Abstract
Studies evaluating the effect of reperfusion on ischemic edema in acute stroke described conflicting results. Net water uptake (NWU) per brain volume is a new quantitative imaging biomarker of space-occupying ischemic edema, which can be measured in computed tomography (CT). We sought to investigate the effects of vessel recanalization on the formation of ischemic brain edema using quantitative NWU. In this multicenter observational study, acute ischemic stroke patients with a large vessel occlusion (LVO) in the anterior circulation were consecutively screened. Patients with vessel recanalization (thrombolysis in cerebral infarction (TICI) 2 b or 3) versus persistent vessel occlusion (no thrombectomy, TICI 0-1) were compared. Lesion-NWU was quantified in multimodal admission CT and follow-up CT (FCT), and ΔNWU was calculated as difference. Of 194 included patients, 150 had successful endovascular recanalization and 44 persistent LVO. In FCT after treatment, the mean (standard deviation) ΔNWU was 15.8% (5.7) in patients with persistent LVO and 9.8% (5.8) with vessel recanalization (p < 0.001). In multivariate regression analysis, vessel recanalization was independently associated with a lowered ΔNWU by 6.3% compared to LVO (95% confidence interval: 3.7–9.0, p < 0.001). Successful vessel recanalization was associated with a significantly reduced formation of ischemic brain edema. Quantitative NWU may be used to compare the treatment effects in acute stroke.
Keywords: Biomarker, brain edema, computed tomography, stroke, thrombectomy
Introduction
Endovascular thrombectomy is of benefit to most patients with acute ischemic stroke caused by an occlusion of the proximal anterior circulation.1–6 However, studies on the association between reperfusion and ischemic edema described conflicting results.7–10 Tissue water uptake after stroke onset caused by a large vessel occlusion (LVO) follows a characteristic course as recently described.11,12 Early after onset of ischemia, osmotically active molecules move from the interstitial spaces into the intracellular compartment causing oncotic cell swelling (i.e. cytotoxic edema). This rearrangement of parenchymal water does not involve any net uptake of water into the brain tissue but generates a new gradient of Na+ across the blood–brain barrier that promotes the influx of water (i.e. ionic edema). Subsequently, the blood–brain barrier gets disrupted as a result of the breakdown of endothelial junctions causing the formation of vasogenic edema with further net water uptake (NWU).8 In large space-occupying infarctions, progressive ischemic edema may lead to severe complications and malignant mass effect with mortality up to 80%.13,14 Lately, it was described that mechanical thrombectomy was associated with reduced brain edema, but these findings were only derived indirectly by magnetic resonance imaging-based measurements of midline shift or swelling volume.15,16 A recent study described a novel computed tomography (CT)-based method to quantify the volume of water uptake in infarct lesions due to ischemic edema.17 Primarily, this method has been developed based on the relationship of density and volume changes in CT. It was observed that the edematous component of infarct lesion volume, i.e. the net volume of water uptake per total volume of infarct lesion can be calculated using densitometric measurements.17 We used this quantitative imaging biomarker of ischemic edema to detect the potential treatment effects of endovascular recanalization with respect to lesion water uptake in CT imaging before and after treatment. The purpose of this study was to investigate how vessel recanalization affects volumetric infarct growth exclusively attributed to edematous water uptake. We hypothezised that successful recanalization reduces the progression of ischemic brain edema.
Methods
Patients
We analyzed de-identified data of all ischemic stroke patients with an acute LVO in the territory of the middle cerebral artery (MCA) admitted between January 2015 and August 2017 in three German stroke centers. Only anonymized data were analyzed after specific ethical review board approval, and no informed consent was necessary after institutional review board review due to the retrospective character of the study (Ethik Kommission der Ärztekammer Hamburg, WF-04/13).
The patients were screened consecutively based on a priori defined inclusion criteria: (1) acute ischemic stroke with occlusion of the M1 segment of the MCA or distal occlusion of the internal carotid artery; (2) initially performed multimodal CT protocol with CT angiography (CTA) and perfusion CT (CTP) performed within 12 h after known onset; (3) follow-up CT (FCT) available 24 h after symptom onset with no signs of hemorrhage; (4) visually evident early infarct lesion as indicated by ischemic hypoattenuation in admission non-enhanced CT (NECT) and/or perfusion lesion with reduced cerebral blood volume (CBV) in CTP; (5) Alberta Stroke Program Early CT Score (ASPECTS) of 5–10 at admission rated by an experienced neuroradiologist and controlled, and if necessary adjusted, by an attending neuroradiologist; (6) National Institutes of Health Stroke Scale (NIHSS) score above 3; (7) patients were included by status of vessel recanalization: (a) vessel recanalization after mechanical thrombectomy (thrombolysis in cerebral infarction (TICI) scale 2 b or 3) and (b) persistent LVO in patients who did not receive treatment or insufficient recanalization after mechanical thrombectomy (TICI 0–1). Patients with TICI 2 a were not included. In patients who did not receive endovascular treatment, persistence of LVO was confirmed via dense artery sign in FCT and/or transcranial color-coded duplex ultrasonography; (8) no intracranial hemorrhage; no preexisting thromboembolic or hemodynamic infarctions in admission NECT; and no preexisting significant carotid stenosis. Baseline clinical characteristics and demographic information were extracted from the medical records.
The study was conducted in accordance with the ethical guidelines of the Ethics Committee of the University of Hamburg Chamber of Physicians, Hamburg, Germany. All study protocols and procedures were conducted in accordance with the Declaration of Helsinki. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Image acquisitions
All patients received a comprehensive stroke imaging protocol at admission with NECT, CTA, and dynamic time resolved CTP performed in equal order on 128 or 256 dual-slice scanners (Philips iCT 256, Siemens Somatom Definition Flash). NECT: 120 kV, 280–340 mA, 5.0-mm slice reconstruction, 1-mm increment, 0.6-mm collimation, 0.8 pitch, H20f soft kernel; CTA: 120 kV, 175–300 mA, 1.0-mm slice reconstruction, 1-mm increment, 0.6-mm collimation, 0.8 pitch, H20f soft kernel, 80 mL highly iodinated contrast medium and 50 mL NaCl flush at 4 mL/s; and CTP: 80 kV, 200–250 mA, 5-mm slice reconstruction (max. 10 mm), slice sampling rate 1.50 s (min. 1.33 s), scan time 45 s (max. 60 s), biphasic injection with 30 mL (max. 40 mL) of highly iodinated contrast medium with 350 mg iodine/mL (max. 400 mg/mL) injected with at least 4 mL/s (max. 6 mL/s) followed by 30 mL sodium chloride chaser bolus. All perfusion data sets were inspected for quality and excluded in case of severe motion artifacts.
Revascularization protocol
Intravenous lysis was administered to patients within 4.5 h after symptom onset. Laboratory and conventional clinical inclusion and exclusion criteria for intravenous thrombolysis were applied. Mechanical thrombectomy was performed via a femoral artery approach under general anesthesia or conscious sedation. Endovascular procedures were performed standardized in each center using either clot retrieval or direct aspiration. The choice of thrombectomy device was left to the operator.
Image analysis
All CT imaging material was de-identified at an external imaging core laboratory for blinded analysis and was segmented manually using commercially available software (Analyze 11.0, Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). The ASPECTS in the admission CT was rated by two experienced neuroradiologists separately with subsequent consensus reading.
Lesion water uptake quantification
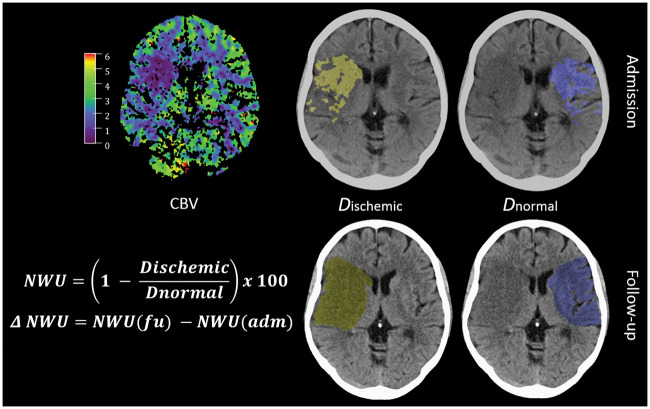
CT-based NWU has recently been introduced as quantitative imaging biomarker of edema in ischemic brain lesions.9,12,17,18 In the present study, we compared NWU per volume of infarct in the FCT with NWU captured before treatment in the admission CT to calculate ΔNWU. In brief, the early hypoattenuated infarct (ischemic core) in NECT was assessed by densitometric measurements (Dischemic). A mirrored region of interest (ROI) was placed within normal tissue of the contralateral hemisphere (Dnormal). CTP was used to improve the precision of the ROI defining the core lesion (early infarct) by simultaneously presenting CBV parameter maps at a fixed window between 0 and 6 mL/100 mL.9,12 ROIs in NECT were segmented with semiautomatic edge detection and sampled between 20 and 80 Hounsfield Units (HU; Analyze 12.0, AnalyzeDirect). Both density measurements (Dnormal and Dischemic) were then used to calculate the percentage of NWU per total volume of early infarct (equation (1)). Likewise, NWU was quantified in FCT using semiautomatic segmentation of the hypoattenuated infarct lesion (Dischemic) with subsequent mirroring of this ROI to determine Dnormal as described above (Figure 1). Progression of edema was defined as the absolute difference between NWU percentages in FCT and admission CT (ΔNWU, equation (2)). Total infarct volume was captured using semiautomatic segmentation of the hypoattenuated infarct lesion in the FCT.12,17
| (1) |
| (2) |
Statistical analysis
Due to the cluster structure of the data (three centers provided data), we first checked whether there is a significant cluster effect. Linear mixed models were fitted with t tests using Satterthwaite’s method. It was than tested whether a random effect for “center” resulted in an improved model fit using analysis of variance-like table for random effects. The p value was 1; no cluster effect could be found and therefore all observations are treated as independent. We presented absolute and relative frequencies for all patient characteristics, separately for patients with vessel recanalization and persistent LVO. Continuous variables are presented as means; confidence intervals (CIs) of means, standard deviations (SDs), or medians; and interquartile ranges (IQRs). Kolmogorov–Smirnov tests were used to determine whether the data sets were well modeled by a normal distribution. To compare the groups (patients with endovascular recanalization or persistent LVO) Student t tests, Mann–Whitney U tests with 95% CIs or IQR were used to determine differences of the acquired parameter. To examine the impact of recanalization status, age, sex, ASPECTS, and NIHSS on ischemic edema (as determined using quantitative NWU), multivariate regression analysis was performed (Figure 3). To test the impact of recanalization on clinical outcome modulated by ΔNWU, we performed a regression analysis for all patients with documented modified Rankin Scale (mRS) score after 90 days according to recanalization status (Figure 4). A statistcally significant difference was accepted at a p value of less than 0.05. Analyses were performed using MedCalc (version 11.5.1.0; Mariakerke, Belgium) and R (R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria, 2017).
Figure 1.
Quantification of NWU. Quantification of NWU as recently published.12,17,18 NWU is determined in admission computed tomography (CT) using CBV parameter maps as template for region of interest definition in the non-enhanced CT (upper images). NWU in follow-up CT is quantified in non-enhanced CT (lower images) and its difference to admission CT is calculated (ΔNWU). NWU: net water uptake; CBV: cerebral blood volume.
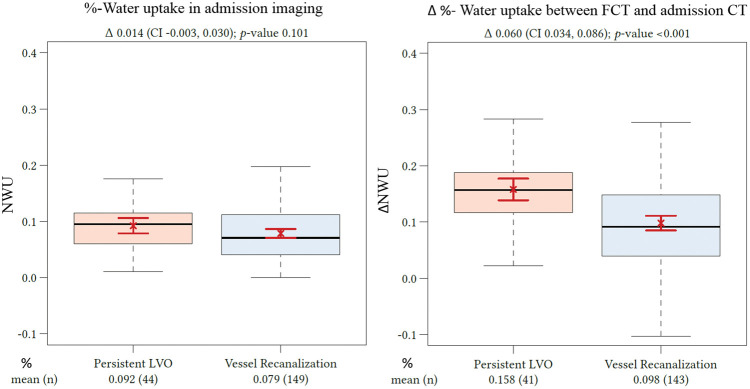
Figure 2.
Difference in ΔNWU between follow-up and admission imaging in patients with persistent LVO versus patients with vessel recanalization. Boxplots representing NWU for both patient groups at admission imaging (left side) and ΔNWU in follow-up imaging (difference in NWU in FCT—admission CT). NWU: net water uptake; CT: computed tomography; CI: confidence interval; LVO: large vessel occlusion; FCT: follow-up CT.
Figure 3.
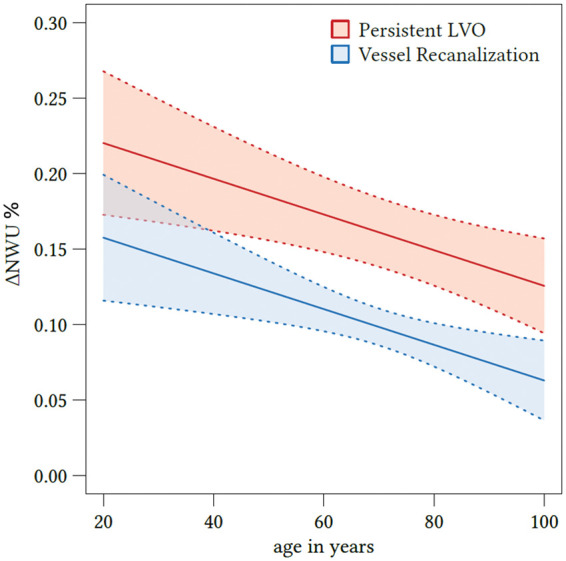
Effect plot of recanalization on ΔNWU and age. Based on multivariate regression analysis, ΔNWU (y-axis) is displayed separately for patients with persistent LVO (upper red line) and vessel recanalization (lower blue line). Age is plotted on the x-axis. LVO: large vessel occlusion; NWU: net water uptake.
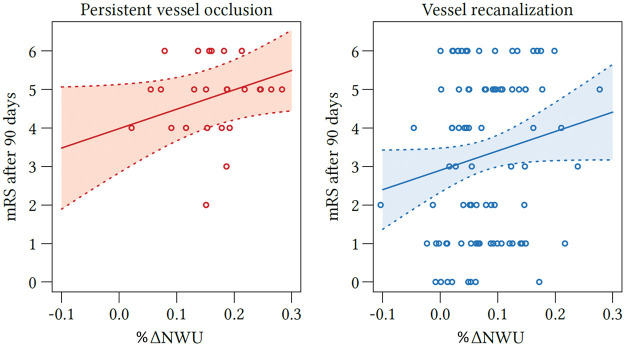
Figure 4.
Regression analysis showing the relationship of ΔNWU and mRS score after 90 days according to recanalization status. mRS after 90 days according to ΔNWU (x-axis) and recanalization status (persistent vessel occlusion on the left side and vessel recanalization on the right side). NWU: net water uptake; mRS: modified Rankin Scale.
Results
Two hundred forty-one patients were returned from our database, and 150 patients were acquired from two external institutions (82 and 68 patients). One hundred ninety-nine patients were excluded after first screening (see Figure 5 for flow chart). One hundred ninety-four patients met all inclusion criteria and were analyzed. The patient characteristics are listed in Table 1. One hundred fifty patients received successful endovascular recanalization (TICI 2 b or 3), and 44 patients had a persistent vessel occlusion. The median ASPECTS was 8 in both groups with an IQR of 7–8 in patients with persistent LVO and 5–9 in patients with successful recanalization. The median NIHSS was 15 (IQR: 12–19) for persistent LVO and 16 (IQR: 14–19) in recanalized patients (p = 0.2). The median time from onset to imaging was 3.2 h (IQR: 2.0–4.5) in patients with vessel recanalization and 3.7 h (IQR: 2.8–5.0) in patients with persistent vessel occlusion, which was not different (p = 0.06). There were no differences in age, sex, or administration of intravenous lysis comparing both patient groups. At admission imaging, the mean (SD) NWU was not different in both patient groups: 9.2% (4.5) in patients with persistent LVO and 7.9% (4.9) in patients with vessel recanalization (p = 0.1).
Table 2.
Multivariate regression analysis to show the impact on ΔNWU for the parameter as captured below.
| Parameter | Coefficient | 2.5% | 97.5% | p value |
|---|---|---|---|---|
| Vessel recanalization | −0.063 | −0.09 | −0.037 | <0.001 |
| Age | −0.012 | −0.02 | −0.004 | 0.005 |
| Sex, male | 0.004 | −0.019 | 0.027 | 0.721 |
| ASPECTS | 0.002 | −0.005 | 0.009 | 0.605 |
| NIHSS | 0.001 | −0.001 | 0.003 | 0.451 |
NIHSS: National Institutes of Health Stroke Scale; ASPECTS: Alberta Stroke Program Early CT Score.
Figure 5.
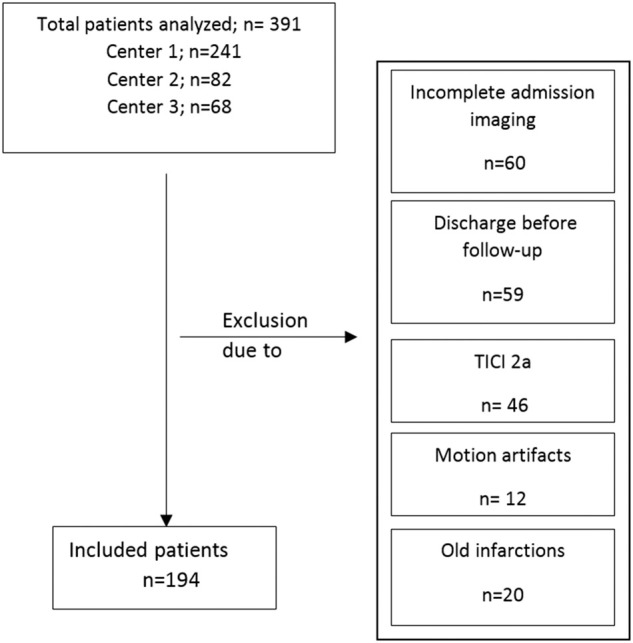
Flow chart with application of exclusion criteria. TICI: thrombolysis in cerebral infarction.
Table 1.
Patient characteristics.
| Baseline characteristics | Endovascular recanalization | Persistent LVO | Group comparison p value |
|---|---|---|---|
| Subjects, n (%) | 150 (77.3) | 44 (22.7) | |
| Age in years, median (IQR) | 75 (62–81) | 74 (63–84) | 0.300 |
| Female sex, n (%) | 71 (47) | 24 (55) | 0.400 |
| Admission NIHSS, median (IQR) | 16 (14–19) | 15 (12–19) | 0.183 |
| ASPECTS, median (IQR) | 8 (5–9) | 8 (7–8) | 0.785 |
| Time from onset to imaging in h, median (IQR) | 3.2 (2–4.5) | 3.7 (2.8–5) | 0.060 |
| Administered intravenous lysis, n (%) | 101 (67) | 24 (55) | 0.120 |
| Follow-up infarct volume in mL, median (IQR) | 22.4 (7.7–71.5) | 71.2 (42.3–124.5) | <0.001 |
| mRS after 90 days available n (%) | 96 (64) | 28 (64) | |
| mRS, median (IQR) | 4 (1–5) | 5 (4–6) | 0.002 |
| mRS 0–2, n (%) | 38 (40) | 1 (4) | <0.001 |
| mRS 3–4, n (%) | 18 (19) | 7 (25) | 0.390 |
| mRS 5–6, n (%) | 40 (41) | 20 (71) | 0.011 |
LVO: large vessel occlusion; IQR: interquartile range; NIHSS: National Institutes of Health Stroke Scale; ASPECTS: Alberta Stroke Program Early CT Score; mRS: modified Rankin Scale.
Of 44 patients with a persistent LVO, 29 patients received an intra-arterial procedure with a TICI score of 0–1 and 15 patients did not receive an intra-arterial procedure. Of these patients, the mean (SD) NWU was 24.9% (5.7) on follow-up in FCT. Of 150 patients with vessel recanalization, the mean (SD) NWU was significantly lower at 17.6% (8.3%) (p < 0.0001). Mean (SD) ΔNWU was 15.8% (5.7) in patients with persistent LVO and 9.8% (5.8) in patients with vessel recanalization (p < 0.001; Figure 2).
The impact of the acquired parameter (recanalization status, age, sex, ASEPCTS, and NIHSS) on ΔNWU was examined using multivariate regression models. The only parameter with significant impact on ΔNWU were recanalization status and age (Figure 3). Successful recanalization was associated with a mean ΔNWU decrease of 6.3% compared to patients with persistent LVO (95% CI: 3.7–9.0, p < 0.001). Increasing age by 10 years was associated with a ΔNWU decrease of 1.2% (95% CI: 0.4–2.0, p < 0.001; Table 2).
The median infarct volume measured in the FCT was 22.4 mL (IQR: 7.7–71.5 mL) in patients with vessel recanalization and 71.2 mL (IQR: 42.3–124.5 mL) in patients with persistent LVO, which was significantly higher (p < 0.0001). The mRS after 90 days was available in 124 patients (64%). The median mRS in patients with persistent vessel occlusion was 5 (IQR: 4–6) and 4 in patients with vessel recanalization (IQR: 1–5), which was significantly lower (p = 0.002). In linear regression analysis, a ΔNWU increase of 10% was associated with an increase of mRS score of 0.78 (95% CI: 0.3–12.6, p = 0.002) (Figure 3). To show the impact of recanalization on clincial outcome, multivariate regression analysis was performed (Figure 4). Vessel recanalization was associated with a mRS decrease of 1.1 (95% CI: 0.1–2.0, p = 0.03).
Discussion
The aim of this study was to investigate the impact of endovascular recanalization on the progression of ischemic brain edema using measurements of lesion water uptake as quantitative CT imaging biomarker. Therefore, ΔNWU was calculated based on the difference between NWU of infarct after treatment in the 24-h FCT and NWU of early infarct in admission CT. The main finding of this study was that reperfusion was independently associated with a lowered ΔNWU by 6.3% compared to LVO (absolute difference in NWU percentages).
The classic CT imaging end point to investigate the effects of vessel recanalization on acute ischemic brain injury has been primarily based on assessing lesion volume. The effects of recanalization on total infarct volume are well known. Correspondingly in this study, the initial ASPECTS was similar in both patient groups, but the total infarct volume was significantly lower in patients with vessel recanalization on follow-up.
Using a quantitative imaging marker of edema (i.e. NWU) introduces a second dimension of classifying infarct lesions besides volume. This may have important implications on differentiating total lesion growth by its two volumetric components: (1) lesion growth by progressing infarct into tissue-at-risk versus (2) lesion growth by edema progression. Our study investigated how vessel recanalization affects the second dimension of lesion characteristic, i.e. the volumetric change exclusively attributed to the formation of edema. The results suggest that vessel recanalization has an effect on the edematous component of acute infarct lesions. A potential advantage may be improved monitoring of therapeutic effects of potential agents specifically targeting the inhibition of edema (e.g. gliburide19–21). Furthermore, prediction of clinical end points, such as development of malignant edema, may be improved.9
In multivariate analysis, only two parameters had an impact on lesion water uptake: recanalization status and age (see Figure 3). Vessel recanalization was associated with reduced progression of ischemic edema by 6.3%. In a recent study, NWU was examined as predictor for malignant infarction, and a 1% increase of NWU was associated with a 1.27 times chance of developing a malignant infarction (95% CI: 1.14–1.45).9 Therefore, the modulating effect of vessel recanalization on NWU in patients with large early infarct lesions could reduce progressing edematous mass effects below a critical level which might contribute to an improved functional outcome.5,22 In eight patients (all of them were successfully recanalized), we observed slightly negative ΔNWU meaning that early edema progression may be stopped entirely, if not reversed. While it is certain that any visible CT ischemic hypoattenuation (due to vasogenic edema) is irreversible, there is evidence of halted edema progression in case of rapid reperfusion and potentially reversible edema might exist beyond recognizable levels for the human eye.8 Therefore, quantitative NWU might be a tool for objectively measuring early brain edema in hyperacute multimodal stroke CT.12
We found a multivariate effect of patient age so that for every decade passing ΔNWU was reduced by 1.2%. Therefore, early infarct lesions in younger patients may show higher levels edema formation on follow-up. This negative effect of a lower age may be in part a result of lower intracranial volume reserve due to higher brain volume.23 NWU in patients with lower intracranial volume reserve might indicate higher intracranial pressure and elevated resistance of arterioles. This again would contribute to a further increase of interstitial pressure and worsening ischemic edema.24
To our knowledge, the present study is the first to investigate the impact of vessel recanalization in patients with LVO on ischemic lesion edema progression using a quantitative imaging biomarker. Lesion water uptake based on CT densitometry has recently been described as a new imaging method that directly quantifies the edematous component of the infarct lesion.9,12,17,18 The method is based on the contingent relationship of the expanding volume of infarct lesions by water uptake due to ischemic edema and decreasing CT density.17 Recently, the effects of mechanical thrombectomy on brain edema were described using midline shifts measurements or swelling volumes, and it was concluded that succesful reperfusion was associated with reduced ischemic edema, which is in accordance with the results of the present study.15,16 The quantitative analysis of our study validated these prior observations using NWU as imaging biomarker that directly describes the volume of water uptake and might therefore offer more precision than measuring midline shift or swelling volumes of the infarct lesion considering that swelling volumes do not differentiate between volume of infarcted tissue and edema.16,17 The application of lesion water uptake as imaging biomarker may directly serve future efforts to implement quantifiable image parameters of cerebral edema for automated algorithms in acute stroke triage or clinical trials.25
Although the proportion of patients with known and documented clinical outcome after 90 days was limited in this study, we still observed a significantly improved outcome in patients with vessel recanalization compared to patients with persistent vessel occlusion, which is in accordance with recent prospective stroke trials (see Table 1).1,5,6,26 Moreover, higher ΔNWU was associated with higher mRS scores after 90 days which may also reflect a higher occurrence of malignant infarctions.9 As patients with low initial ASPECTS were excluded in this study to prevent selection bias among the two patient groups, further research is needed for this particular group of patients.27
Our study has limitations. First, the number of patients with persistent LVO was relatively low and consisted of patients with the intention to treat (resulting in TICI score 0–1) as well as patients who did not receive endovascular treatment (15/144). Decisions for no endovascular treatment was made on an individual basis in cases where there was no significant tissue-at-risk in CTP beyond early infarct, older patient age, extended time windows, and low NIHSS. We excluded patients with TICI score 2a, as we intended to obtain a clean cohort of patients with vessel recanalization versus persistent vessel occlusion. Further methodological limitations might arise from inaccuracies of CT density measurements, which can be imprecise especially in very early admission NECT with small lesions.28 For this reason, we used CTP to improve the precision of the ROI defining the early infarct in NECT.12 Inconsistent CBV core lesion definition of the ROI capturing the early infarct in NECT might be overestimated due to a truncated tissue attenuation curve.12 Finally, prospective validation is needed to reliably investigate the relationship between revascularization and ischemic brain edema.
Endovascular recanalization is associated with a significantly reduced formation of ischemic brain edema compared to patients with a persistent vessel occlusion. Quantitative NWU is a promising biomarker that may be used to compare the treatment effects on edema progression in acute stroke.
Authors’ contribution
GB: Study design; acquisition of data; image processing; data analysis; statistical analysis; drafting the manuscript and revising it critically.
FF: Data analysis; acquisition of data; drafting the manuscript and revising it critically.
UH: Data analysis; drafting the manuscript and revising it critically.
GS: Data analysis; statistical analysis; drafting the manuscript and revising it critically.
PS: Acquisition of data; drafting the manuscript and revising it critically.
JM: Data analysis; drafting the manuscript and revising it critically.
JF: Study design; drafting the manuscript and revising it critically.
AK: Study design; acquisition of data; image analysis; data analysis; drafting the manuscript and revising it critically.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JF: Consultant for Acandis, Boehringer Ingelheim, Codman, Microvention, Sequent, Stryker. Speaker for Bayer Healthcare, Bracco, Covidien/ev3, Penumbra, Philips, Siemens. Grants from Bundesministeriums für Wirtschaft und Energie, Bundesministerium für Bildung und Forschung, Deutsche Forschungsgemeinschaft, European Union, Covidien, Stryker (THRILL study), Microvention (ERASER study), Philips. AK: research collaboration agreement: Siemens Healthcare. All other authors have nothing to disclose.
References
- 1.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 2.Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol 2016; 15: 1138–1147. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Berkhemer OA, Palesch YY, et al. Endovascular therapy is effective and safe for patients with severe ischemic stroke: pooled analysis of interventional management of stroke III and multicenter randomized clinical trial of endovascular therapy for acute ischemic stroke in the Netherlands data. Stroke 2015; 46: 3416–3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilgen MD, Klimek D, Liesirova KT, et al. Younger stroke patients with large pretreatment diffusion-weighted imaging lesions may benefit from endovascular treatment. Stroke 2015; 46: 2510–2516. [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 6.Yoo AJ, Berkhemer OA, Fransen PSS, et al. Effect of baseline Alberta Stroke Program Early CT Score on safety and efficacy of intra-arterial treatment: a subgroup analysis of a randomised phase 3 trial (MR CLEAN). Lancet Neurol 2016; 15: 685–694. [DOI] [PubMed] [Google Scholar]
- 7.Pillai DR, Dittmar MS, Baldaranov D, et al. Cerebral ischemia-reperfusion injury in rats—a 3 T MRI study on biphasic blood-brain barrier opening and the dynamics of edema formation. J Cereb Blood Flow Metab 2009; 29: 1846–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dzialowski I, Klotz E, Goericke S, et al. Ischemic brain tissue water content: CT monitoring during middle cerebral artery occlusion and reperfusion in rats. Radiology 2007; 243: 720–726. [DOI] [PubMed] [Google Scholar]
- 9.Broocks G, Flottmann F, Scheibel A, et al. Quantitative lesion water uptake in acute stroke computed tomography is a predictor of malignant infarction. Stroke 2018; 49: 1906–1912. [DOI] [PubMed] [Google Scholar]
- 10.Kidwell CS, Saver JL, Starkman S, et al. Late secondary ischemic injury in patients receiving intraarterial thrombolysis. Ann Neurol 2002; 52: 698–703. [DOI] [PubMed] [Google Scholar]
- 11.Stokum JA, Gerzanich V, Simard JM. Molecular pathophysiology of cerebral edema. J Cereb Blood Flow Metab 2016; 36: 513–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minnerup J, Broocks G, Kalkoffen J, et al. Computed tomography-based quantification of lesion water uptake identifies patients within 4.5 hours of stroke onset: a multicenter observational study. Ann Neurol 2016; 80: 924–934. [DOI] [PubMed] [Google Scholar]
- 13.Berrouschot J, Sterker M, Bettin S, et al. Mortality of space-occupying (‘malignant’) middle cerebral artery infarction under conservative intensive care. Intensive Care Med 1998; 24: 620–623. [DOI] [PubMed] [Google Scholar]
- 14.Hacke W, Schwab S, Horn M, et al. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol 1996; 53: 309–315. [DOI] [PubMed] [Google Scholar]
- 15.Kimberly WT, Dutra BG, Boers AMM, et al. Association of reperfusion with brain edema in patients with acute ischemic stroke: a secondary analysis of the MR CLEAN Trial. JAMA Neurol 2018; 75: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irvine HJ, Ostwaldt AC, Bevers MB, et al. Reperfusion after ischemic stroke is associated with reduced brain edema. J Cereb Blood Flow Metab 2017; 38: 1807–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broocks G, Flottmann F, Ernst M, et al. Computed tomography-based imaging of voxel-wise lesion water uptake in ischemic brain: relationship between density and direct volumetry. Invest Radiol 2018; 53: 207–213. [DOI] [PubMed] [Google Scholar]
- 18.Broocks G, Faizy TD, Flottmann F, et al. Subacute infarct volume with edema correction in computed tomography is equivalent to final infarct volume after ischemic stroke: improving the comparability of infarct imaging endpoints in clinical trials. Invest Radiol 2018; 53: 472–476. [DOI] [PubMed] [Google Scholar]
- 19.Sheth KN, Kimberly WT, Elm JJ, et al. Pilot study of intravenous glyburide in patients with a large ischemic stroke. Stroke 2014; 45: 281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheth KN, Petersen NH, Cheung K, et al. Long-term outcomes in patients aged </=70 years with intravenous glyburide from the phase II GAMES-RP study of large hemispheric infarction: an exploratory analysis. Stroke 2018; 49: 1457–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheth KN, Elm JJ, Molyneaux BJ, et al. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2016; 15: 1160–1169. [DOI] [PubMed] [Google Scholar]
- 22.Sporns PB, Minnerup J, Warneke N, et al. Impact of the implementation of thrombectomy with stent retrievers on the frequency of hemicraniectomy in patients with acute ischemic stroke. Clin Neuroradiol 2017; 27: 193–197. [DOI] [PubMed] [Google Scholar]
- 23.Minnerup J, Wersching H, Ringelstein EB, et al. Prediction of malignant middle cerebral artery infarction using computed tomography-based intracranial volume reserve measurements. Stroke 2011; 42: 3403–3409. [DOI] [PubMed] [Google Scholar]
- 24.Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke 2017; 48: 2621–2627. [DOI] [PubMed] [Google Scholar]
- 25.Nowinski WL, Gupta V, Qian G, et al. Automatic detection, localization, and volume estimation of ischemic infarcts in noncontrast computed tomographic scans: method and preliminary results. Invest Radiol 2013; 48: 661–670. [DOI] [PubMed] [Google Scholar]
- 26.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 27.Mourand I, Abergel E, Mantilla D, et al. Favorable revascularization therapy in patients with ASPECTS </= 5 on DWI in anterior circulation stroke. J Neurointerv Surg 2018; 10: 5–9. [DOI] [PubMed] [Google Scholar]
- 28.Fabritius MP, Kazmierczak PM, Thierfelder KM, et al. Reversal of CT hypodensity in chronic ischemic stroke: a different kind of fogging. Clin Neuroradiol 2017; 27: 383–384. [DOI] [PubMed] [Google Scholar]