Abstract
Thromboembolism (TE) is a complex disease caused by various acquired and inherited factors. The common mutations; factor V Leiden G1691A (FVL G1691A), prothrombin G20210A (PTG20210A), and methylene tetrahydrofolate reductase C677T (MTHFR C677T) are important inherited causes in both venous and arterial thrombosis. The association between ABO blood groups and thrombophilia has been noted by researchers. We aimed to determine the frequency and association of ABO blood groups as a risk factor along with 3 thrombophilia mutations and another 3 thrombophilia markers in a group of patients with unstimulated thrombosis. In a prospective case-control study, we focused on 100 samples, 50 patients with documented thrombosis as well as 50 healthy age-matched controls. Multiplex polymerase chain reaction and reverse hybridization to oligonucleotide particular probes were employed to detect FVL G1691A, PT G20210A, and MTHFR C677T mutations. Analysis of other thrombophilia markers including protein C (PC), protein S (PS), and antithrombin (AT) assays was also performed. ABO blood group typing was done according to standard methods. Non-O blood group was significantly more frequent among cases than controls (76% vs 54%) with high odds of TE (odds ratio [OR] = 2.69). Positivity for at least 1 thrombophilia marker was more in cases (60%) than controls (34%; OR = 2.9). The combined effect of non-O blood group and thrombophilia markers raised the risk of TE (OR = 4.16, P = .001), particularly FVL (OR = 6.76). This study illustrates that harboring the non-O blood group poses an additive effect with other thrombophilia markers in the causation of TE.
Keywords: ABO blood group, thrombophilia mutations, factor V Leiden, MTHFRC677T, prothrombin G20210A, inherited thrombophilia
Introduction
Thromboembolism (TE) results from either acquired or inherited factors or both, it can occur inside the vein or artery.1 The most common hereditary determinants are mutations in factor V (FVL G1691A), prothrombin (PTG20210A), and methylene tetrahydrofolate reductase (MTHFR C677T).2,3 Resistance to activated protein C (APC), in the majority of cases, after effects of missense mutations actuated by the substitution of guanine by adenine at the position of nucleotide 1691 located in exon 10 of the FV gene (G1691A), known as FVL. This transformation makes the substitution of arginine by glutamine at amino acid 506, situated at one of the sites where FV is recognized, cleaved and inactivated by the APC. As a result, activated FV is not sufficiently inactivated, and thus predisposes thrombosis.2 The substitution of guanine by adenine at nucleotide 20210 in the 3′ untranslated locale of the prothrombin gene (G20210A), increases the messenger RNA stability, bringing about raised prothrombin plasma levels.4 Furthermore, mild to moderate hyperhomocyteinemia is a well-known risk factor for arterial and venous thrombosis.3,5 The role of deficiency of natural anticoagulants, antithrombin (AT), protein C (PC), and protein S (PS) has been settled in thrombophilia.6,7 The association of ABO blood groups and thrombosis has been described in many works of literature,8 yet to the best of our knowledge, no study has been done in our locality. Our study aimed to investigate the association and risk of having different ABO blood groups along with 3 thrombophilia mutations, as well as 3 thrombophilia markers in a group of patients with unstimulated thrombosis in comparison to healthy subjects.
Patients and Methods
This is a prospective case–control study in which a consecutive eligible newly diagnosed patients with documented thrombosis (using ultrasonography, color Doppler, venography-high resolution computed tomography studies) attending the Sulaymaniyah public health laboratory, main governmental laboratory in the city, for thrombophilia screening were enrolled in this study through 6-month period from the beginning of December 2017 to the end of May 2018.
We excluded patients with acquired causes such as a recent history of trauma or surgery (less than 3 months), smoking, prolonged bed rest, pregnancy, women on oral contraceptives, patients on oral anticoagulants, patients with positive markers for lupus anticoagulant, and patients with liver disorders. Consenting healthy age-matched unrelated volunteers from the same region, referred to in the same study period, were enrolled as a control group. They had no history of thrombosis, not smokers, were not having any illness of significance and not taken any drugs at the time of sampling.
The approval of the local ethical committee was obtained at the beginning of the study, and verbal consent from both of the patients and control was claimed. The recommendations and rules of the Helsinki Declaration were followed throughout the conduction of the study. Both EDTA and citrated blood samples were retrieved from the patients and the control group. ABO blood group phenotyping was performed according to standard agglutination methods on EDTA samples, then EDTA samples were deeply frozen until the time of DNA extraction (usually within 1 week from sample collection), the citrated samples were centrifuged within 1 hour, and plasma extracted and deeply frozen for PC, PS, and AT analysis.
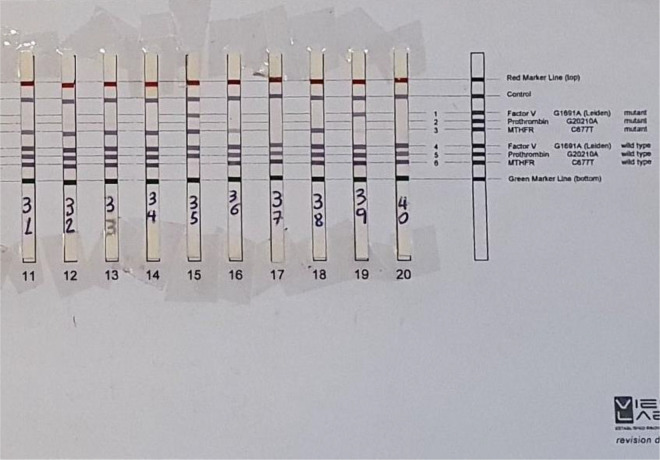
Peripheral blood leukocytes were the source of DNA extraction using reagents provided by the assay kit, followed by polymerase chain reaction amplification and oligo-specific probes were used for reverse hybridization according to the Vienna Lab kits-Austria manufacturer protocol (http://www.viennalab.com). Following genomic DNA extraction from peripheral blood leucocytes, the DNA samples were quality and quantity checked using UV spectrophotometry (nanodrop) and were verified by 1% agarose gel electrophoresis. DNA isolation procedure was repeated for any sample giving a concentration of < 50 ng/µL or optical density (260 of 280) of <1.7 or >2. The FV-PTH-MTHFR Strip Assay kit (Vienna Lab Diagnostics, Austria) gives reagents for in vitro amplification utilizing biotinylated primers, followed by hybridization of amplification products to a test strip that contains oligonucleotide probes for the targeted allele which is then immobilized as an array of parallel lines. From that point, bound biotinylated sequences are recognized utilizing both color substrates and streptavidin-alkaline phosphatase. This assay procedure covers 3 mutations: FVL G1691A, PTG20210A, and MTHFRC677T. Figure 1 shows examples of the strip assay for the studied mutations. Analysis of PC, PS, and AT was performed using STA COMPACT fully automated coagulation analyzer (Diagnostica Stago).
Figure 1.
Examples of reverse hybridization strip assay for the studied thrombophilia mutations. Cases No. 31, 32, 33, 34, 36, and 38 are heterozygous MTHFRC677T; case No. 35 is combined heterozygous FVL and homozygous MTHFRC677T, case No. 39 is heterozygous FVL, while cases No. 37 and 40 are non-carriers for any of the 3 mutations. FVL indicates Factor V Leiden; MTHFRC, methylene tetrahydrofolate reductase.
Statistical Analysis
SPSS version 25.0 for Windows was used for data analysis. Means and SD were calculated in continuous data and the independent t test was used for differences. The frequency proportion for categorical data and χ2 with an odds ratio (OR) provided with a 95% CI was computed to test the risk between different mutations and thrombosis. P value was considered significant at a level of less than .05.
Results
Of all cases referred to our laboratory for thrombophilia testing during the period of the study, only 50 eligible consenting patients according to our criteria mentioned earlier were analyzed for thrombophilia markers. These patients were proven to have different venous or arterial thrombosis. The investigation was also extended to involve 50 age-matched healthy people.
The patients’ group was comprised of 32 females and 18 male patients with a mean age of 34 years (18-49 years). The sex ratio in the control group was more balanced (21 female vs 29 male). The demographic differences and other thrombophilia data in both groups are summarized in Table 1. Most of the cases were of deep vein thrombosis (DVT; n = 22), followed by abortion (n = 11) and ischemic stroke (n = 10).
Table 1.
Thrombophilia Mutations, Sites, and Demographic Parameters in Cases and Controls.
| Controls, n = 50 | Cases, n = 50 | |
|---|---|---|
| Age/years (mean, range) | 37 (25-49) | 34 (18-49) |
| Gender | ||
| Male | 29 (58) | 18 (36) |
| Female | 21 (42) | 32 (64) |
| Thrombosis | ||
| Ischemic stroke | – | 10 (20) |
| Portal vein thrombosis | – | 4 (8) |
| Deep vein thrombosis | – | 22 (44) |
| Pulmonary embolism | – | 2 (4) |
| Abortion | – | 11 (22) |
| Myocardial infarction | – | 1 (2) |
| Thrombophilia markers | ||
| FVL (total)a | 3 (6) | 11 (22) |
| MTHFRC677T (total)a | 15 (30) | 21 (42) |
| PTG20210A (total)a | 1 (2) | 2 (4) |
| PC deficiency | 0 | 0 |
| AT deficiency | 0 | 0 |
| PS deficiency | 0 | 5 (10) |
| Combined thrombophilia | 2 (4)b | 9 (18)c |
Abbreviations: AT, antithrombin; FVL, factor V Leiden; MTHFRC, methylene tetrahydrofolate reductase; PC, protein C; PS, protein S.
a Total: including homozygous and heterozygous mutations.
b Both healthy subjects had FVL + MTHFRC677 T mutations.
c FVL + MTHFRC677T mutations were found in 3 patients; PS deficiency + MTHFRC677T mutation were found in 4 patients; PTG20210A + MTHFRC677T mutations were found in 2 patients.
Eleven carriers of FVL were found in the patients’ group against only 3 in the control group, while 21 for MTHFR and 2 for PTG20210A in the cases compared to 15 and 1 in the control group, respectively. Deficiency of PS (n = 5) was found only among the cases, while no AT or PC deficiencies could be found in any group.
The distribution of ABO blood group phenotypes among patients’ group was as follows: group O (n = 12); group A (n = 19); group B (n = 11), and group AB (n = 8). The non-O group was significantly higher among cases than controls, that is, 38 versus 27, respectively with OR of 2.69 as shown in Table 2. The highest odds of TE were in cases with AB blood group with an OR of 4.57 (P = .046). Cases with A blood group also had higher odds of TE (OR = 1.43), but this increase was found to be of no significance (P = .398) as shown in Table 2. Positivity for at least 1 marker of thrombophilia among cases (n = 30) was higher than the controls (n = 17) with an OR of 2.90 (P = .009; Table 3).
Table 2.
ABO Blood Group Distribution in Cases and Controls.
| ABO blood group | Controls (%) | Cases (%) | Odds ratio (95% CI) | P value |
|---|---|---|---|---|
| O | 23 (46) | 12 (24) | – | Reference |
| A | 15 (30) | 19 (38) | 1.43 (0.62-3.28) | .398 |
| B | 10 (20) | 11 (22) | 1.12 (0.43-2.95) | .806 |
| AB | 2 (4) | 8 (16) | 4.57 (0.91-22.73) | .046 |
| Total non-O | 27 (54) | 38 (76) | 2.69 (1.14-6.34) | .021 |
Table 3.
The Presence of Thrombophilia Markers in the Whole Sample and Association With ABO Blood Group.
| Controls, n (%) | Cases, n (%) | Odds ratio (95% CI) | P value | |
|---|---|---|---|---|
| Without thrombophiliaa | 33 (66) | 20 (40) | – | Reference |
| With thrombophiliaa | 17 (34) | 30 (60) | 2.90 (1.29-6.57) | .009 |
| More than one thrombophilia* | 2 (4) | 9 (18) | 5.26 (1.07-25.77) | .025 |
| O without thrombophilia | 16 (32) | 8 (16) | – | Reference |
| O with thrombophilia | 7 (14) | 4 (8) | 0.53 (0.14-1.95) | .534 |
| Non-O without thrombophilia | 16 (32) | 11 (22) | 0.59 (0.24-1.46) | .260 |
| Non-O with thrombophilia | 11 (22) | 27 (54) | 4.16 (1.74-9.93) | .001 |
a Thrombophilia in the whole sample.
Odds of TE were further increased in cases that had positivity of more than 1 thrombophilia markers (OR = 5.26, 95% CI: 1.07-25.77, P = .025). The thrombophilia markers were present in 27 cases and 11 controls among non-O blood group samples leading to an OR of 4.16 (95% CI: 1.74-9.93, P = .001; Table 3). In our study, the highest odds of TE was found in a combination of non-O blood group cases and FVL with an OR of 6.76 (P = .007; Table 4) followed by non-O blood group cases and MTHFR with an OR of 2.79 (P = .026).
Table 4.
Association of ABO Blood Groups With Presence or Absence of Individual Thrombophilia Markers.
| Controls, n (%) | Cases, n (%) | Odds ratio (95% CI) | P value | |
|---|---|---|---|---|
| O without FVL | 22 (44) | 12 (24) | – | Reference |
| O with FVL | 1 (2) | 0 | 0.49 (0.40-0.60) | .315 |
| Non-O without FVL | 25 (50) | 27 (54) | 1.17 (0.53-2.57) | .689 |
| Non-O with FVL | 2 (4) | 11 (22) | 6.76 (1.41-32.36) | .007 |
| O without MTHFR | 17 (34) | 10 (20) | – | Reference |
| O with MTHFR | 6 (12) | 2 (4) | 0.306 (0.05-1.59) | .140 |
| Non-O without MTHFR | 18 (36) | 19 (38) | 1.09 (0.48-2.45) | .836 |
| Non-O with MTHFR | 9 (18) | 19 (38) | 2.79 (1.11-7.00) | .026 |
| O without PTG20210A | 23 (46) | 12 (24) | – | Reference |
| O with PTG20210A | 0 | 0 | – | – |
| Non-O without PTG20210A | 26 (52) | 36 (72) | 2.37 (1.03-5.44) | .039 |
| Non-O with PTG20210A | 1 (2) | 2 (4) | 2.04 (0.17-23.26) | .559 |
| O without PS deficiency | 23 (46) | 11 (22) | – | Reference |
| O with PS deficiency | 0 | 1 (2) | 0.49 (0.40-0.60) | .315 |
| Non-O without PS deficiency | 27 (54) | 34 (68) | 1.81 (0.80-4.08) | .151 |
| Non-O with PS deficiency | 0 | 4 (8) | 0.47 (0.38-0.59) | .041 |
Abbreviations: FVL, factor V Leiden; MTHFRC, methylene tetrahydrofolate reductase; PS, protein S.
Discussion
Our study showed that non-O blood group frequency was much higher among cases (76%) than controls (54%) with an OR of 2.69 (95% CI: 1.14-6.34). This figure is similar to a meta-analysis study conducted by Dentali et al showing that DVT patients were seen significantly more in non-O blood group patients in comparison with that of healthy blood donors (70.6 vs 53.9%; P < .001) with pooled OR of 2.09.8 While the ABO blood group distribution among our controls (Table 2) was comparable to a study of frequency of blood group among blood donors in Sulaymaniyah city9 and that of the population of the surrounding region.10
Few studies stated against the relationship between ABO blood group and thrombosis, for example, Ketch et al in his study disputed this association in a cohort of patients followed for short- and long-term mortality and outcomes after vascular surgery.11
The risk of having TE in our sample is almost 3 folds increased in those with any thrombophilia markers positivity regardless of ABO blood groups (OR = 2.9, 95% CI: 1.29-6.57 P = .009) and this risk is further increased (more than 5 folds) if more than one thrombophilia markers are present in the patients (OR = 5.26, 95% CI: 1.07-25.77; P = .025). These findings were consistent with other studies such as in a Caucasian population.12
In our study, FVL was discovered in 22% (n = 11) of the cases with TE in comparison to 6% of the controls which is much lower than frequencies reported among Western countries (64%)13 but is more than that in Indian TE healthy subjects (12%-12.5%).14
In a study done by Lijfering et al,15 the risk of having TE was summarized as a relative risk (2- to 5-fold) in AT deficiency to high risk (40-folds) in FVL patients. The frequency of having PC, PS, and AT among TE healthy subjects is varied globally but has ranged from 5%-10% for both PC and PS while it is 2%-5% for AT deficiency. Studies in Indian TE patients have also reported a comparable prevalence of PC deficiency as 9.5%-21.1%, PS deficiencies as 6.5%-19.0% and AT deficiency as 2.6%-6.4%.16,17 However, in our study, deficiency of PS was 10% (Table 1) and no deficiency of PC or AT was reported; probably, this is attributed to ethnic differences of the populations.
Methylene tetrahydrofolate reductase C677T mutation was the commonest thrombophilia mutation in our cases 21 (42%) of 50 and control group 15 (30%) of 50. It has been demonstrated that elevated levels of homocysteine associated with MTHFR C677T have been found to confer an increased risk of TE (2-4 times the normal person’s risk).18 Yet, the isolated mutation is not a cause for thrombosis except if being associated with other hereditary or acquired risk factor or non-O blood group as exhibited in this study similar to other related studies.5 Some studies uncover that this affiliation is disputable.19 In our sample, it was significantly associated with increased risk (OR = 2.79, P =.026) in non-O blood group patients.
In the current study, PTG20210A mutation was double in patients than controls and was found to impart a risk factor in TE but of no statistical significance. These data were similar to a meta-analysis study conducted by Wu et al20 which is probably attributed to a lower frequency of this mutation in the population.
This study demonstrated that even though the non-O blood group and the presence of thrombophilia markers separately comprised a risk factor, the existence of both simultaneously raised (about 2-folds) the odds of TE (OR = 4.16, P = .001) as shown in Table 3. These outcomes point toward a synergistic relationship of non-O blood group and thrombophilia abnormalities in our cases as described in similar studies.21–23
In our patients, the existence of FVL mutation in the non-O blood group presents the most noteworthy risk of thrombosis among cases (OR = 6.76, 95% CI: 1.41-32.36, P = .007).
The attribution of non-O blood group with other thrombophilia mutations in the causation of TE is explained by the higher levels of factor VIII in non-O group individuals.24,25 The diminished responsiveness to APC with high FVIII levels, even in the absence of FVL, has been reported as a cause of TE.26 Besides, the existence of PC or PS deficiencies in non-O blood group individuals with the consequent loss of its neutralizing capacity further leads to a prolonged procoagulable state.
Limitations of this study include the small number of patients and controls, in that way a large-scale study may help to overcome the low frequency of pro-thrombotic carriers among participants. Also, it is important to mention that in many instances it was not feasible to apply thrombophilia testing in our control group.
In conclusion, data extracted from the study revealed that harboring non-O blood group poses an additive effect with other thrombophilia markers in the causation of TE and inclusion of ABO group testing in the management of those patients can help to identify patients at high risk, suitable for counseling, further testing or closer monitoring.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Aveen M. Raouf Abdulqader  https://orcid.org/0000-0002-4301-6470
https://orcid.org/0000-0002-4301-6470
References
- 1. Tripodi A, Mannucci PM. Laboratory investigation of thrombophilia. Clin Chem. 2001;47(2):1597–1606. [PubMed] [Google Scholar]
- 2. Martinelli I, De Stefano V, Mannucci PM. Inherited risk factors for venous thromboembolism. Nat Rev Cardiol. 2014;11(3):140–156. [DOI] [PubMed] [Google Scholar]
- 3. Xuan C, Bai XY, Gao G, et al. Association between polymorphism of methylenetetrahydrofolate reductase (MTHFR) C677 T and risk of myocardial infarction: a meta-analysis for 8,140 cases and 10,522 controls. Arch Med Res. 2011;42(5):677–685. [DOI] [PubMed] [Google Scholar]
- 4. Monsuez JJ, Bouali H, Serve E, et al. Deep venous thrombosis associated with factor V Leiden, G20210A mutation, and protein S deficiency. Am J Med. 2003;114(5):421–422. [DOI] [PubMed] [Google Scholar]
- 5. Şenol S, Kargün K. Methylenetetrahydrofolate reductase polymorphisms in acute deep vein thrombosis. Turk J Vasc Surg. 2019;28(2):69–72. [Google Scholar]
- 6. Bayan K, Tüzün Y, Yılmaz Ş, et al. Analysis of inherited thrombophilic mutations and natural anticoagulant deficiency in patients with idiopathic portal hypertension. J Thrombosis Thrombolysis. 2008;28(1):57–62. [DOI] [PubMed] [Google Scholar]
- 7. Fisher NC, Wilde JT, Roper J, et al. Deficiency of natural anticoagulant proteins C, S, and antithrombin in portal vein thrombosis: a secondary phenomenon? Gut. 2000;46(4):534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dentali F, Sironi A. Non-O blood type is the commonest genetic risk factor for VTE: results from a meta-analysis of the literature. Thromb Hemost. 2012;38(5):535–548. [DOI] [PubMed] [Google Scholar]
- 9. Hisham AG, Shaema SA, Najmaddin Kh, et al. Distribution of red cell antigens according to ABO, Rh and other rare blood group systems in Kurdish ethnicity. Iraqi J Hematology. 2016;5(1):55–80. [Google Scholar]
- 10. Chandra T, Gupta A. Frequency of ABO and rhesus blood groups in blood donors. Asian J Transfus Sci. 2012;6(1):52–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ketch TR, Turner SJ, Sacrinty MT, et al. ABO blood types: influence on infarct size, procedural characteristics, and prognosis. Thromb Res. 2008;123(2):200–205. [DOI] [PubMed] [Google Scholar]
- 12. Schick AP, Editor C, Besa EC. Hypercoagulability-hereditary thrombophilia and lupus anticoagulants associated with venous thrombosis and emboli. Medscape. 2015:6–9. [Google Scholar]
- 13. Ruiz Leonardo GP, Oliveira Maria Gabriela L, Ruiz Adriana LZ, et al. Hereditary thrombophilia by factor V Leiden G1691A (heterozygous) and FII prothrombin G20210A (homozygous) mutations in a patient with ischemic cerebrovascular accident. J Bras Patol Med Lab. 2018;54(2):92–94. [Google Scholar]
- 14. Bhattacharyya M, Kannan M, Chaudhry VP, et al. Venous thrombosis: prevalence of pro-thrombotic defects in north Indian population. Indian J Pathol Microbiol. 2003;46(4):621–624. [PubMed] [Google Scholar]
- 15. Lijfering WM, Rosendaal FR, Cannegieter SC, et al. Risk factors for venous thrombosis-current understanding from an epidemiological point of view. Br J Haematol. 2010;149(3):824–833. [DOI] [PubMed] [Google Scholar]
- 16. Garewal G, Das R, Varma S, et al. Heterogeneous distribution of factor V Leiden in patients from North India with venous thromboembolism. J Thromb Haemost. 2003;1(5):1329–1330. [DOI] [PubMed] [Google Scholar]
- 17. Saman S, Moncef Z, Johan ELF, et al. Clinical implications of different risk factor profiles in patients with mesenteric venous thrombosis and systemic venous thromboembolism: a population-based study. J Thrombosis and Thrombolysis. 2019;47(3):572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liew SC, Gupta ED. Methylenetetrahydrofolate reductase (MTHFR) C677 T polymorphism: epidemiology, metabolism and the associated diseases. Eur J Med Genet. 2015;58(1):1–10. [DOI] [PubMed] [Google Scholar]
- 19. Naushad S, Jamal NJ, Angalena R, et al. Hyperhomocysteinemia and the compound heterozygous state for methylene hydro folate reductase are independent risk factors for deep vein thrombosis among South Indians. Blood Coagul Fibrinolysis. 2007;18(2):113–117. [DOI] [PubMed] [Google Scholar]
- 20. Wu O, Bayoumi N, Vickers MA, et al. ABO(H) blood groups and vascular disease: a systematic review and meta-analysis. J Thromb Haemost. 2008;6(4):62–69. [DOI] [PubMed] [Google Scholar]
- 21. Jukic I, Bingulac-Popovic J, Dogic V, et al. ABO blood groups and genetic risk factors for thrombosis in Croatian population. Croat Med J. 2009;50(6):550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vasan SK, Rostgaard K, Majeed A, et al. ABO blood group and risk of thromboembolic and arterial disease clinical perspective. Circulation. 2016;133(15):1449–1457. [DOI] [PubMed] [Google Scholar]
- 23. Kristensen SR, Overvad K, Steffensen R, et al. Interaction between blood type, smoking and factor V Leiden mutation and risk of venous thromboembolism: a Danish case-cohort study. J Thromb Haemostasis. 2012;10(4):2191–2193. [DOI] [PubMed] [Google Scholar]
- 24. Lima MBPLV, De Oliveira-Filho AB, Campos JF, et al. Increased risk of venous thrombosis by AB alleles of the ABO blood group and factor V Leiden in a Brazilian population. Genet Mol Biol. 2009;32(2):264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohira T, Cushman M. ABO blood group, other risk factors and incidence of venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE). J Thromb Haemostat. 2007;5(7):1455–1461. [DOI] [PubMed] [Google Scholar]
- 26. Cetin MS, Ozcan Cetin EH, Aras D, et al. Non-O blood groups can be a prognostic marker of in-hospital and long-term major adverse cardiovascular events in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Thromb Res. 2015;136(3):599–605. [DOI] [PubMed] [Google Scholar]