Abstract
Optical mapping has become a widely used and important method in cardiac electrophysiology. The method typically uses voltage-sensitive fluorescent dyes and high-speed cameras to image propagation of electrical waves. However, signals are highly susceptible to artifact caused by motion of the target organ. Consequently, cardiac optical mapping is traditionally performed in isolated, perfused organs whose contraction has been pharmacologically arrested. This has prevented optical mapping from being used to study interactions between electrical and mechanical motion. However, recently, a number of groups have developed methods to implement cardiac optical mapping in the presence of motion. These methods employ two basic strategies: (1) compensate for motion by measuring it or (2) ratiometry. In ratiometry, two signals are recorded from each site. The signals have differing sensitivity to membrane potential, but common motion artifact, which can be cancelled by taking the ratio of the two signals. Some methods use both of these strategies. Methods that measure motion have the additional advantage that this information can be used to quantify the organ’s mechanical function. Doing so enables combined “electromechanical mapping,” which allows optical study of electromechanical interactions. By allowing recording in the presence of motion, the new methods open the door to optical recording in in-vivo preparations. In addition, it is possible to implement electromechanical optical mapping techniques in organ systems other than the heart. For example, it was recently shown that optical mapping of slow wave propagation in the swine stomach is feasible. Such studies have the potential to uncover new information on the role of dysrhythmic slow wave propagation in gastric motility disorders.
Impact statement
Electrical and mechanical functions in the heart are bidirectionally coupled, yet are usually studied separately because of the different instrumentation technologies that are used in the two areas. Optical mapping is a powerful and widespread tool for imaging electrical propagation, but has traditionally required mechanical function to be arrested. Recently new methods have been devised that enable optical mapping to be performed in beating hearts and also to simultaneously quantify mechanical function. These new technologies promise to yield new information about electromechanical interactions in normal and pathological settings. They are also beginning to find application in other organ systems such as the gastrointestinal tract where they may provide new insight into motility disorders.
Keywords: Voltage sensitive dye, strain, electrophysiology, arrhythmias, gastroenterology, cardiac
Introduction
Electrical excitation and mechanical function are integral parts of the physiological activity of organs such as the heart, stomach, and intestine.1,2 Over the last century, technological innovations have enabled researchers to more completely describe the independent roles of electrophysiology and mechanical strain in normal and pathological physiology. However, electrical and mechanical functions are coupled bidirectionally. In the heart, electrical excitation regulates mechanical contraction (excitation–contraction coupling),1 and mechanical deformation can modulate electrical activity, potentially triggering ectopic electrical excitation (mechanosensitivity).3 Similar mechanisms operate in the gastrointestinal tract: the rhythmic slow wave regulates peristaltic contraction4 and the interstitial cells of Cajal respond to stretch.5
In recent decades, clinical observations, animal experiments, and computational models have suggested that cardiac mechanosensitivity may play a role in cardiac arrhythmia onset.6–9 Until recently, the experimental study of such mechanisms in intact organs was restricted by technical limitations. For example, optical mapping is a powerful method for imaging electrical wave propagation. However, because wall motion creates artifacts that obscure signals, contraction is typically abolished by electromechanical uncoupling agents such as blebbistatin.10 This practice eliminates the possibility of using optical mapping to investigate mechanical modulation of electrical activity. However, by taking advantage of solid state lighting, high speed imaging, and image processing techniques, optical mapping of electromechanics in intact organs is emerging as a tool for simultaneous, high resolution, quantification of electrical and mechanical activity.11–15 In this article, we will briefly review electromechanical optical mapping by first discussing optical mapping, then mechanical mapping techniques, and finally integrated electromechanical optical mapping.
Optical mapping
Optical mapping is a high-resolution tool for imaging physiological parameters such as membrane potential and intracellular calcium concentration. First developed in the late 1970s as a non-contact method of measuring membrane potential at higher resolution than practical with microelectrodes, the technique has since greatly influenced the fields of neuroscience and cardiac electrophysiology, driving greater understanding of cellular mechanisms and organ level physiology.16,17 Current methods typically utilize high-speed cameras to measure fluorescence emitted by reporters that transduce the parameter of interest.
Electrophysiology
Potentiometric dyes
We will focus on optical mapping of electrical propagation. Optical mapping usually utilizes fast response potentiometric probes to quantify variations in spatial and temporal membrane potentials across cells and tissue. First developed over 30 years ago, these dyes exhibit a shift in fluorescent spectra in response to a change in membrane potential via an electrochromic mechanism.18 Although the spectral shifts are slight, with appropriate choice of excitation and emission bands, voltage-dependent emission light intensity change is measurable and large enough for researchers to obtain signals proportional to membrane potential.18 It has also become possible to genetically encode potentiometric fluorescent proteins, a powerful technique emerging in some experimental settings.19
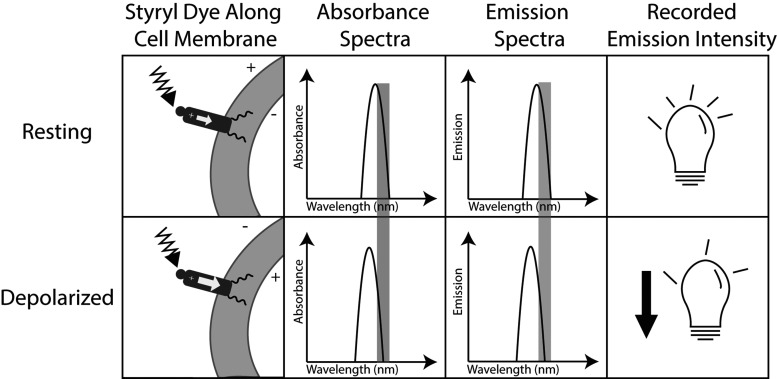
For optical mapping, the dyes have been designed to align parallel to the electric field across the cell membrane to maximize sensitivity to membrane potential. Dyes within the styryl family, specifically di-4-ANEPPS and di-8-ANEPPS, have emerged as popular voltage sensitive dyes, especially for blood free preparations. Styryl dyes are typically anchored in the cell membrane by hydrocarbon chains and oriented by a hydrophilic group that aligns the fluorophore perpendicular to the membrane interface and parallel to the electric field across the cell membrane.20 When the fluorophore is excited by a photon of excitation light, an electric charge moves across the molecule. Potentiometric shifts of the excitation and emission spectra result because the energy required to move this charge depends on the transmembrane potential.20 This family of dyes also tends to have a Stokes shift greater than 200 nm, helping improve the signal-to-noise ratio.20 Figure 1 illustrates how this type of dye transduces membrane potential. A limitation of di-4-ANEPPS and di-8-ANEPPS has been the excitation wavelength range of 450–550 nm, in which endogenous molecules, such as hemoglobin, interfere with excitation light absorption.21 Investigators have introduced variants of the styryl fluorophore with large Stokes shifts and excitation wavelengths greater than 700 nm.21–23 New dye development has enabled deeper tissue probing and optical mapping of blood-perfused preparations, including in vivo studies.24
Figure 1.
Schematic mechanism of dye voltage sensitivity. Column 1: fluorophores orient parallel to the membrane potential. When the fluorophore is excited by absorbing a photon, a positive charge moves across the molecule. This requires a higher energy photon when the cell is depolarized because the fluorophore’s excited state is no longer stabilized by the membrane potential. Column 2: because more energy is required to excite the fluorophore when cells are depolarized, the absorbance spectrum shifts to the left (shorter, more energetic wavelengths). The wavelength of excitation light is often chosen to lie on the right flank of the absorbance spectrum. Therefore, when the spectrum shifts, less excitation light is absorbed by the stained tissue. Column 3: the Stokes shift is fixed, so the emission spectrum also shifts to shorter wavelengths when cells are depolarized. The band of emission light that is recorded is also typically chosen to lie on the right flank of the emission spectrum. Column 4: because both absorbance and emission spectra shift “out from under” the respective excitation and emission bands, less light reaches the photodetector during depolarization. A fluorescence signal recorded from a single site will therefore have inverted action potentials.
Mitigating motion artifact
While optical mapping offers advantages relative to electrical mapping (an older technique in which extracellular potentials are recorded from arrays of electrodes), the technique is susceptible to motion-induced artifacts when recording from tissue that moves or deforms. In optical mapping’s simplest form, transmembrane potential is indirectly measured by observing the change in fluorescence relative to background fluorescence. This change is proportional to membrane potential. However, the background fluorescence varies spatially due to heterogeneities of effective dye concentration or excitation light intensity. Consequently, when tissue moves beneath a photodetector pixel, background fluorescence is not constant and simply subtracting background is insufficient to determine the potentiometric fluorescence change. Traditionally, this issue is avoided simply by arresting the movement of the heart. For this purpose, the electromechanical uncoupling agent blebbistatin is frequently used. It prevents the heart from contracting, but the electrophysiology of the heart is largely maintained.10 Other pharmacological agents, such as 2,3-butanedione monoxime, have also been used to arrest the heart’s movement, but are known to alter the electrophysiology.25 Although this method is useful for studying electrophysiology in isolation, the interaction between mechanical and electrical activity cannot be studied using this method. Furthermore, the use of electromechanical uncoupling agents precludes optical mapping in vivo unless complex methods such as cardiopulmonary bypass are used to support the animal.26
A few motion artifact mitigation techniques have been developed to study the beating heart. Image registration performs post-processing on images to correct for motion artifact. To track the heart’s deformation while beating, each frame of a video is transformed to match a selected reference frame. Rohde et al. employed affine transformation, a technique that uses linear transformations like translation, scaling, and rotation,27 while others have used a variety of elastic methods to transform the target image to match the reference.28 Ratiometry attempts to minimize the effects of motion artifact by taking the ratio of two signals acquired using either two excitation wavelengths (excitation ratiometry) or two emission wavelengths (emission ratiometry).29,30 Such schemes are designed so that the two signals have markedly different sensitivity to membrane potential. However, motion artifacts are common to both signals, so taking the ratio should preserve the voltage signal, while canceling out artifacts. In a typical excitation ratiometry design, the two bands of excitation light are on opposite flanks of the absorbance spectrum and are switched with every camera frame. A single emission band is recorded and the two signals from the two excitation bands are temporally interleaved. In emission ratiometry, there is one excitation band, but two emission wavelengths are recorded—often with two carefully aligned cameras. The emission bands are usually chosen to be on opposite flanks of the emission spectrum. Recently Bourgeois et al. combined image marker tracking with excitation ratiometry to not only eliminate motion artifact but also enable the simultaneous study of bidirectional coupling of electrical and mechanical functions of the heart.11 Zhang et al. later extended this method from tracking a limited number of discrete sites to mapping membrane potential as well as strain across a continuous region.15
Mechanics
Mechanical organ mapping has been studied in parallel to electrophysiological mapping to more deeply understand the differences in structure and dynamics between normal and abnormal hearts. Over the past century, advancement in high-speed imaging technologies permitted high spatiotemporal assessment of cardiac mechanical deformation. In the 1980s and 1990s, a series of animal experiments were carried out to quantify the mechanical deformation of the heart using bi-plane X-ray.31–33 The adoption of the concept of finite strain provided a powerful mathematical tool for quantifying myocardial deformation.32–34 Finite strain is a concept from continuum mechanics that precisely characterizes deformation in three dimensions. It requires knowledge of the motion of material points.35
Currently, ultrasound tissue-Doppler speckle tracking and tagged magnetic resonance imaging (MRI) are the most prevalent techniques for evaluating cardiac mechanical function. Tissue-Doppler speckle imaging tracks speckles, formed from ultrasound beam interference, as tissue material markers while the heart is deforming over time. Speckle displacement is used to determine strain.36 Tagged MRI tracks material markers created by specialized pulse-sequences and calculates strain in three-dimensions.37 While both methods have high spatial resolution, they are limited by temporal resolution of 10–35 ms.38 Recently, a variant of speckle tracking with higher temporal resolution has emerged. Electromechanical wave imaging, an ultrasound-based technique, divides the heart into segments that are imaged on consecutive heart beats and uses radio frequency speckle cross correlation to create strain maps with temporal resolution fine enough to track cardiac waves (∼2 ms), provided the rhythm is repeatable.39 Laughner et al. attempted to improve the spatial and temporal resolution of mechanical imaging using structured light imaging, a technique capable of 1 ms temporal resolution and spatial resolution of 87 µm.38 While structured light offers higher resolution than the other methods, it does not directly measure the motion of material points, and instead uses a geometric registration approach to approximate this information. In addition, the technique does not provide transmural information while tissue-Doppler speckle tracking and tagged-MRI can.
Electromechanics
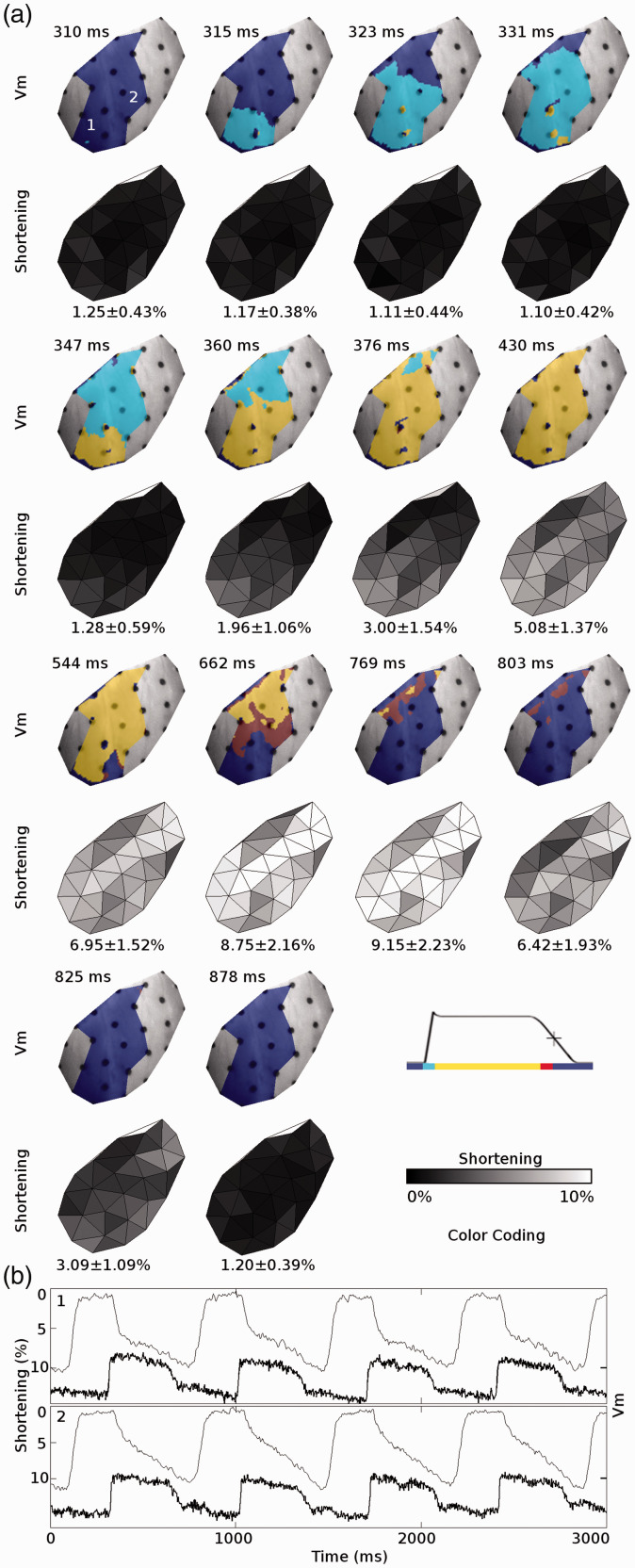
While the tools discussed above have been foundational for our knowledge of the heart as both an electrical and mechanical system, there is much to be learned about the effects of bidirectional electromechanical coupling in the heart during pathological states. In 1993, Evans et al. performed an early study to simultaneously image electrical and mechanical functions by combining biplane x-ray and a dense array of epicardial electrodes.13 More recently, developments in camera technology, motion tracking techniques, and ratiometry for motion artifact mitigation have inspired a plethora of new methods, several of which have incorporated motion tracking along with optical mapping.11,14,15,40 Seo et al. identified the relationship between strain heterogeneity and the initiation of focal excitation by applying known stretch to ventricular tissue preparations and whole hearts.14 They tracked deformation with fiducial markers and mapped electrophysiology with potentiometric dye and emission ratiometry.14 Bourgeois et al. built on this technique by developing a method in which membrane potential was optically acquired from tissue encircled by ring-shaped markers.11 At the same time, epicardial strain was measured by tracking the relative motion of the markers.11 This method used excitation ratiometry to suppress motion artifact and was implemented in whole isolated large animal hearts.11 Zhang et al. extended Bourgeois et al.’s methods to increase spatial resolution by two orders of magnitude.15 Markers were tracked with a binocular camera arrangement, which enabled strain to be computed without artifact caused by motion perpendicular to the camera plane. They used a subpixel marker tracking algorithm and interpolated deformation between markers to produce continuous, high resolution maps of strain and electrical propagation. The method corrects motion sufficiently to be used in vigorously beating working hearts and has sufficient spatiotemporal resolution to image electromechanical function during complex arrhythmias.15 Figure 2 shows an example of electromechanical mapping data acquired with this system. A key limitation of these methods is an inability to measure strain and membrane potential transmurally. In 2018, Christoph et al. utilized ultrasound speckle tracking to image strain transmurally in isolated swine hearts.41 At the same time, they optically mapped electrical propagation on the epicardial surface using a marker-free motion correction algorithm to suppress motion artifact. They studied reentrant waves during ventricular fibrillation and using the two modalities were able to correlate mechanical “vortex filaments” within the heart wall with electrical phase singularities on the epicardium. Phase singularities are the epicardial manifestation of the intersection of a vortex filament with the heart’s surface.41
Figure 2.
Electrical and mechanical functions mapped simultaneously in a beating ex-vivo pig heart using the method of Zhang et al.15 (a: upper rows) Propagation of membrane depolarization and repolarization for a single apically paced beat. (a: lower rows) The resulting contraction. The mapped region includes the anterior left ventricle. The left anterior descending coronary artery is along the top-left edge of each image and the apex is at the bottom. (b) Membrane potential (Vm, bold line) and mechanical shortening (fine line) recorded from the two sites indicated in (a). Shortening is defined as the most negative eigenvalue of the stretch tensor. Reproduced from Zhang et al.15 with permission.
Future directions
Electromechanical mapping of the intact heart has seen great advancements over the past decade. With innovations in motion artifact mitigation and the development of longer wavelength potentiometric dyes, researchers are initiating in vivo studies as a potential platform for studying neural and endocrine influences on the heart. Lee et al. performed one of the first in vivo optical mapping studies earlier this year.24 During ventricular fibrillation, when cardiac motion is not pronounced, they were able to image cardiac wavefronts using excitation ratiometry without any additional motion tracking. Future in-vivo studies could incorporate mechanical mapping as well, offering a more physiologically relevant model. Many investigators have implemented dual optical mapping of membrane potential and intracellular calcium concentration.42 It is possible in principle to combine this with mechanical measurements. We can also expect to see electromechanical mapping applied to other electromechanical organ systems such as the gastrointestinal tract. Zhang et al. optically mapped gastric slow wave propagation for the first time in 2019, setting the stage for a better understanding gastric motility disorders through optical mapping.43 In this study, they mapped in vivo swine stomach. Dye was delivered to a local region of the stomach wall through a cannula placed in the gastroepiploic artery. To record in the presence of stomach wall motion, which was not suppressed, they used a variation of Zhang et al.’s cardiac mapping method15 that employed both fiducial marker tracking and excitation ratiometry to minimize motion artifact. We expect further developments will be made to simultaneously map the electromechanics of in vivo models and in additional organ systems.
Author contributions
All authors contributed to writing and editing the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by NIH grants R21HL140998 (JMR) and R56EB027120 (JMR).
ORCID iDs
Hanyu Zhang https://orcid.org/0000-0003-1810-7677
Jack M Rogers https://orcid.org/0000-0002-3236-4154
References
- 1.Bers DM. Cardiac excitation–contraction coupling. Nature 2002; 415:198–205 [DOI] [PubMed] [Google Scholar]
- 2.Cheng LK, O'Grady G, Du P, Egbuji JU, Windsor JA, Pullan AJ. Gastrointestinal system. Wiley Interdiscip Rev Syst Biol Med 2010; 2:65–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohl P, Sachs F, Franz Mr, Kohl P. Cardiac mechano-electric coupling and arrhythmias. 2nd ed. Oxford/New York, NY: Oxford University Press, 2011, p.xxx, 477 p. [Google Scholar]
- 4.Sanders KM. Rhythmic electrical activity and regulation of gut motility. Eur Rev Med Pharmacol Sci 2008; 12:129–31 [PubMed] [Google Scholar]
- 5.Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci USA 2005; 102:14913–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coronel R, Wilms-Schopman FJG, deGroot JR. Origin of ischemia-induced phase 1b ventricular arrhythmias in pig hearts. J Am Coll Cardiol 2002; 39:166–76 [DOI] [PubMed] [Google Scholar]
- 7.Maron BJ, Estes NA., 3rd. Commotio cordis. N Engl J Med 2010; 362:917–27 [DOI] [PubMed] [Google Scholar]
- 8.Quinn TA, Jin H, Lee P, Kohl P. Mechanically induced ectopy via stretch-activated cation-nonselective channels is caused by local tissue deformation and results in ventricular fibrillation if triggered on the repolarization wave edge (commotio cordis). Circ Arrhythm Electrophysiol 2017; 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trayanova NA, Constantino J, Gurev V. Models of stretch-activated ventricular arrhythmias. J Electrocardiol 2010; 43:479–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedorov V, Lozinsky I, Sosunov E, Anyukhovsky E, Rosen M, Balke C, Efimov IR. Application of blebbistatin as an excitation–contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 2007; 4:619–26 [DOI] [PubMed] [Google Scholar]
- 11.Bourgeois EB, Bachtel AD, Huang J, Walcott GP, Rogers JM. Simultaneous optical mapping of transmembrane potential and wall motion in isolated, perfused whole hearts. J Biomed Opt 2011; 16:096020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christoph J, Schroder-Schetelig J, Luther S. Electromechanical optical mapping. Prog Biophys Mol Biol 2017; 130:150–69 [DOI] [PubMed] [Google Scholar]
- 13.Evans TZ, McCulloch AD, Waldman LK. Distributed activation and deformation in ventricular epicardium: effects of ventricular pacing. In: 15th annual international conference of the IEEE engineering in medicine and biology society, 1993, pp.885–86. October 31, 1993, San Diego, CA, USA
- 14.Seo K, Inagaki M, Nishimura S, Hidaka I, Sugimachi M, Hisada T, Sugiura S. Structural heterogeneity in the ventricular wall plays a significant role in the initiation of stretch-induced arrhythmias in perfused rabbit right ventricular tissues and whole heart preparations. Circ Res 2010; 106:176–84 [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Iijima K, Huang J, Walcott GP, Rogers JM. Optical mapping of membrane potential and epicardial deformation in beating hearts. Biophys J 2016; 111:438–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loew LM, Bonneville GW, Surow J. Charge shift optical probes of membrane potential. Theory. Biochemistry 1978; 17:4065–71 [DOI] [PubMed] [Google Scholar]
- 17.Salama G, Morad M. Merocyanine 540 as an optical probe of transmembrane electrical activity in the heart. Science 1976; 191:485–7 [DOI] [PubMed] [Google Scholar]
- 18.Loew LM. Potentiometric dyes: imaging electrical activity of cell membranes. Pure Appl Chem 1996; 68:1405–9 [Google Scholar]
- 19.Yang HH, St-Pierre F. Genetically encoded voltage indicators: opportunities and challenges. J Neurosci 2016; 36:9977–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loew LM. Design and use of organic voltage sensitive dyes. Adv Exp Med Biol 2015; 859:27–53 [DOI] [PubMed] [Google Scholar]
- 21.Matiukas A, Mitrea BG, Qin M, Pertsov AM, Shvedko AG, Warren MD, Zaitsev AV, Wuskell JP, Wei MD, Watras J, Loew LM. Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium. Heart Rhythm 2007; 4:1441–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan P, Acker CD, Zhou WL, Lee P, Bollensdorff C, Negrean A, Lotti J, Sacconi L, Antic SD, Kohl P, Mansvelder HD, Pavone FS, Loew LM. Palette of fluorinated voltage-sensitive hemicyanine dyes. Proc Natl Acad Sci USA 2012; 109:20443–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wuskell JP, Boudreau D, Wei MD, Jin L, Engl R, Chebolu R, Bullen A, Hoffacker KD, Kerimo J, Cohen LB, Zochowski MR, Loew LM. Synthesis, spectra, delivery and potentiometric responses of new styryl dyes with extended spectral ranges. J Neurosci Methods 2006; 151:200–15 [DOI] [PubMed] [Google Scholar]
- 24.Lee P, Quintanilla JG, Alfonso-Almazan JM, Galan-Arriola C, Yan P, Sanchez-Gonzalez J, Perez-Castellano N, Perez-Villacastin J, Ibanez B, Loew LM, Filgueiras-Rama D. In vivo ratiometric optical mapping enables high-resolution cardiac electrophysiology in pig models. Cardiovasc Res 2019; 115:1659–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kettlewell S, Walker NL, Cobbe SM, Burton FL, Smith GL. The electrophysiological and mechanical effects of 2,3-butane-dione monoxime and cytochalasin-D in the langendorff perfused rabbit heart. Exp Physiol 2004; 89:163–72 [DOI] [PubMed] [Google Scholar]
- 26.Lee P, Taghavi F, Yan P, Ewart P, Ashley EA, Loew LM, Kohl P, Bollensdorff C, Woods CE. In situ optical mapping of voltage and calcium in the heart. PLoS One 2012; 7:e42562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohde GK, Dawant BM, Lin SF. Correction of motion artifact in cardiac optical mapping using image registration. IEEE Trans Biomed Eng 2005; 52:338–41 [DOI] [PubMed] [Google Scholar]
- 28.Khwaounjoo P, Rutherford SL, Svrcek M, LeGrice IJ, Trew ML, Smaill BH. Image-based motion correction for optical mapping of cardiac electrical activity. Ann Biomed Eng 2015; 43:1235–46 [DOI] [PubMed] [Google Scholar]
- 29.Bachtel AD, Gray RA, Stohlman JB, Bourgeois EB, Pollard AE, Rogers JM. A novel approach to dual excitation ratiometric optical mapping of cardiac action potentials with DI-4-ANEPPS using pulsed LED excitation. IEEE Trans Biomed Eng 2011; 58:2120–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knisley SB, Justice RK, Kong W, Johnson PL. Ratiometry of transmembrane voltage-sensitive fluorescent dye emission in hearts. Am J Physiol Heart Circ Physiol 2000; 279:H1421–33 [DOI] [PubMed] [Google Scholar]
- 31.Hashima AR, Young AA, McCulloch AD, Waldman LK. Nonhomogeneous analysis of epicardial strain distributions during acute myocardial ischemia in the dog. J Biomech 1993; 26:19–35 [DOI] [PubMed] [Google Scholar]
- 32.McCulloch AD, Smaill BH, Hunter PJ. Left ventricular epicardial deformation in isolated arrested dog heart. Am J Physiol 1987; 252:H233–41 [DOI] [PubMed] [Google Scholar]
- 33.Waldman LK, Fung YC, Covell JW. Transmural myocardial deformation in the canine left ventricle. Normal in vivo three-dimensional finite strains. Circ Res 1985; 57:152–63 [DOI] [PubMed] [Google Scholar]
- 34.Villarreal FJ, Waldman LK, Lew WY. Technique for measuring regional two-dimensional finite strains in canine left ventricle. Circ Res 1988; 62:711–21 [DOI] [PubMed] [Google Scholar]
- 35.Fung YC. Biomechanics: mechanical properties of living tissues. New York, NY: Springer-Verlag, 1993 [Google Scholar]
- 36.Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Stoylen A, Ihlen H, Lima JA, Smiseth OA, Slordahl SA. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 2006; 47:789–93 [DOI] [PubMed] [Google Scholar]
- 37.Prinzen FW, Hunter WC, Wyman BT, McVeigh ER. Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol 1999; 33:1735–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laughner JI, Zhang S, Li H, Shao CC, Efimov IR. Mapping cardiac surface mechanics with structured light imaging. Am J Physiol Heart Circ Physiol 2012; 303:H712–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Provost J, Lee WN, Fujikura K, Konofagou EE. Imaging the electromechanical activity of the heart in vivo. Proc Natl Acad Sci USA 2011; 108:8565–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garrott K, Kuzmiak-Glancy S, Wengrowski A, Zhang H, Rogers J, Kay MW. KATP channel inhibition blunts electromechanical decline during hypoxia in left ventricular working rabbit hearts. J Physiol 2017; 595:3799–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christoph J, Chebbok M, Richter C, Schroder-Schetelig J, Bittihn P, Stein S, Uzelac I, Fenton FH, Hasenfuss G, Gilmour RF, Jr, Luther S. Electromechanical vortex filaments during cardiac fibrillation. Nature 2018; 555:667–72 [DOI] [PubMed] [Google Scholar]
- 42.Herron TJ, Lee P, Jalife J. Optical imaging of voltage and calcium in cardiac cells & tissues. Circ Res 2012; 110:609–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Yu H, Walcott GP, Paskaranandavadivel N, Cheng LK, O'Grady G, Rogers JM. High-resolution optical mapping of gastric slow wave propagation. Neurogastroenterol Motil 2019; 31:e13449 [DOI] [PMC free article] [PubMed] [Google Scholar]