Abstract
Patient: Female, 64-year-old
Final Diagnosis: Plasmacytoid urothelial carcinoma of the bladder
Symptoms: Nausea • vomiting
Medication:—
Clinical Procedure: Cystoscopy
Specialty: Oncology
Objective:
Rare disease
Background:
Plasmacytoid urothelial carcinoma (PUC) is a rare and aggressive variant of urothelial cancers. Herein, we report a patient with CDH-1 mutated PUC who presented with disseminated peritoneal metastasis and high levels of CA 19-9.
Cases Report:
A 65-year-old female presented to the hospital with vomiting, obstructive jaundice, and acute renal failure. Imaging studies showed bilateral hydronephrosis, bladder wall thickening without masses, and dilation of both common bile and pancreatic ducts without pancreatic masses. Carbohydrate antigen (CA) 19-9 was elevated at >17 000 U/mL. Repeated cystoscopies demonstrated no masses within the bladder, but with nodularity and inflamed mucosa, and random biopsies were obtained and showed PUC. Ascitic fluid cytology revealed meta-static PUC. A targeted exome next-generation sequencing (NGS) revealed pathogenic mutations in TP53, CDH-1, RB1, and ARIDA1A. The patient was debilitated, and hospice care was recommended. She passed away after 2 months of her initial presentation.
Conclusions:
PUC is a rare and aggressive histological variant of bladder cancer. Advanced stage at diagnosis and high relapse rates after treatment with cytotoxic regimens are common. At the molecular level, somatic alterations in cadherin-1 (CDH-1) gene seem to be characteristic. Exploring the molecular sphere of this disease is prudent to identify possible new therapeutic targets.
MeSH Keywords: CA-19-9 Antigen, CDH-1, Plasmacytoid, Transitional Cell Carcinoma, Urinary Bladder Neoplasms, Urothelial Carcinoma
Background
Urinary bladder cancer is the ninth most common malignancy worldwide [1]. Urothelial carcinoma (UC), also referred to as transitional cell carcinoma, is the predominant histo-logical type in the western world, representing 90% of bladder cancers. Plasmacytoid urothelial carcinoma (PUC) is a rare and aggressive histological variant of urothelial cancers. Advanced stage at diagnosis is common, and the peritoneum is the most common site of early metastasis. PUC is associated with worse overall survival and a higher propensity to relapse after treatment in comparison to UC [2–6]. Loss of epithelial cadherin (E-cadherin) is a consistent feature of the tumor cells on immunohistochemistry staining. Given the poor outcomes of PUC with the current chemotherapeutic regimens, alternative approaches focusing on molecular targets are indispensable. In one report, most cases of PUC harbor pathognomonic somatic alterations in the cadherin-1 (CDH-1) gene. Other mutations that are common in UC were also described in PUC [7,8]. In terms of tumor markers, few reports showed that PUC could produce carbohydrate antigen (CA) 19-9 [9,10]. Herein, we report a patient with CDH-1 mutated PUC who presented with disseminated peritoneal metastasis and had high levels of CA 19-9.
Case Report
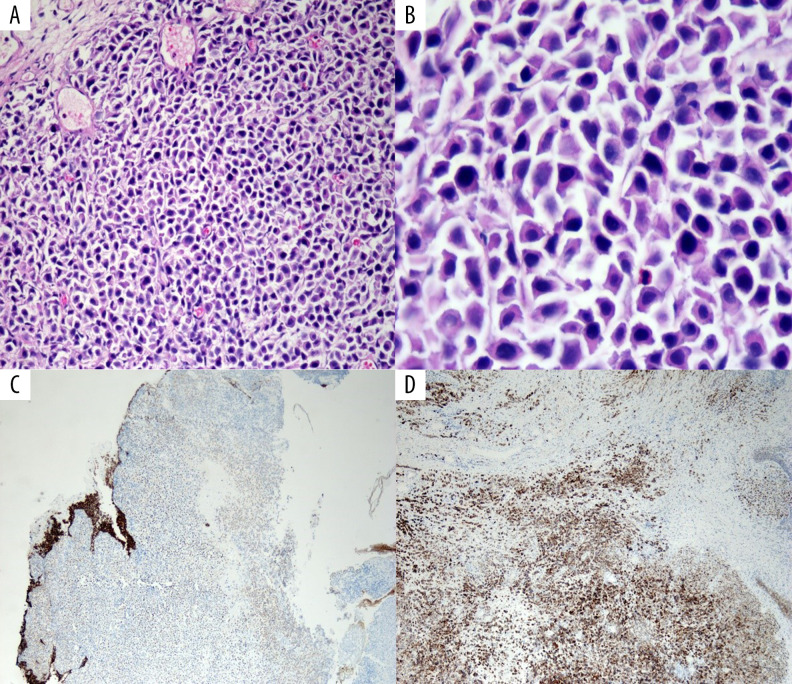
A 65-year-old female presented to the hospital with nausea, vomiting, and fatigue. She was an active smoker with more than 20 pack per year smoking history. She denied having any urinary symptoms. She did not receive prior radiation to the pelvis or have a history of kidney stones, recurrent urinary tract infections, or significant pelvic surgeries. Her medical history included generalized anxiety disorder, hypothyroidism, gastroesophageal reflux disease and history of hysterectomy at the age of 40 years for menorrhagia. Initial blood work showed acute renal failure with a creatinine of 9.3 mg/dL. A computerized tomography (CT) scan of the abdomen and pelvis showed severe bilateral hydronephrosis and bladder wall thickening (Figure 1). Magnetic resonance imaging (MRI) of the abdomen and pelvis confirmed the bladder wall thickness without any masses, lymphadenopathy, or retroperitoneal fibrosis. Cystoscopy with ureteroscopy revealed no masses to biopsy, but she had bilateral mid to distal ureteral stenosis, and bilateral ureteral stents were placed. Her renal function and creatinine slowly improved to 1.4 mg/dL. After 2 weeks, she developed progressive generalized edema, which needed diuresis. Repeat cystoscopy showed marked large dilated vessels of the bladder mucosa as well as bullous edema but without evidence of tumors or stones. The ureteral stents were removed, and bilateral nephrostomy tubes were placed. She had a repeat cystoscopy with bilateral retrograde pyelograms and antegrade nephrostogram a couple of weeks later, which demonstrated a small capacity and poorly compliant rigid bladder. There were no visible papillary masses within the bladder, but there was nodularity of the bladder wall with inflamed and friable mucosa. Random resections for biopsy were done. Within a week of the procedure, the patient was admitted with severe vomiting, sepsis, and obstructive jaundice. Her bilirubin was elevated at 7.0 mg/dL with elevated aspartate aminotransferase (AST) at 135 U/L, alanine aminotransferase (ALT) at 58 U/L, and alkaline phosphatase (ALP) at 820 U/L. CT scan of the chest, abdomen, and pelvis revealed dilation of both the common bile duct (CBD) and pancreatic duct (“double-duct” sign) without masses in the pancreas; there was omental thickening and nodularity, abnormal wall thickening through all the duodenum, and large volume ascites. No masses, lesions or lymphadenopathy were seen (Figure 2). At that time, pathology report of the bladder wall biopsy revealed plasmacytoid urothelial carcinoma that was positive for pan-cytokeratin and cytokeratin CAM 5.2 and that was focally positive for CD138. There was a loss of E-cadherin on tumor cells. The tumor cells showed high proliferative activity with high Ki-67 (Figure 3A–3D). Carbohydrate antigen (CA) 19-9 was elevated >17 000 U/mL, carcinoembryonic antigen (CEA) was also elevated at 132 ng/mL. Endoscopic ultra-sound (EUS) was done and the head of the pancreas was normal without masses. The patient then underwent paracentesis, and ascitic fluid cytology revealed metastatic high-grade urothelial carcinoma compatible with metastatic PUC (Figure 4). Extended molecular testing of the bladder wall biopsy was done with targeted exome next-generation sequencing (NGS) using the Illumina NextSeq platform. NGS showed pathogenic mutations in TP53, CDH-1, RB1, and ARIDA1A (Table 1). The patient was severely debilitated and bed-bound at that time and had an Eastern Cooperative Oncology Group (ECOG) score of 4. The patient continued to decline clinically, and she decided on best supportive care with hospice service. She passed away 2 months of her initial presentation.
Figure 1.
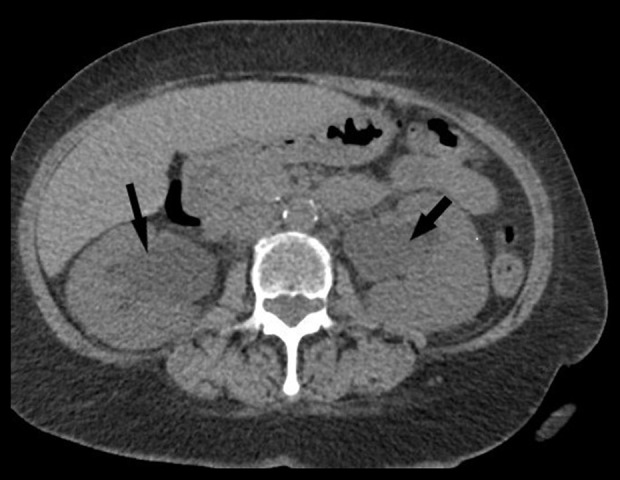
Computed tomography scan of abdomen and pelvis showing bilateral hydronephrosis (arrows).
Figure 2.
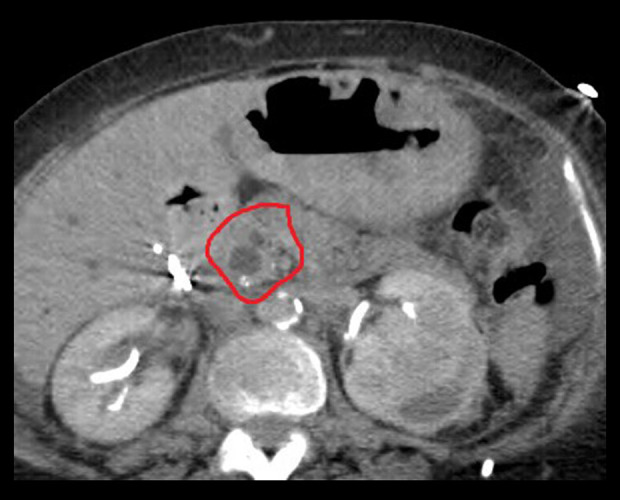
Computed tomography scan of the abdomen showing dilated common bile duct and pancreatic ducts, “double-duct” sign (circle).
Figure 3.
(A) Low power hematoxylin and eosin stain showing sheets of tumor cells. (B) High power hematoxylin and eosin stain showing discohesive plasmacytoid tumor cells with eccentric nuclei and round contour. (C) E-cadherin stain showing tumor cells with loss of E-cadherin. (D) CD138 satin showing tumor cells positive for CD138.
Figure 4.
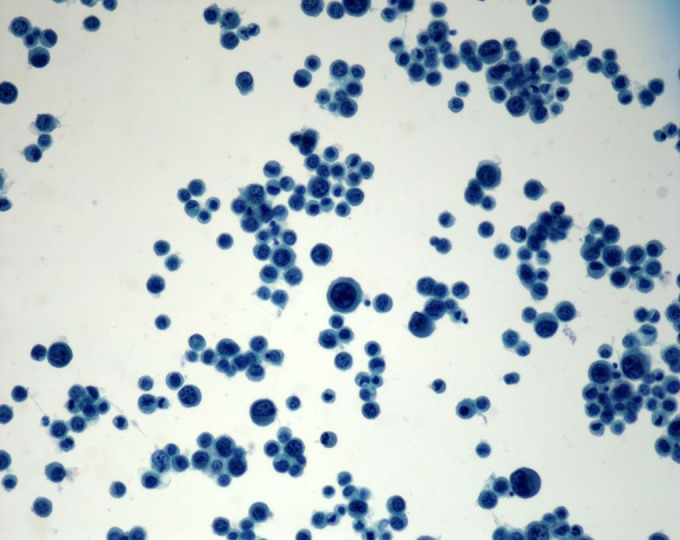
Ascitic fluid cytology showing sheets of plasmacytoid tumor cells.
Table 1.
Molecular profile of the tumor with targeted exome next-generation sequencing, using illumina NextSeq platform.
| Gene | Amino acid change | Allele frequency | Type |
|---|---|---|---|
| CDH-1 | p.R335* | 43.0% | Pathogenic |
| TP53 | p.S215G | 47.0% | Pathogenic |
| RB1 | p.Q217* | 41.0% | Pathogenic |
| ARID1A | p.P320fs*75 | 29.0% | Likely pathogenic |
| NOTCH2 | p.A3F | 17.0% | Uncertain significance |
| FH | p.G424A | 5.24% | Uncertain significance |
| EXO1 | p.G141R | 51.0% | Uncertain significance |
| MSH2 | p.Q324E | 24.0% | Uncertain significance |
| ERBB4 | p.P574T | 50.0% | Uncertain significance |
| ARAF | p.L86F | 27.0% | Uncertain significance |
Discussion
Plasmacytoid urothelial carcinoma (PUC) was initially described in 1991 by Sahin et al. as a tumor resembling multiple myeloma [11]. Mai et al. reported a 2.7% incidence of PUC in a large series of muscle-invasive UC cases [12]. PUC is an aggressive histological variant that frequently presents at an advanced stage at diagnosis. While PUC might initially respond to cytotoxic chemotherapies, it is associated with worse overall survival in comparison to UC and relapses are common [2–6].
Therefore, alternative approaches that target this aggressive disease, particularly at the molecular level, are needed. In one report, up to 80% of PUC cases harbored pathognomonic somatic alterations in the CDH-1 gene. Other mutations that are common to UC have also been described [8]. Our patient presented with disseminated peritoneal metastatic PUC that harbored a CDH-1 mutation at an allele frequency of 43.0%.
The median patient age of PUC diagnosis is patients in their 60s, with a male predominance. Most PUC patients are smokers [3,13]. Clinically, PUC typically presents as advanced disease, and symptoms and signs might be related to metastatic disease. Unlike conventional UC, hematuria is typically a late manifestation and some patients might not encounter any urinary symptoms, which might lead to the advanced disease at diagnosis. The peritoneum is the most common site of metastasis, which can lead to ascites [3,5]. Other reported sites of metastasis include the duodenum, stomach, retroperitoneal area, ribs, skull, brain, heart, and penis [6,11,14–17].
Almost all cases have been reported to occur in the urinary bladder, with only a few cases reported in the upper urinary tract [18–20]. Rarely, urine cytology might be positive [21]. On cystoscopic examination, solitary or multiple masses can be seen. However, mucosal induration and thickened bladder wall without masses are common, which can lead to diagnostics pitfalls [22]. Our patient presented with disseminated peritoneal disease without urinary symptoms. She had 3 cystoscopic examinations, and no masses were seen. The diagnosis was made after mucosal resection was performed.
On morphological examination, PUC is characterized by sheets of poorly differentiated discohesive round or oval cells with eccentric nuclei and abundant amphophilic to eosinophilic cytoplasm resembling plasma cells [23,24]. Three distinctive morphological types have been described for PUC, classic, desmoplastic, and pleomorphic; the desmoplastic morphology seems to be associated with worse survival [2].
The plasmacytoid component of the tumor might be focal or diffuse and varies from less than 30% and up to 100%. Other co-existing morphologies include conventional urothelial carcinoma, sarcomatoid, micropapillary, nested, small cell carcinoma or urothelial carcinoma in situ [23,25].
Immunohistochemistry is crucial for definitive diagnosis. The tumor cells usually stain positive for typical urothelial markers such as cytokeratin-7 (CK7), pan-CK, CK8, CK20, p63, epithelial membrane antigen (EMA), CD56, endothelial transcription factor 3 (GATA3), and uroplakin II [25]. They also can test positive for p53 and p16 [26]. They are negative for neuroendocrine markers (synaptophysin and chromogranin), and S-100. The tumor cells demonstrate a high proliferation index with Ki-67 above 60% [26].
PUC cells typically stain positive for plasma cell marker CD138. However, common leukocyte antigen (LCA) and multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF4), κ or λ light chains stains, and vimentin are negative, differentiating PUC from plasmacytoma. In few reports, PUC cells tend to be negative for programmed death-1 (PD-1) and its ligand PD-L1 [25,27].
The loss of E-cadherin protein, encoded by the CDH-1 gene, is characteristic for PUC [22,25]. Cadherins are a group of trans-membrane proteins that are involved in cell-to-cell adhesion and cell-to-stroma interaction. The cadherin family includes N-cadherin, P-cadherin, and E-cadherin. Loss of E-cadherin expression is associated with a loss of cellular differentiation and increased cellular invasiveness and metastatic potential [26].
Other possible diagnostic mimickers for PUC include plasma-cytoma/myeloma, lymphoma, urothelial carcinoma with rhabdoid features, neuroendocrine tumors, paraganglioma, melanoma, rhabdomyosarcoma, and metastatic diffuse gastric and lobular breast adenocarcinomas. Due to the occasional signet ring appearance and peritoneal metastasis, primary metastatic signet ring cells adenocarcinomas are also in the differential. PUC is not associated with mucin production, in contrast to other signet ring cell tumors which are typically associated with extracellular mucin [23,24].
Tumor cells might produce serum carbohydrate antigen CA 19-9 [10] and the human chorionic gonadotropin (β-HCG), even without the presence of trophoblastic differentiation [9].
Our patient tumor cells showed classic PUC morphology and tested positive for pan-cytokeratin, CAM 5.2, GATA3, and CD138. There was a loss of E-cadherin on tumor cells. The tumor cells showed high proliferative activity with high Ki-67. CA 19-9 was elevated >17 000 U/mL.
At the molecular level, CDH-1 alterations seem to be characteristic. This is supported by the fact that loss of E-cadherin protein is a hallmark for this tumor, in addition to the previous observations which show that alterations in the CDH-1 gene are found in most of the reported cases. The gene CDH-1, which is the coding gene for E-cadherin, is located on chromo-some 16q22.1. Germline mutations in CDH-1 are associated with hereditary diffuse gastric cancer (HDGC) and increased risk of lobular breast adenocarcinoma.
A report by Al-Ahmadie et al. showed that CDH-1 alterations, either truncating mutations or promoter hypermethylation, are present in 30 out of 31 patients with plasmacytoid carcinoma, whereas no CDH-1 alterations were observed in 183 patients with non-plasmacytoid-variant [8]. In contrast to the germ-line CDH-1 alteration associated with HDGC and breast cancer, there were no germline CDH-1 alterations in the plasma-cytoid-variant bladder cancers [8]. Aside from CDH-1 mutation, the genetic alterations in PUC were similar to that of urothelial carcinoma, with mutations in TP53, RB1, ARID1A, ERBB2, PIK3CA, and TERT promotor, and others [7,8,28].
Our patient had a somatic nonsense mutation in the CDH-1 gene c.1003C>T with amino acid change p.R335* with allele frequency (AF) of 43%. This mutation has been reported in other tumors but to our knowledge, not in PUC. Our patient tumor also had pathogenic mutations in other genes that are common to urothelial carcinoma and not characteristic to PUC (Table 1).
The optimal treatment of PUC has not been established due to the infrequency of this variant disease. In general, a management approach similar to UC is followed. However, relapses are frequent, and survival is worse in comparison to UC [2,4–6,13]. While most of the cases are diagnosed at an advanced stage, Kimura et al. reported a successful conservative approach for a patient who desired bladder conservation, with confirmed pT1 disease using intravesical instillation of 40 mg of mitomycin C weekly for 6 weeks [29]. PUC had not recurred at 26 months since the initial diagnosis [29]. Kawaharra et al. described the successful treatment of pT1 disease with transurethral resection of the tumor, followed by adjuvant chemo-radiation with no recurrence for 2 years [30].
For more advanced non-metastatic disease, surgery with radical cystectomy with pelvic lymph node dissection is the primary therapy. Chemotherapy can be used as neoadjuvant or adjuvant with regimens like MVAC (methotrexate, vinblastine, doxorubicin, cisplatin) or gemcitabine and cisplatin. Neoadjuvant chemotherapy might lead to complete pathological response and long-term disease-free survival [6,31–33].
For metastatic disease, systemic chemotherapy is used with an overall response rate exceeding 50%, complete responses have also been described [6,11,15,32]. Our patient presented with disseminated peritoneal disease at diagnosis and poor overall performance status. Thus, she was not a candidate for aggressive treatment. She was managed with best supportive care, and she passed away within 2 months of her initial presentation.
PD1 and PD-L1 are usually negative in PUC. Therefore, the efficacy of the checkpoint inhibitor is uncertain, and the responses are short-lived [2,34]. Exploring the molecular landscape of PUC is essential to identify possible targetable mutations that might eventually improve the treatment outcomes of this aggressive histology.
Conclusions
PUC is a rare and aggressive histological variant of bladder cancer. Advanced stage at diagnosis and high relapse rates after treatment with cytotoxic regimens are common, leading to inferior survival in comparison to urothelial carcinoma. At the molecular level, somatic alterations in CDH-1 seems to be characteristic. Exploring the molecular sphere of this disease is prudent to identify possible new therapeutic targets that might improve patients’ outcomes.
References:
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Hashemi-Sadraei N, Perrino CM, Monn MF, et al. Plasmacytoid urothelial carcinoma: A clinicopathological study. J Clin Oncol. 2018;36:482. [Google Scholar]
- 3.Li Q, Assel M, Benfante NE, et al. The impact of plasmacytoid variant histology on the survival of patients with urothelial carcinoma of bladder after radical cystectomy. Eur Urol Focus. 2019;5(1):104–8. doi: 10.1016/j.euf.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cockerill PA, Cheville JC, Boorjian SA, et al. Outcomes following radical cystectomy for plasmacytoid urothelial carcinoma: Defining the need for improved local cancer control. Urology. 2017;102:143–47. doi: 10.1016/j.urology.2016.09.053. [DOI] [PubMed] [Google Scholar]
- 5.Kaimakliotis HZ, Monn MF, Cary KC, et al. Plasmacytoid variant urothelial bladder cancer: Is it time to update the treatment paradigm? Urol Oncol. 2014;32:833–38. doi: 10.1016/j.urolonc.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Dayyani F, Czerniak BA, Sircar K, et al. Plasmacytoid urothelial carcinoma, a chemosensitive cancer with poor prognosis, and peritoneal carcinoma-tosis. J Urol. 2013;189:1656–61. doi: 10.1016/j.juro.2012.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palsgrove DN, Taheri D, Springer SU, et al. Targeted sequencing of plasmacytoid urothelial carcinoma reveals frequent TERT promoter mutations. Hum Pathol. 2019;85:1–9. doi: 10.1016/j.humpath.2018.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Ahmadie HA, Iyer G, Lee BH, et al. Frequent somatic CDH1 loss-of-function mutations in plasmacytoid variant bladder cancer. Nat Genet. 2016;48:356–58. doi: 10.1038/ng.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimada K, Nakamura M, Ishida E, Konishi N. Urothelial carcinoma with plasmacytoid variants producing both human chorionic gonadotropin and carbohydrate antigen 19-9. Urology. 2006;68:891.e7–10. doi: 10.1016/j.urology.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 10.Makise N, Morikawa T, Takeshima Y, et al. Urinary bladder urothelial carcinoma with concurrent plasmacytoid and micropapillary differentiations: A report of two cases with an emphasis on serum carbohydrate antigen 19-9. Pathol Int. 2015;65:495–500. doi: 10.1111/pin.12314. [DOI] [PubMed] [Google Scholar]
- 11.Sahin AA, Myhre M, Ro JY, et al. Plasmacytoid transitional cell carcinoma. Report of a case with initial presentation mimicking multiple myeloma. Acta Cytol. 1991;35:277–80. [PubMed] [Google Scholar]
- 12.Mai KT, Park PC, Yazdi HM, et al. Plasmacytoid urothelial carcinoma of the urinary bladder report of seven new cases. Eur Urol. 2006;50:1111–14. doi: 10.1016/j.eururo.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 13.Keck B, Wach S, Stoehr R, et al. Plasmacytoid variant of bladder cancer defines patients with poor prognosis if treated with cystectomy and adjuvant cisplatin-based chemotherapy. BMC Cancer. 2013;13:71. doi: 10.1186/1471-2407-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brustmann H. Plasmacytoid urothelial carcinoma of the urinary bladder metastatic to the duodenum: A case report-diagnostic relevance of GATA3 immunohistochemistry. Case Rep Pathol. 2017;2017:5209059. doi: 10.1155/2017/5209059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messina C, Zanardi E, Dellepiane C, et al. A case of plasmacytoid variant of bladder cancer with a single penile metastasis and a complete response to carboplatin-based chemotherapy and review of the literature. Clin Genitourin Cancer. 2016;14:e139–42. doi: 10.1016/j.clgc.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Peck JR, Hitchcock CL, Maguire S, et al. Isolated cardiac metastasis from plasmacytoid urothelial carcinoma of the bladder. Exp Hematol Oncol. 2012;1:16. doi: 10.1186/2162-3619-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nabbout P, Furr J, Paari M, Slobodov G. Plasmacytoid urothelial carcinoma of the bladder metastatic to the stomach: A case report. Case Rep Urol. 2012;2012:715951. doi: 10.1155/2012/715951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jibril A, Stevens AC. Plasmacytoid urothelial carcinoma of ureter with retroperitoneal metastasis: A case report. Am J Case Rep. 2018;19:158–62. doi: 10.12659/AJCR.906679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang IW, Hsu CT, Huang CY, Tsai JW. Plasmacytoid urothelial carcinoma: First case reported in the ureter. Pathol Int. 2013;63:73–76. doi: 10.1111/pin.12026. [DOI] [PubMed] [Google Scholar]
- 20.Keck B, Giedl J, Kunath F, et al. Clinical course of plasmacytoid urothelial carcinoma of the upper urinary tract: A case report. Urol Int. 2012;89:120–22. doi: 10.1159/000335519. [DOI] [PubMed] [Google Scholar]
- 21.Zhang XM, Elhosseiny A, Melamed MR. Plasmacytoid urothelial carcinoma of the bladder. A case report and the first description of urinary cytology. Acta Cytol. 2002;46:412–16. doi: 10.1159/000326742. [DOI] [PubMed] [Google Scholar]
- 22.Fritsche HM, Burger M, Denzinger S, et al. Plasmacytoid urothelial carcinoma of the bladder: histological and clinical features of 5 cases. J Urol. 2008;180:1923–27. doi: 10.1016/j.juro.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 23.Nigwekar P, Tamboli P, Amin MB, et al. Plasmacytoid urothelial carcinoma: Detailed analysis of morphology with clinicopathologic correlation in 17 cases. Am J Surg Pathol. 2009;33:417–24. doi: 10.1097/PAS.0b013e318186c45e. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Beltran A, Requena MJ, Montironi R, et al. Plasmacytoid urothelial carcinoma of the bladder. Hum Pathol. 2009;40:1023–28. doi: 10.1016/j.humpath.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Fox MD, Xiao L, Zhang M, et al. Plasmacytoid urothelial carcinoma of the urinary bladder: A clinicopathologic and immunohistochemical analysis of 49 Cases. Am J Clin Pathol. 2017;147:500–6. doi: 10.1093/ajcp/aqx029. [DOI] [PubMed] [Google Scholar]
- 26.Raspollini MR, Sardi I, Giunti L, et al. Plasmacytoid urothelial carcinoma of the urinary bladder: Clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of a case series. Hum Pathol. 2011;42:1149–58. doi: 10.1016/j.humpath.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Ohtaka M, Kawahara T, Kumano Y, et al. Invasive urothelial carcinoma, lymphoma-like/plasmacytoid variant, successfully treated by radical cystectomy with adjuvant chemotherapy: A case report. J Med Case Rep. 2016;10:48. doi: 10.1186/s13256-016-0806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Lu S, Lombardo K, et al. ARID1A alteration in aggressive urothelial carcinoma and variants of urothelial carcinoma. Hum Pathol. 2016;55:17–23. doi: 10.1016/j.humpath.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Kimura H, Uemura Y, Megumi Y, Fukuzawa S. [A case of pt1 plasmacytoid variant bladder cancer treated by bladder conserving therapy] Hinyokika Kiyo. 2018;64:369–72. doi: 10.14989/ActaUrolJap_64_9_369. [in Japanese] [DOI] [PubMed] [Google Scholar]
- 30.Kawahara T, Oshiro H, Sekiguchi Z, et al. High-grade invasive urothelial carcinoma with focal plasmacytoid differentiation successfully treated by transurethral resection followed by chemoradiotherapy. Int J Urol. 2011;18:851–53. doi: 10.1111/j.1442-2042.2011.02880.x. [DOI] [PubMed] [Google Scholar]
- 31.Gunaratne DA, Krieger LE, Maclean F, et al. Neoadjuvant chemotherapy with gemcitabine and cisplatin for plasmacytoid urothelial bladder cancer: A case report and review of the literature. Clin Genitourin Cancer. 2016;14:e103–5. doi: 10.1016/j.clgc.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi T, Tanigawa G, Fujita K, et al. Two cases of plasmacytoid variant of urothelial carcinoma of urinary bladder: Systemic chemotherapy might be of benefit. Int J Clin Oncol. 2011;16:759–62. doi: 10.1007/s10147-011-0240-4. [DOI] [PubMed] [Google Scholar]
- 33.Kohno T, Kitamura M, Akai H, et al. Plasmacytoid urothelial carcinoma of the bladder. Int J Urol. 2006;13:485–86. doi: 10.1111/j.1442-2042.2006.01338.x. [DOI] [PubMed] [Google Scholar]
- 34.Hunter L, Moser J, Sturge C, et al. First-line pembrolizumab therapy in a cisplatin-ineligible patient with plasmacytoid urothelial carcinoma: A case report. J Oncol Pharm Pract. 2020;26(1):216–19. doi: 10.1177/1078155219835006. [DOI] [PubMed] [Google Scholar]