Abstract
Background
Growing evidence suggests that long non-coding RNAs (lncRNAs), as decoys of microRNAs (miRNAs), are involved in osteoarthritis (OA) progression, but the potential mechanism of lncRNA SNHG15 in OA remains unknown. Thus, the present study explored the molecular mechanism of SNHG15 in OA progression.
Material/Methods
OA chondrocytes were created by 20 ng/ml IL-1β stimulation, and the experimental OA model was created by destabilization of the medial meniscus (DMM) surgery. Cartilage histomorphology was observed by safranin and fast green double dyeing. The relationships between SNHG15 and miR-7, KLF4, and miR-7 were determined by dual-luciferase assay or RNA immunoprecipitation (RIP). Immunofluorescence was used to detect the expressions of Ki67, collagen II, and Aggrecan. Moreover, SNHG15, miR-7, KLF4, MMP3, ADAMTS5, COL2A1, Aggrecan, and β-catenin expressions were assessed by qRT-PCR or Western blot. The methylation status of SNHG15 promoter was evaluated by MS-PCR.
Results
Underexpression of KLF4 and SHNG15 and overexpression of miR-7 were found in human OA knee cartilage tissues and IL-1β-stimulated OA chondrocytes. SHNG15 overexpression significantly inhibited ECM degradation and promoted chondrocyte formation of OA chondrocytes. Furthermore, SNHG15 regulated KLF4 expression by sponging miR-7. Further analysis found that SNHG15 significantly inhibited β-catenin in OA chondrocytes. SNHG15 had a higher level of methylation in human OA tissues than in normal cartilage tissues.
Conclusions
Our results revealed that SNHG15 alleviated OA progression by regulating ECM homeostasis, which provides a promising target for OA therapy.
MeSH Keywords: beta Catenin, Chondrocytes, Extracellular Matrix, Osteoarthritis
Background
OA has become the most prevalent chronic degenerative joint disease in the elderly [1]. Patients with OA usually experience joint pain and deformities, and even chronic disability. As the pathogenesis of OA remains unknown, the current treatment is mainly to relieve symptoms [2]. Therefore, a deeper understanding of the molecular mechanisms of OA can help determine appropriate therapeutic targets for OA.
lncRNAs are non-coding RNA transcripts with over 200 nucleotides [3]. Growing findings suggest that lncRNAs can regulate various biological processes like cell proliferation, migration, and invasion [4]. It has been confirmed that dysregulated lncRNAs participate in OA progression. For example, lncRNA HOTAIR can promote OA progression by regulating the miR-17-5p/FUT2/β-catenin axis [5]. It has been recognized that inflammation and cartilage degeneration are 2 major mechanisms for OA [1]. Inflammation in the synovium and cartilage induces secretion of inflammatory factors, including IL-1β [6]. Furthermore, the secretion of inflammatory factors stimulates chondrocytes and negatively mediates cartilage maintenance and self-renewal [7]. OA is characterized by articular cartilage destruction that is induced by the imbalance of extracellular matrix (ECM) components. ECM components are mainly composed of collagen II and Aggrecan. MMP-13 and ADAMTS5 are responsible for ECM degradation. Therefore, inhibiting MMP-13 and ADAMTS5 expression and promoting collagen II and Aggrecan expression in cartilage are potential strategies for treating OA. Dysregulation of lncRNAs has been found to mediate the imbalance of ECM components in chondrocytes of OA. As an example, lncRNA-CIR knockdown can accelerate apoptosis of chondrocytes and induce cartilage deterioration [8]. Increasing evidence suggests that lncRNA SNHG15 can mediate many human cancers by sponging several miRNAs [9–11]. A recent study has reported that SNHG15 is downregulated in OA cartilage tissue and IL-1β-induced chondrocytes [12]. Furthermore, inhibition of SNHG15 can reduce chondrocyte apoptosis and ECM degradation and ameliorate articular cartilage damage, indicating that SNHG15 is involved in the progression of OA. However, the molecular mechanism of SNHG15 in OA is unknown.
KLF4 is a zinc finger transcription factor involved in various biological processes like cell differentiation and tissue formation [13,14]. KLF4 has been confirmed to be downregulated in human OA cartilage tissues [15]. Astragalus polysaccharide, a traditional Chinese medicine, can ameliorate LPS-induced chondrocyte damage via upregulation of KLF4 [16]. miRNAs, small non-coding RNAs with about 22 nucleotides in length, can bind with the 3′UTR of their target mRNAs, thereby regulating gene expression at the post-transcriptional level. Previous studies found that KLF4 expression is regulated by several miRNAs, such as miR-32-5p [17], miR-9-5p [18], and miR-92a [19], but the molecular mechanism of KLF4 in OA remains to be further clarified.
In the present study, the function of lncRNA SNHG15 in OA progression was explored. The results revealed that lncRNA SNHG15 can alleviate the progression of OA by modulating ECM homeostasis. Mechanistically, SNHG15 regulates the miR-7/KLF4/β-catenin axis and could become a novel target for OA.
Material and Methods
Bioinformatics analysis
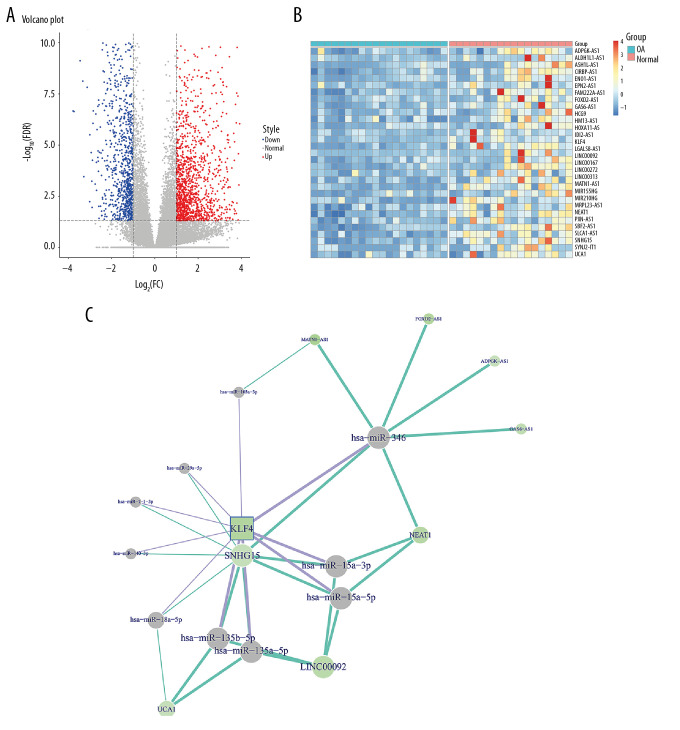
RNA-seq data were obtained from the Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo; accession number: GSE114007), including 8 normal and 20 human OA knee cartilage tissues [15]. Differential expression analyses were performed using the limma package in R [20]. mRNAs and lncRNAs with |log2FC| >1 and FDR<0.05 were considered as differentially expressed mRNAs or lncRNAs. Among them, KLF4 has been confirmed to be involved in the development of OA. We predicted 164 miRNAs targeting KLF4 using the ENCORI database (http://starbase.sysu.edu.cn/). Among them, 8 KLF4-binding miRNAs were verified in previous studies: hsa-miR-346 [21], hsa-miR-140-3p [22], hsa-miR-18a [23], hsa-miR-15a [24], hsa-miR-103a [25], hsa-miR-7 [26], hsa-miR-135 [27], and hsa-miR-29a [28]. Furthermore, targeted miRNAs of downregulated lncRNAs were predicted using the InCeDb database (http://gyanxet-beta.com/lncedb/). After integration of lncRNA-miRNA and KLF4-miRNA pairs, a ceRNA network related with KLF4 was visualized by use of Cytoscape 3.6.1. Co-expressed proteins of KLF4 were predicted using the STRING database (https://string-db.org/), followed by functional enrichment analysis.
Tissue specimens
OA cartilage tissues were harvested from 30 patients who underwent total knee arthroplasty at West China Hospital, Sichuan University from 2016 to 2018. Normal cartilage tissues were collected from 15 amputees without OA or rheumatoid arthritis. This study was approved by the Ethics Committee of West China Hospital, Sichuan University (HXYY20160026). All patients signed written informed consent.
Isolation and culture of chondrocytes
Synovial and fibrous tissues were removed from cartilage tissues under sterile conditions, followed by shearing to 1-mm3 slices. The slices were digested using 0.05% type II collagenase for 30 min, centrifuged at 1000 rpm, and oscillated for 60–100 min at 37°C using 0.1% type II collagenase and 0.25% pancreatin. After that, the slices were dissolved with DMEM medium containing 20% FBS. Finally, isolated chondrocytes were grown at 37°C and 5% CO2.
Cell transfection and IL-1β stimulation
pCDNA3.1-SNHG15 and vector were purchased from Shanghai Generay Biotech Co. (Shanghai, China). miR-7 mimics and inhibitor, SNHG15-shRNAs (sh-SNHG15) or KLF4-shRNAs (sh-KLF4) and corresponding scramble shRNAs (sh-NCs) were purchased from RiboBio (Guangzhou, China). Chondrocytes were transfected via Lipofectamine 3000 (Invitrogen) for 24 h. Finally, chondrocytes were stimulated with 20 ng/ml IL-1β for 24 h, as previously described [29].
OA animal model
All male C57BL/6J mice (10-week-old) from Hunan Slake Jingda Experimental Animal Co., China, were randomly divided into an experimental OA model group (n=10) and a sham operation group (n=30). All mice were housed at 23±2°C, 50±10% humidity, and a 12-h light: 12-h dark cycle. The experimental OA model was constructed by DMM surgery, as described previously [30]. For the DMM surgery group, mice were anesthetized with 1% pentobarbital (50mg/kg). Then, surgery was performed to induce OA by transecting the medial meniscotibial ligament on the right and left knees. For the sham operation group, the medial meniscotibial ligament was visualized but not transected. Three weeks after surgery, an intra-articular injection of 10 μl SNHG15 overexpression lentiviruses or miR-7 lentiviruses was performed on the operation group mice (5 per group) by a trans-patellar tendon. All mice were euthanized 8 weeks after surgery. Our study was approved by the Ethics Committee of West China Hospital, Sichuan University (HXYY20160026).
Immunofluorescence staining
We used 4% paraformaldehyde to fix IL-1β-stimulated chondrocytes for 20 min. After that, chondrocytes were blocked with 5% BSA for 1 h, followed by incubation with primary antibodies. Primary antibodies included anti-Ki67 (ab15580, Abcam), anti-COL2A1 (ab34712, Abcam), and anti-Aggrecan (13880-1-AP, ProteinTech). Nuclei were stained by DAPI (Solarbio).
Safranin-O and fast green staining and immunohistochemistry assay
After fixing with 4% paraformaldehyde, cartilage tissues were decalcified with 10% EDTA for 4 weeks and embedded into paraffin. The thickness of the sections was 5 μm. Dewaxing was performed with xylene. Afterwards, the sections were hydrated with a series of ethanol, followed by staining with hematoxylin for 1 min, 0.02% fast green for 5 min, and 0.1% Safranin-O for 30 min. The severity of OA articular cartilage degeneration was evaluated using the OARSI scores and modified Mankin scales [31,32]. For immunohistochemistry assay, paraffin-embedded tissue sections were dewaxed to water and incubated with primary antibodies including anti-COL2A1 (ab34712, Abcam) and anti-Aggrecan (13880-1-AP, ProteinTech) at 4°C overnight, followed by biotin-labeled secondary antibodies at 37°C for 15 min.
Luciferase reporter assay
Wild-type and mutated KLF4 or SNHG15 3′UTR luciferase vectors were created. Chondrocytes were transfected with vectors via Lipofectamine 3000 (Invitrogen). After 48 h, the dual-luciferase reporter assay kit (Promega) was used to assess the luciferase activity.
RIP assay
RIP lysis buffer (Solarbio) was used to lyse chondrocytes. Then, lysate was incubated with RIP buffer plus magnetic beads conjugated to human anti-Ago2 antibody (Millipore), followed by proteinase K. Finally, qRT-PCR was used to examine enriched RNA levels.
qRT-PCR
Treated chondrocytes (1000 cells/well) were seeded for this assay. RNA extraction was performed using TRIzol reagent (Invitrogen). We reverse transcribed 2 μg RNA into 40 ng cDNA. qRT-PCR was carried out using the miScript SYBR-Green PCR Kit (Qiagen). The primer sequences of target genes are listed in Table 1. U6 or β-actin was used as a control. The results were determined with the 2−ΔΔCT method.
Table 1.
The primer information for qRT-PCR assay.
| Gene | Name | Sequence |
|---|---|---|
| KLF4 | Forward | 5′-GTCCCGGGGATTTGTAGCTC-3′ |
| Reverse | 5′-TGTAGTGCTTTCTGGCTGGG-3′ | |
| Aggrecan | Forward | 5′-CAGACCATGACAACTCGCTG-3′ |
| Reverse | 5′-GCAGCACTACCTCCTTCTCC-3′ | |
| MMP3 | Forward | 5′-TGAGGACACCAGCATGAACC-3′ |
| Reverse | 5′-ACTTCGGGATGCCAGGAAAG-3′ | |
| COL2A1 | Forward | 5′-GCAGGATGGGCAGAGGTATAA-3′ |
| Reverse | 5′-CGAGGTCAGTTGGGCAGATG-3′ | |
| ADAMTS5 | Forward | 5′-CTCGGGAGGATTTATGTG-3′ |
| Reverse | 5′-ATGCTGGTAAGGATGGAA-3′ | |
| SNHG15 | Forward | 5′-CTAGTCATCCACCGCCATCC-3′ |
| Reverse | 5′-TTTGAGACCGTCACCTCAGC-3′ | |
| β-catenin | Forward | 5′-GCGCCATTTTAAGCCTCTCG-3′ |
| Reverse | 5′-GGCCATGTCCAACTCCATCA-3′ | |
| β-actin | Forward | 5′-AGGGGCCGGACTCGTCATACT-3′ |
| Reverse | 5′-GGCGGCACCACCATGTACCCT-3′ |
Western blot assay
Chondrocytes were lysed using RIPA lysis buffer. The BCA protein kit was used to measure the protein concentration. We isolated 20 μg proteins by 12% SDS-PAGE and transferred them onto PVDF membranes. The membranes were incubated with primary antibodies (including anti-MMP3, anti-ADAMTS5, anti-KLF4, anti-β-catenin, and anti-β-actin) overnight, followed by secondary antibodies. Finally, the blots were visualized with ECL.
MS-PCR
DNA was extracted from chondrocytes by QIAamp DNA Blood Mini Kit (Qiagen, 51104), and genomic DNA was processed by CpGenome DNA kit (Chemicon International, Inc., Temecula, CA). The methylation of SNHG15 promoter was carried out by two-step method [33]. SNHG15 methylation primers (M-F: GTTTTGGTTGGTAGATTTGTATTTC, M-R: ACTAATACCCCCACTCACTACGAC) and SNHG15 non-methylation primers (U-F: TAGTTTTGGTTGGTAGATTTGTATTTT; U-M: ACCACTACACTCCAACCTAAACAAC) were used for MS-PCR, with 1.5% agarose gel electrophoresis. The fragments of methylated and non-methylated MS-PCR products were 209 bp and 212 bp, respectively.
Statistical analysis
Data are expressed as the mean±standard deviation (SD), n ≥3. All data were analyzed using GraphPad Prism 7.0 (San Diego, CA, USA) or R language. The t test or ANOVA was used to evaluate statistical differences. P<0.05 was considered statistically significant.
Results
KLF4 is downregulated both in human OA knee cartilage tissues and IL-1β-induced OA chondrocytes
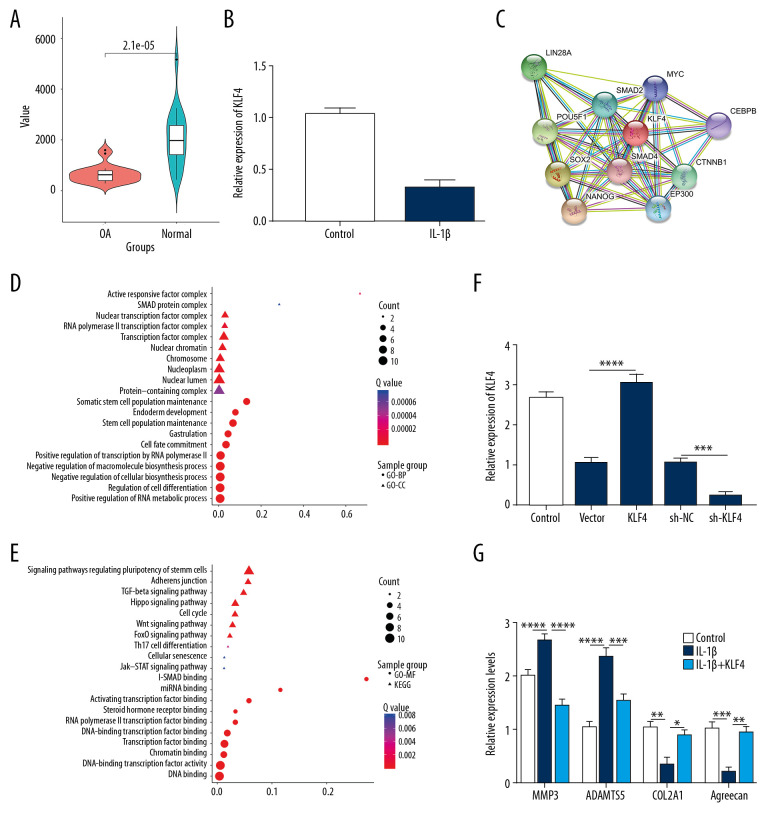
In the GSE114007 dataset, 2247 differentially expressed genes (936 downregulated and 1311 upregulated) were identified between human OA knee cartilage tissues and normal cartilage tissues (Figure 1A). The expression patterns of the top 30 downregulated lncRNAs and KLF4 between OA knee cartilage tissues and normal cartilage tissues are shown in Figure 1B. After integration of miRNAs targeting KLF4 and target miRNAs of downregulated lncRNAs, a ceRNA network was constructed for OA (Figure 1C). As shown in Figure 2A, KLF4 was significantly downregulated in OA. Consistent with bioinformatics results, its low expression was found in IL-1β-stimulated OA chondrocytes (Figure 2B). Using the STRING database, 10 co-expressed proteins of KLF4 were predicted, including LIN28A, POU5F1, SMAD2, MYC, SOX2, SMAD4, CEBPB, NANOG, EP300, and CTNNB1 (Figure 2C). Functional enrichment analysis results showed that these proteins were significantly associated with biological processes (BP) such as somatic stem cell population maintenance, endoderm development, and cell fate commitment (Figure 2D). Furthermore, these proteins were mainly enriched in several important cellular components (CC) like activin responsive factor complex, SMAD protein complex, and nuclear transcription factor complex (Figure 2D). As shown in Figure 2E, these proteins had the molecular function (MF) of miRNA binding, activating transcription factor binding, and DNA binding. KEGG enrichment analysis results showed that these proteins were significantly involved in the TGF-β signaling pathway, Hippo signaling pathway, cell cycle, and Wnt signaling pathway. According to the above analysis, KLF4 plays a key role in the progression of OA. To further explore the functions of KLF4 in OA progression, KLF4 was successfully overexpressed and silenced in chondrocytes (Figure 2F). IL-1β stimulation was used to induce OA phenotype in vitro. As shown in Figure 2G, after IL-1β stimulation, ECM degradation biomarkers including MMP3 and ADAMTS5 were significantly upregulated in chondrocytes, while chondrocyte formation biomarkers including COL2A1 and Aggrecan were significantly downregulated in chondrocytes. However, KLF4 overexpression significantly reversed changes in the above genes induced by IL-1β, indicating that KLF4 can alleviate ECM degradation and promote chondrocyte formation in OA.
Figure 1.
Construction of the ceRNA network of KLF4 for OA. (A) Volcano plot showing differentially expressed mRNAs between human OA and normal cartilage tissues in the GSE114007 dataset. (B) The expression patterns of the top 30 downregulated lncRNAs and KLF4. (C) The ceRNA network of KLF4 for OA.
Figure 2.
Low KLF4 expression in human OA knee cartilage tissues and IL-1β-stimulated OA chondrocytes. (A) Comparison of expression levels of KLF4 between human OA and normal cartilage tissues in GSE114007 dataset. (B) qRT-PCR showing KLF4 expression in IL-1β-stimulated chondrocytes. (C) Protein-protein interaction network of KLF4. (D, E) Functional enrichment analysis results of co-expressed proteins of KLF4. (F) qRT-PCR showing the transfection efficiency of KLF4 overexpression or knockdown in chondrocytes. (G) The expression levels of MMP3, ADAMTS5, COL2A1, and Aggrecan in IL-1β-stimulated chondrocytes transfected by KLF4 overexpression as shown by qRT-PCR. * p<0.05; ** p<0.01, *** p<0.001; **** p<0.0001.
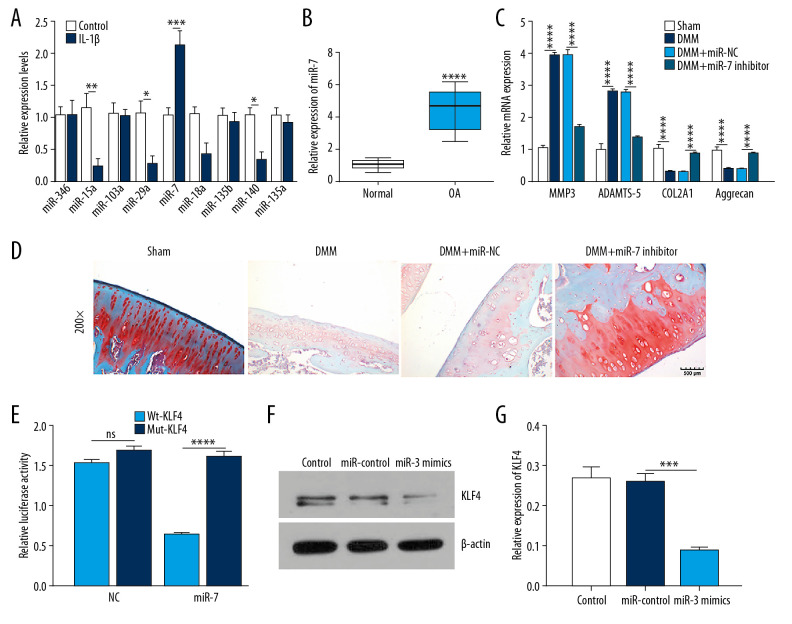
MiR-7 is upregulated in human OA knee cartilage tissues and IL-1β-stimulated OA chondrocytes and directly targets KLF4
Among all miRNAs targeting KLF4, miR-7 had the highest expression level in IL-1β-stimulated chondrocytes (Figure 3A). Similarly, miR-7 had a higher expression level in human OA cartilage tissues than normal cartilage tissues (Figure 3B). The experimental OA model was constructed by DMM surgery. qRT-PCR results showed that MMP3 and ADAMTS5 were upregulated while COL2A1 and Aggrecan were downregulated in OA models (Figure 3C). Three weeks after surgery, an intra-articular injection of miR-7 inhibitor was performed. We found that miR-7 inhibitor significantly reversed MMP3, ADAMTS5, COL2A1, and Aggrecan expression induced by DMM. Also, miR-7 inhibitor ameliorated the morphology changes in OA cartilage tissues (Figure 3D). As shown by luciferase activity assay, miR-7 directly targeted the 3′ UTR of KLF4-mut (Figure 3E). Moreover, miR-7 mimics suppressed KLF4 expression in chondrocytes (Figure 3F, 3G). Collectively, these findings demonstrated that miR-7 is upregulated in IL-1β-stimulated OA chondrocyte and human OA knee cartilage tissues, and KLF4 was directly targeted by miR-7.
Figure 3.
MiR-7 was upregulated in OA and directly targeted KLF4. (A) qRT-PCR showing miR-346, miR-15a, miR-103a, miR-29a, miR-7, miR-18a, miR-135b, miR-140, and miR-135a expression in IL-1β-stimulated chondrocytes. (B) MiR-7 expression in human OA and normal cartilage tissues as shown by qRT-PCR. (C) The expression levels of MMP3, ADAMTS5, COL2A1, and Aggrecan in OA model cartilage tissues injected with miR-7 inhibitor as shown by qRT-PCR. (D) Safranin-O and fast green staining showing the morphology changes of OA model cartilage tissues. Bar: 500 μm. (E) Luciferase activity assay revealed the target relationship between miR-7 and KLF4. (F, G) Western blot results showing KLF4 expression in OA chondrocytes transfected by miR-7 mimics. * p<0.05; ** p<0.01, *** p<0.001; **** p<0.0001.
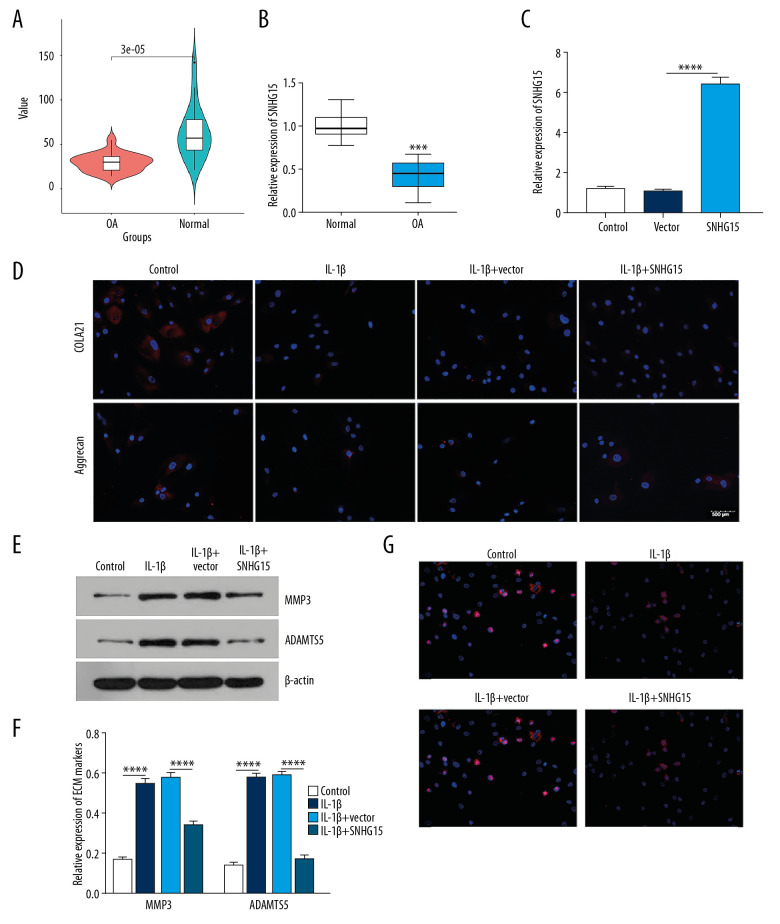
lncRNA SHNG15 is downregulated in human OA knee cartilage tissues and its overexpression alleviates ECM degradation and promotes chondrocyte formation of IL-1β-stimulated OA chondrocytes
lncRNA SHNG15 was downregulated in human OA cartilage tissues as shown by bioinformatics analysis (Figure 4A). Consistently, the results showed that SNHG15 had a lower expression in human OA cartilage tissues than in normal cartilage tissues (Figure 4B). We further assessed whether SNHG15 could affect ECM degradation and chondrocyte formation of OA chondrocytes. qRT-PCR results confirmed that SNHG15 was successfully overexpressed in chondrocytes (Figure 4C). As shown in immunofluorescence results, SNHG15 overexpression promoted the expression of COL2A1 and Aggrecan in OA chondrocytes after IL-1β stimulation (Figure 4D). Furthermore, Western blot results showed that overexpression of SNHG15 significantly inhibited the expression levels of MMP3 and ADAMTS5 in IL-1β-stimulated chondrocytes (Figure 4E, 4F), indicating that SNHG15 overexpression could inhibit ECM degradation of OA chondrocytes. Furthermore, SNHG15 overexpression significantly elevated Ki67 expression in IL-1β-stimulated chondrocytes, indicating that SNHG15 could promote OA chondrocyte proliferation (Figure 4G).
Figure 4.
SHNG15 was downregulated in OA knee cartilage tissues and its overexpression inhibited ECM degradation and promoted chondrocyte formation in IL-1β-induced chondrocytes. (A) SNHG15 expression in human OA and normal cartilage tissues using GSE114007. (B) qRT-PCR showing the expression of SNHG15 in human OA and normal cartilage tissues. (C) Transfection efficiency of SNHG15 overexpression vector in chondrocytes. (D) Immunofluorescence assay results showing the expression levels of COL2A1 and Aggrecan in IL-1β-stimulated chondrocytes transfected by SNHG15 overexpression. (E, F) Western blot assay showing MMP3 and ADAMTS5 expression in IL-1β-stimulated chondrocytes transfected by SNHG15 overexpression. (G) The Ki67 expression in IL-1β-stimulated chondrocytes transfected by SNHG15 overexpression using immunofluorescence assay. *** p<0.001, **** p<0.0001.
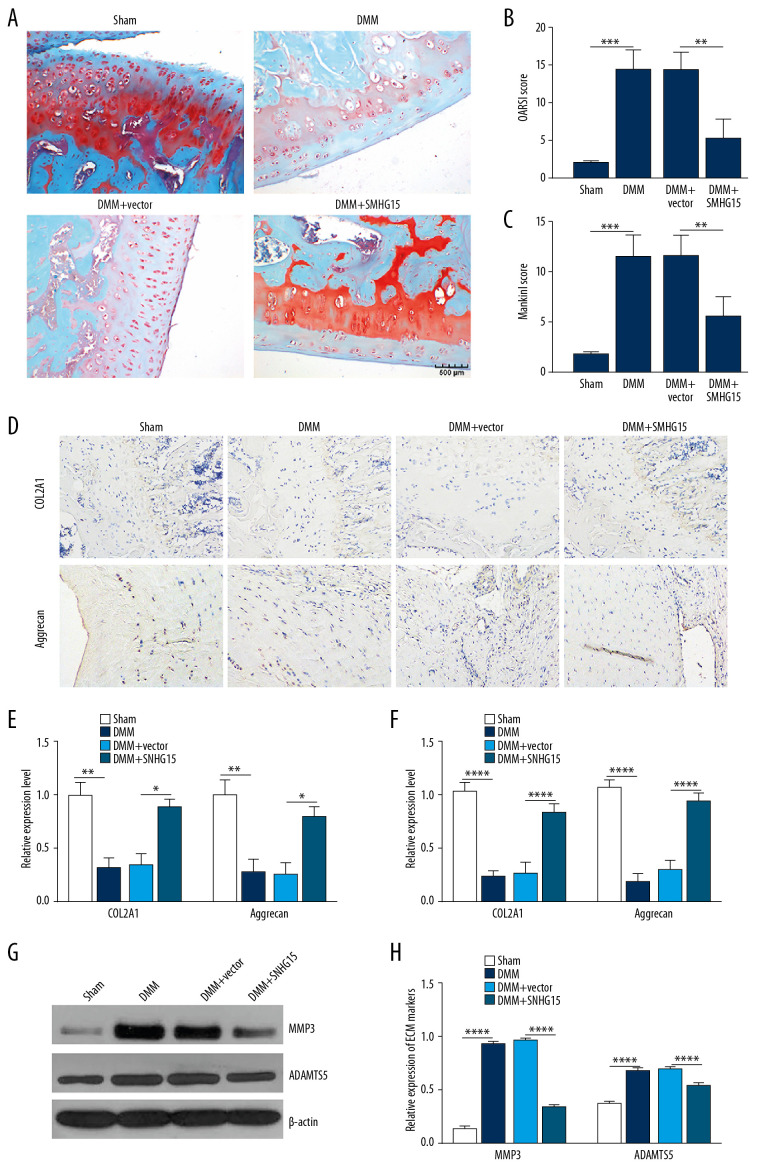
In the experimental OA model, SNHG15 overexpression alleviated the morphology changes in OA cartilage tissues, as shown by Safranine fixation green staining results (Figure 5A). According to the OARSI and modified Mankin scores, SNHG15 overexpression distinctly alleviated OA cartilage damage (Figure 5B, 5C). Immunohistochemistry results showed that SNHG15 overexpression remarkedly increased the expression of COL2A1 and Aggrecan in the OA model (Figure 5D, 5E). At the mRNA levels, SNHG15 overexpression significantly promoted cartilage formation by elevating the expression of COL2A1 and Aggrecan in the OA model (Figure 5F). Furthermore, SNHG15 overexpression significantly alleviated ECM degradation by decreasing the expression levels MMP3 and ADAMTS5 in the OA model at the protein level (Figure 5G, 5H). The above results suggest that SNHG15 overexpression alleviates ECM degradation and promotes chondrocyte formation of OA in vitro and in vivo.
Figure 5.
SNHG15 overexpression inhibited ECM degradation and promoted chondrocyte formation in an experimental OA model. (A) The morphology changes of cartilage tissues in the experimental OA model injected by SMHG15 overexpression using Safranin-O and fast green staining. (B, C) Cartilage destruction was assessed according to the OARSI and Mankin scores. (D) Representative images of immunohistochemistry of COL2A1 and Aggrecan in cartilage tissues of the experimental OA model injected by SMHG15 overexpression. Scale bar: 50 μm. Magnification: 200×. (E) Quantitative results of immunohistochemistry for COL2A1 and Aggrecan. (F) COL2A1 and Aggrecan expression in cartilage tissues of experimental OA model injected by SMHG15 overexpression using qRT-PCR. (G, H) MMP3 and ADAMTS5 expression in cartilage tissues of experimental OA model injected by SMHG15 overexpression using western blot. * p<0.05; ** p<0.01, *** p<0.001; **** p<0.0001.
SNHG15 indirectly regulates KLF4 expression by sponging miR-7
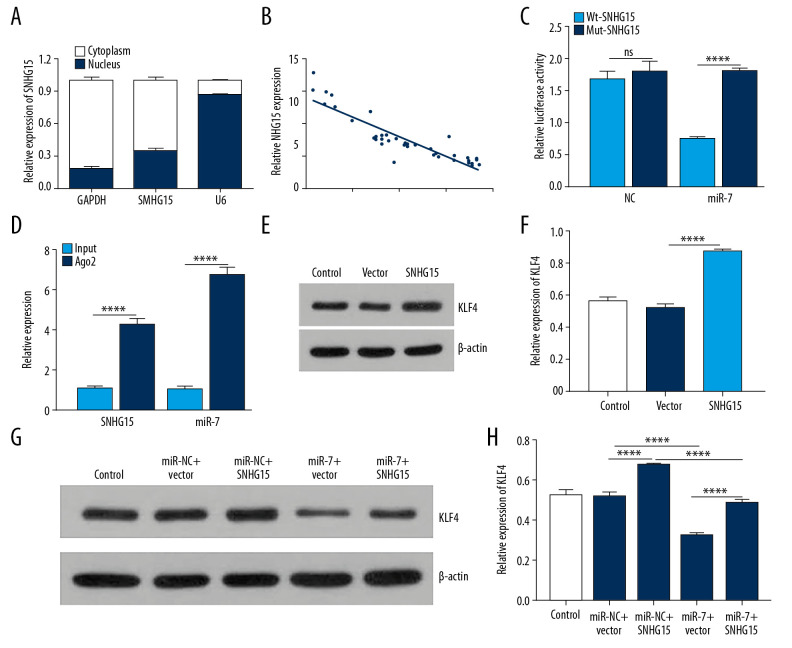
SNHG15 was mainly expressed in the cytoplasm (Figure 6A). Furthermore, its expression had a negative association with miR-7 expression (Figure 6B; p<0.0001, r=−0.8967). Dual-luciferase reporter confirmed that miR-7 was directly targeted by SNHG15 (Figure 6C). Furthermore, RIP assay results showed that SNHG15 and miR-7 were preferentially enriched in the Ago2 pellet. SNHG15 pull-down was mainly enriched in chondrocytes with miR-7 overexpression (Figure 6D). These findings revealed that miR-7 is a target of SNHG15.
Figure 6.
SNHG15 indirectly regulates KLF4 expression by miR-7. (A) The distribution of SNHG15 in subcellular fractions of chondrocytes was evaluated by qRT-PCR. U6 and GAPDH served as nuclear and cytoplasmic markers, respectively. (B) Correlation analysis results showed that SNHG15 was negatively correlated with miR-7. (C, D) Luciferase reporter assay and RIP confirmed that SNHG15 was a target of miR-7. (E, F) SNHG15 overexpression significantly promoted the expression levels of KLF4 in IL-1β-induced chondrocytes as shown by Western blot. (G, H) MiR-7 significantly reversed the elevated expression of SNHG15 on KLF4 expression in IL-1β-induced chondrocytes as shown by Western blot. **** p<0.0001.
In a previous study, KLF4 was confirmed to be a potential target of miR-7 [26]. In this study, we found that SNHG15 overexpression significantly promoted the expression levels of KLF4 in OA chondrocytes (Figure 6E, 6F; p<0.0001), which was reversed by miR-7 overexpression (Figure 6G, 6H). These results suggested that SNHG15 as a ceRNA of miR-7 regulates KLF4 expression.
SNHG15 inhibits β-catenin via miR-7/KLF4 in IL-1β-stimulated chondrocytes
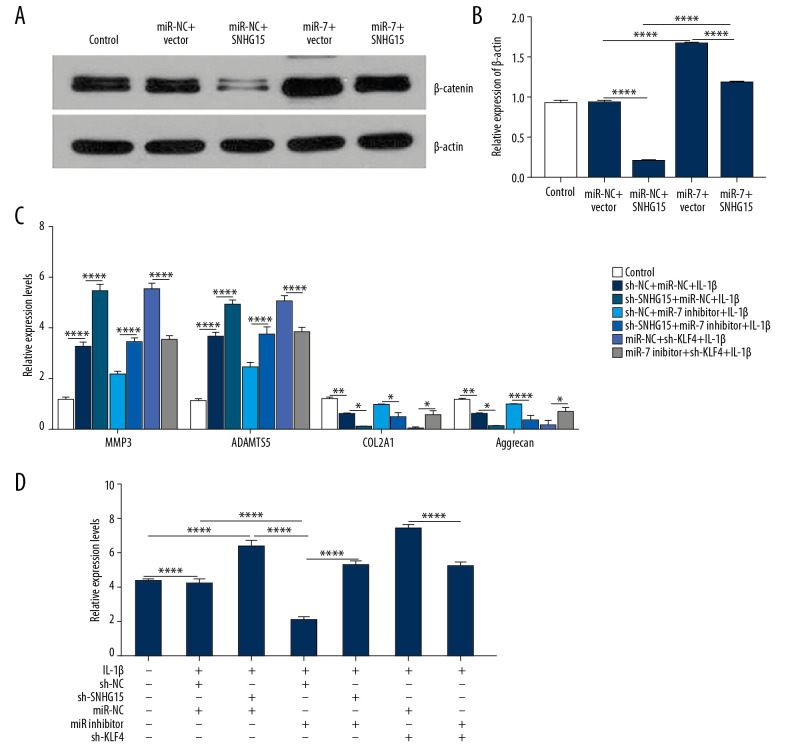
We further conducted rescue experiments to explore whether SNHG15 could inhibit β-catenin via targeting miR-7 in IL-1β-stimulated chondrocytes. Our results showed that overexpression of SNHG15 significantly inhibited β-catenin expression, which was reversed by miR-7 overexpression (Figure 7A, 7B). We also found that miR-7 inhibitor reversed the promotion effects of MMP3 and ADAMTS-5 and inhibitory effects of COL2A1 and Aggrecan induced by SNHG15 knockdown in OA chondrocytes (Figure 7C). Furthermore, KLF4 knockdown significantly suppressed the overexpression of MMP3 and ADAMTS-5 and elevated the downregulation of COL2A1 and Aggrecan induced by miR-7 inhibitor in OA chondrocytes (Figure 7C). Similarly, knockdown of SNHG15 promoted the expression levels of β-catenin, which was blocked by miR-7 inhibitor (Figure 7D). The above results reveal that SNHG15 inhibits β-catenin via miR-7/KLF4 in IL-1β-stimulated chondrocytes.
Figure 7.
SNHG15 inhibits β-catenin via miR-7/KLF4 in IL-1β-stimulated chondrocytes. (A, B) β-catenin expression was inhibited by SNHG15 overexpression in IL-1b-stimulated chondrocytes, which was reversed by miR-7 overexpression as shown by Western blot. (C) MiR-7 overexpression reversed the effects of SNHG15 and KLF4 on ECM degradation and chondrocyte formation in IL-1β-stimulated chondrocytes by qRT-PCR. (D) MiR-7 reversed the effects of SNHG15 and KLF4 on β-catenin expression in IL-1β-stimulated chondrocytes by qRT-PCR. * p<0.05, ** p<0.01, **** p<0.0001.
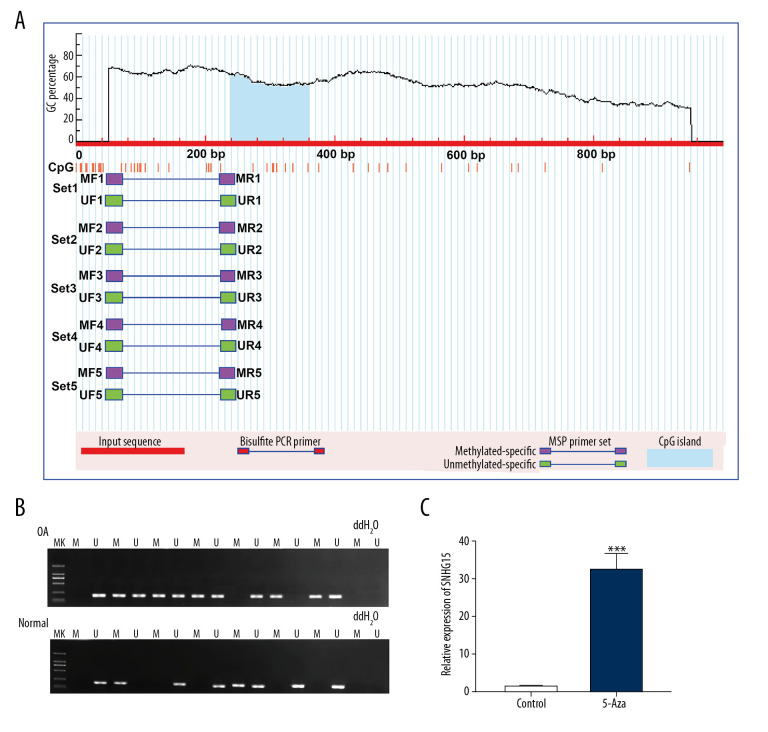
Hypermethylation of SNHG15 promoter region in OA cartilage tissues
The methylation level in the promoter region of SNHG15 was predicted. The results showed that there was a methylation site (Figure 8A) in the promoter region of SNHG15. Furthermore, we detected the methylation levels of SNHG15 in OA and normal cartilage tissues. In MSP results, SNHG15 had a higher level of methylation in OA compared to normal cartilage tissues (Figure 8B). Methylation inhibitor 5-Aza significantly promoted SNHG15 expression in OA chondrocytes (Figure 8C). The above results indicated that hypermethylation of SNHG15 promoter region could inhibit its expression in OA chondrocytes.
Figure 8.
Hypermethylation of SNHG15 promoter region in OA cartilage tissues. (A) Methylation sites of SHNG15 promoter region. (B) Methylation levels of SHNG15 promoter region in OA and normal cartilage tissues as shown by MSP assay; (C) qRT-PCR results showing SHNG15 expression in OA chondrocytes treated with 5-Aza. *** p<0.001.
Discussion
In our study, lncRNA SNHG15 was identified to be downregulated in OA cartilage tissues, which could regulate OA progression by ECM homeostasis. Furthermore, our findings showed SNHG15 could mediate miR-7/KLF4/β-catenin axis in OA, which could provide a novel insight into the pathogenesis of OA.
It has been confirmed that lncRNAs are involved in OA progression [34 35]. As an example, lncRNA CASC2 is upregulated in OA. Overexpression of CASC2 can inhibit chondrocyte proliferation and promote chondrocyte apoptosis by upregulation of IL-17 [36]. lncRNA FOXD2-AS1 overexpression promotes proliferation of chondrocytes via sponging miR-27a-3p in OA [37]. In this study, our findings showed that lncRNA SNHG15 was underexpressed in OA cartilage tissues and IL-1β-induced chondrocytes. Previous studies have found that SNHG15 participates in the progression of several cancers, including colorectal cancer [38], lung cancer [39,40], papillary thyroid carcinoma [10], and renal cell carcinoma [41]. The disruption of ECM homeostasis is a key event in the pathogenesis of OA due to the ability to degrade various ECM components [42]. In our study, we found that overexpression of SNHG15 alleviated ECM degradation and promoted chondrocyte formation. These results indicated that low SNHG15 expression was involved in OA progression.
Accumulating evidence suggests that lncRNA as a ceRNA regulates OA progression by sponging miRNAs [43, 44]. Previously, low HOTAIRM1-1 expression was found to inhibit MSC viability and differentiation via the miR-125b/BMPR2 axis [35]. Our findings confirmed that miR-7 was underexpressed in OA cartilage tissues and IL-1β-induced chondrocytes. KLF4 is a target of miR-7, which is consistent with our bioinformatics analysis results and previous studies [26,45]. Zhou confirmed that miR-7 is underexpressed in IL-1β-induced chondrocytes, and its underexpression inhibits inflammatory cytokine release and cell apoptosis [45]. Zhao found that KLF4 is a target of miR-7 in acute lung injury [46]. In this study, our findings suggested that miR-7 expression was negatively associated with SNHG15, and SNHG15 could directly target miR-7. Additionally, we found that overexpression of miR-7 reversed SNHG15-mediated KLF4 expression in OA chondrocytes. These findings demonstrated that SNHG15 as a ceRNA of miR-7 can inhibit OA progression, and can indirectly stimulate the expression of KLF4, which deepens our understanding of the molecular mechanisms of OA.
In this study, β-catenin was identified as a functional downstream effector of KLF4. Inhibition of β-catenin expression ameliorated OA in an experimental OA model [47,48]. β-catenin is mediated by many factors. For example, tyrosine kinase Fyn can induce OA by activation of β-catenin [49]. We found that KLF4 overexpression could suppress the expression of β-catenin. Our findings revealed that SNHG15/miR-7/KLF4 axis could regulate β-catenin expression in OA chondrocytes. We further explored the cause of the underexpression of SNHG15 in OA. We found that there was a methylation site in the SNHG15 promoter region. High methylation of SNHG15 inhibited its expression. Therefore, our study provides a novel insight into the mechanism of OA.
In this study, the lentiviral vector was used for the overexpression and knockdown of targeted genes. Although the application of lentiviral vectors has theoretical potential for altering the expression of target genes, the long-term safety and effectiveness of these vectors still require further observation. Moreover, other basic and clinical studies are still needed to improve transduction efficiency. In future work, we will continue to evaluate the potential of lentiviral vector in clinical applications for OA treatment.
Conclusions
Our results suggest that lncRNA SNHG15 inhibits the progression of OA by ECM homeostasis, and it regulates the miR-7/KLF4/β-catenin axis, which suggest it may be a novel target for OA therapy.
Abbreviations
- lncRNAs
long non-coding RNAs
- miRNAs
microRNAs
- OA
osteoarthritis
- SNHG15
small nucleolar RNA host gene 15
- DMM
destabilization of the medial meniscus
- ECM
extracellular matrix
- GEO
Gene Expression Omnibus
- RIP
RNA immunoprecipitation
- SD
standard deviation
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Chen D, Shen J, Zhao W, et al. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. doi: 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Z, Li J, Ruan G, et al. Investigational drugs for the treatment of osteoarthritis, an update on recent developments. Expert Opin Investig Drugs. 2018;27:881–900. doi: 10.1080/13543784.2018.1539075. [DOI] [PubMed] [Google Scholar]
- 3.Cen X, Huang XQ, Sun WT, et al. Long noncoding RNAs: A new regulatory code in osteoarthritis. Am J Transl Res. 2017;9:4747–55. [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Chang Y, Lu S, Xiang YY. Downregulation of long noncoding RNA DGCR5 contributes to the proliferation, migration, and invasion of cervical cancer by activating Wnt signaling pathway. J Cell Physiol. 2019;234:11662–69. doi: 10.1002/jcp.27825. [DOI] [PubMed] [Google Scholar]
- 5.Hu J, Wang Z, Shan Y, et al. Long non-coding RNA HOTAIR promotes osteoarthritis progression via miR-17-5p/FUT2/beta-catenin axis. Cell Death Dis. 2018;9:711. doi: 10.1038/s41419-018-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Bosch MHJ. Inflammation in osteoarthritis: Is it time to dampen the alarm(in) in this debilitating disease? Clin Exp Immunol. 2019;195:153–66. doi: 10.1111/cei.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dancevic CM, McCulloch DR. Current and emerging therapeutic strategies for preventing inflammation and aggrecanase-mediated cartilage destruction in arthritis. Arthritis Res Ther. 2014;16:429. doi: 10.1186/s13075-014-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Q, Zhang X, Dai L, et al. Long noncoding RNA related to cartilage injury promotes chondrocyte extracellular matrix degradation in osteoarthritis. Arthritis Rheumatol. 2014;66:969–78. doi: 10.1002/art.38309. [DOI] [PubMed] [Google Scholar]
- 9.Li M, Bian Z, Jin G, et al. LncRNA-SNHG15 enhances cell proliferation in colorectal cancer by inhibiting miR-338-3p. Cancer Med. 2019;8:2404–13. doi: 10.1002/cam4.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu DM, Wang S, Wen X, et al. LncRNA SNHG15 acts as a ceRNA to regulate YAP1-Hippo signaling pathway by sponging miR-200a-3p in papillary thyroid carcinoma. Cell Death Dis. 2018;9:947. doi: 10.1038/s41419-018-0975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye J, Tan L, Fu Y, et al. LncRNA SNHG15 promotes hepatocellular carcinoma progression by sponging miR-141-3p. J Cell Biochem. 2019;120:19775–83. doi: 10.1002/jcb.29283. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Huang CR, Pan S, et al. Long non-coding RNA SNHG15 is a competing endogenous RNA of miR-141-3p that prevents osteoarthritis progression by upregulating BCL2L13 expression. Int Immunopharmacol. 2020;83:106425. doi: 10.1016/j.intimp.2020.106425. [DOI] [PubMed] [Google Scholar]
- 13.Chang SF, Huang KC, Chang HI, et al. 2 dyn/cm(2) shear force upregulates kruppel-like factor 4 expression in human chondrocytes to inhibit the interleukin-1beta-activated nuclear factor-kappaB. J Cell Physiol. 2018;234:958–68. doi: 10.1002/jcp.26924. [DOI] [PubMed] [Google Scholar]
- 14.Chen YJ, Huang SM, Tai MC, et al. Glucosamine impedes transforming growth factor beta1-mediated corneal fibroblast differentiation by targeting Kruppel-like factor 4. J Biomed Sci. 2019;26:72. doi: 10.1186/s12929-019-0566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisch KM, Gamini R, Alvarez-Garcia O, et al. Identification of transcription factors responsible for dysregulated networks in human osteoarthritis cartilage by global gene expression analysis. Osteoarthritis Cartilage. 2018;26:1531–38. doi: 10.1016/j.joca.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan L, Li M, Cao FY, et al. Astragalus polysaccharide ameliorates lipopolysaccharide-induced cell injury in ATDC5 cells via miR-92a/KLF4 mediation. Biomed Pharmacother. 2019;118:109180. doi: 10.1016/j.biopha.2019.109180. [DOI] [PubMed] [Google Scholar]
- 17.Sun J, Hu J, Wang G, et al. LncRNA TUG1 promoted KIAA1199 expression via miR-600 to accelerate cell metastasis and epithelial-mesenchymal transition in colorectal cancer. J Exp Clin Cancer Res. 2018;37:106. doi: 10.1186/s13046-018-0771-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Dong X, Wang F, Xue Y, et al. MicroRNA95p downregulates Klf4 and influences the progression of hepatocellular carcinoma via the AKT signaling pathway. Int J Mol Med. 2019;43:1417–29. doi: 10.3892/ijmm.2019.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu PJ, Ye YX, Wang YX, et al. MiRNA-92a promotes cell proliferation and invasion through binding to KLF4 in glioma. Eur Rev Med Pharmacol Sci. 2019;23:6612–20. doi: 10.26355/eurrev_201908_18550. [DOI] [PubMed] [Google Scholar]
- 20.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao X, Wu X, Shi W. MicroRNA-346 regulates neural stem cell proliferation and differentiation by targeting KLF4. Am J Transl Res. 2017;9:5400–10. [PMC free article] [PubMed] [Google Scholar]
- 22.Kenyon JD, Sergeeva O, Somoza RA, et al. Analysis of -5p and -3p Strands of miR-145 and miR-140 during mesenchymal stem cell chondrogenic differentiation. Tissue Eng Part A. 2019;25:80–90. doi: 10.1089/ten.tea.2017.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Cai X, Liu E, et al. MicroRNA-18a promotes proliferation and metastasis in hepatocellular carcinoma via targeting KLF4. Oncotarget. 2017;8:68263–69. doi: 10.18632/oncotarget.19293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kikkawa Y, Ogura T, Nakajima H, et al. Altered expression of microRNA-15a and Kruppel-Like factor 4 in cerebrospinal fluid and plasma after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2017;108:909–16.e3. doi: 10.1016/j.wneu.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Zheng J, Liu Y, Qiao Y, et al. miR-103 promotes proliferation and metastasis by targeting KLF4 in gastric cancer. Int J Mol Sci. 2017;18:910. doi: 10.3390/ijms18050910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu W, Liu S, Liang Y, et al. MiR-7 inhibits progression of hepatocarcinoma by targeting KLF-4 and promises a novel diagnostic biomarker. Cancer Cell Int. 2017;17:31. doi: 10.1186/s12935-017-0386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin L, He Y, Xi BL, et al. MiR-135a suppresses calcification in senescent VSMCs by regulating KLF4/STAT3 pathway. Curr Vasc Pharmacol. 2016;14:211–18. doi: 10.2174/1570161113666150722151817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Shi J, Chen X. Identification of novel targets for seasonal allergic rhinitis during and outside the pollen season by microarray analysis. Acta Otolaryngol. 2015;135:1330–36. doi: 10.3109/00016489.2015.1067906. [DOI] [PubMed] [Google Scholar]
- 29.Lee SA, Moon SM, Han SH, et al. Chondroprotective effects of aqueous extract of Anthriscus sylvestris leaves on osteoarthritis in vitro and in vivo through MAPKs and NF-kappaB signaling inhibition. Biomed Pharmacother. 2018;103:1202–11. doi: 10.1016/j.biopha.2018.04.183. [DOI] [PubMed] [Google Scholar]
- 30.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–69. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Yamagishi K, Tsukamoto I, Nakamura F, et al. Activation of the renin-angiotensin system in mice aggravates mechanical loading-induced knee osteoarthritis. Eur J Histochem. 2018;62:2930. doi: 10.4081/ejh.2018.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Yu Y, Ye R, et al. An epigenetic biomarker combination of PCDH17 and POU4F2 detects bladder cancer accurately by methylation analyses of urine sediment DNA in Han Chinese. Oncotarget. 2016;7:2754–64. doi: 10.18632/oncotarget.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang LP, Ding JB, Liu ZH, Zhou GJ. LncRNA TUG1 promotes osteoarthritis-induced degradation of chondrocyte extracellular matrix via miR-195/MMP-13 axis. Eur Rev Med Pharmacol Sci. 2018;22:8574–81. doi: 10.26355/eurrev_201812_16620. [DOI] [PubMed] [Google Scholar]
- 35.Xiao Y, Yan X, Yang Y, Ma X. Downregulation of long noncoding RNA HOTAIRM1 variant 1 contributes to osteoarthritis via regulating miR-125b/BMPR2 axis and activating JNK/MAPK/ERK pathway. Biomed Pharmacother. 2019;109:1569–77. doi: 10.1016/j.biopha.2018.10.181. [DOI] [PubMed] [Google Scholar]
- 36.Huang T, Wang J, Zhou Y, et al. LncRNA CASC2 is up-regulated in osteoarthritis and participates in the regulation of IL-17 expression and chondrocyte proliferation and apoptosis. Biosci Rep. 2019;39 doi: 10.1042/BSR20182454. BSR20182454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Cao L, Wang Q, et al. LncRNA FOXD2-AS1 induces chondrocyte proliferation through sponging miR-27a-3p in osteoarthritis. Artif Cells Nanomed Biotechnol. 2019;47:1241–47. doi: 10.1080/21691401.2019.1596940. [DOI] [PubMed] [Google Scholar]
- 38.Huang L, Lin H, Kang L, et al. Aberrant expression of long noncoding RNA SNHG15 correlates with liver metastasis and poor survival in colorectal cancer. J Cell Physiol. 2019;234:7032–39. doi: 10.1002/jcp.27456. [DOI] [PubMed] [Google Scholar]
- 39.Cui HX, Zhang MY, Liu K, et al. LncRNA SNHG15 promotes proliferation and migration of lung cancer via targeting microRNA-211-3p. Eur Rev Med Pharmacol Sci. 2018;22:6838–44. doi: 10.26355/eurrev_201810_16152. [DOI] [PubMed] [Google Scholar]
- 40.Dong YZ, Meng XM, Li GS. Long non-coding RNA SNHG15 indicates poor prognosis of non-small cell lung cancer and promotes cell proliferation and invasion. Eur Rev Med Pharmacol Sci. 2018;22:2671–79. doi: 10.26355/eurrev_201805_14963. [DOI] [PubMed] [Google Scholar]
- 41.Du Y, Kong C, Zhu Y, et al. Knockdown of SNHG15 suppresses renal cell carcinoma proliferation and EMT by regulating the NF-kappaB signaling pathway. Int J Oncol. 2018;53:384–94. doi: 10.3892/ijo.2018.4395. [DOI] [PubMed] [Google Scholar]
- 42.Si HB, Zeng Y, Liu SY, et al. Intra-articular injection of microRNA-140 (miRNA-140) alleviates osteoarthritis (OA) progression by modulating extracellular matrix (ECM) homeostasis in rats. Osteoarthritis Cartilage. 2017;25:1698–707. doi: 10.1016/j.joca.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Sharp RC, Effiom OA, Dhingra A, et al. Enhanced basal autophagy supports ameloblastoma-derived cell survival and reactivation. Arch Oral Biol. 2019;98:61–67. doi: 10.1016/j.archoralbio.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu G, Wang Y, Zhang M, Zhang Q. Long non-coding RNA THRIL promotes LPS-induced inflammatory injury by down-regulating microRNA-125b in ATDC5 cells. Int Immunopharmacol. 2019;66:354–61. doi: 10.1016/j.intimp.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 45.Zhou X, Jiang L, Fan G, et al. Role of the ciRS-7/miR-7 axis in the regulation of proliferation, apoptosis and inflammation of chondrocytes induced by IL-1beta. Int Immunopharmacol. 2019;71:233–40. doi: 10.1016/j.intimp.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 46.Zhao J, Chen C, Guo M, et al. MicroRNA-7 Deficiency Ameliorates the Pathologies of Acute Lung Injury through Elevating KLF4. Front Immunol. 2016;7:389. doi: 10.3389/fimmu.2016.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouaziz W, Sigaux J, Modrowski D, et al. Interaction of HIF1alpha and beta-catenin inhibits matrix metalloproteinase 13 expression and prevents cartilage damage in mice. Proc Natl Acad Sci USA. 2016;113:5453–58. doi: 10.1073/pnas.1514854113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lietman C, Wu B, Lechner S, et al. Inhibition of Wnt/β-catenin signaling ameliorates osteoarthritis in a murine model of experimental osteoarthritis. JCI Insight. 2018;3:e96308. doi: 10.1172/jci.insight.96308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li K, Zhang Y, Zhang Y, et al. Tyrosine kinase Fyn promotes osteoarthritis by activating the beta-catenin pathway. Ann Rheum Dis. 2018;77:935–43. doi: 10.1136/annrheumdis-2017-212658. [DOI] [PubMed] [Google Scholar]