Abstract
Background and Aims:
Efficacy of adalimumab in Crohn’s disease (CD) has not been shown in China. The aim of this study was to evaluate the efficacy and safety of adalimumab in Chinese patients with CD.
Methods:
This 26-week, multicenter, phase III study evaluated patients with moderately to severely active CD and elevated high-sensitivity C-reactive protein (⩾3 mg/l) who were naïve to anti–tumor necrosis factor therapy. Patients were randomized to double-blind adalimumab 160/80 mg at weeks 0/2 and 40 mg at weeks 4/6 or placebo at weeks 0/2 followed by blinded adalimumab 160/80 mg at weeks 4/6. At week 8, all patients received open-label 40 mg adalimumab every other week through week 26. The primary endpoint was clinical remission [CD activity index (CDAI) <150] at week 4. Clinical remission at week 26 was assessed in week-8 responders (decrease in CDAI ⩾70 points at week 8 from baseline) and compared with a clinically meaningful threshold of 30%. Adverse events (AEs) were recorded throughout the study.
Results:
At baseline, 205 patients were enrolled, with mean [standard deviation (SD)] age of 32.9 (9.9) years and CD duration of 2.7 (3.0) years. At week 4, 38/102 patients (37%) receiving adalimumab and 7/103 (7%) receiving placebo (p < 0.001) achieved clinical remission. Among week-8 responders, 93/144 (65%) achieved clinical remission at week 26 (p < 0.001). No unexpected AEs and no malignancies, active tuberculosis, or deaths were reported.
Conclusions:
Adalimumab induced and maintained remission in Chinese patients with CD. Safety results were consistent with the known safety profile of adalimumab.
ClinicalTrials.gov identifier:
Keywords: adalimumab, Chinese, Crohn’s disease
Introduction
The incidence rates of inflammatory bowel disease (IBD), including Crohn’s disease (CD), have recently increased in geographic regions with traditionally low prevalence, such as Asia.1,2 Risk factors in developing nations include environmental exposures in childhood, immunologic factors, hygiene, and diet.2–4 However, there is evidence that a number of risk factors differ between Western and Asian patients with CD. Studies characterizing Asian patients with IBD have found that susceptibility genes differ from those of Western populations.5–7 Although clinical phenotypes are similar in Asian and Western populations, some studies report differences in disease location and surgical rates.5
The increased incidence of CD in Asia was initially theorized to result from better disease awareness and diagnosis.3 However, improved diagnostic techniques and facilities were available before the increase in incidence and have not changed.3 Increased disease awareness is insufficient to account for the incidence increase observed in recent decades.2,3,7 Increased incidence is most likely attributable to environmental and lifestyle factors tied to socioeconomic changes and rapid industrialization.2–4
The standard of care for moderate to severe CD in China has been corticosteroids and immunosuppressants.8 However, these treatments are often not sufficient and can have serious adverse effects, especially if used long term.9–11 Corticosteroids are effective in inducing remission, although prolonged exposure can lead to steroid dependence, steroid resistance, or surgery.9,10,12 Adverse effects are common9,12; long-term prednisone therapy is linked with infection and death in patients with CD.13 Although treatment guidelines recommend immunomodulators as maintenance therapies,14 clinical evidence shows that immunomodulators are associated with suboptimal efficacy.15,16 A recent case-control study of a French registry suggested that the benefits of azathioprine therapy may be offset by the increased risk of treatment-related malignancy.17
An approved treatment for Chinese patients with CD that is refractory to corticosteroids, immunosuppressants, or both is infliximab, an anti–tumor necrosis factor (TNF) antibody.8,18 However, some patients do not respond to treatment, experience loss of response, or become intolerant to the infusions,19,20 suggesting the need to explore alternative options. Adalimumab, a fully human anti-TNF monoclonal antibody, has been shown to induce and maintain remission in global clinical trials of patients with CD,21–23 including a Japanese program,24 but has not been studied in a Chinese CD population in a registration program. The purpose of this study was to evaluate the efficacy and safety of adalimumab in induction and maintenance treatment in adult Chinese patients with moderately to severely active CD.
Methods
Study design
This was a 26-week, randomized, phase III study of adalimumab in patients naïve to anti-TNF therapy conducted between 17 August 2015 and 15 December 2017 at 15 sites in China. The study comprised an 8-week double-blind (DB) period followed by an 18-week open-label (OL) period. At the screening visit, patients were assigned a unique identification number by the interactive response technology (IRT) system. Once enrolled, patients were randomized by the IRT to a treatment group. During the DB period, all study investigators, study site personnel, and patients were blinded to each patient’s treatment. To maintain blinding, prepackaged study drug kits were assigned by the IRT according to the patient’s randomized treatment schedule.
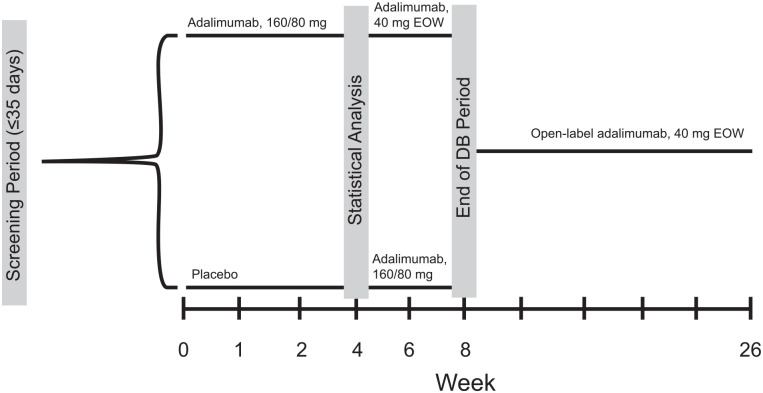
Patients were stratified by Crohn’s disease activity index (CDAI, ⩽300/>300) and systemic corticosteroid use (yes/no) at baseline and randomized 1:1 to adalimumab or placebo at week 0 (Figure 1). Patients randomized to adalimumab received blinded adalimumab 160 mg at week 0, 80 mg at week 2, and 40 mg at weeks 4 and 6. Patients randomized to placebo received blinded placebo (same number of injections) at weeks 0 and 2, then blinded adalimumab 160 mg at week 4 and 80 mg at week 6. Patients originally randomized to adalimumab received matching placebo injections at weeks 4 and 6 so that all patients received the same number of injections through week 6. At week 8, all patients were enrolled in an 18-week OL period and received 40 mg adalimumab every other week (EOW). At week 4, patients receiving oral corticosteroids underwent mandatory corticosteroid dose tapering at a rate of 5 mg/day/week or 2.5 mg/day/week for patients receiving either >10 mg/day or ⩽10 mg/day prednisone (or equivalent), respectively, and at a rate of 3 mg/day/week for patients receiving ⩽9 mg/day budesonide. Doses of other CD-related medications (e.g., immunosuppressants, aminosalicylates, and CD-related antibiotics) were to remain stable during the study except if treatment-related toxicities were considered moderate to severe in the opinion of the investigator. At or after week 12, patients who experienced an inadequate response to adalimumab could increase their dosage to 80 mg EOW. An inadequate response was defined as CDAI ⩾200 and at least one of the following criteria: an increase of high-sensitivity C-reactive protein (hs-CRP) from baseline of ⩾1 mg/l or hs-CRP ⩾5 mg/l. If the patient continued to show an inadequate response after dose escalation, the patient was discontinued from the study.
Figure 1.
Study design. At week 4, patients receiving systemic corticosteroids had their corticosteroid dose tapered. At or after week 12, patients who experienced an inadequate response could increase their dosage to 80 mg EOW.
DB, double blind; EOW, every other week.
Before study initiation, all investigators obtained approval from their respective independent ethics committee or institutional review board at each study site. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guideline. Written informed consent was obtained from each participant before screening for the study occurred.
One amendment was made to the original protocol in October 2015 after enrollment of 51 patients. The remaining 154 patients were enrolled under the protocol amendment that clarified screening requirements and windows for anti–HIV-1, hepatitis B virus, and anti-hepatitis C to reduce unnecessary testing and align with the seroconversion window of the immunoassay used by the central laboratory. The amendment also updated doses of immunosuppressants in inclusion criteria to add that half-tablet formulations should be considered when rounding to determine dose, removed efalizumab from the exclusion criteria because this therapy was no longer available, and specified the location of the dysplasia exclusion criterion to clarify that only gastric dysplasia and not dysplasia of the gastrointestinal tract was exclusionary to allow broader enrollment of patients. No changes were made to study objectives, choice or timing of endpoints, entry criteria, planned statistical analyses, or any other aspect of the study that would impact interpretation of results.
Patient selection
Patients were eligible for inclusion if they were adults (aged ⩾18–⩽70 years) naïve to anti-TNF therapy with moderately to severely active CD (CDAI ⩾220 and ⩽450) and elevated hs-CRP (⩾3 mg/l) who did not improve with conventional therapy of oral corticosteroids and/or immunosuppressants. Elevated hs-CRP was used as a biomarker to ensure patients had active inflammatory CD at the time of enrollment in lieu of performing endoscopy.25 During screening, patients needed to have negative QuantiFERON-TB Gold (QFT) test results as assessed by a central laboratory and chest imaging results to rule out the presence of active and latent tuberculosis (TB). Patients were excluded if they had undergone surgical bowel resection within 6 months of screening or planned surgical bowel resection; symptomatic known obstructive strictures; internal or external fistula (with the exception of anal fistula without dysplasia; or had undergone ostomy or ileoanal pouch surgery.
Outcome measures
The primary endpoint was the proportion of patients who achieved clinical remission (defined as CDAI < 150 points) at week 4. The key secondary endpoint was patients who achieved clinical remission at week 26 among week-8 responders compared with the prespecified threshold of 30%. Week-8 responders were defined as patients with a decrease in CDAI ⩾ 70 points at week 8 from baseline. Two key secondary endpoints assessed at week 4 were a decrease in CDAI ⩾70 points and a decrease in CDAI ⩾70 plus a reduction in hs-CRP of 30% from baseline. Steroid-free clinical remission and steroid-free clinical remission plus hs-CRP reduction of 50% from baseline were assessed at week 26 in week-8 responders. Clinical remission plus hs-CRP reduction of 50% from baseline, clinical remission plus hs-CRP < 3 mg/l, and clinical remission plus hs-CRP < 3 mg/l and fecal calprotectin (FC) <250 µg/g were assessed at weeks 4 and 26. Health-related quality of life was assessed using the Inflammatory Bowel Disease Questionnaire (IBDQ, which was validated in Chinese) at weeks 4 and 26 in week-8 responders.26–28 The 32-item IBDQ score ranges from 32 (worst) to 224 (best). IBDQ remission was defined as an IBDQ score ⩾170.
Immunogenicity and pharmacokinetics
Patient blood samples were collected immediately before dosing at specified time points and analyzed by enzyme-linked immunosorbent assay to determine anti-adalimumab antibody (AAA) levels (WuXi AppTec, Shanghai, China). A patient was considered AAA+ if they had ⩾1 AAA concentration >20 ng/ml. Samples with a quantifiable AAA concentration (>20 ng/ml) underwent confirmatory assays to determine the specificity of the AAA response. Adalimumab serum concentration was measured using a validated electrochemiluminescence ligand-binding assay method. The lower limit of quantification for adalimumab was established at 50 ng/ml in undiluted serum.
Safety
Adverse events (AEs) were collected throughout the study and for 70 days after the last dose of study drug. AEs assessed by the investigator to be of unknown severity were counted as severe, and AEs of unknown relationship to study drug were counted as study drug related. Treatment-emergent AEs (TEAEs) during the DB, placebo-controlled period (weeks 0–4) were defined as any AE with an onset date on or after the first dose of study drug and up to the first dose at week 4. Screening for TB was conducted throughout the study at specified time points or if a patient discontinued prematurely from the study; screening was performed using the QFT test (weeks 2, 4, 6, 8, 12, 16, 20, and 26) and chest X-rays or computed tomographic (CT) scans (weeks 8, 16, and 26). Patients who presented with repeated indeterminate or a positive QFT test result or findings of active or latent TB on chest radiograph or CT scan during the study participation were to be discontinued from the study and were reported as having an AE of latent TB. These patients were required to consult a TB specialist regarding appropriate management.
Statistical analyses
All statistical analyses were conducted with SAS version 9.2 or later (SAS Institute, Inc., Cary, NC, USA). Analysis of the primary and secondary endpoints between adalimumab and placebo was performed using the Cochran-Mantel-Haenszel test and stratified by the randomization factors. The key secondary endpoint of clinical remission at week 26 was compared with a clinically meaningful threshold of 30% using the 1-sample exact test. Based on the assumptions of a 47% remission rate for the adalimumab 160/80-mg treatment group and 24% remission rate for the placebo group at week 4, a sample size of 100 patients per group provided approximately 90% power to detect the expected 23% treatment difference between treatment groups for the primary endpoint using a 2-sided Fisher exact test at a 0.05 significance level. Similarly, a sample size of 100 patients provided ⩾99% power to detect a remission rate of 52% at week 26 against a clinically meaningful threshold of 30% using a 1-sample exact test at a 2-sided, 0.05 significance level.
The intent-to-treat population included all randomized patients. The any-adalimumab set comprised all randomized patients who received ⩾1 dose of adalimumab. For analyses of change from baseline for hs-CRP, CDAI, and FC, the baseline for the DB period (weeks 0–4) was defined as induction baseline for the intent-to-treat population. For the any-adalimumab data set, the baseline was relative to the first dose of adalimumab.
Missing continuous values were imputed using last observation carried forward (LOCF) and observed case (OC). Missing categorical variables were imputed using non-responder imputation (NRI). Data from patients whose doses were escalated were analyzed using NRI or LOCF as appropriate. Subgroup analyses of patients achieving the primary endpoint of clinical remission were performed for baseline variables, including corticosteroid use, immunosuppressant use, disease duration, FC, and hs-CRP. A post hoc logistic regression analysis was performed with the primary endpoint of clinical remission as the dependent variable and the protocol prespecified baseline demographic and characteristic variables (treatment, sex, age, corticosteroid use, immunosuppressant use, hs-CRP, CDAI, weight, albumin, disease duration, disease location, and investigator site) as risk factors. To retain variables in the model, a backward-elimination process was performed with a significance level of 0.1.
Results
Patient demographics and baseline characteristics
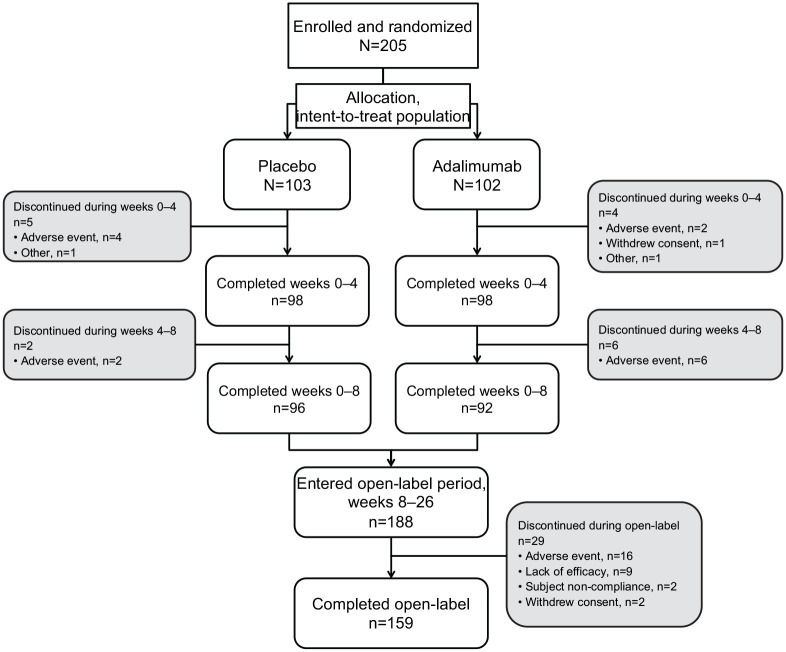
In total, 205 patients were randomized and received study drug (Figure 2, Supplemental Table S1). The majority of patients were men (n = 140; 68%). The mean [standard deviation (SD)] age was 32.9 (9.9) years, with a mean disease duration of 2.7 (3.0) years. The baseline characteristics were well balanced between the treatment groups, including corticosteroid use, with 30% of patients (n = 31) in the adalimumab group and 31% (n = 32) in the placebo group reporting systemic corticosteroid use. However, the mean (SD) duration of CD was significantly longer for those in the adalimumab group at 3.1 (3.2) years compared with 2.3 (2.7; p = 0.040) years for those in the placebo group (Table 1).
Figure 2.
Patient disposition.
Table 1.
Patient demographics and baseline characteristics (ITT).
| Placebo N = 103 |
Adalimumab N = 102 | Total N = 205 |
p value | |
|---|---|---|---|---|
| Men, n (%) | 73 (71) | 67 (66) | 140 (68) | 0.425 |
| Age, mean (SD), y | 32.6 (9.5) | 33.2 (10.2) | 32.9 (9.9) | 0.672 |
| Weight, mean (SD), kg | 53.0 (9.9) | 53.3 (9.1) | 53.2 (9.5) | 0.861 |
| FC, mean (SD), µg/g | 1435 (766) | 1482 (809) | 1458 (786) | 0.682 |
| hs-CRP, mean (SD), mg/L | 27.1 (31.5) | 23.9 (24.6) | 25.5 (28.3) | 0.422 |
| Corticosteroid use, n (%) | 32 (31) | 31 (30) | 63 (31) | 0.916 |
| IMM use, n (%) | 65 (63) | 61 (60) | 126 (62) | 0.627 |
| Azathioprine | 59 (57) | 60 (59) | 119 (58) | |
| Mercaptopurine | 2 (2) | 0 | 2 (1) | |
| Methotrexate | 4 (4) | 1 (1) | 5 (2) | |
| CDAI, mean (SD) | 274.7 (49.1) | 272.1 (48.1) | 273.4 (48.5) | 0.695 |
| Disease duration, mean (SD), y | 2.3 (2.7) | 3.1 (3.2) | 2.7 (3.0) | 0.040 |
| Disease location, n (%)* | ||||
| Colonic | 24 (23) | 19 (19) | 43 (21) | |
| Ileal | 19 (18) | 22 (22) | 41 (20) | |
| Ileal-colonic | 60 (58) | 60 (59) | 120 (59) | |
| Upper disease | 10 (10) | 9 (9) | 19 (9) | |
| CD surgical history, n (%) | ||||
| Any surgery before baseline | 26 (25) | 24 (24) | 50 (24) | |
| Surgery within 2 y of baseline | 8 (8) | 11 (11) | 19 (9) | |
CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; FC, fecal calprotectin; hs-CRP, high-sensitivity C-reactive protein; IMM, immunosuppressant medication; ITT, intent-to-treat population consisting of all randomized patients.
Patients could have multiple CD locations. Patients with both colonic and ileal CD were categorized as ileal-colonic. The locations of colonic, ileal, and ileal-colonic did not overlap.
Of 205 patients, 196 (96%) completed the 4-week, DB, placebo-controlled induction period; 188 (92%) entered the OL period; and 159 (78%) completed the OL period to week 26 (Figure 2). Nine patients (4%) discontinued during the 8-week DB period, six because of AEs (four from the placebo group and two from the adalimumab group). During the OL period, 29 patients (15%) discontinued prematurely; the most common reasons were AEs (n = 16; 9%) and lack of efficacy (n = 9; 5%). Between weeks 12 and 26 of the OL period, 21% of patients (41/200) in the any-adalimumab set had their adalimumab dose escalated from 40 mg to 80 mg EOW.
Outcome measures
Primary endpoint
At week 4, clinical remission (CDAI < 150) was achieved by 38/102 patients (37%) in the adalimumab group compared with 7/103 patients (7%) in the placebo group (p < 0.001, NRI; Figure 3). Similar results were observed when LOCF and OC methods of handling missing data were used. The treatment difference between adalimumab and placebo for achievement of CDAI < 150 at week 4 showed a consistent effect size for baseline variables analyzed by subgroup. (Supplemental Figure S1).
Figure 3.
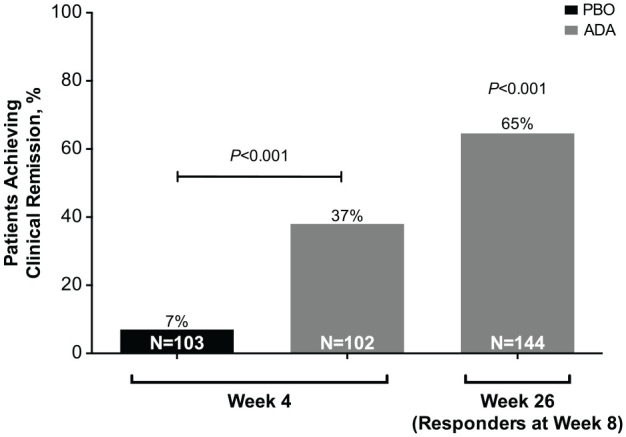
Clinical remission at weeks 4 and 26, intent-to-treat population. Patients with response (decrease in CDAI ⩾70 from baseline; NRI) at week 8 were compared at week 26 with a prespecified, clinically relevant threshold of 30%.
ADA, adalimumab; CDAI, Crohn’s disease activity index; NRI, non-responder imputation; PBO, placebo.
Results from the logistic regression analysis indicated that treatment group (adalimumab, placebo) and baseline CDAI (⩽300, >300) were significant factors retained in the final model; all other variables were not significant based on a backward-elimination process with a significance level of 0.1. The odds ratio estimates for the two remaining variables were 8.5 and 3.4, respectively. In a post hoc subgroup analysis by disease location, a significantly higher proportion of patients with ileal-colonic disease in the adalimumab group (42%, 25/60) achieved clinical remission at week 4 compared with those in the placebo group (5%, 3/60; p < 0.001).
Secondary endpoints
There were 144/205 responders (70%) at week 8. Among 144 responders at week 8, 93 (65%) achieved clinical remission (CDAI < 150) at week 26 (Figure 3). The key secondary endpoint of clinical remission at week 26 in week-8 responders compared with the prespecified, clinically relevant threshold of 30% was met (p < 0.001; Figure 3). Furthermore, the mean change from baseline in CDAI decreased over time in patients receiving adalimumab compared with those receiving placebo (Figure 4A). Similarly, the mean change from baseline in hs-CRP decreased over time in the adalimumab group compared with that in the placebo group (Figure 4B).
Figure 4.
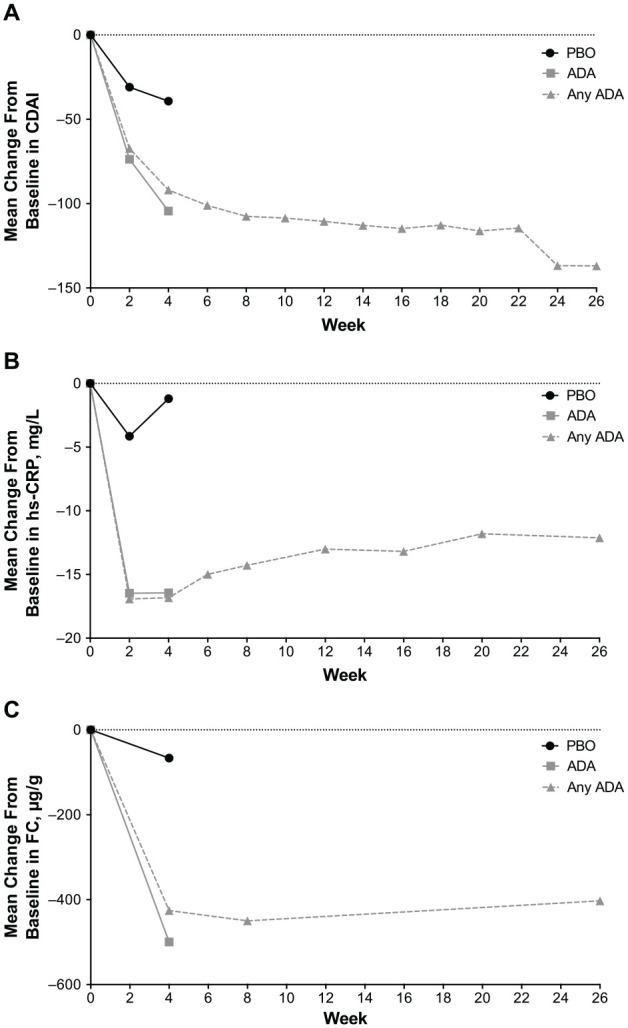
Mean change from baseline in (A) CDAI (LOCF), (B) hs-CRP (LOCF), and (C) FC (LOCF). For the ADA and PBO treatment groups, change from baseline at weeks 2 and 4 was analyzed for the intent-to-treat population. For the any-adalimumab set (any ADA), baseline and visits were relative to the first dose of ADA. FC was collected at baseline and weeks 4, 8, and 26.
ADA, adalimumab; CDAI, Crohn’s disease activity index; FC, fecal calprotectin; hs-CRP, high-sensitivity C-reactive protein; LOCF, last observation carried forward; PBO, placebo.
At week 4, 10% of patients receiving adalimumab achieved clinical remission plus hs-CRP < 3 mg/l and FC < 250 µg/g compared with 0% of patients receiving placebo (p < 0.01; Table 2). In addition, the proportion of patients receiving adalimumab with a total IBDQ score ⩾170 points (IBDQ remission) was 2-fold greater than patients receiving placebo, 40% (n = 41/102) versus 20% (n = 21/103; p < 0.01; Table 2). Compared with baseline, there were also significant improvements in the adalimumab group versus the placebo group in clinical remission plus hs-CRP reduction of 50%, clinical remission plus hs-CRP < 3 mg/l, clinical response, and clinical response plus hs-CRP reduction of 30% (all p < 0.001; Table 2). Mean (SD) change from baseline for hs-CRP at week 4 was −16.4 (19.8) mg/l for the adalimumab group, significantly greater than the change from baseline in the placebo group of −1.2 (18.7) mg/l (p < 0.001). A significantly greater change from baseline was also observed for FC at week 4 for the adalimumab group, which had a mean (SD) change of −500 (869) µg/g compared with −66 (845) µg/g change from baseline in the placebo group (p < 0.001; Figure 4C).
Table 2.
Secondary endpoints (NRI).
| Week 4 | ITT | Placebo N = 103 n (%) |
Adalimumab N = 102 n (%) |
p value |
|---|---|---|---|---|
| Clinical remission plus hs-CRP reduction of 50% from baseline | 0 | 34 (33) | <0.001 | |
| Clinical response (decrease in CDAI ⩾70 from baseline) | 28 (27) | 69 (68) | <0.001 | |
| Clinical response plus hs-CRP reduction of 30% from baseline | 12 (12) | 63 (62) | <0.001 | |
| Clinical remission plus hs-CRP < 3 mg/L | 0 | 28 (28) | <0.001 | |
| Clinical remission plus hs-CRP < 3 mg/L and FC < 250 µg/g | 0 | 10 (10) | <0.01 | |
| IBDQ remission (IBDQ ⩾170) | 21 (20) | 41 (40) | <0.01 | |
| Week 26 | Week-8 Responders (Decrease in CDAI ⩾ 70 From Baseline) | Adalimumab n/N (%) |
||
| Clinical remission plus hs-CRP reduction of 50% from baseline | 66/120* (55) | |||
| Steroid-free clinical remission | 27/43† (63) | |||
| Steroid-free clinical remission plus hs-CRP reduction of 50% from baseline (NRI) | 19/33‡ (58) | |||
| Clinical remission plus hs-CRP < 3 mg/L | 53/144 (37) | |||
| Clinical remission plus hs-CRP < 3 mg/L and FC < 250 µg/g | 23/144 (16) | |||
| IBDQ remission (IBDQ ⩾170) | 74/144 (51) | |||
CDAI, Crohn’s Disease Activity Index; FC, fecal calprotectin; hs-CRP, high-sensitivity C-reactive protein; IBDQ, Inflammatory Bowel Disease Questionnaire; ITT, intent-to-treat population consisting of all randomized patients; NRI, non-responder imputation.
Week-8 responders with ⩾30% reduction from baseline in hs-CRP.
Week-8 responders with corticosteroid use at baseline.
Week-8 responders with ⩾30% reduction from baseline in hs-CRP and corticosteroid use at baseline.
At week 26, 51% of week-8 responders achieved a total IBDQ score ⩾170 points, 63% of week-8 responders who used corticosteroids at baseline achieved clinical remission and were steroid-free, and 58% were steroid-free plus experienced a reduction in hs-CRP of 50% from baseline (Table 2). Of week-8 responders, 37% had clinical remission plus hs-CRP < 3 mg/l and 16% had clinical remission plus hs-CRP < 3 mg/l and FC < 250 µg/g. Mean (SD) change from baseline for hs-CRP and FC at week 26 was −12.1 (24.2) mg/l and −403 (980) µg/g, respectively, for patients who received any adalimumab during the study.
Immunogenicity and pharmacokinetics
All enrolled patients were AAA negative throughout the study. Only two patients had non-zero AAA concentrations; however, both were below the established threshold (20 ng/ml) to be considered AAA positive. All other patients had AAA concentrations of 0 ng/ml. At week 4, among the patients randomized to adalimumab, the mean (SD) adalimumab concentration was 14.3 (5.8) µg/ml for patients achieving remission (n = 37) and 11.5 (5.1) µg/ml for those not in remission (n = 62).
Safety
During the DB period, a similar number of patients in both groups experienced an AE: 38/103 (37%) for placebo and 38/102 (37%) for adalimumab (Table 3). There were two serious AEs and no serious infections in patients receiving adalimumab during the DB period, and one serious AE and no serious infections in patients receiving placebo. During the entire 26-week course, there were 36 reports of serious AEs and 8 reports of serious infections in seven patients receiving any adalimumab. Six events of serious infection were considered by the investigator to be not related to study drug. Two events of serious infection (abdominal abscess and lung infection) were assessed by the investigator to have a reasonable possibility of being related to study drug. No malignancies, active TB, or deaths were reported during the study. The most common TEAEs among all adalimumab-treated patients included a decreased white blood cell (WBC) count (20%), the majority of which were associated with concomitant thiopurine use. Of the 39 patients with decreased WBC count, 5 received azathioprine before study entry and 30 received azathioprine concomitantly during the study. Additionally, 29 of the 39 patients entered the study with normal WBC; of these patients, decreased WBC resolved in 27 patients before the end of their study participation. The other most common TEAEs were upper respiratory tract infection (12%), CD (11%), leukopenia (11%), and viral upper respiratory tract infection (10%; Table 4).
Table 3.
TEAEs during the DB, placebo-controlled period (weeks 0–4).
| Placebo N = 103 n (%) |
Adalimumab N = 102 n (%) |
|
|---|---|---|
| Any AE | 38 (37) | 38 (37) |
| Any serious AE | 1 (1) | 2 (2) |
| Any AE leading to discontinuation of study drug | 5 (5) | 3 (3) |
| Any severe AE | 2 (2) | 1 (1) |
| Any AE with reasonable possibility of being related to study drug* | 17 (17) | 18 (18) |
| Any infection | 9 (9) | 8 (8) |
| Any serious infection | 0 | 0 |
| Any active TB | 0 | 0 |
| Any latent TB† | 3 (3) | 1 (1) |
| Any malignancy | 0 | 0 |
| Any AE leading to death | 0 | 0 |
AE, adverse event; CT, computed tomography; DB, double blind; QFT, QuantiFERON-TB Gold; TB, tuberculosis; TEAE, treatment-emergent adverse event.
Assessed by the investigator.
Serial QFT testing and chest radiographs or CT scans were performed during the study. Patients with positive or repeated indeterminate QFT result or findings of active/latent TB on chest radiograph/CT scan were to be discontinued from the study. These instances were reported as an AE of latent TB.
Table 4.
TEAEs reported by ⩾5% of patients (any adalimumab).
| Patients with TEAEs | Any adalimumab N = 200 n (%) |
|---|---|
| WBC count decreased* | 39 (20) |
| Upper respiratory tract infection | 24 (12) |
| Crohn’s disease | 22 (11) |
| Leukopenia | 22 (11) |
| Viral upper respiratory tract infection | 20 (10) |
| Cough | 16 (8) |
| Pyrexia | 15 (8) |
| Influenza | 13 (7) |
| Mycobacterium tuberculosis complex test positive† | 13 (7) |
| Dizziness | 12 (6) |
| Abdominal pain | 11 (6) |
| Oropharyngeal pain | 11 (6) |
| Hematochezia | 10 (5) |
AE, adverse event; CT, computed tomography; QFT, QuantiFERON-TB Gold; TB, tuberculosis; TEAE, treatment-emergent AE; WBC, white blood cell.
Medical history of “white blood cell count decreased” was reported in 12 patients.
Serial QFT testing and chest radiographs or CT scans were performed during the study. Patients with repeat indeterminate or a positive QFT result or findings of active/latent TB on chest radiograph or CT scan were to be discontinued from the study. These instances were reported as an AE of latent TB.
Discussion
In this study, Chinese patients with moderately to severely active CD and elevated hs-CRP who received adalimumab achieved clinical remission in significantly greater proportions compared with patients receiving placebo at week 4 and compared with the prespecified clinically relevant threshold of 30% at week 26. These results were supported by the secondary endpoints, including IBDQ remission and cutoffs for the objective disease biomarkers of hs-CRP and FC. A regression analysis evaluating predictors of clinical remission identified treatment group and baseline CDAI as significant factors. The results of this study are comparable with, or better than, those observed with adalimumab in global clinical trials conducted in patients with CD.21,22
In CLASSIC I, the Western CD study most comparable with this study because it enrolled a biologic - naïve population, week-4 remission rate of the adalimumab group was 36% compared with 12% in the placebo group.22 In the current study, 37% of patients in the adalimumab group achieved remission compared with 7% in the placebo group. Similar remission rates were observed, although the patients in the current study had higher mean hs-CRP baseline levels than patients in CLASSIC I because of an additional inclusion criterion that ensured active inflammation at the time of enrollment.22 The effect size in this study was greater than that observed with the 160/80-mg dose in the Japanese adalimumab study (33%), which enrolled a population more similar in weight to the patients in this study.24 In the CHARM study, 40% of patients who responded to induction therapy receiving adalimumab 40 mg EOW maintained remission at week 26 compared with 17% of patients receiving placebo (similar results were seen in the Japanese study).21,24 In the current study, 65% of patients achieved maintenance of remission at week 26. However, in the CHARM and Japanese studies, the maintenance periods were DB and placebo-controlled, whereas in the current study, weeks 8–26 were an OL period, which may have contributed to a higher proportion of patients maintaining remission.21,24
Patient demographics in the current study were broadly similar to those of patient populations in Western and Japanese CD studies.21,22,24 Some differences were expected, such as the lower mean body weight of Chinese patients compared with patients in the Western studies, reflecting general differences between Western and Asian populations.21,22 Typical of Asian CD studies was a slight male predominance in the Chinese patient population compared with Western populations.7,21,22,24 Baseline clinical characteristics reflected a population with moderately to severely active CD that was comparable to Western CD populations. The exception was elevated hs-CRP levels in the Chinese study, reflecting the additional inclusion criterion and indicating more inflammatory CD in the Chinese patients. The rates of concurrent corticosteroid use were similar to those observed in global populations, but there was a slightly higher rate of immunosuppressant use, possibly reflecting evolving treatment patterns since the global studies were conducted.
The overall rate of AEs during the DB period was similar to that in placebo, and the overall safety profile observed in this study was similar to that observed in the global studies.29 In the current study, serious infections of Chinese patients occurred at a rate of 9/100 patient-years. Combined data from global CD studies showed serious infection rates of 6.7/100 patient-years.29 There were no cases of active TB, malignancies, or deaths reported during the study. Because latent TB infection is endemic in the Chinese general population (approximately a 13–24% prevalence),30,31 the trial design included more stringent TB monitoring than in previous studies, including repeat TB screening. Patients with a positive or repeated indeterminate QFT screening test result during the study were discontinued and reported to have an AE of latent TB. In the any-adalimumab set, 5.5% discontinued adalimumab because of a positive TB test result. Overall, of the 205 patients enrolled in the study, 22% discontinued. The most common reasons for discontinuation were AEs (15%) and lack of efficacy (4%). The most frequent AE in the any-adalimumab set was a decrease in WBC count, the majority of which was associated with concomitant thiopurine use. In Asian populations, including Chinese patients with CD, the incidence of thiopurine-induced leukopenia is higher than in individuals of European descent, which may explain the rate of leukopenia observed in this study.32–34 No new safety signals with adalimumab treatment were observed.
Chinese patients with CD receiving infliximab were reported as having beneficial results,19,35,36 indicating that TNF-α plays an intrinsic role in the pathophysiology of CD despite differing ethnic or genetic backgrounds, although a randomized, DB, placebo-controlled trial has not been reported in Chinese patients. In a short-term, OL study, 14 Chinese patients with CD refractory to standard treatments all achieved clinical remission after treatment with three doses of infliximab.35 In a retrospective report of 106 Chinese patients with CD receiving an induction regimen of infliximab, 58% achieved clinical remission and an additional 16% achieved a clinical response.19 In a longer term (>14 weeks) retrospective study with 26 Chinese patients with CD, treatment with infliximab was effective in inducing clinical remission in 62% of patients at 14 weeks and 54% of patients at the end of follow up.36
Limitations of this study included the DB period, which was relatively short at 4 weeks; however, this matches the time point of the primary analysis of the adalimumab global studies in the Western and Japanese populations.22,24 Only patients who were anti-TNF–naïve were included. However, studies conducted in Western and Japanese patients who lost response to previous treatment with infliximab have shown that adalimumab effectively induced remission.23,24 Finally, endoscopic assessment was not performed in this study. However, an inclusion criterion of elevated hs-CRP was implemented to ensure patients had active inflammatory CD at study entry, given its high association with disease activity in patients with CD.25 The reduction in hs-CRP and FC shown in adalimumab-treated patients in this study indicates objective biologic evidence of reduction in inflammation. These results are consistent with those shown in the adalimumab study EXTEND, which demonstrated effects on mucosal healing, although endoscopy was not performed.37
The results of this randomized, multicenter, phase III study showed that adalimumab induced and maintained clinical remission in Chinese patients with moderately to severely active CD and elevated hs-CRP. These results were consistent with those from global CD clinical trials. The safety profile of adalimumab was generally comparable with that of placebo. No unexpected safety findings were observed in this Chinese population compared with the known safety profile of adalimumab.
Supplemental Material
Supplemental material, China_Ethics_Committees-April2020 for Efficacy and safety of adalimumab in Chinese patients with moderately to severely active Crohn’s disease: results from a randomized trial by Baili Chen, Xiang Gao, Jie Zhong, Jianlin Ren, Xuan Zhu, Zhanju Liu, Kaichun Wu, Jasmina Kalabic, Zhuqing Yu, Bidan Huang, Nisha Kwatra, Thao Doan, Anne M. Robinson and Min-Hu Chen in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_Material_revision_3 for Efficacy and safety of adalimumab in Chinese patients with moderately to severely active Crohn’s disease: results from a randomized trial by Baili Chen, Xiang Gao, Jie Zhong, Jianlin Ren, Xuan Zhu, Zhanju Liu, Kaichun Wu, Jasmina Kalabic, Zhuqing Yu, Bidan Huang, Nisha Kwatra, Thao Doan, Anne M. Robinson and Min-Hu Chen in Therapeutic Advances in Gastroenterology
Acknowledgments
The authors thank the patients who participated in the study and all study investigators for their contributions. Medical writing support was provided by Catherine DeBrosse and Lauriaselle Afanador of ICON (North Wales, PA, USA), and was funded by AbbVie Inc. (North Chicago, IL, USA).
Footnotes
Conflict of interest statement: B. Chen, X. Gao, J. Zhong, J. Ren, X. Zhu, Z. Liu, K. Wu, and M. H. Chen have nothing to disclose. J. Kalabic, Z. Yu, B. Huang, N. Kwatra, T. Doan, and A. M. Robinson are AbbVie employees and may own AbbVie stock and/or options.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: AbbVie funded the publication. AbbVie designed the study, conducted research, analysis, data collection, and participated in interpretation of data or writing, reviewing, and approving of the publication.
ORCID iDs: Zhanju Liu  https://orcid.org/0000-0002-0326-543X
https://orcid.org/0000-0002-0326-543X
Min-Hu Chen  https://orcid.org/0000-0001-9925-135X
https://orcid.org/0000-0001-9925-135X
Data sharing: AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (e.g., protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Baili Chen, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Xiang Gao, The Sixth Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Jie Zhong, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Jianlin Ren, Zhongshan Hospital Xiamen University, Xiamen, China.
Xuan Zhu, The First Affiliated Hospital of Nanchang University, Nanchang, China.
Zhanju Liu, Shanghai Tenth People’s Hospital, Shanghai, China.
Kaichun Wu, The First Affiliated Hospital of Fourth Military Medical University, Xi’an, China.
Jasmina Kalabic, AbbVie Deutschland GmbH & Co. KG, Ludwigshafen, Germany.
Zhuqing Yu, AbbVie Inc., North Chicago, IL, USA.
Bidan Huang, AbbVie Inc., North Chicago, IL, USA.
Nisha Kwatra, AbbVie Inc., North Chicago, IL, USA.
Thao Doan, AbbVie Inc., North Chicago, IL, USA.
Anne M. Robinson, AbbVie Inc., North Chicago, IL, USA
Min-Hu Chen, Department of Gastroenterology and Hepatology, The First Affiliated Hospital of Sun Yat-sen University, 58 Zhongshan Road II, Guangzhou, China.
References
- 1. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46–54.e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 2. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-Pacific Crohn’s and colitis epidemiology study. Gastroenterology 2013; 145: 158–165.e152. [DOI] [PubMed] [Google Scholar]
- 3. Leong RW, Lau JY, Sung JJ. The epidemiology and phenotype of Crohn’s disease in the Chinese population. Inflamm Bowel Dis 2004; 10: 646–651. [DOI] [PubMed] [Google Scholar]
- 4. Ng SC, Tang W, Leong RW, et al. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut 2015; 64: 1063–1071. [DOI] [PubMed] [Google Scholar]
- 5. Thia KT, Loftus EV, Jr, Sandborn WJ, et al. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol 2008; 103: 3167–3182. [DOI] [PubMed] [Google Scholar]
- 6. Ng SC, Tsoi KK, Kamm MA, et al. Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflamm Bowel Dis 2012; 18: 1164–1176. [DOI] [PubMed] [Google Scholar]
- 7. Prideaux L, Kamm MA, De Cruz PP, et al. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol 2012; 27: 1266–1280. [DOI] [PubMed] [Google Scholar]
- 8. Chinese Cooperative Group for the Study on IBD, Chinese Society of Gastroenterology, Ouyang Q, et al. Consensus on the management of inflammatory bowel disease in China in 2007. J Dig Dis 2008; 9: 52–62. [DOI] [PubMed] [Google Scholar]
- 9. Waljee AK, Wiitala WL, Govani S, et al. Corticosteroid use and complications in a US inflammatory bowel disease cohort. PLoS One 2016; 11: e0158017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Selinger CP, Parkes GC, Bassi A, et al. A multi-centre audit of excess steroid use in 1176 patients with inflammatory bowel disease. Aliment Pharmacol Ther 2017; 46: 964–973. [DOI] [PubMed] [Google Scholar]
- 11. Kwak MS, Kim DH, Park SJ, et al. Efficacy of early immunomodulator therapy on the outcomes of Crohn’s disease. BMC Gastroenterol 2014; 14: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siegel CA. What options do we have for induction therapy for Crohn’s disease? Dig Dis 2010; 28: 543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol 2012; 107: 1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol 2018; 113: 481–517. [DOI] [PubMed] [Google Scholar]
- 15. Cosnes J, Bourrier A, Laharie D, et al. Early administration of azathioprine vs conventional management of Crohn’s disease: a randomized controlled trial. Gastroenterology 2013; 145: 758–765.e2; quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 16. Panes J, Lopez-Sanroman A, Bermejo F, et al. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn’s disease. Gastroenterology 2013; 145: 766–774.e1. [DOI] [PubMed] [Google Scholar]
- 17. Camus M, Seksik P, Bourrier A, et al. Long-term outcome of patients with Crohn’s disease who respond to azathioprine. Clin Gastroenterol Hepatol 2013; 11: 389–394. [DOI] [PubMed] [Google Scholar]
- 18. Remicade®, https://www.xian-janssen.com.cn/en/product/brand/68/296 (2015, accessed 8 January 2019).
- 19. Yu L, Yang X, Xia L, et al. Infliximab preferentially induces clinical remission and mucosal healing in short course Crohn’s disease with luminal lesions through balancing abnormal immune response in gut mucosa. Mediators Inflamm 2015; 2015: e793764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002; 359: 1541–1549. [DOI] [PubMed] [Google Scholar]
- 21. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007; 132: 52–65. [DOI] [PubMed] [Google Scholar]
- 22. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology 2006; 130: 323–333. [DOI] [PubMed] [Google Scholar]
- 23. Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med 2007; 146: 829–838. [DOI] [PubMed] [Google Scholar]
- 24. Watanabe M, Hibi T, Lomax KG, et al. Adalimumab for the induction and maintenance of clinical remission in Japanese patients with Crohn’s disease. J Crohns Colitis 2012; 6: 160–173. [DOI] [PubMed] [Google Scholar]
- 25. Lichtenstein GR, McGovern DP. Using markers in IBD to predict disease and treatment outcomes: rationale and a review of current status. Am J Gastroenterol Suppl 2016; 3: 17–26. [Google Scholar]
- 26. Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989; 96: 804–810. [PubMed] [Google Scholar]
- 27. Leong RW, Lee YT, Ching JY, et al. Quality of life in Chinese patients with inflammatory bowel disease: validation of the Chinese translation of the Inflammatory Bowel Disease Questionnaire. Aliment Pharmacol Ther 2003; 17: 711–718. [DOI] [PubMed] [Google Scholar]
- 28. Ren WH, Lai M, Chen Y, et al. Validation of the mainland Chinese version of the Inflammatory Bowel Disease Questionnaire (IBDQ) for ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis 2007; 13: 903–910. [DOI] [PubMed] [Google Scholar]
- 29. Colombel JF, Sandborn WJ, Reinisch W, et al. Long-term safety of adalimumab in clinical trials in adult patients with Crohn’s disease or ulcerative colitis. Aliment Pharmacol Ther 2018; 47: 219–228. [DOI] [PubMed] [Google Scholar]
- 30. Gao L, Lu W, Bai L, et al. Latent tuberculosis infection in rural China: baseline results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis 2015; 15: 310–319. [DOI] [PubMed] [Google Scholar]
- 31. Liu Y, Huang S, Jiang H, et al. The prevalence of latent tuberculosis infection in rural Jiangsu, China. Public Health 2017; 146: 39–45. [DOI] [PubMed] [Google Scholar]
- 32. Ding L, Zhang FB, Liu H, et al. Hypoxanthine guanine phosphoribosyltransferase activity is related to 6-thioguanine nucleotide concentrations and thiopurine-induced leukopenia in the treatment of inflammatory bowel disease. Inflamm Bowel Dis 2012; 18: 63–73. [DOI] [PubMed] [Google Scholar]
- 33. Zhu X, Wang XD, Chao K, et al. NUDT15 polymorphisms are better than thiopurine S-methyltransferase as predictor of risk for thiopurine-induced leukopenia in Chinese patients with Crohn’s disease. Aliment Pharmacol Ther 2016; 44: 967–975. [DOI] [PubMed] [Google Scholar]
- 34. Chao K, Wang X, Cao Q, et al. Combined detection of NUDT15 variants could highly predict thiopurine-induced leukopenia in Chinese patients with inflammatory bowel disease: a multicenter analysis. Inflamm Bowel Dis 2017; 23: 1592–1599. [DOI] [PubMed] [Google Scholar]
- 35. Zheng JJ, Zhi P, Wang YM, et al. Short-term study of infliximab treatment for Crohn’s disease in China. J Dig Dis 2011; 12: 105–109. [DOI] [PubMed] [Google Scholar]
- 36. Lu X, Yin A, Ding Y. Clinical efficacy of infliximab in Chinese patients with Crohn’s disease: a retrospective study. Biomedical Research 2016; 27: 1228–1230. [Google Scholar]
- 37. Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology 2012; 142: 1102–1111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, China_Ethics_Committees-April2020 for Efficacy and safety of adalimumab in Chinese patients with moderately to severely active Crohn’s disease: results from a randomized trial by Baili Chen, Xiang Gao, Jie Zhong, Jianlin Ren, Xuan Zhu, Zhanju Liu, Kaichun Wu, Jasmina Kalabic, Zhuqing Yu, Bidan Huang, Nisha Kwatra, Thao Doan, Anne M. Robinson and Min-Hu Chen in Therapeutic Advances in Gastroenterology
Supplemental material, Supplementary_Material_revision_3 for Efficacy and safety of adalimumab in Chinese patients with moderately to severely active Crohn’s disease: results from a randomized trial by Baili Chen, Xiang Gao, Jie Zhong, Jianlin Ren, Xuan Zhu, Zhanju Liu, Kaichun Wu, Jasmina Kalabic, Zhuqing Yu, Bidan Huang, Nisha Kwatra, Thao Doan, Anne M. Robinson and Min-Hu Chen in Therapeutic Advances in Gastroenterology