Abstract
Background
This was a prospective comparative study of mixed reality (MR) technology assisted lumbar pedicle screws placement and traditional lumbar pedicle screws placement.
Material/Methods
Fifty cases of lumbar pedicle screws placement were randomly divided into 2 groups: 25 cases with MR technology in group A, and 25 cases without MR technology in group B. All patients had their scores on the Oswestry disability index (ODI) of low back pain and the visual analog scale (VAS) of the affected lower limb recorded at pre-operation. Blood loss, operative duration, success rate of first penetration by tap, and number of times C-arm fluoroscopy was performed were recorded at intraoperation. The postoperative drainage was recorded. The ODI of low back pain and VAS of the affected lower limb were recorded at 1, 3, and 6 months after operation.
Results
Group A had less bleeding, shorter operation time, higher success rate of first penetration by tap, and fewer times using C-arm fluoroscopy at intraoperation (P<0.05). There was significant difference in ODI scores and VAS scores at 1 mouth after operation (P<0.05). The postoperative drainage of group A was less than group B (P<0.05). The implantation accuracy of group A was higher than group B (P<0.05). The postoperative recovery rate of low back pain of group A was faster than group B (P<0.05).
Conclusions
The safety of spinal surgery and implantation accuracy of pedicle screw fixation system could be increased by MR technology.
MeSH Keywords: Lumbar Surgery, Mixed Reality, Pedicle Screws
Background
The pedicle screw internal fixation system has been proven to be the best internal fixation material to stabilize the spine and has become the most commonly used surgical method for spinal surgeons to stabilize the spine [1]. At present, spinal surgeons mainly perform pedicle screw placement, according to their experience, based on anatomical signs, preoperative x-ray, computed tomography (CT) and intraoperative C-arm fluoroscopy observation and positioning [2–4]. However, complex tissue structure, rich vasculature and nerves around the vertebral pedicle, and individual anatomical differences undoubtedly increase the difficulty and risk of surgical screw placement. According to a large number of studies, the penetration rate of the pedicle wall in the traditional unarmed nailing method is 12.5% to 72.4% [5,6], the improper placement of a pedicle screw will cause complications in nerve, viscera, blood vessels, and other aspects, with serious consequences.
How to insert the pedicle screw quickly and accurately has become a focus and difficulty for spine doctors. With the development of science, advanced manufacturing technology and digital technology have been applied to the field of medicine. Among them, three-dimensional (3D) Print is also known as rapid prototyping technology or additive manufacturing technology, which through CT scanning, 3D solid parts can be directly printed without being restricted by the complexity of parts’ shapes. Guide plates can be prepared for use in spinal surgery [7,8], to improve the success rate of the operation [9]. However, 3D printing technology is still relatively abstract, with a high degree of integration between guide plate and vertebra, which cannot be closely combined with the operation process in real time. Mixed reality (MR) technology is a technology that breaks the boundary between the digital virtual world and the physical real world, and realizes the precise combination of 3D virtual objects and the real world. In the aspect of spinal surgery, digital technology, 3D modeling technology, and intraoperative navigation technology are used to make individualized plans before surgery and check the structure of the lesion site at any time during surgery, so as to reduce the difficulty and risk of surgery and reduce the exposure of radiation to both doctors and patients, effectively improve the safety and accuracy of surgery, shorten the operation time [10–12]. In this study, randomized controlled trials were prospectively included to compare the differences in surgical operation and efficacy between MR-assisted thoracolumbar pedicle screw implantation and traditional thoracolumbar pedicle screw implantation, in order that provide clinicians to selecting surgical methods.
Material and Methods
Inclusion and exclusion criteria
The study inclusion criteria were as follows, 1) Course of disease ≥6 months, all patients with lumbago back pain, radiation pain of lower limb, lumbar disc herniation diagnosed with or without spinal stenosis. 2) The course of disease ≥6 months, all patients had lumbar back pain, accompanied by or without intermittent claudication, and the diagnosis of lumbar spondylolisthesis was clear, but the vertebral isthmus was not broken. 3) The patient’s symptoms were consistent with the imaging examination results, failed to respond to conservative treatment, and met the surgical indications. 4) The lesion segment is no more than 3 vertebral bodies. 5) Preoperative improvement of x-ray, CT, magnetic resonance imaging (MRI) examination. 6) Patients without active bleeding and female patients not in menstrual period. 7) According to the patient’s condition, the following surgical methods should be adopted: lumbar posterior decompression, nucleus pulposus extraction, artificial intervertebral disc implantation, bone graft fusion and internal fixation.
The study exclusion criteria were as follows. 1) Patients with lumbar acute infection, lumbar tuberculosis, tumor and other diseases. 2) Patients with severe cardiopulmonary insufficiency, intolerance to surgery, diabetes and poor blood glucose control, combined with coagulation dysfunction and other contraindications. 3) Vertebral fracture patients. 4) Bone mineral density (BMD) examination: BMD was measured by dual-energy x-ray absorptiometry at the lumbar spine (L2~L4) and femoral head, and BMD and T values were observed. Abnormal bone mass was excluded according to: T >−1 standard deviation (SD) [13]. 5) Patient had disc herniation with severe lumbar degenerative disease but does not require nucleus pulposus extraction.
Research methods and ethics
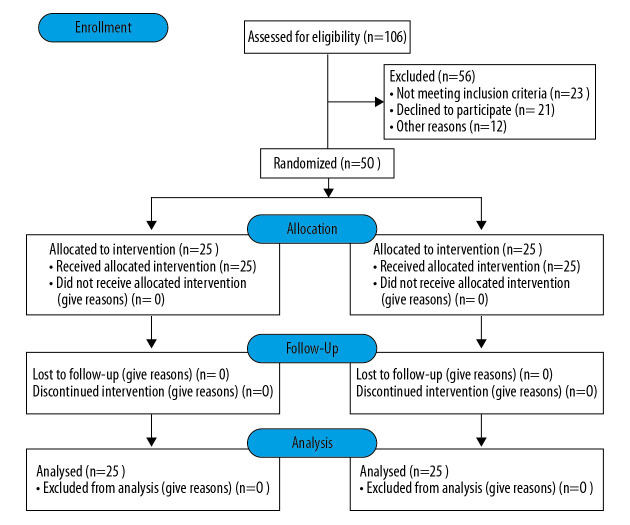
This study was conducted by a prospective, randomized controlled method. Here is flow diagram (Figure 1). Patients were informed of surgical risks, technical and theoretical advantages, uncertainties and clinical research plans preoperative. All patients signed informed consent forms. This study was approved by the ethics review committee of Nanjing First Hospital and the implementation was supervised. The operation place was Nanjing First Hospital. This study did not increase the burden on patients and did not cause additional harm to patients. Study data are only used in this study to ensure patient privacy. The investigator had detailed personal information of all patients participating in this study, and informed the patients for outpatient follow-up 1 week before the follow-up, and informed them again 1 day before the follow-up and confirmed that the patients could be followed up on time. No patient follow-up loss was found in this study. The author guarantees the accuracy and integrity of the data.
Figure 1.
The ethics.
General information
According to the aforementioned criteria, 50 patients with lumbar disc herniation and/or lumbar spondylolisthesis admitted to the orthopedic department of Nanjing First Hospital from January 2017 to October 2018 were selected for inclusion in the study. All patients underwent surgery within 1 week after admission. The patients were followed up for 6 months, and the latest study follow-up date was April 2019. According to the random number table method, patients were divided into group A and group B with 25 cases in each group.
In group A, there were 11 males and 14 females in the group. The average age was 44.32±3.78 years old. Low back pain measured by the Oswestry disability index (ODI) score was 50.35±9.27% and lower extremity visual analog scale (VAS) score was 7.16±1.40 preoperative.
In group B, there were 13 males and 12 females in the group. The average age was 45.56±3.93 years old. Low back pain ODI score was 51.75±9.18% and lower limb VAS score was 7.12±1.26 preoperative.
Comparison of preoperative general conditions between the 2 groups showed no statistically significant difference (P>0.05) (Table 1).
Table 1.
Preoperative general information.
| Gender | Age (years) | Low back pain ODI score (%) | VAS score of lower extremity of affected side (score) | Number of lumbar spondylolisthesis | ||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| Group A | 11 | 14 | 44.32±3.78 | 50.35±9.27 | 7.16±1.40 | 7 |
| Group B | 13 | 12 | 45.56±3.93 | 51.75±9.18 | 7.12±1.26 | 9 |
| χ2/t value | 0.3205 | 1.1354 | 0.5366 | 0.1062 | 0.3682 | |
| P values | 0.5713 | 0.2618 | 0.5941 | 0.9159 | 0.5446 | |
ODI – Oswestry disability index; VAS – visual analog scale.
Design and production of personalized programs
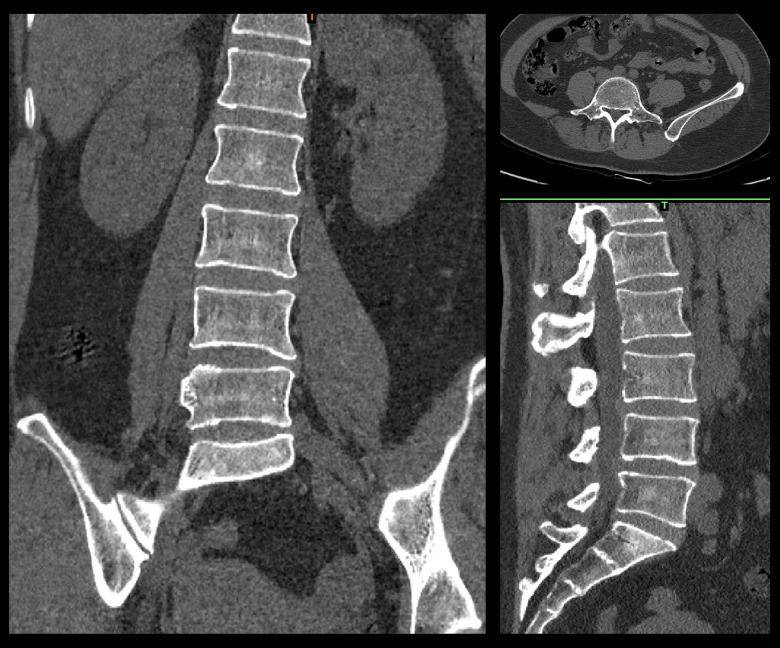
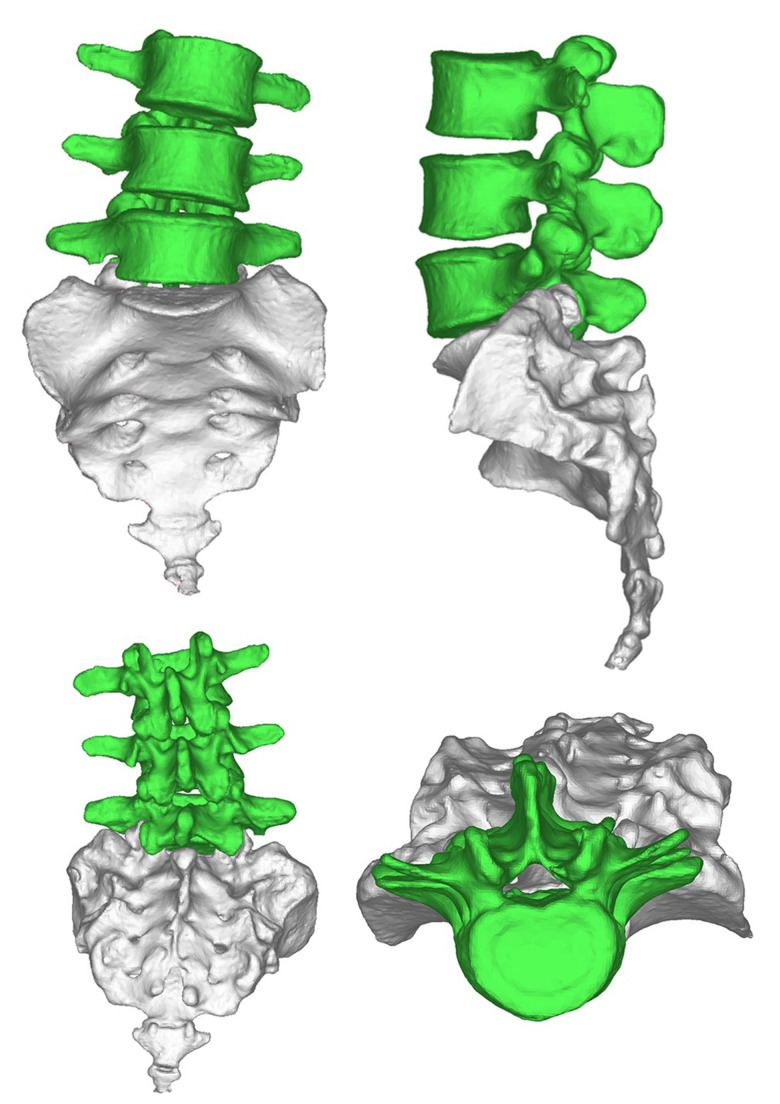
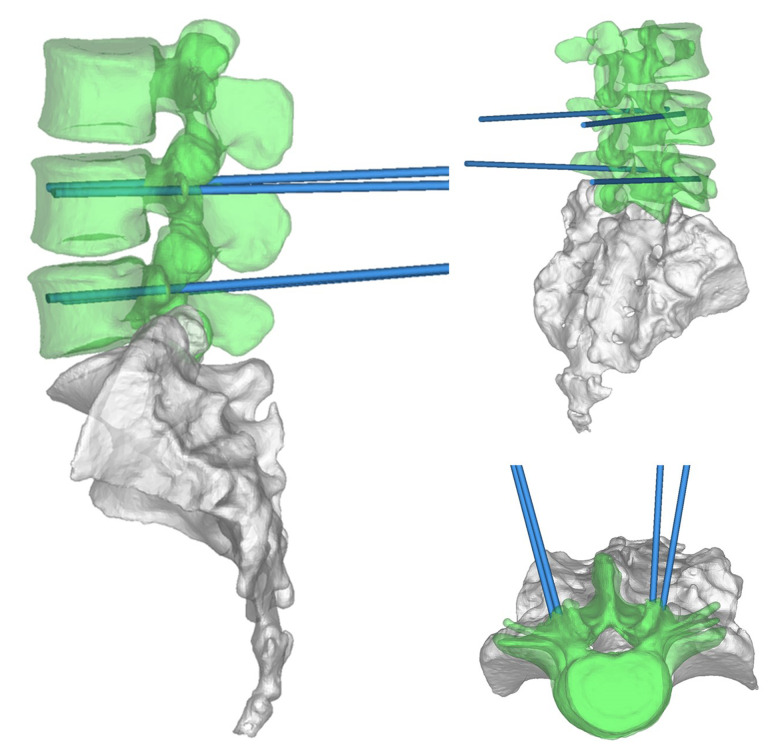
The scheme was completed by doctors and engineers. Doctors determine shape, matching areas, and all parameters for each patient’s condition. An engineer was responsible for 3-dimensional (3D) reconstruction, Midivi 3D reconstruction software (Bahulu, Shanghai Front Computing Company, China) was used to directly read the original DICOM (Digital Imaging and Communications in Medicine) images by double source 64 row helical CT (Siemens Sensation, Germany) technology and based on a 0.5 mm thick target continuous fault vertebral body image (Figure 2). Different masks were generated according to different threshold settings. The image of each layer was processed by edge segmentation, selective editing and hole filling, and remove redundant data establish the 3D structure of the target vertebra, then obtain the computer-aided design (CAD) images (Figure 3). The design of lumbar pedicle screw track was carried out in the corresponding module (Figure 4).
Figure 2.
Computed tomography scan results.
Figure 3.
The spinal image after 3-dimensional reconstruction.
Figure 4.
Design and simulation of screw implantation trajectory after 3-dimensional reconstruction
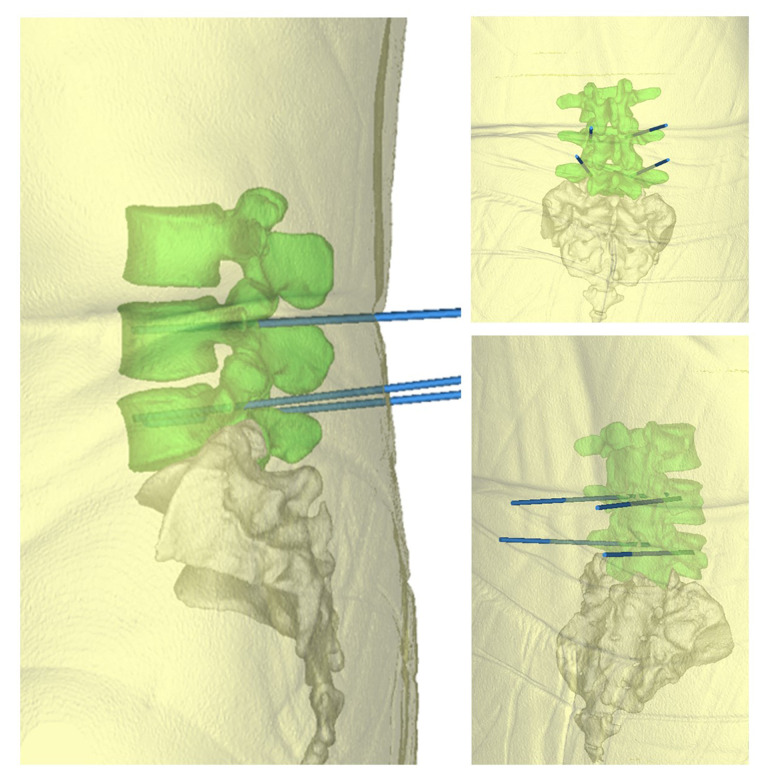
The doctors and engineers confirmed the design results through preoperative imaging data and made the module successfully (Figure 5); skin and other soft tissues were added. The blue point seen in Figure 5 is the needle entry point. The rod as trajectory is the needle entry angle and direction. Finally, the relevant information was introduced into the system for intraoperative use.
Figure 5.
Trajectory of screw implantation after soft tissue addition (this track only represents the direction and position of screw feeding, not the screw itself.)
Surgical methods
Both groups were completed by the same group of physicians.
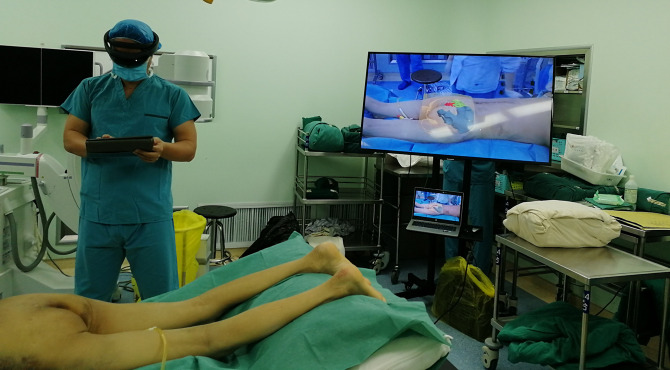
Group A procedure was as follows: the surgeon wore HoloLens II (Microsoft, USA) glasses connected to the MR system. Under general anesthesia, the patient was in a prone position on the operating table (Figure 6). The lesion segments were clearly identified by HoloLens II glasses using the standard posterior approach. The lesion space, upper and lower lamina, and facet joints were fully exposed. Under the guidance of the MR navigation system, the simulated positioning point and puncture angle could be seen. The physician performed positioning and threading, and then a C-arm fluoroscopy was used to check the tapping position, and successively place the bilateral pedicle screws. A C-arm fluoroscopy was used to examine the screw placement and found no medial pedicle fracture. The vertebral plate was opened to expose the compressed nerve root, the prominent nucleus pulposus was removed, and the compressed nerve root was fully loosened. The upper and lower cartilaginous plates of the affected vertebra were scraped off one by one with a hob until the bony endplate. According to the MR navigation system, an appropriate artificial cage was placed at a suitable angle and intervertebral bone grafting was performed. A C-arm fluoroscopy was used to confirm that the cage was in a suitable position. The pedicle screw was pressurized, the tail was broken, and transverse fixation was used. After a C-arm fluoroscopy was used to finally check whether there was anything abnormal, hemostasis and washing were performed, and a negative pressure drainage tube was placed and sutured layer by layer.
Figure 6.
The preoperative 3-dimensional reconstructed image was presented in the surgeon’s glasses HoloLens II intraoperatively, which accurately imaged the corresponding part of the body.
Group B procedure was as follows: the surgeon followed a routine regimen. Under general anesthesia, the patient was in a prone position on the operating table. A C-arm fluoroscopy was used to locate the affected vertebra and fully expose the lesion space, upper and lower lamina, and facet joints. Bone-biting forceps were used to remove the growths. Positioning and threading were performed according to the experience of physician after positioning according to preoperative imaging data and confirmed by C-arm fluoroscopy, then bilateral pedicle screws were placed successively after the tapping position was checked by C-arm fluoroscopy. Screw placement was examined by C-arm fluoroscopy, and no medial pedicle fracture was found. The vertebral plate was opened to expose the compressed nerve root, the prominent nucleus pulposus was removed, and the compressed nerve root was fully loosened. The upper and lower cartilaginous plates of the affected vertebra were scraped off one by one with a hob until the bony endplate. An appropriate cage was implanted, and intervertebral bone grafting was performed according to the experience of the physician, and a C-arm fluoroscopy was used to confirm that the cage was in a suitable position. The next steps in the procedure were the same as group A.
The 2 groups used the same pedicle screw system and fusion device (Shandong Weigao Medical Equipment Co., Ltd.). Patients in both groups were given routine prophylactic antibiotics and anticoagulant drugs, the drainage tube was removed 24–48 hours after the operation, and the waist brace was worn to the ground 24 hours after the removal of the drainage tube. The same rehabilitation plan was adopted after the operation.
Efficacy evaluation
Intraoperative time, blood loss, success rate of first tapping and number of C-arm fluoroscopy irradiation were recorded. After extubation, the anterior and lateral spine x-ray and CT were taken. Total postoperative drainage volume was recorded. The ODI score of lower back pain and VAS score of lower limbs of the affected side were collected at 1 day, 1 week, 1 month, 3 months, and 6 months after the operation. Grade I is considered excellent according to the standard of Gertzbein-Robbins (grade I, self-contained complete within the pedicle nail; grade II, self-contained nail through 2 mm below the cortex; grade III, self-contained nail through 2 mm or greater, cortex <4 mm; nail through 4 mm or higher cortex; grade IV, self-contained, <6 mm; grade V, self-contained nail through 6 mm or higher cortex) [14]. The precision of screw implantation was evaluated by the excellent and good rate of screw placement in both groups.
Statistical treatment
SPSS 20.0 statistical software was used for statistical analysis. Comparison between the 2 groups was performed using the t-test of comparison of the mean of 2 samples. The enumeration data were evaluated by Fisher’s exact survey test or chi-2 test. The rank sum test was used for paired design difference and grade data. Test level alpha was set at 0.05 on both sides.
Results
All patients underwent surgery successfully, and no spinal cord, nerve, or blood vessel injuries were observed. There were no pulmonary embolisms, incision infections, nerve injuries, or other complications.
Compared with group B, group A had less bleeding, shorter operation time, higher success rate of first threading, and fewer intraoperative C-arm x-ray irradiation Table 2), with statistically significant differences (P<0.05).
Table 2.
Comparison of intraoperative conditions.
| Intraoperative blood loss (mL) | Operation time (min) | Success rate of tapping first penetration (%) | Number of intraoperative C-arm fluoroscopy irradiation (times) | Total postoperative drainage volume (mL) | |
|---|---|---|---|---|---|
| Group A | 382.27±95.75 | 96.00±11.93 | 95.07 | 5.76±0.83 | 104.09±12.95 |
| Group B | 449.76±91.69 | 120.09±13.14 | 88.40 | 6.60±1.29 | 125.24±11.08 |
| χ2/t value | 2.5454 | 6.7868 | 4.1230 | 2.7359 | 6.2018 |
| P values | 0.0142 | <0.001 | 0.0423 | 0.009155 | <0.001 |
The efficacy of the 2 groups was significant. One month after surgery, the ODI score of lumbago and VAS score of lower limbs were significantly lower than those before surgery (Table 3), and the differences were statistically significant (P<0.05). Postoperative drainage volume in the group A was lower than that in group B, the difference was statistically significant (P<0.05) (Table 2).
Table 3.
Comparison of efficacy.
| Low back pain ODI score | VAS scores of lower limbs on the affected side | |||
|---|---|---|---|---|
| Group A | Group B | Group A | Group B | |
| Preoperative | 50.35±9.27 | 51.75±9.18 | 7.16±1.40 | 7.12±1.26 |
| One month after surgery | 34.44±8.50 | 43.03±9.30 | 3.48±1.00 | 3.88±1.20 |
| T value | 6.3230 | 3.4243 | 10.0558 | 9.2710 |
| P values | <0.001 | 0.0013 | <0.001 | <0.001 |
ODI – Oswestry disability index; VAS – visual analog scale.
The screw placement accuracy in group A was higher than that in the t-psi group according to the Gertzbein-Robbins criteria, the difference was statistically significant (P<0.05) (Table 4). Low back pain recovery was faster in group A than in group B postoperative, with statistically significant difference (P<0.05). By 6 months after surgery, ODI scores of the 2 groups were not statistically significant (P>0.05) (Table 5). Both groups had significant effect on nerve compression relief. VAS scores of lower limbs on the affected side showed no statistically significant difference (P>0.05) (Table 6).
Table 4.
Effect of screw placement.
| Total number of screws (PCS) | Classification criteria for Gertzbein-Robbins | Excellent and good rate (%) | ||
|---|---|---|---|---|
| Class I | Level II and above | |||
| Group A | 142 | 136 | 6 | 95.77 |
| Group B | 138 | 123 | 15 | 89.13 |
| χ2 value | 4.4534 | |||
| P values | 0.0348 | |||
χ2 value and P value are the comparison of excellent and good rates between the 2 groups.
Table 5.
Postoperative lumbago ODI score (%).
| 1 month after surgery | 3 months after surgery | 6 months after surgery | |
|---|---|---|---|
| Group A | 34.44±8.50 | 16.94±5.15 | 17.15±5.21 |
| Group B | 43.03±9.30 | 20.91±4.31 | 16.19±4.75 |
| T value | 3.4086 | 2.9506 | 0.68195 |
| P values | <0.001 | 0.004951 | 0.4986 |
Table 6.
VAS scores of lower limb on the affected side after operation.
| 1 month after surgery | 3 months after surgery | 6 months after surgery | |
|---|---|---|---|
| Group A | 3.48±1.00 | 1.92±0.49 | 1.24±0.83 |
| Group B | 3.88±1.20 | 2.08±0.86 | 1.12±0.66 |
| T value | 1.2769 | 0.80539 | 0.5636 |
| P values | 0.208 | 0.4256 | 0.5758 |
Discussion
The comparison of MR assisted lumbar pedicle screws placement and traditional operation shows that MR assisted technique improved the safety, timeliness, and accuracy of pedicle screw placement in spine surgery.
Degenerative diseases of the spine usually manifest as insidious, symptoms are not always present early and may progress due to factors such as trauma or excessive exercise. Lumbar discectomy pedicle screw internal fixation bone graft fusion is the gold standard for the treatment of lumbar degenerative diseases [15]. The key of pedicle screw placement technique lies in the position of screw entry point, the direction of screw entry and the proper selection of screw diameter and length. At present, the selection of screw diameter and length is not a major problem with the popularity of imaging examination, but the position of screw placement has always been a difficulty. In the event of screw placement failure, no more screws can be placed in this area, or the nerve roots below the pedicle can be damaged [16–22]. With the degeneration of the human spine, vertebral rotation and scoliosis are inevitable, which adds insult to injury for the difficult screw placement technique. This method inevitably causes a certain rate of misplacement. Due to the different operation modes of different doctors, the misplacement rate of the screw is greatly different [23,24]. Even though the probability of serious complications due to poor screw position is very low, the screw position deviation may cause some long-term complications due to the weakening of screw holding force [25,26]. Mixed reality technology changes the bottleneck of the difficulty of pedicle screw fixation, and it reproduces and reduces the lesion location more intuitively in front of doctors through the preoperative 3D CT reconstruction of patients.
During the operation, the principal surgeon and assistant surgeon wear HoloLens II glasses and obtain holographic 3D image information through star map, so that the virtual 3D digital model of the patient completely overlaps with the lesion site of the patient, enabling the doctor to have a completely new operation mode [27]. IN our study, short-term efficacy of MR-assisted surgery was obvious by comparison of ODI score of lumbago before and after surgery and VAS score of lower extremity of the affected side, which was helpful to accelerate postoperative recovery of patients. Intraoperative blood loss was significantly reduced compared with traditional methods, and postoperative drainage was also reduced, which could reduce postoperative complications. We believe that this may be related to the surgical trauma being smaller than that of traditional surgery which is a result of the fact that the surgeon has more anatomical information about the patient’s site before surgery and the target is clearer when the incision is exposed. The lower level of blood loss found in group A (Table 2), may be related to the shorter operation time and clearer incision size and location. Because of the clearer size and location of the incision, the scope of exposure was reduced, and soft tissue injury was reduced. In addition, shorter operative time can also reduce the amount of anesthesia used during surgery and the likelihood of postoperative infection. The effect of screw placement was significantly improved compared with the traditional method. In particular, some patients suffered from severe vertebral lesions, which were accompanied by mild scoliosis, rotation, osteophyte formation, etc., which increased the difficulty of operation and made it difficult to control the insertion point and direction. However, with the assistance of MR, virtual positioning can be carried out to provide the correct positioning point and needle direction for the physician, making screw placement accurate, safe, and easy to operate. Elmi-Terander et al. [28] showed that in the process of thoracolumbar pedicle screw implantation or vertebral body puncture, 3D image navigation technology is superior to intraoperative x-ray or fluoroscopy, with higher accuracy and security. In our study, the x-ray irradiation frequency of C-arm fluoroscopy in group A was less than that in group B. In the past, C-arm fluoroscopy has been used to determine the position of each intraoperative positioning and used several times, which can cause great harm to doctors. However, the use of MR navigation technology can significantly reduce the number of intraoperative uses of C-arm fluoroscopy, which can significantly reduce the harm to doctors. Some scholars have applied rapid prototyping guidance template technology to posterior spinal screw setting, which improves its accuracy and achieves good surgical results [29]. The safety, timeliness, and accuracy of screw placement were improved in this study, and good surgical effect was also achieved.
MR has many advantages, but it also has some limitations in auxiliary surgery. Firstly, errors will occur in the design and production process, affecting the accuracy of screw placement [30]. Secondly, individualized treatment programs require higher requirements for preoperative CT imaging data collected, and most hospital equipment cannot meet the requirements. Other limitations include surgical design, required preparation time and cost. In addition, this technology cannot be used for emergency surgery, and there is a delay phenomenon when using this technique. Moreover, due to the instability of the system itself, the rotation of the field of vision may occur easily after the change of posture of the physician, so the matching should be adjusted again. Besides, the HoloLens II glasses worn by doctors are heavy, which may cause great physical exertion and limited neck movement if worn for a long time. In addition, if there is an accident (such as excision of fracture ends caused by external displacement, pathological histomorphological changes of intraoperative traction, etc.) at the patient’s operative site after preoperative 3D imaging, the error between the actual situation and 3D imaging can also lead to an unsuccessful operation. Therefore, the operation cannot be completely carried out in accordance with the virtual surgical scheme, thus affecting surgical accuracy [31]. But these problems will be solved as the technology matures.
As an emerging technology, mixed reality (MR) technology, closely combined with clinical practice, marks an important development direction for modern medicine. Due to its obvious advantages, and ability to overcome shortcomings of traditional model surgery, MR technology shows a wide range of potential applications. The development of MR technology can better promote the growth of new, young spine surgeons if they can visually view the location of lesions from multiple angles during operation and conduct virtual operation by rebuilding the 3D structure of the spine [32,33]. At the same time, it is hoped that a tracker can be installed on the patient during the operation for real-time follow-up, so as to reduce the influence of pathological changes at the site of the operation [34]. The limitations of this study are the small number of cases and short follow-up time. Patients with lumbar disc herniation and lumbar spondylolisthesis were included in the study using the same technique. Although there was no statistical difference in the randomized grouping of patients with these 2 diseases, no statistical comparison was made in the early postoperative efficacy of these 2 diseases. Therefore, it remains to be further demonstrated whether the statistical difference in the early postoperative efficacy of the 2 groups would be affected. Prospective randomized controlled studies of this method and other screw placement methods are needed to collect large number of cases and statistically analyze the registration correlation between virtual and real screw placement, so as to further explain the superiority and promotion significance of this method. Finally, it is hoped that MR technology can solve the aforementioned problems and be better applied in clinical practice.
Conclusions
In clinical application, MR technology is an effective navigation and positioning method. Compared with the traditional operation methods, it improves the accuracy of pedicle screw implantation, shortens the operation time, and results in better clinical outcomes. We consider this technology as an appropriate application for spine surgery.
Acknowledgements
We thank all the participants that took part in this research for their time and help.
Footnotes
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of interest
None.
Source of support: This work was supported by National Natural Science Foundation of China (81771985), Key Research Program of Science and Technology of Jiangsu Province (BE2015613) and Nanjing Science and Technology Development Foundation (201805001)
References
- 1.Fuster S, Vega A, Barrios G, et al. Accuracy of pedicle screw insertion in the thoracolumbar spine using image-guided navigation. Neurocirugia (Astur) 2010;21(4):306–11. doi: 10.4321/s1130-14732010000400003. [DOI] [PubMed] [Google Scholar]
- 2.Van de Kelft E, Costa F, Van der Planken D, Schils F. A prospective multicenter registry on the accuracy of pedicle screw placement in the thoracic, lumbar, and sacral levels with the use of the O-arm imaging system and Stealth Station Navigation. Spine. 2012;37(25):E1580–87. doi: 10.1097/BRS.0b013e318271b1fa. [DOI] [PubMed] [Google Scholar]
- 3.Tian NF, Huang QS, Zhou P, et al. Pedicle screw insertion accuracy with different assisted methods: A systematic review and meta-analysis of comparative studies. Eur Spine J. 2011;20(6):846–59. doi: 10.1007/s00586-010-1577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinesh SK, Tiruchelvarayan R, Ng I. A prospective study on the use of intraoperative computed tomography (iCT) for image-guided placement of thoracic pedicle screws. Br J Neurosurg. 2012;26(6):838–44. doi: 10.3109/02688697.2012.690917. [DOI] [PubMed] [Google Scholar]
- 5.Bergeson RK, Schwend RM, DeLucia T, et al. How accurately do novice surgeons place thoracic pedicle screws with the free hand technique. Spine. 2008;33(15):501–7. doi: 10.1097/BRS.0b013e31817b61af. [DOI] [PubMed] [Google Scholar]
- 6.Smorgick Y, Millgram MA, Anekstein Y, et al. Accuracy and safety of thoracic pedicle screw placement in spinal deformities. Spinal Disord Tech. 2005;18(6):522–26. doi: 10.1097/01.bsd.0000154448.90707.a8. [DOI] [PubMed] [Google Scholar]
- 7.Drstvensek I, Hren IH, Strojnik T, et al. Applications of rapid prototyping in cranio-maxilofacial surgery procedures. Int J Biol Biomed Eng. 2008;(1):29–38. [Google Scholar]
- 8.Marengo N, Matsukawa K, Monticelli M, et al. Cortical bone trajectory screw placement accuracy with a patient-matched 3-dimensional printed guide in lumbar spinal surgery: A clinical study. Neurosurg. 2019;130:e98–e104. doi: 10.1016/j.wneu.2019.05.241. [DOI] [PubMed] [Google Scholar]
- 9.Vardiman AB, Wallace DJ, Crawford NR, et al. Pedicle screw accuracy in clinical utilization of minimally invasive navigated robot-assisted spine surgery. J Robot Surg. 2020;14(3):409–13. doi: 10.1007/s11701-019-00994-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keitaro M, Takashi K, Yuichiro A. Accuracy of cortical bone trajectory screw placement using patient-specific template guide system. Neurosurg Rev. 2019 doi: 10.1007/s10143-019-01140-1. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Sayari AJ, Pardo C, Basques BA, Colman MW. Review of robotic-assisted surgery: What the future looks like through a spine oncology lens. Ann Transl Med. 2019;7:224–34. doi: 10.21037/atm.2019.04.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richter M, Ploux D. Spinal navigation for posterior cervical and cervicothoracic instrumentation. Oper Orthop Traumatol. 2019;31(4):263–74. doi: 10.1007/s00064-019-0610-z. [DOI] [PubMed] [Google Scholar]
- 13.Melton LR. Perspective: How many women have osteoporosis now. J Bone Miner Res. 1995;10(2):175–77. doi: 10.1002/jbmr.5650100202. [DOI] [PubMed] [Google Scholar]
- 14.Gertzbein SD, Robbins SE. Accuracy of pedicular screw placement in vivo. Spine (Phila Pa 1976) 1990;15(1):11–14. doi: 10.1097/00007632-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Parker SL, McGirt MJ, Farber SH, et al. Accuracy of freehand pedicle screws in the thoracic and lumbar spine: Analysis of 6816 consecutive screws. Neurosurgery. 2011;68(1):170–78. doi: 10.1227/NEU.0b013e3181fdfaf4. [DOI] [PubMed] [Google Scholar]
- 16.Baluch DA, Patel AA, Lullo B, et al. Effect of physiological loads on cortical and traditional pedicle screw fixation. Spine (Phila Pa 1976) 2014;39(22):E1297–302. doi: 10.1097/BRS.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 17.Hojo Y, Ito M, Suda K, et al. A multicenter study on accuracy and complications of freehand placement of cervical pedicle screws under lateral fluoroscopy in different pathological conditions: CT based evaluation of more than 1 000 screws. Eur Spine J. 2014;23(10):2166–74. doi: 10.1007/s00586-014-3470-0. [DOI] [PubMed] [Google Scholar]
- 18.Esses SI, Sachs BL, Dreyzin V. Complications associated with the technique of pedicle screw fixation. A selected survey of ABS members. Spine (Phila Pa 1976) 1993;18(15):2231–38. doi: 10.1097/00007632-199311000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Matsukawa K, Yato Y, Kato T, et al. Cortical bone trajectory for lumbosacral fixation: Penetrating S-1 endplate screw technique: technical note. J Neurosurg Spine. 2014;21(2):203–9. doi: 10.3171/2014.3.SPINE13665. [DOI] [PubMed] [Google Scholar]
- 20.Calvert GC, Lawrence BD, Abtahi AM, et al. Cortical screws used to rescue failed lumbar pedicle screw construct: A biomechanical analysis. J Neurosurg Spine. 2015;22(2):166–72. doi: 10.3171/2014.10.SPINE14371. [DOI] [PubMed] [Google Scholar]
- 21.Marengo N, Ajello M, Pecoraro MF, et al. Cortical bone trajectory screws in posterior lumbar interbody fusion: Minimally invasive surgery for maximal muscle sparing – a prospective comparative study with the traditional open technique. Biomed Res Int. 2018;2018 doi: 10.1155/2018/7424568. 7424568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marengo N, Berjano P, Cofano F, et al. Cortical bone trajectory screws for circumferential arthrodesis in lumbar degenerative spine: Clinical and radiological outcomes of 101 cases. Eur Spine J. 2018;27(Suppl 2):213–21. doi: 10.1007/s00586-018-5599-8. [DOI] [PubMed] [Google Scholar]
- 23.Amiot LP, Lang K, Putzier M, et al. Comparative results between conventional and computer-assisted pedicle screw installation in the thoracic, lumbar, and sacral spine. Spine (Phila Pa 1976) 2000;25(5):606–14. doi: 10.1097/00007632-200003010-00012. [DOI] [PubMed] [Google Scholar]
- 24.Kosmopoulos V, Schizas C. Pedicle screw placement accuracy: A meta-analysis. (Phila Pa 1976) 2007;32(3):E111–20. doi: 10.1097/01.brs.0000254048.79024.8b. [DOI] [PubMed] [Google Scholar]
- 25.Putzier M, Strube P, Cecchinato R, et al. A new navigational tool for pedicle screw placement in patients with severe scoliosis: A pilot study to prove feasibility, accuracy, and identify operative challenges. Clin Spine Surg. 2017;30(4):E430–39. doi: 10.1097/BSD.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 26.Aichmair A, Moser M, Bauer MR, et al. Pull-out strength of patient-specific template-guided vs. free-hand fluoroscopically controlled thoracolumbar pedicle screws: A biomechanical analysis of a randomized cadaveric study. Eur Spine J. 2017;26(11):2865–72. doi: 10.1007/s00586-017-5025-7. [DOI] [PubMed] [Google Scholar]
- 27.Ghanai S, Marmulla R, Wiechnik J, et al. Computer-assisted three-dimensional surgical planning: 3D virtual articulator: technical note. Int J Oral Maxillofac Surg. 2010;39(1):75–82. doi: 10.1016/j.ijom.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Elmi-Terander A, Nachabe R, Skulason H, et al. Feasibility and accuracy of thoracolumbar minimally invasive pedicle screw placement with augmented reality navigation technology. Spine (Phila Pa 1976) 2018;43(14):1018–23. doi: 10.1097/BRS.0000000000002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goffin J, Van Brussel K, Martens K, et al. Three-dimensional computed tomography-based, personalized drill guide for posterior cervical stabilization at C1–C2. Spine. 2001;26(12):1343–47. doi: 10.1097/00007632-200106150-00017. [DOI] [PubMed] [Google Scholar]
- 30.Jain A, Hassanzadeh H, Strike SA, et al. Pelvic fixation in adult and pediatric spine surgery: historical perspective, indications, and techniques: AAOS exhibit selection. J Bone J Surg Am Vol. 2015;97(18):1521–28. doi: 10.2106/JBJS.O.00576. [DOI] [PubMed] [Google Scholar]
- 31.Naddeo F, Fontana C, Naddeo A, et al. Novel design for a customized, 3D-printed surgical template for thoracic spinal arthrodesis. Int J Med Robot. 2019;15(4):e2005. doi: 10.1002/rcs.2005. [DOI] [PubMed] [Google Scholar]
- 32.Luciano CJ, Banerjee PP, Bellotte B, et al. Learning retention of thoracic pedicle screw placement using a high-resolution augmented reality simulator with haptic feedback. Neurosurgery. 2011;69(1 Suppl Operative):14–19. doi: 10.1227/NEU.0b013e31821954ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaliya-Perumal AK, Soh T, et al. Spinal navigation during orthopedic residency training: A double-edged sword? Clin Orthop Surg. 2019;11(2):170–75. doi: 10.4055/cios.2019.11.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elmi-Terander A, Skulason H, Soderman M, et al. Surgical navigation technology based on augmented reality and integrated 3d intraoperative imaging: A spine cadaveric feasibility and accuracy study. Spine (Phila Pa 1976) 2016;41(21):E1303–11. doi: 10.1097/BRS.0000000000001830. [DOI] [PMC free article] [PubMed] [Google Scholar]