Short abstract
Stroke is a leading cause of mortality and morbidity, with long-term debilitating effects. Accumulating evidence from experimental studies as well as observational studies in patients suggests a cross talk between the brain and kidney after stroke. Stroke may lead to kidney dysfunction which can adversely impact patient outcome. In this review article, we discuss the epidemiology and mechanisms of brain–kidney interaction following ischemic stroke. Specifically, we discuss the role of the central autonomic network, autoregulation, inflammatory and immune responses, the role of extracellular vesicles and their cargo microRNA, in mediating brain–kidney interaction following stroke. Understanding the bidirectional nature of interaction between the brain and kidney after cerebral injury would have clinical implications for the treatment of stroke and overall patient outcome.
Keywords: Acute kidney injury, chronic kidney disease, extracellular vesicles, immune response, ischemic stroke
Introduction
The brain and kidney act in concert to maintain normal homeostasis of the extracellular fluid by controlling sodium and water balance. Both organs exhibit several common physiological characteristics: first, the kidney and brain are highly perfused organs and are exposed to a relatively large volume of blood flow during the cardiac cycle; second, with variations in arterial blood pressure, both the brain and kidney strive to maintain constant perfusion via autoregulation; and third, there is hemodynamic parallelism between the vascular beds of the two organs. Chronic conditions such as diabetes mellitus and hypertension are high risk factors for cerebrovascular and cardiovascular disease, diabetic nephropathy, and hypertensive nephropathy. While these common physiological characteristics set the stage for an exploration of how the brain and kidney are inextricably linked under normal and disease states, it is likely that the complex interactions between the brain and kidney transcend these shared characteristics and risk factors. Until now, the research focus on brain and kidney cross talk has focused on the role of kidney impairment in inducing brain dysfunction. However, the effects of brain injury on kidney function remain relatively unexplored. This article focuses on the mechanisms of stroke-induced kidney dysfunction, and briefly discusses the impact of renal disease on stroke.
Cerebrovascular diseases can stimulate kidney dysfunction as kidney activity is regulated by the brain through neural pathways. The term organ cross talk can be defined as mutual effects on distant organs via biological communication through central neural and peripheral humoral pathways. The central pathway of brain–kidney interaction may be via the central autonomic network (CAN) and sympathetic nervous system (Figure 1). The peripheral signaling pathways of organ cross talk may be regulated by inflammatory responses, autoregulation, neuroendocrine system as well as by extracellular vesicles (EVs) (Figure 2). While several mechanistic links between brain–kidney interactions are associative and experimental evidence is emerging, in this review, we will discuss the CAN, neuroendocrine system, inflammatory and immune responses, and EVs that may contribute to brain–kidney interaction after stroke.
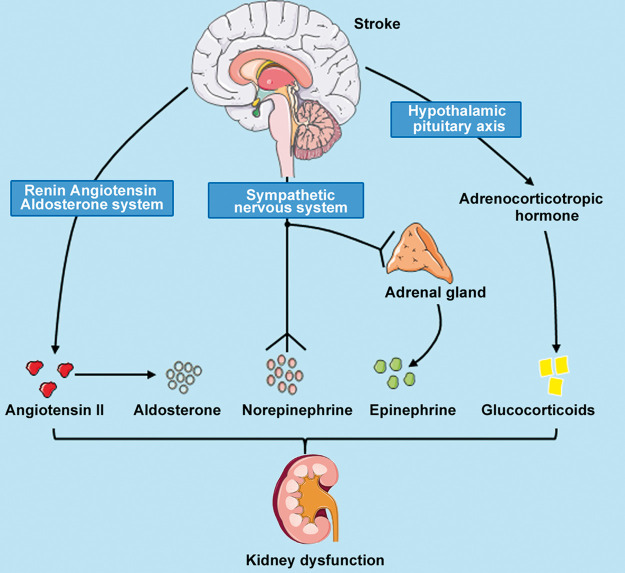
Figure 1.
Role of central autonomic network in mediating kidney dysfunction after ischemic stroke. Stroke-induced activation of HPA axis, sympathetic nervous system, and the RAAS may alter hormone and neurotransmitter release, thereby inducing kidney dysfunction.
HPA axis: hypothalamic–pituitary–adrenal axis; RAAS: renin–angiotensin–aldosterone system.
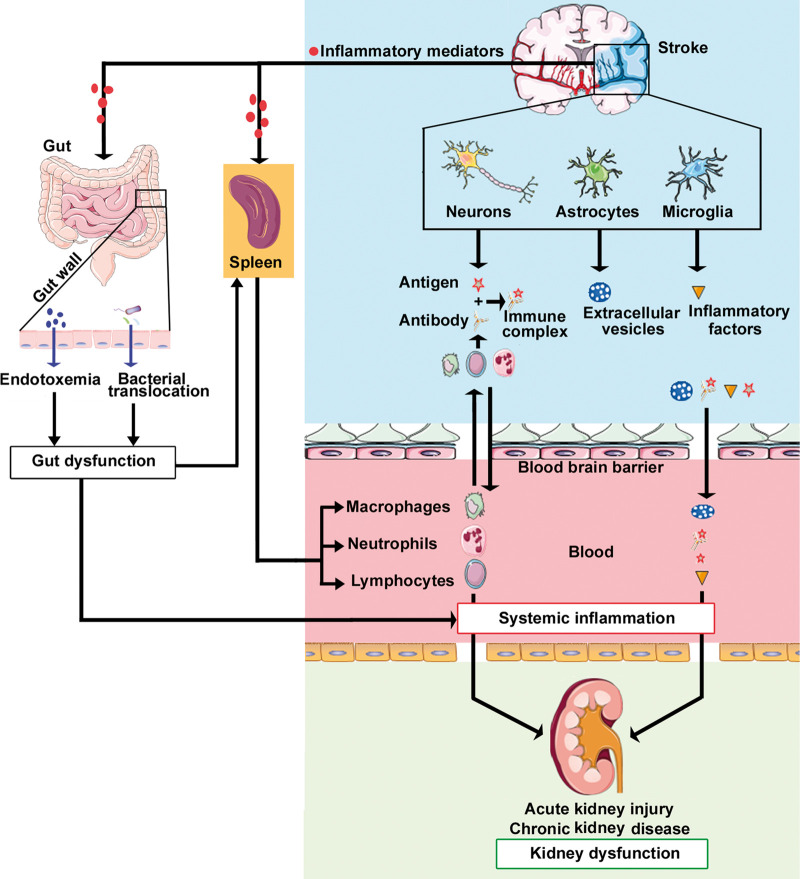
Figure 2.
Inflammatory and immune responses mediate kidney dysfunction after stroke. Release of inflammatory mediators by injured brain cells as well as stroke-induced gut dysbiosis can increase systemic inflammation. Systemic inflammation and immune responses mediated by the spleen likely play a central role in promoting kidney dysfunction after brain injury.
Epidemiology: Brain and kidney interaction
Chronic kidney disease and ischemic stroke
Chronic kidney disease (CKD) typically develops over time and includes five stages of disease ranging from mild damage to complete kidney failure. CKD increases vascular dysfunction, vascular calcification, and arterial stiffness which increases risk of stroke as well as exacerbates pathogenesis of stroke.1 CKD is often diagnosed by measuring creatinine clearance, proteinuria, cystatin C, albuminuria, estimated glomerular filtration rate (eGFR), urinalysis to evaluate for leukocytes and red blood cells, serum electrolytes, serum calcium, and parathyroid hormone levels. Among these parameters, eGFR is the most commonly used by clinicians to identify the stage of CKD. GFR is usually indirectly assessed from serum levels of endogenous filtration markers, such as creatinine or cystatin C. Measuring proteinuria is helpful in CKD diagnosis, assessing prognosis, and is an independent predictor of progression of renal disease.2
CKD may be a frequently encountered problem in post ischemic stroke patients. In a large-scale multicenter hospital-based study, approximately 35% of first ever stroke patients exhibited symptoms of CKD such as proteinuria or low eGFR, and multivariate analyses after adjusting for confounding factors indicated that patients with proteinuria had significantly (p < 0.001) increased risks of worse neurologic deficits and in-hospital mortality.3 In a nationwide study of Medicare aged (≥65 years) acute ischemic stroke patients, low eGFR and dialysis status on admission were independent and strong predictors of poor outcomes with high risk of 30-day and 1-year mortality and rehospitalization.4 Low admission eGFR (<15 mL/min/1.73 m2) increased the risk of short-term (1 month) mortality by threefold and long-term (1 year) mortality by fourfold compared to patients with eGFR ≥90 mL/min/1.73 m2 or on dialysis.5 Other studies have reported that proteinuria, but not low eGFR, is an independent predictor of high risk of neurological deterioration, disability, and death.6 In a group of post ischemic stroke survivors (66.42% of 352 recruited patients survived) in Poland, renal impairment indicated by decreased eGFR or increased urine albumin/creatinine ratio was found at 4 months after stroke onset in 40.38% of patients.7 Post stroke albuminuria was predicted by older age, comorbidity with diabetes, and severe functional deficits, and increased albumin/creatinine ratio was more frequently encountered in women.7 This study found a higher frequency of renal dysfunction with increasing age and atheromatic and lacunar stroke subtype.7 In patients with lacunar infarctions, decreased eGFR and proteinuria have been associated with white matter hyper-intensities, cerebral microbleeds, and enlargement of perivascular spaces.8,9 Kidney damage indicated by proteinuria and renal insufficiency was found to be differentially dependent on stroke subtype.10 Patients who suffered a stroke of cardioembolic subtype displayed kidney damage and renal insufficiency, while hemorrhagic stroke (subcortical and subarachnoid) patients primarily exhibited proteinuria.10 Proteinuria after stroke was found to be an independent risk factor for worse neurologic deficits and mortality.3 In acute ischemic stroke patients of large artery atherosclerotic subtype, approximately 41% patients had low eGFR which was associated with increased stroke severity, increased 6-month mortality rate, and worse neurological functional outcome.11 The incidence of albuminuria was found to be significantly higher among ischemic stroke survivors compared to the general population.7 These studies indicate that kidney dysfunction is an independent predictor of worse post stroke outcome. The TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification categorizes ischemic stroke into the following subtypes: (1) large-artery atherosclerosis, (2) cardioembolism, (3) small-vessel occlusion, (4) stroke of other determined etiology, and (5) stroke of undetermined etiology. The different kidney abnormalities associated with various stroke subtypes are summarized in Table 1.
Table 1.
Summary of the different kidney abnormalities associated with various stroke subtypes.
| Stroke subtype | Kidney abnormalities | Ref |
|---|---|---|
| Acute ischemic stroke |
|
13–17 |
| Lacunar stroke |
|
8,9 |
| Cardioembolic stroke |
|
10 |
| Hemorrhagic stroke (subcortical and subarachnoid) |
|
10 |
| Large artery atherosclerotic stroke |
|
11 |
eGFR: estimated glomerular filtration rate.
Acute kidney injury and ischemic stroke
Acute kidney injury (AKI) is an abrupt onset of renal dysfunction involving structural damage and functional impairment that develops within few hours or days.12 AKI can result from decreased renal perfusion, nephrotoxicity, or damage to glomeruli, tubules, interstitium or renal vasculature.12 AKI is commonly encountered in patients with critical illness and identified by decreased eGFR, albuminuria, acute rise in serum creatinine, increased serum cystatin C, and oliguria, i.e. low urine output.12 Serum creatinine levels do not accurately reflect acute changes in kidney function and can vary widely with age, gender, etc. making it difficult to determine reference points.12 A meta-analysis of eight studies indicated that AKI is a common complication of acute ischemic stroke, with a pooled prevalence rate of 12.9% patients developing AKI, and AKI is associated with increased mortality rate.13 In a cohort of 40 Hispanic stroke patients (mean age 69.13 years, 90% of patients had ischemic stroke), 62.5% patients developed AKI.14 In a 10-year follow-up study of a large cohort of first-ever acute stroke patients, approximately 27% of patients were found to develop AKI and had higher mortality rate than stroke patients without AKI.15 In stroke patients treated with or without tPA, AKI prevalence did not differ significantly (35.5% in tPA treated vs 33.89% in non-tPA treated patients developed AKI), but patients who developed AKI had a significantly higher in-hospital mortality rate (50.0% mortality in patients with AKI vs 3.4% in patients without AKI).16 In a 7-year follow-up study, acute stroke patients with reduced admission calculated creatinine clearance, increased serum creatinine and urea concentrations, and increased ratio of urea to creatinine had increased short-term and long-term mortality rates.17 Symptoms of AKI such as decreased eGFR and elevated serum levels of uric acid have been reported to present within 72 h of stroke.14,16 Largely, stroke patients with severe neurological deficits, cardiac abnormalities such as heart failure, atrial fibrillation and ischemic heart disease, hyperglycemia, hypertension, low eGFR, or advanced age were more susceptible to developing AKI.15,17 While the incidence of AKI after acute ischemic stroke varies widely based on AKI defining criteria and coding definitions, an increased mortality risk has been reported among patients who develop AKI after stroke.15,16 Therefore, acute management of kidney dysfunction after stroke may be important to improve post stroke outcome and decrease mortality rates.
Hemorrhagic stroke-induced renal dysfunction
A meta-analysis including 12 studies reported that AKI is a common complication of both acute ischemic stroke and intracerebral hemorrhage (ICH).13 In a prospective cohort study including both ischemic stroke (27/52 patients) and hemorrhagic stroke (25/52 patients), patients with hemorrhagic stroke had lower admission eGFR compared to ischemic stroke patients, and 64% patients in the hemorrhagic stroke subgroup and 33.3% patients in the ischemic stroke subgroup developed renal impairment.18 In this cohort, the number of patients with hypertension (newly diagnosed or uncontrolled) was significantly higher in hemorrhagic stroke patients compared to ischemic stroke, although the mean arterial pressure at admission in patients who developed renal impairment from those who did not was not statistically significant.18 In another study, at the time of hospital admission, patients with ischemic stroke subtype had a significantly higher mean serum creatinine level compared to patients with hemorrhagic stroke.19 Nearly 30% of ICH patients have CKD, and CKD patients were likely to be older, female, and present with comorbidities such as diabetes.20 In ICH patients, risk of AKI is not significantly different between patients with normal kidney function or CKD at admission; however, mortality rates were significantly higher in ICH patients with normal kidney function compared to ICH patients with CKD at admission.20,21 Maximum systolic blood pressure reduction was dichotomized to 90 mmHg and found to increase the AKI risk in ICH patients with normal renal function.21 AKI in ICH patients has been associated with hyperosmolality caused by administration of mannitol, an osmotic agent used to decrease intracranial pressure.22 AKI with transient elevation of urea and creatinine was more frequent and more severe in ICH patients receiving higher mannitol infusion rates (≥1.34 g/kg/day) than in ischemic stroke patients.22 Additionally, risk of AKI in ICH patients receiving mannitol increased with advancing age, hypertension, and low GFR.22 In a large prospective study of patients with aneurysmal subarachnoid hemorrhage, decreases in creatinine clearance were associated with significantly worse 3-month outcomes.23 Renal impairment after ICH was not dependent on the location or size of hematoma.18 However, in ICH patients with moderate to severe CKD, renal impairment was found to be associated with larger lobar hematomas and poor outcome.24 ICH significantly increases intracranial pressure which may lead to kidney dysfunction via sympathetic activation.25 Renal dysfunction after ICH is usually transient and rarely requires renal replacement therapy.18,25 In this review article, we will focus on ischemic stroke-induced renal dysfunction.
Effects of renal dysfunction on ischemic stroke
There is a significant increase in risk of stroke and post-stroke mortality in patients with CKD and with end-stage renal disease.26 Proteinuria is associated with severe neurological deficits and higher in-hospital mortality after stroke.3 Hemorrhagic stroke is a leading cause of death in patients with CKD. The risk of atherosclerotic cardiovascular diseases such as myocardial infarction and stroke is 5–30 times higher in CKD patients with greater risk associated with patients on dialysis.27 Patients with end-stage renal disease undergoing peritoneal dialysis or hemodialysis have a high risk of stroke, with an increased risk of hemorrhagic stroke among hemodialysis patients.28 In a large-scale study involving 4315 patients over an average follow-up period of 3.36 years, the risk of de novo stroke, stroke severity, and stroke-related mortality was found to be significantly elevated among AKI patients who recovered following dialysis treatment (regardless of subsequent progression to CKD) compared to the non-AKI group.29 In patients with kidney disease undergoing dialysis, the increased risk of stroke may be attributed at least partially to impaired cerebral perfusion resulting from the sudden decrease in systemic blood pressure during dialysis.29 However, other mechanisms may also contribute to renal dysfunction-induced cerebral injury. Severe ischemic AKI in mice increases proinflammatory chemokine expression in the brain, increases neuronal pyknosis, induces microgliosis and astrogliosis in the brain, disrupts the blood brain barrier (BBB), and impairs locomotor function compared to sham control mice.30 In CKD mice, comorbidity with ischemic stroke significantly increases infarct volume, worsens neurological deficits, and significantly increases inflammatory responses such as increased M1 microglia/macrophage and inflammatory cytokine expression in the ischemic brain.31 In addition to chronic inflammation, increased oxidative stress, decreased nitric oxide production, endothelial dysfunction, platelet aggregation, and vascular injury may contribute to stroke in CKD patients.26 The mechanisms underlying CKD induced aggravated stroke pathogenesis, and worse stroke outcomes have been recently reviewed.32 Comorbidities such as hypertension may account partially for increased stroke risk in CKD patients.27 Hypertension is known to increase the risk of stroke and worsen stroke outcome.33 Hypertension may be a cause as well as a consequence of kidney dysfunction, with kidney dysfunction increasing blood pressure and sustained high blood pressure accelerating renal dysfunction. Hypertension can result from sympathetic activation34 as well as increased activity of the rennin angiotensin aldosterone system.35 Hypertension increases vascular shear stress, endothelial dysfunction, and increases arterial stiffness which can affect cerebral autoregulation and lead to stroke.33 Animals subjected to a renovascular model of chronic arterial hypertension not only developed spontaneous brain lesions but also developed larger brain lesions when subject to ischemia.36 Blood pressure management may be an important therapeutic strategy for primary and secondary stroke prevention in CKD patients.
Effects of age and sex in brain–kidney interaction after stroke
In addition to comorbidities such as hypertension and diabetes, age and sex may be factors affecting brain–kidney interaction. The prevalence of CKD rises dramatically with age and while 38% of elderly (>65 years) population have moderate or severe CKD, only 13% of middle-aged adults (45–64 years) and 7% young adults (18–44 years) have CKD.37 While the kidneys are affected by aging, the elderly with low eGFR usually present with comorbidities such as arteriosclerosis and hypertension while healthy aging adults have modest decline in GFR.38 The rate of progression from CKD to end-stage renal disease with patients needing dialysis or kidney replacement increases with advancing age.39 CKD is more common in women (15%) compared to men (12%), and the progression of CKD to end-stage renal disease is faster in men than in women; however, women with CKD are at a higher risk of fatal strokes compared to men with CKD.37 AKI indicated by high serum creatinine levels within 48 hours of admission after stroke, is associated with increased mortality.19 At the time of hospital admission, while the mean serum creatinine level did not differ between ischemic and hemorrhagic stroke subtypes in men, the mean serum creatinine level was significantly higher in women with ischemic stroke compared to women with hemorrhagic stroke.19 Interestingly, age but not gender varied significantly between patients with ischemic or hemorrhagic subtype—with older patients (mean age 72) presenting with ischemic stroke and younger patients (mean age 65) presenting with hemorrhagic stroke.19 The elderly population who develop CKD are highly susceptible to AKI due to their reduced renal function reserve.38 Risk factors associated with AKI in the elderly include age-related structural and functional changes in the kidney, presence of comorbidities, and diagnostic (contrast agents) and pharmacological interventions.40 Post stroke incidence of AKI was significantly elevated in elderly patients after acute ischemic stroke.15 However, the risk of AKI after ischemic stroke did not differ between elderly men and women.15 Further studies are warranted to investigate effects of age and sex on brain–kidney interaction after stroke.
Mechanisms of kidney dysfunction following stroke
CAN mediates kidney dysfunction after stroke
The CAN is an integral internal regulation system that controls visceral motor and neuroendocrine responses. Lesions of the brain parenchyma affecting CAN or compression around the CAN after brain injury stimulate the superior sympathetic center, the visceral motor pathway, and finally the sympathetic nerve endings which then release norepinephrine. The role of CAN in mediating kidney dysfunction after stroke is discussed below and summarized in Figure 1. CAN consists of the insular cortex, medial prefrontal cortices, hypothalamus, periaqueductal gray matter, amygdala, ventrolateral medulla, nucleus of the tractus solitaries, and part of primary motor cortex and the rostromedial motor area. The primary and rostromedial cortex are autonomic control centers of the kidney and influence kidney functioning. Physiological experiments in rodents have demonstrated that electrical stimulation of primary motor cortex and contiguous areas of premotor cortex leads to changes in visceromotor function including a reduction in renal blood flow.41 Therefore, brain damage to primary and rostromedial motor area may affect renal function. Since studies on the visceral motor pathways are mostly based on the study of static and dead animals, there is a lack of in vivo techniques for real-time observation of the visceral motor pathway. Prospective and retrospective cohort studies of the changes in the renal function after frontal lobe injury are needed. The hypothalamus is an important component of the limbic system and innervates the sympathetic preganglionic neurons which mainly dwell on the intermediolateral column of the spinal cord and have synaptic connections with renal postganglionic neurons. Studies have shown that disinhibition of the paraventricular nucleus of the hypothalamus decreases eGFR, urinary sodium excretion, urine flow, and renal plasma flow.42 Therefore, injury to the brain disrupting these thoracic segments and the fiber tracts projecting to the hypothalamus may result in kidney dysfunction. The insular, medial prefrontal, and other regions in the prefrontal cortex are high-order autonomic control centers. The insular lobe plays an important role in regulating visceral function. Both insular infarction and insular surgical resection cause heart disease (myocardial necrosis, cardiac arrhythmias, and ECG abnormalities).43 Following a model of insular cortical lesion in normotensive rats, renal sympathetic nerve discharge immediately increased at 10 min after insular cortex damage.44 However, renal dysfunction following damage to CAN components has not been investigated.
Neuroendocrine system
Hypothalamic–pituitary axis and sympathetic pathway
The hypothalamus–pituitary–adrenal axis regulates glucocorticoid release from the adrenal glands and can be directly activated following injury to the brain such as stroke, thereby altering serum glucocorticoid levels.45 Glucocorticoids affect renal function by influencing glomerular and tubular function.46 Short-term administration of glucocorticoids leads to eGFR increase, but chronic exposure of glucocorticoids may decrease GFR.46 In patients with acute cerebral ischemia, peripheral sympathetic nerve excitation stimulates the adrenal glands to secrete catecholamines into the bloodstream resulting in elevated plasma norepinephrine levels.47 Adrenergic receptors are expressed in juxtaglomerular cells, renal tubules, and in the vasculature. Norepinephrine and epinephrine released by the sympathetic nerve innervating the kidney and in plasma, can bind to these adrenergic receptors. High intensity and long duration of sympathetic excitation leads to the binding of catecholamine and angiotensin II to their receptors of the renal artery, and prompts constriction and contraction of the renal artery leading to renal ischemia.48 Angiotensin II participates in renal fibrosis formation.49 Furthermore, acute cerebral injury is often involved with elevated serum catecholamines and visceral sympathetic nervous system activity which lead to hypertension.50 Therefore, prolonged elevation of catecholamines may lead to kidney disease.
Renin–angiotensin–aldosterone system
Angiotensin II is involved in the pathogenesis of stroke, and is increased in the plasma of patients with mild stroke.51 The renin–angiotensin–aldosterone system may play an important role in the pathogenesis of glomerular and interstitial fibrosis in the kidney.49 In vitro, several cell types (mesangial and tubular cells) respond to angiotensin II stimulation by increasing transforming growth factor (TGF) expression and synthesis.52 Infusion of angiotensin II into different biological species contributes to renal injury and progression to renal fibrosis.53 Angiotensin II promotes the secretion of vasopressin or antidiuretic hormone (ADH), which regulates vasoconstriction and water retention in the body. In response to ischemic stress after stroke, sympathetic nervous system activity increases, which also increases ADH generation.54 In vivo experiments have suggested several mechanisms of angiotensin II-mediated kidney dysfunction. For instance, direct stimulation of fibroblasts by angiotensin II may contribute to the pathogenesis of renal interstitial fibrosis by inducing the expression of TGF and angiotensinogen genes.55 Both mouse and human studies suggest that angiotensin II induces interleukin-6 (IL-6) production in the kidney, and that increased IL-6 may play an important pathogenic role in CKD by inducing fibrotic gene expression and Endothelin-1 gene expression.53 Moreover, in vivo, angiotensin II infusion increases glomerular TGF-p production.52 Angiotensin II can directly activate glomerular expression of cytokines, inflammatory, and fibrotic factors that alter renal hemodynamics.56 In addition to direct actions on renal glomerular and tubular cells, angiotensin II stimulates macrophage accumulation in the glomerulus and tubule cells.56 Aldosterone regulates kidney sodium and potassium handling and blood pressure. High aldosterone level has been associated with worse stroke outcome in patients with and without hypertension.57 Aldosterone stimulates the production of reactive oxygen species (ROS), inflammation and fibrosis of kidney.58 Therefore, the renin–angiotensin–aldosterone system may be involved in molecular mechanisms of stroke-induced kidney dysfunction.
ADH hormone
ADH is normally synthesized in the supraoptic and paraventricular nuclei of hypothalamus, but can also be released in response to sympathetic activation. Ischemic stroke, as a stressor, can increase the activity of sympathetic nerves. The combination of ADH released into the blood and the V1 receptor and V2 receptor expressed in the kidney lead to an increase of corresponding water channel proteins. The V2 receptor activation leads to increased numbers of aquaporin 2 channels in the apical membrane, and aquaporin 2 channel syntheses causes increased water reabsorption.42 V1a receptor activation increases the renal water reabsorption and osmotic gradient. In addition, activation of V1a receptors expressed in the glomeruli causes reduced proximal tubular filtrate flow, leading to increased renal tubular sodium reabsorption and mesangial constriction with reduced glomerular blood flow.59 Thus, stroke may affect kidney function by interaction between ADH and V1 and V2 reception.
Role of autoregulation in mediating kidney dysfunction after brain injury
Autoregulation of blood flow is a mechanism that maintains constant and continuous perfusion during changes in blood pressure. Autoregulation is present in both the cerebral and renal vasculature. The vasculature of the kidney and brain are low resistance arterial beds where autoregulation is maintained by myogenic and metabolic mechanisms. Cerebral autoregulation can be modulated by sympathetic nervous activity, the vascular renin–angiotensin system, and other factors that alter cerebral blood flow (CBF). Cerebral autoregulation is compromised following acute ischemic stroke.60 In stroke patients with ischemia in the middle cerebral artery territory, dynamic cerebral autoregulation and kidney function indicated by eGFR were evaluated within 6 h of stroke onset, and stroke outcome was measured using modified Rankin Scale at 3 months after stroke.60 This study found a significant correlation between reduced eGFR values and reduced autoregulation in the brain.60 In addition, both reduced eGFR and decreased cerebral autoregulation were associated with increased white matter lesion burden, and were predictors of poor stroke outcome at 3 months after stroke.60 Secondary complications such as hemorrhagic transformation and cerebral edema are also associated with reduced eGFR and worse cerebral autoregulation and may contribute to worse stroke outcome.60 Many of the patients had diabetes (30%), dyslipidemia (59%), and hypertension (61%); however, these vascular risk factors were not related to cerebral autoregualtion.60
Role of inflammatory and immune responses in mediating kidney dysfunction after brain injury
Inflammation and immune responses play an important role in the pathological progression of stroke. Injury to the brain increases the expression of inflammatory factors such as C-reactive protein (CRP), IL-6, IL-1β, ROS, tumor necrosis factor-α (TNF-α), matrix metalloproteinase-9 (MMP9), and IL-33 in the brain tissue and blood, as well as increases the infiltration of immune cells, including adaptive immune cells and innate immune cells into the brain. Inflammation and immune system activation are vital factors in the development of AKI and CKD. The innate immune response is nonspecific and is often the first response to invading pathogens. Macrophages express many cytotoxic moieties, including proteolytic enzymes, and pro-inflammatory cytokines and chemokines, and ROS. Macrophage-derived inflammatory cytokines such as ROS and interleukins (IL-1, IL-6, IL-23) play a key role in mediating kidney impairment in experimental models of kidney injury.56 Following infiltration into the kidney, macrophages may promote inflammatory injury and renal fibrosis or tissue repair depending on their phenotype (M1 vs M2).61 In a mouse model of renal ischemia–reperfusion injury, natural killer (NK) cells can promote apoptosis in tubular epithelial cells, and depletion of NK cells protects against ischemia–reperfusion injury.62 Recruitment of neutrophils into the kidney is hallmark in AKI pathogenesis and is observed in ischemia–reperfusion injury as well as sepsis-induced AKI models in mice.63 Neutrophil degranulation leads to the release of oxygen free radicals, proteases, myeloperoxidase and several cytokines, which amplify renal tissue injury.64 Systemic inflammation and immune responses mediated by the spleen likely play a central role in promoting kidney dysfunction after brain injury, and is discussed below and summarized in Figure 2.
C-reactive protein
CRP is a protein synthesized and secreted primarily by the liver hepatocytes into the bloodstream in response to inflammatory signals released by the macrophages and adipocytes.65 CRP can also be synthesized by smooth muscle cells, macrophages, endothelial cells, lymphocytes, and adipocytes.65 Plasma CRP levels are increased after ischemic stroke, CKD, and kidney failure and serve as a biomarker of disease.66,67 Compared to healthy controls, ischemic stroke patients have elevated blood CRP levels in the acute phase of stroke as well as at their 3-month follow-up.66 Experimental studies have shown that injection of CRP increases infarct volume after stroke in rats,68 and high levels of CRP increase paracellular permeability and can pass the BBB in mice.69 Upon entering the ischemic brain, CRP bind to ligands exposed in damaged brain tissue and aggravates inflammation by activating the complement pathway.68 High CRP levels after stroke decrease renal filtration particularly in patients with high body mass; as high body fat-induced early inflammatory processes may predispose these patients to glomerular hyperfiltration related renal functional loss.70 Significantly elevated CRP plasma levels are found in patients with chronic renal failure.67 Elevated levels of CRP contribute to aggravated macrophage and T-cell infiltration and macrophage polarization to M1 macrophage phenotype, and is associated with increased expression of adhesion molecules (intercellular adhesion molecule 1), proinflammatory cytokines (IL-1β and TNF-α), and chemokines (monocyte chemoattractant protein-1).71 Elevated CRP levels also promote renal fibrosis including myofibroblast accumulation and collagen I and III production and deposition, which are likely to be associated with increased TGF-β/SMAD signaling.72 In rats subject to ischemic stroke, TGF-β1 and SMAD3 were both significantly upregulated in ischemic brain tissue.73 It is noteworthy that TGF-β1/SMAD3 may exert neuroprotective effects against ischemia-induced neuronal apoptosis.73 Increased TGF-β signaling promotes renal fibrosis through its downstream SMAD signaling.74 In addition, CRP induces IL-6 mRNA expression and protein release from HK-2 cells.75 TGF-1 is highly expressed in HK-2 cells, which suggests that CRP plays a critical role in renal fibrosis.75 Therefore, CRP is a likely mediator of brain injury-induced kidney dysfunction.
Interleukin-6
In the CNS, many cells can secrete IL-6 including neurons, astrocytes, microglia, and endothelial cells, and IL-6 can pass the BBB into the blood stream. Ischemic stroke is known to increase plasma levels of IL-6, a multifunctional proinflammatory cytokine, compared to healthy control subjects.76 The kidney takes part in the elimination of IL-6 from the systemic circulation.77 Plasma IL-6 is also increased in patients with creatinine clearance rate < 20 mL/min, chronic renal failure, or CKD.78 In both humans and mice, elevated IL-6 has been shown to directly induce fibrotic gene expression and facilitate progression of renal fibrosis.53 A number of in vitro studies have demonstrated that elevated IL-6 may give rise to epithelial cell apoptosis and damage, mesangial cell proliferation, and proliferation and infiltration of leukocytes which are associated with the pathophysiology of kidney disease.79 IL-6 also promotes the upregulation of multiple fibrotic genes including α2-procollagen, TGF-β, and plasminogen activator inhibitor-1 in the mouse kidney which are associated with kidney fibrosis.79 IL-6 induces CRP synthesis by the liver. Therefore, IL-6 is also a suitable candidate to mediate stroke-induced kidney dysfunction. However, whether elevated plasma IL-6 post stroke can penetrate the endothelial and epithelial cell layers into the mesangial region and tubular interstitial area, respectively, is unclear.
Reactive oxygen species
Increased ROS promote tissue destruction and cell death in patients with stroke.80 In the acute phase after stroke, there is a rapid increase in ROS production which impairs antioxidant defense mechanisms and causes additional tissue damage via promoting autophagy, apoptosis, and necrosis in the ischemic brain region.80 ROS have also been implicated in ischemia reperfusion injury, during which rapid restoration of CBF increases tissue oxygenation levels and triggers a second burst of ROS.80 BBB disruption following brain injury enables entry of ROS into the bloodstream.81 In the kidney, ROS increases endothelial cell permeability, which facilitates entry of high-molecular-weight compounds, inflammatory mediators, immune cells, and immune complexes into the endothelium of glomerular filtration membrane.82 ROS degrade the glomerular basement membrane and impair glomerular and tubular cell functions.82 ROS molecules may have key roles in facilitating TGF-β-induced SMAD2/3 activation which has an important role in renal fibrosis.83
Interleukin-1 beta
IL-1β is produced and secreted by activated microglia and infiltrating macrophages after stroke.84 IL-1β is generated as an acute inflammatory response after injury to the brain, and plays a vital pathogenic role in several chronic diseases.85 For instance, mice lacking IL-1α and IL-1β have significantly decreased ischemic infarct volume and edema compared to wild-type mice subjected to a model of stroke,86 and a selective IL-1β antibody treatment decreased ischemic injury.87 Serum levels of inflammatory cytokines IL-1β were markedly increased in the acute period after ischemic stroke in patients.76 In pigs subjected to a model of brain death, 6 h after brain death, there was significantly elevated expression of plasma cytokines including IL-6, IL-1β, and TNF-α.88 Interestingly, IL-6 and IL-1β were also significantly increased in peripheral organs such as kidney, heart, and lungs.88 IL-1β also plays a key role in kidney injury and fibrosis. Pro-inflammatory M1 macrophages induce kidney fibrosis by secreting IL-1β.89 Furthermore, there is a twofold increase in IL-1β levels following acute tubular necrosis induction, and an increase in neutrophil recruitment.90 Following AKI, IL-1β deficient mice show markedly less infiltration of inflammatory cells compared with controls.91 IL-1β increases the expression of ICAM-1 (CD54) on endothelial cells, which interacts with integrin molecules of neutrophils and monocytes to promote endothelial adherence.92 Treatment with monoclonal antibodies directed against ICAM-1 reduced kidney damage in ischemic acute tubular necrosis model.92 Therefore, increased circulating inflammatory factors such as IL-1β in response to stroke may contribute to kidney dysfunction.
Tumor necrosis factor-α
In preclinical stroke models, expression of the pro-inflammatory cytokine TNF-α was significantly increased in ischemic neurons and facilitated inflammatory cell infiltration into brain, thereby exacerbating brain tissue damage in the acute phase after stroke.93 In addition, brain parenchymal cells such as astrocytes, microglia, and endothelial cells also produce TNF-α.94 In the acute phase after ischemic stroke in mice, activated spleen cells secrete significantly increased levels of TNF-α which may explain elevated TNF-α expression in the peripheral circulation.95 On the other hand, TNF-α can exert protective and regenerative effects after ischemic stroke.96,97 TNF knockout mice or transgenic mice lacking TNF or the TNF receptor that are subjected to ischemic injury have increased ischemic infarct volume compared to corresponding wild-type control mice, indicating a protective role of TNF-α.96,97 The neuroprotective and neurotoxic roles of inflammatory cytokines such as TNF-α, IL-1β, and IL-6 have previously been discussed in detail.98 TNF-α is normally absent in the kidney, but under a disease state, TNF-α expression in kidney increases with infiltration of activated monocytes and macrophages and may contribute to progression of AKI to CKD.99 Recent studies have found that the expression level of TNF-α in serum and kidney in AKI correlated with the severity of kidney injury,100 and inhibition of the TNF-α protects against cisplatin-induced AKI.101 Therefore, whether increase in circulating TNF-α following stroke may contribute to kidney dysfunction warrants further investigation.
Interleukin-33
IL-33 is a pro-inflammatory cytokine and a member of the IL-1 family that is expressed by numerous cell types, including dendritic cells, macrophages, and endothelial cells. Serum IL-33 is significantly elevated in patients with acute ischemic stroke compared to healthy controls.102 Serum IL-33 is also elevated in cisplatin-induced AKI, and IL-33 promotes development of AKI through CD4+ T cell-mediated production of CXCL1.103 Therefore, it is likely that IL-33 contributes to the acute renal injury following stroke.
Matrix metalloproteinase-9
MMP9 is expressed in the hippocampus, cerebellum, and cortex in the brain, with limited expression in astrocytes and microglia and predominant expression in neurons. Ischemic stroke significantly increases plasma and brain MMP9 expression and mediates neuronal damage and apoptosis, inflammatory cascade and BBB disruption.104 In the adult kidney tissue, MMP9 is mainly expressed in collecting duct cells and podocytes.105 MMPs are a family of endopeptidases that degrade all components of the extracellular matrix, and under pathological conditions regulate remodeling of renal tissues.106 Elevated circulating MMP9 is associated with resistant albuminuria and CKD progression in patients.107 Therefore, it is possible that MMP9 also contributes to CKD following brain injury.
T cells
As a part of adaptive immune response to ischemic stroke in patients, peripheral blood T cells are activated, particularly, circulating T cells specific for central nervous system antigens are activated.108 The release of inflammatory mediators by activated T cells can infiltrate the kidney and cause renal dysfunction. T cells play a key role in the development of glomerular nephritis and CKD.109 CD8+ T cells can be activated by specific renal autoantigens which cause kidney damage and contribute to renal disease and inflammation.110 In this setting, activated CD8+ T cells can release other renal-specific autoantigens that cause further activation of CD8+ cells and greater renal damage.110 CD4+Th cells are mainly activated by inflammatory cytokines and antigen presenting cells. CD4+Th cells can be further classified into different subsets such as Th1 and Th17 cells. Th1 cells take part in tissue injury by the releasing inflammatory cytokines such as interferon-γ, IL-2, and TNF-α.111 The Th1 subset promotes the activation of macrophages and CD8+ T cells which lead to tissue injury.111 In experimental models of AKI, activation of pro-inflammatory Th1 and Th17, the phenotype of effecter memory CD4+T cell, directly contributes to kidney tissue damage via the action of released cytokines and chemokines.112 Th17 cytokines can drive renal inflammation partly by increasing TNF-α expression and upregulating chemokines which cause the infiltration of immune cells into the renal tissue.113
Immune complexes
In general, immune complexes mediate the pathogenesis of kidney disease by two ways: (1) deposition of soluble antigen antibody complex from blood circulation and other tissue in the glomeruli and (2) via the formation of in situ immune complexes. A recent study demonstrated that anti-brush border antibodies present in inflammatory bowel disease patients cross-react with renal proximal tubular brush-border membrane antigens.114 Other studies suggest an antigenic association between the human colon, human kidney, and the common antigen of entero-bacteriaceae.115 It is unknown whether the brain and kidney also have common antigens. Elevated levels of circulating neurofilament antibodies were found at 1, 3, and 6 months after stroke in patients.116 Therefore, injured brain cells release brain antigens to the extracellular milieu and the circulation which can then reach the kidney and cross-react with renal antigens and cause kidney disease. The antigens remaining in the glomerular filtration membrane and the corresponding antibody from the brain and circulation can react with the antigens to form immune complexes. Such formation of in situ immune complexes and their subsequent deposition in the glomeruli can activate inflammatory mediators. In stroke patients, serum concentration of immune complexes is significantly elevated.117 Immune complexes promote the synthesis of collagen fibers and enhance infiltration of monocytes and lymphocytes.118 If immune complexes released into blood remain in the glomerular filtration barrier, they may function similarly to in situ immune complexes and cause renal lesions.119
Extracellular vesicles and microRNAs mediate brain–kidney interaction
EVs are cell-derived membrane particles ranging from 30 to 5000 nm in size and include exosomes, microparticles, and apoptotic bodies. EVs mediate cell–cell communication and affect recipient cell function by carrying and delivering complex cargos of biomolecules (nucleic acids such as DNAs, ribosomal RNAs, circular RNAs, long noncoding RNAs and microRNAs (miRs), proteins, and lipids). MiRs are a class of small noncoding RNAs which regulate gene expression by degrading or blocking translation of mRNA targets at the post-transcriptional level. Over the past few years, the important role of miRs in human diseases is being unraveled. Upon injury to the brain, EVs released by endothelial cells, neurons, astrocytes, microglia, and other cells can mediate a “Yin-Yang” effect such as injury and repair mechanisms in the brain as well as be transported to distal organs. In our previous review article, we summarized evidence of systemic organ interaction in modulating neurological outcome after stroke and discussed the effects of stroke on other organs such as the heart, gut, spleen, and lungs in addition to kidney.120,121 Treating secondary injury to peripheral organs after stroke may be an important means to promote overall recovery, and future studies are warranted to elucidate the communication axes among the various organs.120
Role of EVs in mediating kidney dysfunction after injury to the brain
EVs participate in kidney development and normal physiology and in the pathogenesis of AKI, CKD, diabetic nephropathy, renal fibrosis, glomerular diseases, and end-stage renal disease.122 EVs transfer functional receptors and promote kinin-associated inflammation, as well as promote thrombosis, inflammation, and immune-mediated disease and regulate kidney dysfunction.123 Compared with normal control mice, AKI and CKD mice secrete high levels of EVs from kidneys and in urine, which contain elevated levels of inflammatory cytokine mRNA, particularly the chemokines chemokine (C-C motif) ligand 2 (CCL2).124 EV-mediated transfer of CCL2 mRNA from tubular epithelial cells to macrophages plays a critical role in albumin-induced tubule-interstitial inflammation.124 Inflammatory responses in the brain activate a systemic acute-phase response, which is dependent on the release of EVs into the circulation. A clinical study reported that levels of endothelial/platelet microparticles are increased in anti-coagulated atrial fibrillation patients, and endothelial/platelet microparticles levels are correlated with CKD identified by eGFR and serum creatinine.125 Circulating microparticles provide a pro-coagulant aminophospholipid surface and promote thrombosis and coagulation.126 Acute ischemic stroke increases circulating platelet EVs which are significantly correlated with stroke severity, and these platelet EVs increase thrombin generation.127 Systemic coagulation leads to small thrombi which can cause vascular obstruction, renal ischemia, and kidney dysfunction.
Role of miRs in mediating kidney dysfunction after injury to the brain
MiRs may play key roles in mediating inter-organ cross talk and peripheral organ injury after damage to the brain.120 It has recently been reported that cardiac dysfunction after ischemic stroke in mice may be induced by decreased expression of miR-126, an miR known to maintain vascular integrity and regulate angiogenesis.128 Similarly, stroke-induced alterations in miR expression levels may be directed to adjust cytokines, chemokines, and adhesion molecules to control the occurrence and development of renal disease.129 MiR expression is chronically affected after stroke, and dysregulated miRs have been detected in blood samples collected from stroke patients at 6–18 months after ischemia onset.130 In the peripheral blood of young stroke patients, 138 miRs were found to be upregulated while 19 miRs were downregulated.130 Among these differentially regulated miRs, miR-21, miR-29c, and miR-200b/c can potentially provoke kidney dysfunction. MiR-21 is a TGF-β1-induced miR that has been shown to promote renal fibrosis by positively regulating the expression of extracellular matrix and α-smooth muscle actin in tubular epithelial cells and fibrotic kidneys.131 In response to TGF-β, miR-21 is upregulated in tubular epithelial cells, which is mediated by SMAD3 signaling and results in increased renal fibrosis.131 In-vitro, human tubular epithelial cells cultured with TGF-β to induce fibrosis and treated with quercetin significantly attenuated fibrosis via suppressing the miR-21 expression and increasing the expression of its target genes such as phosphatase and tensin homolog and tissue inhibitor of metalloproteinases-3.132 In another study, by employing astragaloside IV treatment in diabetic mice, inhibition of miR-21 decreased podocyte dedifferentiation and mesangial cell activation thereby improving renal function and attenuating renal fibrosis.133 Elevated miR-29c promotes cell apoptosis and increases extracellular matrix protein accumulation in mice with diabetic nephropathy.134 In addition, knockdown of miR-29c in diabetic mice using a specific antisense oligonucleotide significantly attenuated renal impairment indicated by decreased albuminuria and kidney mesangial matrix accumulation.134 MiR-200b/c expression was significantly increased in glomeruli of type 1 and type 2 diabetic mice as well as in vitro in mouse mesangial cells treated with TGF-β1.135 Upregulation of miR-200b/c increases collagen type I alpha2 and collagen type IV alpha 1 expressions which are the main constituents of the extracellular matrix of kidney.135 MiR-181a appears to be a robust and stable biomarker, being significantly decreased by approximately 200-fold in exosomes of CKD patients compared to healthy controls.136 Overexpression of miR-181a activates TLR/NF-κB signaling thereby attenuating CKD symptoms such as glomerulosclerosis and renal tubular epithelial cell apoptosis.137 Overall, miRs may contribute to renal dysfunction after stroke, and further studies are warranted to investigate their use as novel biomarkers as well as therapeutic targets.
Future directions
Role of spleen
Leukocytes of spleen include various subsets of T cells, B cells, dendritic cells, and macrophages. There is mobilization of splenocytes after stroke, and the spleen releases immune cells such as T cells, macrophages and neutrophils and chemokines into the circulation following stroke.138 It has been shown that following stroke, immune cells migrate to heart tissue and induce cardiac dysfunction.139 A similar mechanism may also contribute to kidney dysfunction after stroke and requires investigation.
Role of gut microbiome
Emerging evidence indicates that the gut microbiota interact with the immune system, CNS, and kidney under normal as well as disease conditions. Systemic inflammation and immune responses are altered in response to stroke, kidney diseases, and by the gut microbiome.140 Stroke alters gut permeability which enables recognition of antigens of microbiota by the immune cells.141 Activated immune cells can then attack common antigens of colon and kidney leading to kidney injury. T cells are known to increase kidney disease142 and may mediate interaction of gut, brain, and kidney. Stroke also induces gut dysbiosis, which alters immune homeostasis, including reduced IL-17-producing T cells which accumulate in the meninges and take part in brain injury.143 Both experimental and clinical evidence have shown that IL-17-producing T cells contribute to renal diseases.144 Whether decreased IL-17-producing T cells in gut induced by stroke participate in kidney disease remains to be studied.
Role of imaging contrast agents
Early vascular imaging using computed tomographic angiography (CTA) and conventional angiography aids the diagnosis and management of acute stroke patients; however, there may be an associated risk of iodinated contrast dyes inducing AKI. A retrospective study comparing CTA and noncontrasted head computed tomography (NCHCT) in patients with acute ischemic stroke was conducted to evaluate the renal safety of using contrast agents as well as timeliness of tPA treatment.145 The study included 289 patients admitted to the University of Virginia Medical Center Emergency Department among which 54.8% were women, mean age of patients was 68.8 years, and 157 had CTA imaging and 132 had NCHCT only. The change in serum creatinine levels from presentation to 24–48 h later was used to assess AKI. Overall, 6.3% of ischemic stroke patients developed AKI, and there was no significant difference in the number of patients developing AKI between the patient groups subjected to CTA and NCHCT.145 Also, there was no significant difference in the time from admission to tPA administration between the two patient groups.145 Similarly, a large population-based stroke incidence study and a systematic review and meta-analysis evaluating AKI incidence in acute ischemic stroke patients undergoing CT imaging, also reported that the risk of developing AKI or worsening of existing renal disease after contrast dye was relatively small.146,147 A retrospective study conducted in China studied the correlation between proteinuria and contrast-induced AKI in stroke patients undergoing cerebral angiography (total 2015 patients with mean age of 52 years and 49.4% women).148 AKI was defined as ≥25% increase in baseline serum creatinine at 48 h after exposure to contrast agent. Contrast-induced AKI was observed in 4.2% of patients (82 out of 2015 patients), and proteinuria increased the risk of developing contrast-induced AKI by fivefold as well as increased 1-year mortality rate.148 Among the patients who developed contrast agent-induced AKI, there was a trend of advanced age, greater neurological severity, lower eGFR, and higher prevalence of proteinuria and anemia compared to patients who did not develop AKI.148 In the clinic, for patients presenting with ischemic stroke, the risk of contrast agent-induced AKI must be weighed against the diagnostic and interventional benefits of rapid and early assessment of vascular anatomical structures using imaging. It may be important to detect potential risks of renal dysfunction (proteinuria, serum creatinine, eGFR, etc.) to identify risk of contrast agent-induced AKI in stroke patients and device management plans.
Conclusions
Based on the cross talk between organs and related epidemiological data, this review summarizes mechanisms that may underlie stroke-induced renal dysfunction. Importantly, whether stimulation to the nerve center is sufficient to cause kidney disease remains to be confirmed by experimental preclinical studies. There is limited evidence of the mechanisms underlying direct brain–kidney interaction and kidney dysfunction after injury to the brain. The current evidence is largely associative, and further experiments investigating renal dysfunction after stroke are required to verify the pathological changes and to identify molecular mechanisms which may enable development of novel therapeutic interventions.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD
Qiang Zhao https://orcid.org/0000-0002-8383-913X
Poornima Venkat https://orcid.org/0000-0001-8938-663X
References
- 1.Toyoda K, Ninomiya T.Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol 2014; 13: 823–833. [DOI] [PubMed] [Google Scholar]
- 2.Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 1997; 51: 1908–1919. [DOI] [PubMed] [Google Scholar]
- 3.Kumai Y, Kamouchi M, Hata J, et al. Proteinuria and clinical outcomes after ischemic stroke. Neurology 2012; 78: 1909–1915. [DOI] [PubMed] [Google Scholar]
- 4.El Husseini N, Fonarow GC, Smith EE, et al. Association of kidney function with 30-day and 1-year poststroke mortality and hospital readmission. Stroke 2018; 49: 2896–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang IK, Liu CH, Yen TH, et al. Renal function is associated with 1-month and 1-year mortality in patients with ischemic stroke. Atherosclerosis 2018; 269: 288–293. [DOI] [PubMed] [Google Scholar]
- 6.Ovbiagele B, Sanossian N, Liebeskind DS, et al. Indices of kidney dysfunction and discharge outcomes in hospitalized stroke patients without known renal disease. Cerebrovasc Dis 2009; 28: 582–588. [DOI] [PubMed] [Google Scholar]
- 7.Chwojnicki K, Król E, Wierucki Ł, et al. Renal dysfunction in post-stroke patients. PloS One 2016; 11: e0159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao L, Lan W, Sun W, et al. Chronic kidney disease in patients with lacunar stroke. Stroke 2015; 46: 2081–2086. [DOI] [PubMed] [Google Scholar]
- 9.van Overbeek EC, Staals J, van Oostenbrugge RJ.Decreased kidney function relates to progression of cerebral microbleeds in lacunar stroke patients. Int J Stroke 2016; 11: 695–700. [DOI] [PubMed] [Google Scholar]
- 10.Kudo K, Konta T, Degawa N, et al. Relationship between kidney damage and stroke types in Japanese patients. Clin Exp Nephrol 2012; 16: 564–569. [DOI] [PubMed] [Google Scholar]
- 11.Yeh SJ, Jeng JS, Tang SC, et al. Low estimated glomerular filtration rate is associated with poor outcomes in patients who suffered a large artery atherosclerosis stroke. Atherosclerosis 2015; 239: 328–334. [DOI] [PubMed] [Google Scholar]
- 12.Makris K, Spanou L.Acute kidney injury: Definition, pathophysiology and clinical phenotypes. Clin Biochem Rev 2016; 37: 85–98. [PMC free article] [PubMed] [Google Scholar]
- 13.Zorrilla-Vaca A, Ziai W, Connolly ES, Jr, et al. Acute kidney injury following acute ischemic stroke and intracerebral hemorrhage: A meta-analysis of prevalence rate and mortality risk. Cerebrovasc Dis 2018; 45: 1–9. [DOI] [PubMed] [Google Scholar]
- 14.Olguín-Ramírez LA, Martínez HR, Gongora-Rivera F, et al. Acute kidney injury in acute stroke. A preliminary study in Hispanic population (P5.041). Neurology 2017; 88. [Google Scholar]
- 15.Tsagalis G, Akrivos T, Alevizaki M, et al. Long-term prognosis of acute kidney injury after first acute stroke. Clin J Am Soc Nephrol 2009; 4: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gadalean F, Simu M, Parv F, et al. The impact of acute kidney injury on in-hospital mortality in acute ischemic stroke patients undergoing intravenous thrombolysis. PloS One 2017; 12: e0185589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacWalter RS, Wong SY, Wong KY, et al. Does renal dysfunction predict mortality after acute stroke? A 7-year follow-up study. Stroke 2002; 33: 1630–1635. [DOI] [PubMed] [Google Scholar]
- 18.Shrestha P, Thapa S, Shrestha S, et al. Renal impairment in stroke patients: A comparison between the haemorrhagic and ischemic variants. F1000Res 2017; 6: 1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snarska K, Kapica-Topczewska K, Bachórzewska-Gajewska H, et al. Renal function predicts outcomes in patients with ischaemic stroke and haemorrhagic stroke. Kidney Blood Press Res 2016; 41: 424–433. [DOI] [PubMed] [Google Scholar]
- 20.Ovbiagele B, Schwamm LH, Smith EE, et al. Hospitalized hemorrhagic stroke patients with renal insufficiency: Clinical characteristics, care patterns, and outcomes. J Stroke Cerebrovasc Dis 2014; 23: 2265–2273. [DOI] [PubMed] [Google Scholar]
- 21.Burgess LG, Goyal N, Jones GM, et al. Evaluation of acute kidney injury and mortality after intensive blood pressure control in patients with intracerebral hemorrhage. J Am Heart Assoc 2018; 7: e008439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim MY, Park JH, Kang NR, et al. Increased risk of acute kidney injury associated with higher infusion rate of mannitol in patients with intracranial hemorrhage. J Neurosurg 2014; 120: 1340–1348. [DOI] [PubMed] [Google Scholar]
- 23.Zacharia BE, Ducruet AF, Hickman ZL, et al. Renal dysfunction as an independent predictor of outcome after aneurysmal subarachnoid hemorrhage: A single-center cohort study. Stroke 2009; 40: 2375–2381. [DOI] [PubMed] [Google Scholar]
- 24.Molshatzki N, Orion D, Tsabari R, et al. Chronic kidney disease in patients with acute intracerebral hemorrhage: Association with large hematoma volume and poor outcome. Cerebrovasc Dis 2011; 31: 271–277. [DOI] [PubMed] [Google Scholar]
- 25.Misra UK, Kalita J, Srivastava M, et al. Transient renal impairment in acute intracerebral haemorrhage. J Neurol 1996; 243: 417–420. [DOI] [PubMed] [Google Scholar]
- 26.Cherng Y-G, Lin C-S, Shih C-C, et al. Stroke risk and outcomes in patients with chronic kidney disease or end-stage renal disease: Two nationwide studies. PloS One 2018; 13: e0191155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longenecker JC, Coresh J, Powe NR, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: The CHOICE Study. J Am Soc Nephrol 2002; 13: 1918–1927. [DOI] [PubMed] [Google Scholar]
- 28.Boonpheng B, Thongprayoon C, Cheungpasitporn W.The comparison of risk of stroke in patients with peritoneal dialysis and hemodialysis: A systematic review and meta-analysis. J Evid Based Med 2018; 11: 158–168. [DOI] [PubMed] [Google Scholar]
- 29.Wu VC, Wu PC, Wu CH, et al. The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc 2014; 3. DOI: 10.1161/jaha.114.000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu M, Liang Y, Chigurupati S, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol 2008; 19: 1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henaut L, Grissi M, Brazier F, et al. Cellular and molecular mechanisms associated with ischemic stroke severity in female mice with chronic kidney disease. Sci Rep 2019; 9: 6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chelluboina B, Vemuganti R.Chronic kidney disease in the pathogenesis of acute ischemic stroke. J Cereb Blood Flow Metab 2019; 39(10): 1893–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cipolla MJ, Liebeskind DS, Chan SL.The importance of comorbidities in ischemic stroke: Impact of hypertension on the cerebral circulation. J Cereb Blood Flow Metab 2018; 38: 2129–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neumann J, Ligtenberg G, Klein II, et al. Sympathetic hyperactivity in chronic kidney disease: Pathogenesis, clinical relevance, and treatment. Kidney Int 2004; 65: 1568–1576. [DOI] [PubMed] [Google Scholar]
- 35.Riet LT, van Esch JH, Roks AJ, et al. Hypertension: Renin–angiotensin–aldosterone system alterations. Circ Res 2015; 116: 960–975. [DOI] [PubMed] [Google Scholar]
- 36.Menard B, Chazalviel L, Roussel S, et al. Two-kidney one-clip is a pertinent approach to integrate arterial hypertension in animal models of stroke: Serial magnetic resonance imaging studies of brain lesions before and during cerebral ischemia. J Cereb Blood Flow Metab 2018; 38: 1769–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CDC. Chronic kidney disease in the United States, 2019, https://www.cdc.gov/kidneydisease/publications-resources/2019-national-facts.html (accessed 8 August 2019).
- 38.Denic A, Glassock RJ, Rule AD.Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis 2016; 23: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prakash S, O’Hare AM.Interaction of aging and chronic kidney disease. Semin Nephrol 2009; 29: 497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yokota LG, Sampaio BM, Rocha EP, et al. Acute kidney injury in elderly patients: Narrative review on incidence, risk factors, and mortality. Int J Nephrol Renovasc Dis 2018; 11: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green HD, Hoff EC.Effects of faradic stimulation of the cerebral cortex on limb and renal volumes in the cat and monkey. Am J Physiol 1937; 118: 641–658. [Google Scholar]
- 42.Haselton JR, Vari RC.Neuronal cell bodies in paraventricular nucleus affect renal hemodynamics and excretion via the renal nerves. Am J Physiol 1998; 275: R1334–R1342. [DOI] [PubMed] [Google Scholar]
- 43.de Morree HM, Rutten GJ, Szabo BM, et al. Effects of Insula resection on autonomic nervous system activity. J Neurosurg Anesthesiol 2016; 28: 153–158. [DOI] [PubMed] [Google Scholar]
- 44.Butcher KS, Cechetto DF.Insular lesion evokes autonomic effects of stroke in normotensive and hypertensive rats. Stroke 1995; 26: 459–465. [DOI] [PubMed] [Google Scholar]
- 45.Mracsko E, Liesz A, Karcher S, et al. Differential effects of sympathetic nervous system and hypothalamic-pituitary-adrenal axis on systemic immune cells after severe experimental stroke. Brain Behav Immun 2014; 41: 200–209. [DOI] [PubMed] [Google Scholar]
- 46.Smets P, Meyer E, Maddens B, et al. Cushing’s syndrome, glucocorticoids and the kidney. Gen Comp Endocrinol 2010; 169: 1–10. [DOI] [PubMed] [Google Scholar]
- 47.Meyer JS, Stoica E, Pascu I, et al. Catecholamine concentrations in CSF and plasma of patients with cerebral infarction and haemorrhage. Brain 1973; 96: 277–288. [DOI] [PubMed] [Google Scholar]
- 48.Fujii T, Kurata H, Takaoka M, et al. The role of renal sympathetic nervous system in the pathogenesis of ischemic acute renal failure. Eur J Pharmacol 2003; 481: 241–248. [DOI] [PubMed] [Google Scholar]
- 49.López-Novoa JM, Rodríguez-Peña AB, Ortiz A, et al. Etiopathology of chronic tubular, glomerular and renovascular nephropathies: Clinical implications. J Transl Med 2011; 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nongnuch A, Panorchan K, Davenport A.Brain-kidney crosstalk. Crit Care 2014; 18: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masaki M, Masakazu K, Kana T, et al. Serum levels of renin-angiotensin system components in acute stroke patients. Geriatr Gerontol Int 2014; 14: 793–798. [DOI] [PubMed] [Google Scholar]
- 52.Kagami S, Border WA, Miller DE, et al. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Investig 1994; 93: 2431–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang W, Wang W, Yu H, et al. Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension 2012; 59: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joynt RJ, Feibel JH, Sladek CM.Antidiuretic hormone levels in stroke patients. Ann Neurol 1981; 9: 182–184. [DOI] [PubMed] [Google Scholar]
- 55.Ruiz-Ortega M, Egido J.Angiotensin II modulates cell growth-related events and synthesis of matrix proteins in renal interstitial fibroblasts. Kidney Int 1997; 52: 1497–1510. [DOI] [PubMed] [Google Scholar]
- 56.Imig JD, Ryan MJ.Immune and inflammatory role in renal disease. Compr Physiol 2013; 3: 957–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dinh QN, Arumugam TV, Young MJ, et al. Aldosterone and the mineralocorticoid receptor in the cerebral circulation and stroke. Exp Transl Stroke Med 2012; 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown NJ.Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol 2013; 9: 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davenport A.The brain and the kidney–organ cross talk and interactions. Blood Purif 2008; 26: 526–536. [DOI] [PubMed] [Google Scholar]
- 60.Castro P, Azevedo E, Rocha I, et al. Chronic kidney disease and poor outcomes in ischemic stroke: Is impaired cerebral autoregulation the missing link? BMC Neurol 2018; 18: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ricardo SD, van Goor H, Eddy AA.Macrophage diversity in renal injury and repair. J Clin Invest 2008; 118: 3522–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang ZX, Wang S, Huang X, et al. NK cells induce apoptosis in tubular epithelial cells and contribute to renal ischemia-reperfusion injury. J Immunol 2008; 181: 7489–7498. [DOI] [PubMed] [Google Scholar]
- 63.Herter JM, Rossaint J, Spieker T, et al. Adhesion molecules involved in neutrophil recruitment during sepsis-induced acute kidney injury. J Innate Immun 2014; 6: 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berry M, Clatworthy MR.Immunotherapy for acute kidney injury. Immunotherapy 2012; 4: 323–334. [DOI] [PubMed] [Google Scholar]
- 65.Sproston NR, Ashworth JJ.Role of C-reactive protein at sites of inflammation and infection. Front Immunol 2018; 9: 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ladenvall C, Jood K, Blomstrand C, et al. Serum C-reactive protein concentration and genotype in relation to ischemic stroke subtype. Stroke 2006; 37: 2018–2023. [DOI] [PubMed] [Google Scholar]
- 67.Pecoits-Filho R, Heimbürger O, Bárány P, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis 2003; 41: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 68.Gill R, Kemp JA, Sabin C, et al. Human C-reactive protein increases cerebral infarct size after middle cerebral artery occlusion in adult rats. J Cereb Blood Flow Metab 2004; 24: 1214–1218. [DOI] [PubMed] [Google Scholar]
- 69.Hsuchou H, Kastin AJ, Mishra PK, et al. C-reactive protein increases BBB permeability: Implications for obesity and neuroinflammation. Cell Physiol Biochem 2012; 30: 1109–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stuveling EM, Hillege HL, Bakker SJ, et al. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int 2003; 63: 654–661. [DOI] [PubMed] [Google Scholar]
- 71.Devaraj S, Jialal I.C-reactive protein polarizes human macrophages to an M1 phenotype and inhibits transformation to the M2 phenotype. Arterioscler Thromb Vasc Biol 2011; 31: 1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li ZI, Chung AC, Zhou L, et al. C-reactive protein promotes acute renal inflammation and fibrosis in unilateral ureteral obstructive nephropathy in mice. Lab Invest 2011; 91: 837–851. [DOI] [PubMed] [Google Scholar]
- 73.Zhu H, Gui Q, Hui X, et al. TGF-β1/Smad3 signaling pathway suppresses cell apoptosis in cerebral ischemic stroke rats. Med Sci Monit 2017; 23: 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang W, Koka V, Lan HY.Transforming growth factor-beta and Smad signalling in kidney diseases. Nephrology (Carlton) 2005; 10: 48–56. [DOI] [PubMed] [Google Scholar]
- 75.Wang HR, Chen DL, Zhao M, et al. C-reactive protein induces interleukin-6 and thrombospondin-1 protein and mRNA expression through activation of nuclear factor-kB in HK-2 cells. Kidney Blood Press Res 2012; 35: 211–219. [DOI] [PubMed] [Google Scholar]
- 76.Wytrykowska A, Prosba-Mackiewicz M, Nyka WM.IL-1beta, TNF-alpha, and IL-6 levels in gingival fluid and serum of patients with ischemic stroke. J Oral Sci 2016; 58: 509–513. [DOI] [PubMed] [Google Scholar]
- 77.Nowak M, Wyczalkowska-Tomasik A, Wlodarczyk Z, et al. The role of the kidney in the systemic elimination of interleukin 6, platelet-derived growth factor and transforming growth factor beta. Cytokine 2012; 59: 258–263. [DOI] [PubMed] [Google Scholar]
- 78.Lee BT, Ahmed FA, Hamm LL, et al. Association of C-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC Nephrol 2015; 16: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grassl C, Luckow B, Schlondorff D, et al. Transcriptional regulation of the interleukin-6 gene in mesangial cells. J Am Soc Nephrol 1999; 10: 1466–1477. [DOI] [PubMed] [Google Scholar]
- 80.Rodrigo R, Fernandez-Gajardo R, Gutierrez R, et al. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol Disord Drug Targets 2013; 12: 698–714. [DOI] [PubMed] [Google Scholar]
- 81.Chodobski A, Zink BJ, Szmydynger-Chodobska J.Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res 2011; 2: 492–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baud L, Ardaillou R.Reactive oxygen species: Production and role in the kidney. Am J Physiol 1986; 251: F765–F776. [DOI] [PubMed] [Google Scholar]
- 83.Rhyu DY, Yang Y, Ha H, et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol 2005; 16: 667–675. [DOI] [PubMed] [Google Scholar]
- 84.Clausen BH, Lambertsen KL, Meldgaard M, et al. A quantitative in situ hybridization and polymerase chain reaction study of microglial-macrophage expression of interleukin-1beta mRNA following permanent middle cerebral artery occlusion in mice. Neuroscience 2005; 132: 879–892. [DOI] [PubMed] [Google Scholar]
- 85.Dinarello CA.A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur J Immunol 2011; 41: 1203–1217. [DOI] [PubMed] [Google Scholar]
- 86.Boutin H, LeFeuvre RA, Horai R, et al. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci 2001; 21: 5528–5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamasaki Y, Matsuura N, Shozuhara H, et al. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke 1995; 26: 676–680; discussion 681. [DOI] [PubMed] [Google Scholar]
- 88.Skrabal CA, Thompson LO, Potapov EV, et al. Organ-specific regulation of pro-inflammatory molecules in heart, lung, and kidney following brain death. J Surg Res 2005; 123: 118–125. [DOI] [PubMed] [Google Scholar]
- 89.Zhang JD, Patel MB, Griffiths R, et al. Type 1 angiotensin receptors on macrophages ameliorate IL-1 receptor-mediated kidney fibrosis. J Clin Invest 2014; 124: 2198–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Faubel S, Lewis EC, Reznikov L, et al. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther 2007; 322: 8–15. [DOI] [PubMed] [Google Scholar]
- 91.Furuichi K, Wada T, Iwata Y, et al. Interleukin-1-dependent sequential chemokine expression and inflammatory cell infiltration in ischemia-reperfusion injury. Crit Care Med 2006; 34: 2447–2455. [DOI] [PubMed] [Google Scholar]
- 92.Kelly KJ, Williams WW, Jr, Colvin RB, et al. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc Natl Acad Sci U S A 1994; 91: 812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu T, Clark RK, McDonnell PC, et al. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke 1994; 25: 1481–1488. [DOI] [PubMed] [Google Scholar]
- 94.Sairanen T, Carpen O, Karjalainen-Lindsberg ML, et al. Evolution of cerebral tumor necrosis factor-alpha production during human ischemic stroke. Stroke 2001; 32: 1750–1758. [DOI] [PubMed] [Google Scholar]
- 95.Offner H, Subramanian S, Parker SM, et al. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab 2006; 26: 654–665. [DOI] [PubMed] [Google Scholar]
- 96.Gary DS, Bruce-Keller AJ, Kindy MS, et al. Ischemic and excitotoxic brain injury is enhanced in mice lacking the p55 tumor necrosis factor receptor. J Cereb Blood Flow Metab 1998; 18: 1283–1287. [DOI] [PubMed] [Google Scholar]
- 97.Bruce AJ, Boling W, Kindy MS, et al. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med 1996; 2: 788–794. [DOI] [PubMed] [Google Scholar]
- 98.Lambertsen KL, Biber K, Finsen B.Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab 2012; 32: 1677–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lech M, Gröbmayr R, Ryu M, et al. Macrophage phenotype controls long-term AKI outcomes—Kidney regeneration versus atrophy. J Am Soc Nephrol 2014; 25: 292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ramesh G, Reeves WB.TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 2002; 110: 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ozkok A, Edelstein CL.Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int 2014; 2014: 967826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qian L, Yuanshao L, Wensi H, et al. Serum IL-33 is a novel diagnostic and prognostic biomarker in acute ischemic stroke. Aging Dis 2016; 7: 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Akcay A, Nguyen Q, He Z, et al. IL-33 exacerbates acute kidney injury. J Am Soc Nephrol 2011; 22: 2057–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chaturvedi M, Kaczmarek L.Mmp-9 inhibition: A therapeutic strategy in ischemic stroke. Mol Neurobiol 2014; 49: 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Piedagnel R, Murphy G, Ronco PM, et al. Matrix metalloproteinase 2 (MMP2) and MMP9 are produced by kidney collecting duct principal cells but are differentially regulated by SV40 large-T, arginine vasopressin, and epidermal growth factor. J Biol Chem 1999; 274: 1614–1620. [DOI] [PubMed] [Google Scholar]
- 106.Tan RJ, Liu Y.Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol 2012; 302: F1351–F1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pulido-Olmo H, Garcia-Prieto CF, Alvarez-Llamas G, et al. Role of matrix metalloproteinase-9 in chronic kidney disease: A new biomarker of resistant albuminuria. Clin Sci (Lond) 2016; 130: 525–538. [DOI] [PubMed] [Google Scholar]
- 108.Wang WZ, Olsson T, Kostulas V, et al. Myelin antigen reactive T cells in cerebrovascular diseases. Clin Exp Immunol 1992; 88: 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sung SS, Bolton WK.T cells and dendritic cells in glomerular disease: The new glomerulotubular feedback loop. Kidney Int 2010; 77: 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Panzer U, Kurts C.T cell cross-talk with kidney dendritic cells in glomerulonephritis. J Mol Med (Berl) 2010; 88: 19–26. [DOI] [PubMed] [Google Scholar]
- 111.Holdsworth SR, Kitching AR, Tipping PG.Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int 1999; 55: 1198–1216. [DOI] [PubMed] [Google Scholar]
- 112.Dong X, Bachman LA, Miller MN, et al. Dendritic cells facilitate accumulation of IL-17 T cells in the kidney following acute renal obstruction. Kidney Int 2008; 74: 1294–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Turner JE, Paust HJ, Steinmetz OM, et al. The Th17 immune response in renal inflammation. Kidney Int 2010; 77: 1070–1075. [DOI] [PubMed] [Google Scholar]
- 114.Skogh T, Heuman R, Tagesson C.Anti-brush border antibodies (ABBA) in Crohn’s disease. J Clin Lab Immunol 1982; 9: 147–150. [PubMed] [Google Scholar]
- 115.Rabin BS, Rogers S.Pathologic changes in the liver and kidney produced by immunization with intestinal antigens. Am J Pathol 1976; 84: 201–210. [PMC free article] [PubMed] [Google Scholar]
- 116.Bornstein NM, Aronovich B, Korczyn AD, et al. Antibodies to brain antigens following stroke. Neurology 2001; 56: 529–530. [DOI] [PubMed] [Google Scholar]
- 117.Tarnacka B, Gromadzka G, Czlonkowska A.Increased circulating immune complexes in acute stroke: The triggering role of Chlamydia pneumoniae and cytomegalovirus. Stroke 2002; 33: 936–940. [DOI] [PubMed] [Google Scholar]
- 118.Hegele RA.The pathogenesis of atherosclerosis. Clin Chim Acta 1996; 246: 21–38. [DOI] [PubMed] [Google Scholar]
- 119.Mathieson PW.What has the immune system got against the glomerular podocyte? Clin Exp Immunol 2003; 134: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Venkat P, Chen J, Chopp M.Exosome-mediated amplification of endogenous brain repair mechanisms and brain and systemic organ interaction in modulating neurological outcome after stroke. J Cereb Blood Flow Metab 2018; 38: 2165–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen Z, Venkat P, Seyfried D, et al. Brain-heart interaction: Cardiac complications after stroke. Circ Res 2017; 121: 451–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang W, Zhou X, Zhang H, et al. Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol 2016; 311: F844–F851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Karpman D, Stahl AL, Arvidsson I.Extracellular vesicles in renal disease. Nat Rev Nephrol 2017; 13: 545–562. [DOI] [PubMed] [Google Scholar]
- 124.Lv LL, Feng Y, Wen Y, et al. Exosomal CCL2 from tubular epithelial cells is critical for albumin-induced tubulointerstitial inflammation. J Am Soc Nephrol 2018; 29: 919–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lau YC, Xiong Q, Blann AD, et al. Relationship between renal function and circulating microparticles, soluble P-selectin and E-selectin levels in atrial fibrillation. J Thromb Thrombolysis 2017; 43: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Puddu P, Puddu GM, Cravero E, et al. The involvement of circulating microparticles in inflammation, coagulation and cardiovascular diseases. Can J Cardiol 2010; 26: 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yao Z, Wang L, Wu X, et al. Enhanced procoagulant activity on blood cells after acute ischemic stroke. Transl Stroke Res 2017; 8: 83–91. [DOI] [PubMed] [Google Scholar]
- 128.Chen J, Cui C, Yang X, et al. MiR-126 affects brain-heart interaction after cerebral ischemic stroke. Transl Stroke Res 2017; 8(4): 374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Trionfini P, Benigni A, Remuzzi G.MicroRNAs in kidney physiology and disease. Nat Rev Nephrol 2014; 11: 23. [DOI] [PubMed] [Google Scholar]
- 130.Tan KS, Armugam A, Sepramaniam S, et al. Expression profile of MicroRNAs in young stroke patients. PloS One 2009; 4: e7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhong X, Chung ACK, Chen H-Y, et al. Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol 2011; 22: 1668–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cao Y, Hu J, Sui J, et al. Quercetin is able to alleviate TGF-beta-induced fibrosis in renal tubular epithelial cells by suppressing miR-21. Exp Ther Med 2018; 16: 2442–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang X, Gao Y, Tian N, et al. Astragaloside IV improves renal function and fibrosis via inhibition of miR-21-induced podocyte dedifferentiation and mesangial cell activation in diabetic mice. Drug Des Devel Ther 2018; 12: 2431–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Long J, Wang Y, Wang W, et al. MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J Biol Chem 2011; 286: 11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kato M, Arce L, Wang M, et al. A microRNA circuit mediates transforming growth factor-|[beta]|1 autoregulation in renal glomerular mesangial cells. Kidney Int 2011; 80: 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Khurana R, Ranches G, Schafferer S, et al. Identification of urinary exosomal noncoding RNAs as novel biomarkers in chronic kidney disease. RNA 2017; 23: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu L, Pang XL, Shang WJ, et al. Over-expressed microRNA-181a reduces glomerular sclerosis and renal tubular epithelial injury in rats with chronic kidney disease via down-regulation of the TLR/NF-kappaB pathway by binding to CRY1. Mol Med 2018; 24: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Offner H, Subramanian S, Parker SM, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol 2006; 176: 6523–6531. [DOI] [PubMed] [Google Scholar]
- 139.Yan T, Chen Z, Chopp M, et al. Inflammatory responses mediate brain-heart interaction after ischemic stroke in adult mice. J Cereb Blood Flow Metab 2018: 271678x18813317. DOI: 10.1177/0271678x18813317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mazmanian SK, Liu CH, Tzianabos AO, et al. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005; 122: 107–118. [DOI] [PubMed] [Google Scholar]
- 141.Singh V, Roth S, Llovera G, et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci 2016; 36: 7428–7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kurts C, Panzer U, Anders HJ, et al. The immune system and kidney disease: Basic concepts and clinical implications. Nat Rev Immunol 2013; 13: 738–753. [DOI] [PubMed] [Google Scholar]
- 143.Benakis C, Brea D, Caballero S, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med 2016; 22: 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim YG, Kim EY, Ihm CG, et al. Gene polymorphisms of interleukin-17 and interleukin-17 receptor are associated with end-stage kidney disease. Am J Nephrol 2012; 36: 472–477. [DOI] [PubMed] [Google Scholar]
- 145.Ehrlich ME, Turner HL, Currie LJ, et al. Safety of computed tomographic angiography in the evaluation of patients with acute stroke. Stroke 2016; 47: 2045–2050. [DOI] [PubMed] [Google Scholar]
- 146.Demel SL, Grossman AW, Khoury JC, et al. Association between acute kidney disease and intravenous dye administration in patients with acute stroke. Stroke 2017; 48: 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brinjikji W, Demchuk AM, Murad MH, et al. Neurons over nephrons. Stroke 2017; 48: 1862–1868. [DOI] [PubMed] [Google Scholar]
- 148.Tao Y, Dong W, Li Z, et al. Proteinuria as an independent risk factor for contrast-induced acute kidney injury and mortality in patients with stroke undergoing cerebral angiography. J Neurointerv Surg 2017; 9: 445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]