Abstract
Cognitive impairment occurs in stroke and hip fracture patients. In mice, bone fracture (BF) exacerbates stroke-related neuronal damage and sensorimotor dysfunction. We hypothesize that BF exacerbates post-stroke cognitive impairment. Adult mice were randomly assigned into BF, stroke, BF+stroke (BF 6 h before stroke), and control (sham operated) groups. Memory function was evaluated weekly for eight weeks by Y maze test and at eight weeks post-surgeries by novel object recognition (NOR) test. The neuronal damage and inflammation in hippocampus were analyzed three days and eight weeks after the surgeries. In Y maze test, BF+stroke mice started making fewer alternations than controls two weeks after the surgeries. Significant difference between BF+stroke and stroke groups started at five weeks post-injury and continued to the end of the experiment. In NOR test, BF+stroke group spent less time on novel objective than that of other groups. Cx3cr1+ cells and CD68+ cells accumulated in the stratum lacunosum moleculare (SLM) on the ipsilateral side of stroke injury in stroke and BF+stroke mice. BF+stroke mice had a higher ratio of ipsilateral/contralateral Cx3cr1+ cell-density than that of stroke mice. Therefore, BF shortly before stroke exacerbates hippocampal inflammation and causes long-lasting memory dysfunction.
Keywords: Ischemic stroke, memory dysfunction, bone fracture, stroke recovery, neuroinflammation
Introduction
Up to 5% of stroke victims sustain a bone fracture (BF).1–5 The risk of stroke doubles after hip fracture.6,7 In US, the hazard ratio of suffering from a hip fracture in the first 24 h after the stroke is increased by ∼4-fold, when compared to a non-stroke population.7 Within the first year of hip surgery, about 4% patients will suffer from stroke. Currently in the US, there are six million hip fractures/year,6,8 that will increase with the population aging. As advanced age is a risk factor for both stroke and fracture, it is imperative to understand the neurobiologic underpinnings for the association of these two conditions on the trajectory of aged-related cognitive decline.
Stroke doubles the risk of developing dementia.9 About one-tenth of stroke patients develop new dementia soon after the onset of stroke, and over one-third became demented after a recurrent stroke.10 The mechanism of post-stroke dementia is not clear. It has been suggested that a B-lymphocyte response to stroke contributes to dementia in some stroke patients.9
Cognitive impairment occurs in nearly half of hip fracture patients.11 The elderly are at significant risk for long-term cognitive dysfunction after aseptic surgical trauma.12,13 Patients with metabolic syndrome, preexisting cognitive decline, such as Alzheimer’s disease or at risk for developing it appeared to have a greater likelihood of having impaired cognitive function.14–17 Animal study showed that, in young adult mice, tibia fracture causes a short-term (<1 week) memory dysfunction.18,19 Post-tibia fracture, neuroinflammation and memory dysfunction are more severe in rats that have metabolic syndrome and can persist up to three months.16 We found in our previous studies that tibia fracture occurring either before or after pMCAO exacerbates infarct sizes and sensorimotor dysfunction.20–23 However, the impact of BF on post-stroke cognitive function has not been studied.
We tested our hypothesis that BF arguments hippocampal inflammation and enhances post-stroke cognitive dysfunction. To test this notion, we monitored memory function of mice subjected to tibia fracture 6 h before ischemic stroke for eight weeks. The tibia fracture was created under aseptic surgery conditions to prevent infection-induced inflammatory/immune responses. To prevent additional tissue damage caused by unstable fracture, a 0.38 mm stainless steel rod was inserted in tibial intramedullary canal through a 0.5 mm hole drilled in the proximal tibia just beneath and medial to the patellar tendon. To ensure the consistency of fracture site, the fibula and the muscles surrounding the tibia were isolated, the periosteum stripped over a distance of 10 mm circumferentially and an osteotomy was performed with scissors at the junction of the middle and distal third of the tibia. We found that BF caused long lasting cognitive dysfunction in stroke mice.
Materials and methods
Animals
C57BL/6 J (WT) male mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Cx3cr1GFP/+/Ccr2RFP/+ transgenic mice that have green fluorescent protein (GFP) gene knocked into one allele of Cx3cr1 gene and red fluorescent protein (RFP) gene knocked into one allele of Ccr2 gene were offered by Israel F. Charo at the University of California, San Francisco, and raised in the animal facility of Zuckerberg San Francisco General Hospital; 24 8–12 weeks old WT male mice and 24 8–12 weeks old male and female Cx3cr1GFP/+/Ccr2RFP/+ mice were randomly assigned to 4 groups: (1) stroke [permanent occlusion of the distal middle cerebral artery (pMCAO)], plus sham tibia fracture (BF)], (2) BF (plus sham pMCAO), (3) BF+Stroke (BF 6 h before pMCAO). (4) control (Sham BF plus sham pMCAO). Brain samples were collected from these mice three days after surgeries. Additional 60 8–12 weeks old male and female WT mice were randomly assigned to above four groups for studying long-term (eight weeks after surgeries) cognitive function. Brain samples from these mice were collected eight weeks after the surgeries for analysis.
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California, San Francisco, and conformed to National Institutes of Health guidelines. All surgeries were performed under anesthesia with 2% isoflurane inhalation and aseptic conditions. Buprenorphine (analgesia, 0.1 mg/kg of body weight) was given at the beginning of and 6 h after each surgery and as needed afterword. Rectal temperature was maintained at 37 ± 0.5℃ using a thermal blanket during surgeries. The following experiments and data are reported following the ARRIVE guidelines for animal experimentation.
BF
BF was performed under aseptic surgical conditions using the method described previously.22 Under anesthesia, the mouse received an open tibia fracture of the right hind limb with an intramedullary fixation. After surgery, the mice were allowed to recover spontaneously from anesthesia in warmed cages. Control mice (BF sham) received hind limb hair shaving with the same amount and duration of anesthesia and analgesia as those of BF mice.
Permanent distal middle cerebral artery occlusion
Permanent occlusion of the left distal middle cerebral artery (MCA) was conducted as described previously.22 Briefly, a 1.0 cm skin incision was made from the left orbit to the ear, followed by a 2 mm2 craniotomy to expose the MCA. The MCA was then permanently occluded by electrically coagulation just proximal to the pyriform branch. The surface cerebral blood flow was monitored by a laser Doppler flow-meter (Vasamedics, Little Canada, MN, USA). Mice were excluded if the reduction of surface cerebral blood flow in the ischemic core is <15% of the baseline or massive bleeding occurred. In this study, 8 mice were excluded and replaced by additional mice. Mice were allowed to recover from anesthesia in warmed and clean cages. Control mice (pMCAO sham) were subjected to craniotomy without arterial occlusion but with the same amount and duration of anesthesia and the same amount of buprenorphine as stroke mice. Mouse blood pressure was measured using CODA Non-Invasive Blood Pressure System (Kent Scientific Corporation, CT,USA), before and after anesthesia was applied, 10 min before and after pMCAO procedure, and after recovery. We did not detect any significant different between Stroke and Stroke+BF mice at any time point (two-way ANOVA, P = 0.99 Supplementary Figure 1).
Y maze spontaneous alternation test
To assess the spatial memory and working memory, mice were placed in the center of a Y-shaped maze (Stoelting, Chicago, IL) that have three white, opaque plastic arms set at a 120° angle from each other and allow to freely explore the three arms for 10 min until 20 entries have been achieved. The maze was sprayed with 70% ethanol, cleaned, and air-dried for 3 min between each test. The exploration processes were recorded by a video camera. Analysis was done by reviewing the videos. Over the course of multiple entries, normal mice will typically exhibit a tendency to visit a new arm in the maze rather than the recently visited arm. An entry was recorded when all four limbs of the mouse were within an arm. The number of arm entries and the number of alternative entries were recorded to determine the percentage of spontaneous alternations.
Novel object recognition test
Seven days prior to the test, the operator hand maneuvered the mice for 5 min each day. The novel object test consists of four days. In the first and second day, each mouse was allowed to explore freely a rectangular arena for 10 min. In the third day, two identical items were placed in the arena. Each mouse was allowed to explore the arena for 5 min. In the last day, one of the objectives was replaced with a different one. The exploration processes were recorded and analyzed using the Ethovision System (Noldus Information Technology Inc. Leesburg, VA, USA). The mice will spend more time exploring the novel object than the familiar one, if their memory is normal. If exploration time of all objects are same, it can be interpreted as a memory dysfunction.24 The preference was determined by dividing the exploration time on the novel object with the whole exploration time. Mouse was excluded if the entire exploration time was less than 5 s. We have removed six mice from normal control group, three from stroke group and one from BF+Stroke group.
Histological and immunohistological stains
After the mice were anesthetized with isoflurane inhalation, the brains of WT mice were collected, frozen in dry ice, and cut into 20 µm thick sections (CM1900 Cryostat, Leica, Wetzlar, Germany). The Cx3cr1GFP/+/Ccr2RFP/+ transgenic mice were perfused with 10% neutralized formalin fixation solution through the left ventricle before their brains were removed. The brain samples of Cx3cr1GFP/+/Ccr2RFP/+ mice were further fixed in 10% neutralized formalin overnight at room temperature, and then frozen on dry ice before sectioned into 20 µm thick sections. Sections between bregma −1.22 and −2.18 mm were used for immunostaining and quantification.
After washing with phosphate-buffered saline (PBS), sections were stained with antibodies specific to CD68 (for activated microglia/macrophage, 1:50, AbD Serotec, MCA1957, Raleigh, NC, USA) or NeuN (for neuronal nuclei, 1:500, Millipore, Bedford, MA) at 4℃ overnight and then with secondary antibody, Alexa Fluor 594-conjugated anti-rag IgG (1:500, Invitrogen, Carlsbad, CA, USA) at room temperature for 2 h. Negative controls were performed by omitting the primary or the secondary antibodies. Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick end-labeling (TUNEL) assay was performed using the dedicated kit (ApopTag, Millipore, Burlington, MA, USA) following the manufacturer’s instructions. Fluoro-Jade C (Millipore, Bedford, MA, USA) staining was performed according to the manufacturer instruction. Sections from the brains of the Cx3cr1GFP/+/Ccr2RFP/+ transgenic mice were directly mounted with a mounting media containing DAPI ((Wectashield HardSet Mounting Medium with Dapi, Vector Laboratories Inc, Burlingam, CA, USA).
Quantifying Cx3cr1+, Ccr2+ and CD68+ cells in the stratum lacunosum moleculare (SLM)
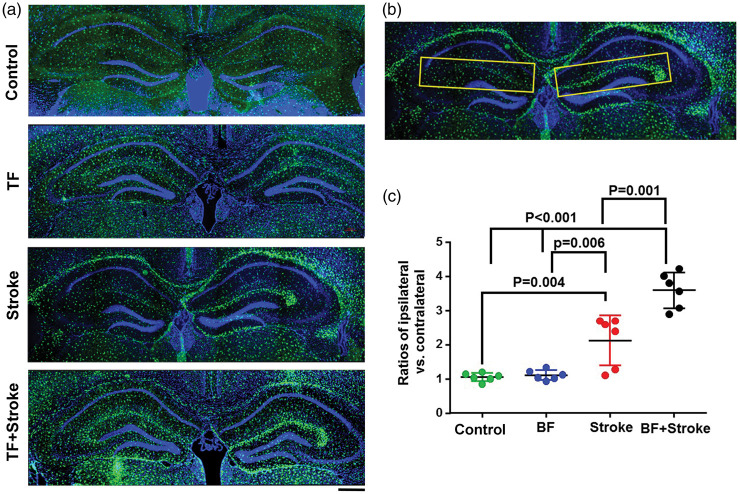
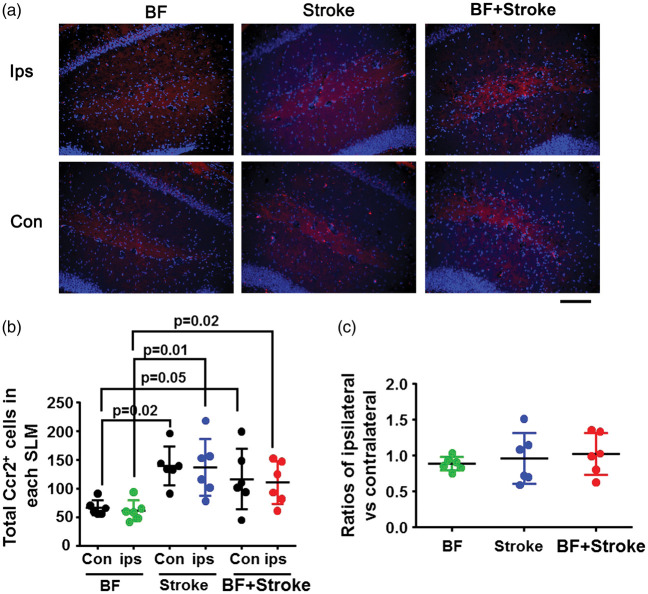
Cx3cr1+ and Ccr2+ cells in the SLM of the hippocampus were quantified in the areas showed in Figure 3(b) using the software Adobe Photoshop CS6 (version 13.0.1). Two coronal sections 200 µm apart that contain hippocampus were selected. The ratios of the density of Cx3cr1+ of ipsilateral/contralateral were calculated.
Figure 3.
More Cx3cr1+ cells in the hippocampal SLMs region ipsilateral to stroke injury in stroke- and BF+stroke-groups. (a) Representative microscopic images. Microglia express GFP (green). The nuclei were counterstained by DAPI (blue). Scale bar: 400 µm. (b) The areas in yellow boxes were used for quantifying Cx3cr1+ cells. (c) The ratios of Cx3cr1+ cells in SLMs of the ipsilateral verse contralateral sides of stroke injury. n = 6.
The Ccr2+ cells and CD68+ cell numbers in the SLM region were counted on two coronal sections 200 µm apart that contain hippocampus.
Quantifying the volumes of granular cell layer in hippocampus
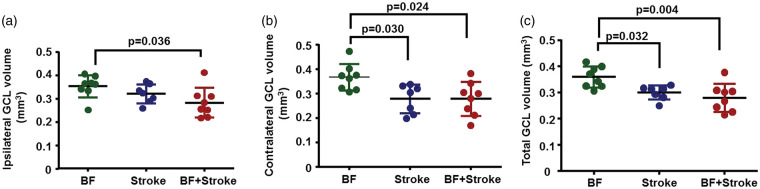
Two representative sections 200 µm apart selected between bregma −1.888 mm to −2.155 mm were mounted with mounting medium that contains DAPI (Wectashield HardSet Mounting Medium with Dapi, Vector Laboratories Inc, Burlingam, CA, USA). GCL volumes were measured using the method described by Cavalieri principle.25 Briefly, the GCL areas were delineated by red lines (Figure 6(a)) and measured using NIH Image J software (version 1.45 J). The GCL volume was calculated using the equation: V = A × T × N × n (V: volume, A: area, T: thickness of a single section, N: number of sections represented by one section, n: number of sections that GCL has). In this case, T = 20 um, N = 10, n = 10. GCL volume = (area 1 + area 2) × 20 × (10/2).
Figure 6.
Stroke and BF+stroke mice had smaller GCLs than control mice eight weeks after injuries. (a) Quantification of ipsilateral GCL volumes. (b) Quantification of contralateral GCL volumes. (c) Quantification of total GCL volumes. BF: n = 8; stroke: n = 7; BF+stroke: n = 8.
Statistical analysis
All quantification analyses were performed by at least two researchers who did not know the group assignment. Sample sizes were estimated according to our previous published effect sizes of infarct size and sensorimotor function in a similar model.20,22 Sample sizes for each experiments were indicated in the figure legends. Data are presented as mean ± SD. All data were analyzed by one-way ANOVA followed by Tukey’s multiple comparisons using GraphPad Prism 6, except Y-Maze and blood pressure, which were analyzed by two-way ANOVA followed by Tukey’s multiple comparisons. A P value < 0.05 was considered to be significant.
Results
BF 6 h before stroke causes long-lasting spatial memory dysfunction
All mice generated in this study showed similar stroke-related brain injuries and sensorimotor dysfunction as we have showed in one of our previous papers that used same models.22 The BF+stroke mice had larger infarct sizes, more inflammatory cell infiltration in the peri-infarct region, and more severe sensorimotor dysfunction than stroke only mice.
In current study, we analyzed memory function using Y-Maze spontaneous alternation test weekly and novel objective recognition tested (NOR) at eight weeks post-injuries. Sham operated mice were tested in parallel as control.
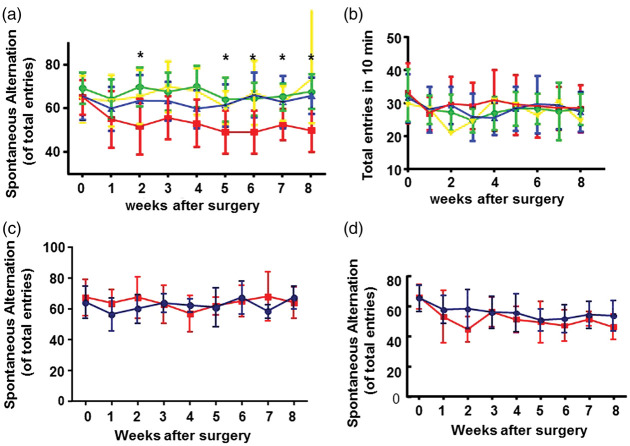
In Y-maze test, two-way ANOVA analysis showed that the differences among groups are significant (P = 0.045). Tukey’s multiple comparisons showed that mice subjected with BF or stroke alone performed similarly to that of normal mice at all-time points, while mice subjected to BF+stroke started to make fewer alternations than normal mice (P = 0.004), BF (P < 0.001) mice and stroke mice (P = 0.006) two weeks after the surgeries (Figure 1(a)). The BF+stroke mice continuously made fewer alternations than control mice from three to eight weeks after the surgeries. Statistically significant differences between BF+stroke group and stroke group started in five weeks post-injury (P = 0.004) and continued to the end of the experiment (eight weeks post-injury, P < 0.001). All mice made similar number of entries at each time-points, indicating no movement deficit (Figure 1(b)). No difference was found between male (n = 8) and female (n = 7) mice in stroke group (two-way ANOVA: P = 0.43, Figure 1(c)) and male (n = 10) and female (n = 8) mice in BF+stroke group in Y maze test (two-way ANOVA: P = 0.6, Figure 1(d)) at base line and at all-time points examined post surgeries.
Figure 1.
Mice subjected to BF+stroke made fewer alternations in Y-maze test than control mice and mice subjected to BF or stroke alone. (a) Percentage of spontaneous alternations before surgeries and one-to-eight weeks after surgeries. Green: BF group (n = 16); Blue: stroke (n = 15); Red: BF+stroke (n = 18); Yellow: control (n = 11). *indicate that the differences between stroke group and BF+stroke group are statistically significant. (b)Total entries of mice in 10 minutes testing period. (c)Comparison of the percentage of spontaneous alternations of male (Blue, n = 8) and female (Red, n = 7) mice in stroke group. (d) Comparison of the percentage of spontaneous alternations of male (Blue, n = 10) and female (Red, n = 9) mice subjected to BF and stroke.
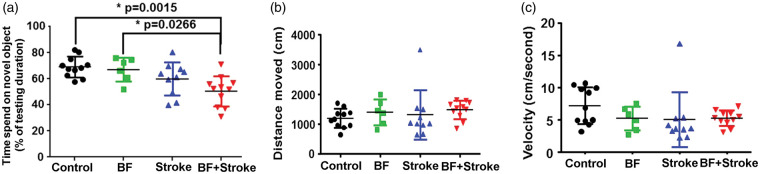
In NOR test, one-way ANOVA analysis showed that the differences among groups are significant (P = 0.0016). Tukey’s multiple comparisons showed that the stroke, BF and control mice used similar time to explore the novel object; however, the BF+stroke mice spent less time on the novel object than control mice (P = 0.002) and mice in BF group (P = 0.03, Figure 2(a)). Although BF+stroke mice trend toward spending less time on novel object than stroke mice (P = 0.28), the difference did not reach statistical significant perhaps due to the limited sample size. The running distances (one-way ANOVA: P = 0.37, Figure 2(b)) and velocities (one-way ANOVA: P = 0.28. Figure 2(c)) are similar among groups. These data suggest that BF shortly before stroke causes long-lasting spatial memory dysfunction.
Figure 2.
Mice subjected to BF+stroke spend less time than control and BF mice on novel objective. (a) Time that mice spend on novel objective. (b) Distance that mice moved during the test. (c) Running velocity of mice during the test. BF: n = 6; Stroke: n = 10; BF+Stroke: n = 11; Control: n = 11.
Cx3cr1+ cells accumulated in the hippocampal SLM ipsilateral to stroke injury in stroke- and BF+stroke-groups
Since previous studies showed that tibia fracture causes macrophage infiltration in the hippocampus,26 we analyzed microglia and macrophage load in the hippocampus three days after injuries using Cx3cr1GFP/+/Ccr2RFP/+ transgenic mice. We found accumulation of Cx3cr1+ cells in the alveus and SLMs ipsilateral to stroke injury of mice in stroke and BF+stroke groups (Figure 3(a)). Since the increase of Cx3cr1+ cells in the alveus was not consistent (Supplementary Figure 2) and not correlated with cognitive functions well, we only quantified the density of Cx3cr1+ cells in the areas defined in Figure 3(b)). that covers the SLM region. One-way ANOVA analysis showed that the differences among groups are significant (P < 0.0001). Tukey’s multiple comparisons showed that the ratios of Cx3cr1+ cell-densities in ipsilateral versus contralateral side SLMs were higher in BF+stroke group than that in the control (P < 0.001), BF (P < 0.001), and stroke alone groups (P = 0.001, Figure 3(c)). Mice subjected to stroke alone also have more Cx3cr1+ cells in the SLMs in the ipsilateral side of stroke injury than control mice (P = 0.004) and BF mice (P = 0.006). The densities of Cx3cr1+ cells in BF group were equal on both sides of SLMs and were similar to that of control mice. The densities of Cx3cr1+ cells in SLMs in the contralateral side were similar among groups.
There is very few Ccr2+ cells in the SLM regions of control mice and only a small numbers of Ccr2+ cells were detected in mice subjected to surgeries. Mice subjected to stroke or BF+stroke had more Ccr2+ cells on both ipsilateral and contralateral SLMs than that of mice subjected to BF alone (one-way ANOVA: P = 0.0017, Figure 4(a) and (b)). There was no difference between stroke and BF+stroke mice. The ratios of the numbers of Ccr2+ cells in the ipsilateral and contralateral side are similar among all groups (one-way ANOVA: P = 0.68, Figure 4(c)).
Figure 4.
More Ccr2+ cells were detected in SLMs of stroke- and BF+stroke mice. (a) Representative microscopic images. Ccr2+ cells express RFP (red). The nuclei were counterstained by DAPI (blue). Scale bar: 100 µm. (b) Quantification of total Ccr2+ cells in the SLM. (c) Ratios of Ccr2+ cells in SLMs ipsilateral verses contralateral to the stroke side. ips: ipsilateral; con: contralateral. n = 6.
More CD68+ cells were presented in the ipsilateral SLMs of mice subjected to stroke or BF+stroke
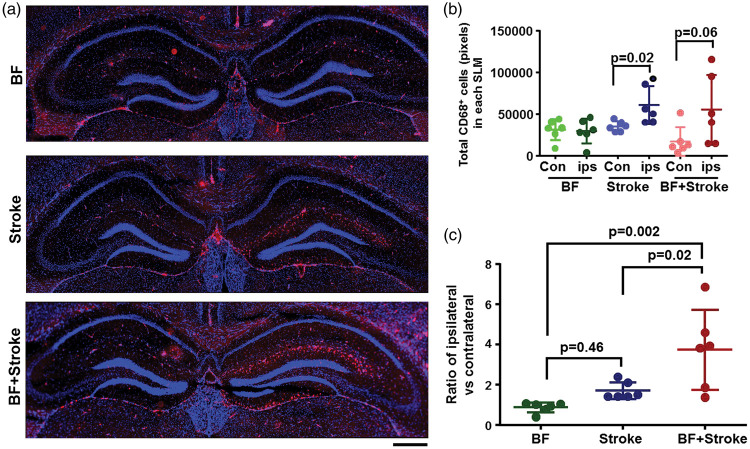
To test wither those Cx3cr1+ cells are activated microglia, the brain sections collected from WT male mice three days after the surgeries were stained with an antibody specific to CD68. One-way ANOVA analysis showed that the ratios of CD68+ cells between ipsilateral and contralateral sides were significantly different among groups (P = 0.0023). Tukey’s multiple comparisons showed that there were more CD68+cells in the ipsilateral sides than contralateral sides of stroke mice (P = 0.02) and BF+stroke mice (P = 0.06). Few CD68+ cells were detected in SLMs of BF mice and the numbers of CD68+ cells were equal in on both sides of SLMs of BF mice (Figure 5).
Figure 5.
More CD68+ cells were detected in the ipsilateral SLMs of stroke- and BF+stroke mice. (a) Representative microscopic images. CD68+ cells were visualized by immunostaining using an anti-CD68+ antibody (red). The nuclei were counterstained by DAPI (blue). Scale bar: 400 µm. (b) Quantification of total CD68+ cells in the SLM. (c) Ratios of CD68+ cells in SLMs ipsilateral verses contralateral to the stroke side. ips: ipsilateral; con: contralateral. n = 6.
BF+stroke mice have smaller GCL volume than that of control mice
To investigate if Cx3cr1+ cell accumulation causes neuronal death in the hippocampus, brain sections were co-stained with an antibody specific to NeuN and Fluoro-Jade C or TUNEL. No degenerated neuron was detected in the hippocampi of brains collected three days or eight weeks after the injuries (Supplementary Figure 3). We then measured the GCL volume. The GCL volumes were similar among all groups both on ipsilateral and contralateral sides three days after the surgeries (one-way ANOVA: P = 0.36). However, at eight weeks after the surgery, one-way ANOVA analysis showed the volumes of ipsilateral GCLs were significantly different among groups (P = 0.045). Tukey’s multiple comparisons showed that the ipsilateral GCLs of BF+stroke mice were smaller than that of BF mice (P = 0.036, Figure 6(a)). One-way ANOVA analysis also showed the volumes of contralateral GCLs were significantly different among groups (P = 0.014). Tukey’s multiple comparisons showed that both stroke (P = 0.03) and BF+stroke (=0.024) mice had smaller GCLs on the contralateral side (Figure 6(b)) and total GCLs (stroke verses BF: P = 0.032; BF+stroke verses BF: P = 0.004, Figure 6(c)) than that of BF mice. Thus, BF caused more severe atrophy of GCLs of stroke animals.
Discussion
In this study, we showed that tibia fracture shortly before ischemic stroke caused long-lasting (≥8 weeks) spatial memory dysfunction in young mice (8–10 weeks of age), which was associated with an accumulation of Cx3cr1+ and CD68+ cells in the hippocampal SLM region ipsilateral to the stroke injury, a phenomenon that has not been reported before.
Dementia and stroke often occur together, and the combination of these injuries significantly increases the mortality and the cost of care.27–29 Stroke survivors are more likely to develop cognitive dysfunction than the general population.29,30 The underlying mechanism(s) for this increased dementia risk is unknown. Doyle et al.9 demonstrated that the delayed cognitive impairment in patients and mice following stroke is associated with a B-lymphocyte-response. Using a mouse model of post-BF stroke, we noted in this study a remarkable increase of Cx3cr1+ and CD68+ cells in the hippocampal SLMs ipsilateral to stroke injury of mice three days after stroke or BF+stroke. The increases of Cx3cr1+ and CD68+ cells in the hippocampal SLMs ipsilateral to stroke injury could be due to the additional effect of BF on increase of blood–brain barrier permeability and the levels of cytokines in the hippocampus,26 as we have observed an increase of extravascular IgG in the ipsilateral SLMs of BF+stroke mice (data not shown). Ccr2+ cells were increased in SLMs both ipsilateral and contralateral to stroke injury. These data suggest that augmented inflammation in hippocampus is one of the mechanisms that contribute to cognitive dysfunction of patients suffering from both injuries. We have showed in our previous study that reduction of systemic inflammation through depletion of macrophages, inhibition of the high-mobility group box chromosomal protein-1 (HMGB1),20 or activation of α7 nicotinic acetylcholine receptor (nAChR)21 reduces neuroinflammation, infarct size and sensorimotor dysfunction in BF+stroke mice. Therefore, modulate inflammation might also reduce the memory dysfunction of BF+ stroke mice.
Stroke and BF are risk factors for cognitive dysfunction. If these conditions present simultaneous, the individuals will have less optimal outcome. Stroke patients have an increased risk of BF.1–3,31 In US, compared to a non-stroke population, the hazard ratio of suffering from a hip fracture in the first 24 h after the stroke is increased by ∼4-fold.7 Within the first year of hip surgery, about 4% patients will suffer from stroke. Although stroke and BF have many common risk factors,5,32,33 strategies for preventing34,35 or treating stroke may adversely influence bone healing.2 Understanding how these two conditions interact with each other will help to develop specific interventions to treat the patients.
Peripheral trauma, including BF, can cause neuroinflammation and cognitive impairment.12,18 For the majority of patients, post-trauma or post-surgery neuroinflammation and cognitive decline resolve promptly with no sequelae. However, for patients with some risk factors, such as metabolic syndrome, Alzheimer’s disease and aging, neuroinflammation can persist and cause persistent and even permanent cognitive dysfunction.17 Cognitive dysfunction increases the risk of mortality.36
Animal studies show that tibia fracture increases circulating and hippocampal inflammatory cytokines and monocytes/macrophages that is associated with a short-term (<7 days) memory dysfunction in young mice.18,19 Post-BF neuroinflammation and memory dysfunction are more severe in rats that have metabolic syndrome and can persist up to three months.16 Here we showed that the spatial memory dysfunction in mice subjected to stroke shortly after BF lasted beyond eight weeks, which is accompanied with an increase of activated microglia/macrophage in the hippocampus. Therefore, the augmented innate immune response from stroke could be one of the mechanisms for long-term cognitive dysfunction.
It has been shown that brain injury is more severe in the elderly after stroke and BF. In a rat forebrain ischemia model, older rats showed greater neocortical and striatal injury than younger rats.37,38 Stroke-induced neurogenesis is reduced in aged animals.39 In addition, the aged brain has an activated basal state of low-grade chronic inflammation40,41 that may lead to more severe and persistent behavioral and cognitive deficits after stroke, and thereby impairing stroke recovery.42,43 We showed previously that the brain’s angiogenic and neurogenic responses to vascular endothelial growth factor (VEGF) are reduced in aged mice,44 which could contribute to impaired stroke recovery observed in old mice, and that compared with 3-month-old mice, 12-month-old mice have larger infarct size, more severe neuroinflammation and behavioral dysfunction after pMCAO.45
Questions need to be investigated further. (1) The number of Cx3cr1+ cells and CD68+ cells was also increased in alveus in some stroke and BF+stroke mice. It is not clear if the accumulation of these cells in alveus has any functional means. (2) It is not clear why the volumes of GCLs were reduced in both stroke and BF+stroke mice eight weeks after the injuries, while the long-term memory dysfunction was only present in BF+stroke mice. (3) We could not detect any TUNEL or Fluoro-Jade C positive neuron in the hippocampus at three days and eight weeks after the injuries. It is not clear why the volumes of GCLs were reduced in both stroke and BF+stroke mice eight weeks after the injuries. The neuronal damage could occur at those time points that we did not covered, or neuron regeneration was reduced after stroke and BF+stroke. (4) Compared to BF mice, stroke and BF+stroke mice had more Ccr2+ cells in both ipsilateral and contralateral SLMs. It is not clear if Ccr2+ accumulation has any roles in the reduction of GCL volumes. (5) As we have discussed in previous paragraph, inhibition of inflammatory through depletion of macrophage, HMGB1 antibody treatment or activation of nAChR reduces the negative impact of BF on stroke-related injuries, such as reduce infarct size, sensorimotor dysfunction and brain edema.21,23 We have not tested if these strategies also reduce the negative impact of BF on post stroke cognitive function yet. We will test them in our future studies.
Patients with both stroke and BF have less optimal outcomes than patients that have single injury. The strategies for preventing or treating stroke can worse bone healing. Due to the absent of biologic understanding of the interaction of these two conditions, there are no specific interventions to prevent post-BF stroke, nor post-stroke BF. Advanced age is a risk factor for both stroke and BF. The neurobiologic underpinnings for the association of these two conditions include the trajectory of age-related cognitive decline. The increase of aging population in the US will increase the number of patients with both stroke and BF, as well as the burden of health care at both the individual and societal levels. Despite the growing prevalence of the number of patients with both injuries, few studies have been designed to address the impact of BF/surgery on stroke, which, if unabated, will lead to an increased burden of these illnesses on individual suffering and healthcare resources. In this study, we used aseptic tibia fracture to mimic long bone and hip fractures in human. We showed that mice subjected to BF shortly before stroke developed long-lasting memory dysfunction, which is associated with an increase of activated microglia/macrophages in the SLMs of hippocampi. Our data suggest that augmented innate immune response could be one of the underlying mechanisms. Therefore, inhibition of innate immune could be developed to prevent or alleviate the cognitive dysfunction of patients that suffer BF and stroke or stroke patients that will undergo elective hip surgery.
Acknowledgements
The current affiliation of Dr. Zhengxi Li is Shanghai Ninth People’s Hospital, Shanghai, China, 200025.
Authors’ contributions
ZL: concept and design, data acquisition, data analysis, manuscript drafting. MW: data acquisition, data analysis. HL: data acquisition, data analysis. KH: data acquisition, data analysis. LW: data acquisition, data analysis. MZ: data acquisition, data analysis. HS: design, data analysis, manuscript revision, finalized manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants to H. S. from the National Institutes of Health (R01 HL122774 and R21 NS070153 and NS083788), and from the Michael Ryan Zodda Foundation and the UCSF Research Evaluation and Allocation Committee (REAC).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Kapral MK, Fang J, Alibhai SM, et al. Risk of fractures after stroke: results from the Ontario Stroke Registry. Neurology 2017; 88: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huo K, Hashim SI, Yong KL, et al. Impact and risk factors of post-stroke bone fracture. World J Exp Med 2016; 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coutinho ES, Fletcher A, Bloch KV, et al. Risk factors for falls with severe fracture in elderly people living in a middle-income country: a case control study. BMC Geriatr 2008; 8: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myint PK, Poole KE, Warburton EA. Hip fractures after stroke and their prevention. QJM 2007; 100: 539–545. [DOI] [PubMed] [Google Scholar]
- 5.Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. JAMA 2009; 302: 1666–1673. [DOI] [PubMed] [Google Scholar]
- 6.Kang JH, Chung SD, Xirasagar S, et al. Increased risk of stroke in the year after a hip fracture: a population-based follow-up study. Stroke 2011; 42: 336–41. [DOI] [PubMed] [Google Scholar]
- 7.Lakshminarayan K, Schissel C, Anderson DC, et al. Five-year rehospitalization outcomes in a cohort of patients with acute ischemic stroke: medicare linkage study. Stroke 2011; 42: 1556–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai CH, Lin CL, Hsu HC, et al. Increased risk of stroke among hip fracture patients: a nationwide cohort study. Osteoporos Int 2015; 26: 645–652. [DOI] [PubMed] [Google Scholar]
- 9.Doyle KP, Quach LN, Sole M, et al. B-lymphocyte-mediated delayed cognitive impairment following stroke. J Neurosci 2015; 35: 2133–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009; 8: 1006–1018. [DOI] [PubMed] [Google Scholar]
- 11.Seitz DP, Adunuri N, Gill SS, et al. Prevalence of dementia and cognitive impairment among older adults with hip fractures. J Am Med Dir Assoc 2011; 12: 556–564. [DOI] [PubMed] [Google Scholar]
- 12.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 1998; 351: 857–861. [DOI] [PubMed] [Google Scholar]
- 13.Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008; 108: 18–30. [DOI] [PubMed] [Google Scholar]
- 14.Tzimas P, Petrou A, Laou E, et al. Impact of metabolic syndrome in surgical patients: should we bother? Br J Anaesth 2015; 115: 194–202. [DOI] [PubMed] [Google Scholar]
- 15.Arora SS, Gooch JL, Garcia PS. Postoperative cognitive dysfunction, Alzheimer's disease, and anesthesia. Int J Neurosci 2014; 124: 236–242. [DOI] [PubMed] [Google Scholar]
- 16.Feng X, Degos V, Koch LG, et al. Surgery results in exaggerated and persistent cognitive decline in a rat model of the Metabolic Syndrome. Anesthesiology 2013; 118: 1098–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salazar F, Donate M, Boget T, et al. Intraoperative warming and post-operative cognitive dysfunction after total knee replacement. Acta Anaesthesiol Scand 2011; 55: 216–222. [DOI] [PubMed] [Google Scholar]
- 18.Cibelli M, Fidalgo AR, Terrando N, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol 2010; 68: 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terrando N, Monaco C, Ma D, et al. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A 2010; 107: 20518–20522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Degos V, Maze M, Vacas S, et al. Bone fracture exacerbates murine ischemic cerebral injury. Anesthesiology 2013; 118: 1362–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han Z, Li L, Wang L, et al. Alpha-7 nicotinic acetylcholine receptor agonist treatment reduces neuroinflammation, oxidative stress and brain injury in mice with ischemic stroke and bone fracture. J Neurochem 2014; 131: 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Kang S, Zou D, et al. Bone fracture pre-ischemic stroke exacerbates ischemic cerebral injury in mice. PLoS One 2016; 11: e0153835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou D, Luo M, Han Z, et al. Activation of alpha-7 nicotinic acetylcholine receptor reduces brain edema in mice with ischemic stroke and bone fracture. Mol Neurobiol 2016; 54: 8278–8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 2012; 13: 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eric M, Anderla A, Stefanovic D, et al. Breast volume estimation from systematic series of CT scans using the Cavalieri principle and 3D reconstruction. Int J Surg 2014; 12: 912–917. [DOI] [PubMed] [Google Scholar]
- 26.Terrando N, Eriksson LI, Ryu JK, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol 2011; 70: 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marengoni A, Corrao S, Nobili A, et al. In-hospital death according to dementia diagnosis in acutely ill elderly patients: the REPOSI study. Int J Geriatr Psychiatry 2011; 26: 930–936. [DOI] [PubMed] [Google Scholar]
- 28.Marengoni A, Nobili A, Romano V, et al. Adverse clinical events and mortality during hospitalization and 3 months after discharge in cognitively impaired elderly patients. J Gerontol A Biol Sci Med Sci 2013; 68: 419–425. [DOI] [PubMed] [Google Scholar]
- 29.Lo Coco D, Lopez G, Corrao S. Cognitive impairment and stroke in elderly patients. Vasc Health Risk Manage 2016; 12: 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bejot Y, Aboa-Eboule C, Durier J, et al. Prevalence of early dementia after first-ever stroke: a 24-year population-based study. Stroke 2011; 42: 607–612. [DOI] [PubMed] [Google Scholar]
- 31.Fisher A, Srikusalanukul W, Davis M, et al. Poststroke hip fracture: prevalence, clinical characteristics, mineral-bone metabolism, outcomes, and gaps in prevention. Stroke Res Treat 2013; 2013: 641943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sathiyakumar V, Avilucea FR, Whiting PS, et al. Risk factors for adverse cardiac events in hip fracture patients: an analysis of NSQIP data. Int Orthop 2016; 40: 439–445. [DOI] [PubMed] [Google Scholar]
- 33.van Staa TP, Dennison EM, Leufkens HG, et al. Epidemiology of fractures in England and Wales. Bone 2001; 29: 517–522. [DOI] [PubMed] [Google Scholar]
- 34.Semenkovich CF. Insulin resistance and a long, strange trip. N Engl J Med 2016; 374: 1378–1379. [DOI] [PubMed] [Google Scholar]
- 35.Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016; 374: 1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gruber-Baldini AL, Hosseini M, Orwig D, et al. Cognitive differences between men and women who fracture their hip and impact on six-month survival. J Am Geriatr Soc 2017; 65: e64–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutherland GR, Dix GA, Auer RN. Effect of age in rodent models of focal and forebrain ischemia. Stroke 1996; 27: 1663–1667; discussion 1668. [DOI] [PubMed] [Google Scholar]
- 38.Suenaga J, Hu X, Pu H, et al. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol 2015; 272: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moraga A, Pradillo JM, Garcia-Culebras A, et al. Aging increases microglial proliferation, delays cell migration, and decreases cortical neurogenesis after focal cerebral ischemia. J Neuroinflammation 2015; 12: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franceschi C, Bonafe M, Valensin S, et al. Inflammaging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000; 908: 244–254. [DOI] [PubMed] [Google Scholar]
- 41.Morganti JM, Riparip LK, Chou A, et al. Age exacerbates the CCR2/5-mediated neuroinflammatory response to traumatic brain injury. J Neuroinflammation 2016; 13: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Badan I, Buchhold B, Hamm A, et al. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J Cereb Blood Flow Metab 2003; 23: 845–854. [DOI] [PubMed] [Google Scholar]
- 43.Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol 2008; 213: 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao P, Shen F, Young WL, et al. Aging-dependent attenuation of angiogenic response in the mouse brain [Abstract]. Stroke 2009; 40: e141. [Google Scholar]
- 45.Shen F, Jiang L, Han F, et al. Increased inflammatory response in old mice is associated with more severe neuronal injury at the acute stage of ischemic stroke. Aging Dis. Epub ahead of print 31 October 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]