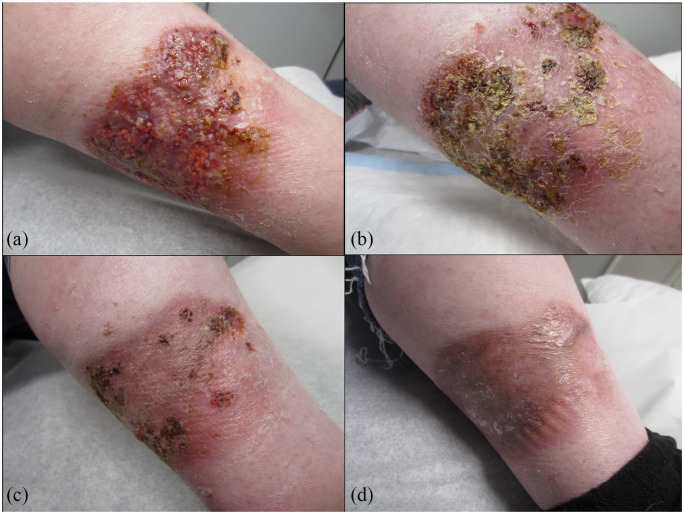
On presentation to dermatology, there was a tender, 14 cm × 9 cm granulating ulcer on the left lower medial leg with irregular, undermined borders (see Figure 1(a)). Laboratory studies, including a complete blood count,metabolic panel, hepatitis panel, rheumatoid factor, and serum protein electrophoresis/urine protein electrophoresis, were all within normal limits. Two 4-mm-punch biopsies were taken for histopathologic examination and culture. Histopathology showed a dense neutrophilic infiltrate predominantly occupying the deep dermis. Tissue culture showed very light growth of Escherichia coli and Coryneform bacteria, but no fungus. A diagnosis of pyoderma gangrenosum was made on the basis of Maverakis criteria.4
Figure 1.
(a) Painful ulcer with undermined borders and purulent discharge diagnosed as pyoderma gangrenosum on the left lower leg before secukinumab; (b) improvement after 1 month; (c) 2 months; and (d) 3 months of secukinumab, started at 300 mg subcutaneous weekly for 5 weeks, followed by monthly maintenance dosing.
The patient was started on oral prednisone 40 mg for 7 days, and then tapered by 5 mg every 5 days. This was supplemented with clobetasol ointment applied daily. The patient had a slight decrease in pain, but the lesion did not change in size. The patient’s treatment regime was changed to prednisone 50 mg for 10 days, tapered by 5 mg every 5 days; however, this also proved nonbeneficial. Unfortunately, the patient did not have drug coverage and was unable to afford cyclosporine. Therefore, treatment with methotrexate 15 mg weekly and folic acid was initiated. After 2 months of treatment, the patient had no improvement.
Following several failed treatment options, the decision was made to attempt a trial of secukinumab, which was obtained on compassionate grounds. Secukinumab was started at 300 mg subcutaneous weekly at weeks 0, 1, 2, 3, and 4, followed by monthly maintenance dosing. Within 4 weeks of secukinumab initiation, the patient’s pain decreased by 70%–80% and she was able to walk easier. The patient had significant improvement after 3 months of treatment; she had no pain and the ulcerated skin had healed completely (see Figure 1(b)–(d)). She remained on this treatment for an additional 2 months before stopping. She has had no recurrence or worsening in the past 3 months.
Discussion
Pyoderma gangrenosum is an inflammatory ulcerative process mediated by neutrophil-predominant infiltrates in the dermis. The pathophysiology of pyoderma gangrenosum remains poorly understood; though, abnormalities in the function of inflammatory cytokines, loss of innate immune regulation, and neutrophil dysfunction are believed to be involved in the pathogenesis of the disease.1,5 A number of cytokines have been found to be elevated in pyoderma gangrenosum lesions, including TNF-α, IL-8, IL-17, chemokines 1, 2, 3, and 16, and matrix metalloproteinase 2 and 9.6
As the complex pathogenesis of pyoderma gangrenosum is further elucidated, therapeutic approaches have expanded to include novel, more targeted therapies. Pyoderma gangrenosum has been reported to respond to multiple different biologic agents, most commonly anti-TNF-α drugs such as infliximab, adalimumab, and etanercept.3 There is emerging evidence for the use of other biologic agents, including IL-12, IL-23, IL-1, and IL-6 antagonists.3
Currently, there are three biologic agents used to target the IL-17A pathway: secukinumab, brodalumab, and ixekizumab. Secukinumab is a recombinant, human IgG1 monoclonal antibody that binds to the protein IL-17A, a cytokine involved in the release of proinflammatory mediators. Secukinumab is approved to treat psoriasis, ankylosing spondylitis, and psoriatic arthritis. It is generally well tolerated, with low reported immunogenicity. The most common side-effects include upper respiratory symptoms, nausea, and diarrhoea.
A recent study found augmented numbers of T helper cells (Th17) in patients with pyoderma gangrenosum, proposing the use of therapies targeting the Th17 pathway, such as IL-17 antagonists, as a possible treatment alternative for pyoderma gangrenosum.7 To date, evidence for the efficacy of secukinumab for pyoderma gangrenosum is extremely limited. To our knowledge, there are only two published accounts of secukinumab being used for the treatment of pyoderma gangrenosum, both resulting in a partial response.8,9 However, several clinical trials are currently evaluating the safety and efficacy of IL-17 inhibitors for pyoderma gangrenosum, including two open-label trials on secukinumab (ClinicalTrials.gov, NCT02733094 and NCT04274166) and a recently completed open-label trial on ixekizumab (NCT03137160).10
Interestingly, there are several recent anecdotal reports of pyoderma gangrenosum being paradoxically induced by IL-17 inhibitors.11–13 Different theories have been proposed to explain these paradoxical reactions to biologic agents, including an imbalance in cytokine production, unopposed production of interferon alpha (IFN-α), and a shift towards a Th1 cytokine profile.14,15 While the IFN-α pathway has been identified as an alternative pathway in paradoxical psoriasis reactions caused by biologic therapy,16 the pathogenesis of IL-17 inhibitor-induced pyoderma gangrenosum is largely unknown and there are likely multiple inflammatory pathways of disease induction.
The marked improvement observed in our patient suggests that secukinumab may be a promising therapeutic option for pyoderma gangrenosum. However, clinicians should be aware of the potentially dual role of IL-17 inhibitors in both treating and paradoxically inducing pyoderma gangrenosum. More research is required to establish the efficacy of secukinumab for pyoderma gangrenosum.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.G.K.—Abbvie; Novartis-Speaker, Advisory Board Member, Consultant; Janssen; Leo Pharma; Pfizer; Sanofi-Genzyme; UCB-Speaker, Advisory Board Member; Celgene, Eli Lilly-Advisory Board Member.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed written consent was obtained for patient information and images to be published.
References
- 1. Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum. Am J Clin Dermatol 2012; 13(3): 191–211. [DOI] [PubMed] [Google Scholar]
- 2. Pereira N, Brites MM, Gonçalo M, et al. Pyoderma gangrenosum: a review of 24 cases observed over 10 years. Int J Dermatol 2013; 52(8): 938–945. [DOI] [PubMed] [Google Scholar]
- 3. Alavi A, French LE, Davis MD, et al. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol 2017; 18(3): 355–372. [DOI] [PubMed] [Google Scholar]
- 4. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatology 2018; 154(4): 461466. [DOI] [PubMed] [Google Scholar]
- 5. Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol 2015; 73(4): 691–698. [DOI] [PubMed] [Google Scholar]
- 6. Marzano A, Fanoni D, Antiga E, et al. Expression of cytokines, chemokines and other effector molecules in two prototypic autoinflammatory skin diseases, pyoderma gangrenosum and Sweet’s syndrome. Clin Exp Immunol 2014; 178(1): 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caproni M, Antiga E, Volpi W, et al. The Treg/Th17 cell ratio is reduced in the skin lesions of patients with pyoderma gangrenosum. Br J Dermatol 2015; 173(1): 275–278. [DOI] [PubMed] [Google Scholar]
- 8. Herberger K, Dissemond J, Brüggestrat S, et al. Biologics and immunoglobulins in the treatment of pyoderma gangrenosum – analysis of 52 patients. J Dtsch Dermatol Ges 2019; 17(1): 32–41. [DOI] [PubMed] [Google Scholar]
- 9. García M, González M, Lobato J. Secukinumab for pyoderma gangrenosum: a case report. Med Clínica 2019; 152(6): 246–246. [DOI] [PubMed] [Google Scholar]
- 10. U.S. National Library of Medicine. ClinicalTrials.gov. [DOI] [PubMed]
- 11. Wollina U, Schönlebe J, Fürll C. Pyoderma gangrenosum induced by secukinumab – a late paradoxical drug reaction. Dermatol Ther 2020; 33(1): e13161. [DOI] [PubMed] [Google Scholar]
- 12. Jin K, Matsuzaki Y, Akasaka E, et al. Pyoderma gangrenosum triggered by switching from adalimumab to secukinumab. J Dermatol 2019; 46(3): e108–e109. [DOI] [PubMed] [Google Scholar]
- 13. Sadik C, Thieme M, Zillikens D, et al. First emergence of pyoderma gangraenosum, palmoplantar pustulosis and sacroiliitis in a psoriasis patient associated with switching from secukinumab to brodalumab. J Eur Acad Dermatol Venereol 2019; 33(11): e406–e407. [DOI] [PubMed] [Google Scholar]
- 14. Munera-Campos M, Ballesca F, Carrascosa J. Paradoxical reactions to biologic therapy in psoriasis: a review of the literature. Actas Dermosifiliogr 2018; 109(9): 791–800. [DOI] [PubMed] [Google Scholar]
- 15. Toussirot Aubin ÉF. Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open 2016; 2(2): e000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wollina U, Hansel G, Koch A, et al. Tumor necrosis factor-[alpha] inhibitor-induced psoriasis or psoriasiform exanthemata: first 120 cases from the literature including a series of six new patients. Am J Clin Dermatol 2008; 9(1): 1–4. [DOI] [PubMed] [Google Scholar]