Abstract
Background
Blood gas analyzers are capable of delivering results on electrolytes and metabolites within a few minutes and facilitate clinical decision‐making. However, whether the results can be used interchangeably with values measured by chemistry analyzers remains controversial.
Blood gas analyzers are capable of delivering results on electrolytes and metabolites within a few minutes and facilitate clinical decision‐making. However, whether the results can be used interchangeably with values measured by chemistry analyzers remains controversial.
Methods
In total, arterial and matched venous blood samples were collected from 200 hospitalized patients. Arterial blood samples were evaluated using a RAPIDPOINT 500 to test electrolyte and glucose levels, then the samples were centrifuged and the same parameters were measured with an AU5800. Venous blood samples were processed and tested in accordance with standard operation procedures. Data were compared by using a paired t test, the agreement between the two analyzers was evaluated by using the Bland‐Altman test, and sensitivity and specificity were calculated.
Results
Paired t tests showed that all parameters tested were significantly different between the two analyzers except chloride. The biases calculated indicated that blood gas analyzers tend to underestimate the parameters, and the linear regression showed a strong correlation between the two analyzers. The sensitivity, specificity and kappa values demonstrated that the diagnostic performance of blood gas analyzers is not satisfactory.
Conclusion
The significant reduction in parameter estimation and diagnostic performance we observed suggested that clinicians should interpret results from blood gas analyzers more cautiously. The reference interval of blood gas analyzers should be adjusted accordingly, given that values are underestimated.
Keywords: agreement, blood gas analyzers, diagnostic performance, electrolytes, glucose
1. INTRODUCTION
Blood gas analysis (BGA) is an effective test that measures the partial pressures of oxygen and carbon dioxide in the blood, as well as oxygen content, oxygen saturation, bicarbonate content, and blood pH.1 It allows clinicians to evaluate a patient's acid‐base disorder and hypoxia; thus, BGA plays a vital role in managing acute respiratory failure, conducting surgery, and managing critically ill patients. Moreover, a certain number of manufacturers have provided additional tests, including assessments that measure electrolyte, lactate, and glucose levels, which can give the clinicians more detailed insights into a patient's status and consequently help with patient management.2
It is generally acknowledged that electrolyte homeostasis is of critical importance in the proper functioning of several metabolic processes and organ functions in human body. Therefore, electrolyte disorders can be found in various medical conditions, such as chronic renal failure3 and diabetes mellitus,4 and even in some cancer patients who receive platinum‐based chemotherapy.5 Glucose measurements are also very common in patients who were unconscious and admitted to the emergency department, as they allow clinicians to determine if the patients were suffering from hypoglycemic coma or the opposite, hyperosmolar coma. The advent of such blood gas analyzers greatly facilitates the management of these patients, especially those who are critically ill. The major advantage of these blood gas analyzers is that the tests do not require centrifugation. Moreover, the time that blood gas analyzers need is usually short as it takes approximately 2 or 3 minutes for blood gas analyzers to finish the whole testing process.6
In contrast, autoanalyzers that are used to conduct biochemical analyses require samples to be centrifuged before testing; this process would take at least 10 minutes. In addition to the preanalytical process, the electrolytes and glucose measurements in autoanalyzers are rather time consuming because they employ sophisticated methods such as the hexokinase method, which requires a certain amount of time to acquire results. In the daily practice of clinical laboratories, the turnaround time for tests in autoanalyzers is often set to 90 minutes, taking time for centrifugation, test processes, and the volume of samples into account. Although the results generated by autoanalyzers are believed to be more precise and reliable by most clinicians and even laboratory technologists, the relatively long turnaround time of autoanalyzers limits the value of these tests in the management of critically ill patients.
A much debated question is that whether these parameters measured by two different kinds of instruments can be used interchangeably because clinicians are often confused by results generated by two different kinds of equipment, especially when they are significantly different from each other. A study conducted by Gavala et al7 pointed out that electrolytes were underestimated by blood gas analyzers, and, compared with autoanalyzers, 30% of the included parameters were out of US Clinical Laboratory Improvement Amendment (USCLIA) accepted biases. Zhang et al8 reported an even higher rate of samples that violated USCLIA among 50 patients; to be more specific, 88% of samples surpassed USCLIA limits in potassium, and 64% surpassed the limit for sodium. However, the sample size in this previously conducted study was rather small. To investigate this matter better, we conducted a study by employing a RAPIDPOINT 500 blood gas analyzer (Siemens Healthcare Diagnostics) and a BECKMAN COULTER chemistry analyzer AU5800 (Beckman Coulter) to measure electrolytes and glucose in patients’ arterial blood samples at the same time, aiming to evaluate the agreement between the two instruments and gain insight into whether these results are interchangeable.
2. MATERIALS AND METHODS
2.1. Study participants
We selected 200 patients who were admitted to Zhongshan Hospital, Xiamen University from September 2018 to March 2019, irrespective of their sex and age. Among these 200 subjects, 33% were admitted for respiratory diseases, 25% were admitted for cardiovascular diseases, 15.5% were admitted for gastrointestinal diseases, 8.5% were admitted for injuries, and 8.5% were chronic renal failure patients. The remaining 9.5% of patients were classified as having other diseases. The present study was approved by the Ethics Committee of Zhongshan Hospital, Xiamen University in accordance with the Helsinki Declaration.
2.2. Instrumentation and procedures
The blood gas analyzer we used in the present study was a RAPIDPOINT 500 (Siemens Healthcare Diagnostics), and we selected a BECKMAN COULTER chemistry analyzer AU5800 to test the blood samples simultaneously. Genuine measurement cartridges for 750 tests were used to determine electrolytes and glucose levels with the RAPIDPOINT 500. Similarly, we also used genuine system reagents for the quantitative determination of glucose (Beckman Coulter, Lot No. 5182) and electrolyte levels (Beckman Coulter, ISE Reference Lot No. 5843, ISE Buffer Lot No. 5846, ISE Mid Standard Lot No. 5757) with a BECKMAN COULTER chemistry analyzer AU5800.
The calibration process and quality control for both analyzers are performed on a daily basis before testing samples. The quality of these instruments, along with the tests, were assured as they underwent both internal and external quality control programs. Internal quality control data were acquired and analyzed in the laboratory information system every day and reviewed every month to assess if any changes were needed. The external quality program was organized by the Chinese National Center for Clinical Laboratories, and the abovementioned parameters were approved in the program both in 2018 and 2019.
Sample collection was conducted before an intravenous injection or administering oral medication to reduce the potential confounding effects. Arterial blood samples were taken by using commercially available plastic arterial blood gas syringes coated with 25 IU/mL of heparin (Becton, Dickinson and Company). After collection, samples were thoroughly mixed, rendered homogeneous and immediately sent to the clinical laboratory. Sodium, potassium, chloride, and glucose levels were measured by using RAPIDPOINT 500 within 30 minutes of collection. After the measurement process in RAPIDPOINT 500, each arterial blood sample was moved to a sterilized plastic tube, and then centrifuged at 1500 g for 10 minutes. After centrifugation, the plasma isolated from arterial blood samples was tested for the abovementioned parameters in a BECKMAN COULTER chemistry analyzer AU5800. A paired venous blood sample was collected by using a lithium heparin tube from each patient at the same time, and then centrifuged at the same speed and duration as mentioned above. To minimize the interpersonal variations, the analyses with the RAPIDPONT 500 and AU5800 were conducted independently by two highly skilled technicians to guarantee consistency. All procedures were conducted in accordance with the manufacturers’ instructions and Chinese National Guide to Clinical Laboratory Procedures.
2.3. Statistical analysis
Data on electrolytes (sodium, potassium, and chloride) and glucose were transferred from the analyzers to a laboratory information system and exported to an Excel file for further data analysis. All parameters are expressed as the mean ± standard deviation. A paired t test was used to analyze the differences in electrolytes and glucose between the two instruments in blood samples. The agreement between RAPIDPOINT500 and AU5800 on the abovementioned parameters was evaluated by using the Bland‐Altman test with 95% confidence interval (CI) limits of agreement (LoA). To further analyze whether parameter measurements acquired by two instruments are correlated, we calculated the Pearson correlation coefficient. A strong correlation was considered if the coefficient was >0.8. We classified each parameter into two categories, namely, normal test results and abnormal test results. The sensitivity and specificity of the RAPIDPOINT500 in identifying abnormal test results were determined, and the test results of venous blood samples based on the AU5800 were used as the “Gold Standard.” Moreover, data on the red blood cell count, creatine level, uric acid level, bilirubin level, and vitamin C level were collected to analyze whether they have an impact on glucose measurements. All statistical analyses were conducted by using IBM SPSS version 25, and a P value <.05 was considered significant. Statistical plots were created by using the R package ggplot2.
3. RESULTS
Table 1 presents the reference interval of each parameter tested by using different instruments, and these intervals are used in the clinical diagnosis of our institution.
Table 1.
Reference interval for investigated parameters
| Parameter | Instrument | Reference interval (mmol/L) |
|---|---|---|
| Sodium | RAPIDPOINT 500 | 136.00‐145.00 |
| AU5800 | 137.00‐147.00 | |
| Potassium | RAPIDPOINT 500 | 3.50‐4.50 |
| AU5800 | 3.50‐5.30 | |
| Chloride | RAPIDPOINT 500 | 98.00‐107.00 |
| AU5800 | 99.00‐110.00 | |
| Glucose | RAPIDPOINT 500 | 3.60‐5.30 |
| AU5800 | 3.90‐6.10 |
The mean, standard deviation, and range of each parameter are provided in Table 2. In addition, we also attempted to conduct two comparisons of each parameter. First, we compared the values of arterial blood samples measured by the RAPIDPOINT 500 and plasma samples isolated from arterial blood samples that were evaluated via the AU5800 simultaneously by using paired t tests. The results suggested that all parameters were significantly different when using different instruments (P < .001), except chloride (P = .517). We also conducted the same comparison between values of arterial blood samples measured by the RAPIDPOINT 500 and venous blood samples measured by the AU5800. In this comparison, all parameters were significantly different between the two instruments. The statistical analysis indicated that these two methods have significant impacts on the results of electrolytes and glucose.
Table 2.
Descriptive statistics and paired t test of investigated parameters
| Parameter | Arterial blood by RAPID POINT 500 | Arterial plasma by AU5800 | Venous plasma by AU5800 | P a | P b | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |||
| Sodium (mmol/L) | 141.10 ± 7.65 | 122.40‐171.90 | 146.42 ± 8.31 | 127.10‐174.20 | 141.71 ± 7.39 | 125.10‐165.90 | <.001* | <.001* |
| Potassium (mmol/L) | 3.86 ± 0.61 | 2.50‐5.93 | 3.94 ± 0.63 | 2.65‐6.14 | 4.01 ± 0.62 | 2.67‐6.33 | <.001* | <.001* |
| Chloride (mmol/L) | 105.08 ± 7.19 | 87.00‐135.00 | 106.58 ± 7.58 | 89.80‐137.90 | 105.15 ± 7.34 | 86.50‐137.10 | .517 | <.001* |
| Glucose (mmol/L) | 7.75 ± 3.09 | 2.80‐22.70 | 7.93 ± 3.21 | 3.13‐22.92 | 8.09 ± 3.18 | 3.36‐22.41 | <.001* | <.001* |
Arterial blood by RAPID POINT 500 vs Arterial plasma by AU5800.
Arterial blood by RAPID POINT 500 vs Venous plasma by AU5800.
P < .05.
We further conducted a Bland‐Altman analysis to evaluate the agreement between RAPIDPOINT 500 and AU5800 measurements, and the corresponding plots are displayed in Figures 1 and 2. Table 3 presents the bias and the LoA between the two analyzers, and, as shown in the table, all parameters measured by the RAPIDPOINT 500 were lower than those acquired by the AU5800 because average biases calculated by conducting two different comparisons are consistently lower than zero, regardless of the parameter and reference. Nevertheless, we found that the average bias of sodium and chloride in comparison A is greater than that in comparison B. Conversely, the corresponding bias of potassium and glucose in comparison A is smaller than that in comparison B (see Table 3). We employed the LoA to analyze the concordance between the two analyzers, and the LoA was set to the average bias ± 1.96 × SD. Based on this criterion, any value that exceeded the LoA was considered inconsistent between the two analyzers. However, the pairs outside the LoA were not entirely related to the intensity of the corresponding average bias. Using sodium as an example, the average bias calculated in comparison B was only −0.60, but the percentage of pairs outside the LoA reached 10%. In contrast, the bias of comparison A was −5.32, but the corresponding percentage was only 3%. Pearson's coefficient was calculated to estimate the correlation between the two analyzers, and similarly, each parameter had two comparisons. Data from the linear regression showed that these parameters had a strong correlation regardless of the sample type and analyzer because all Pearson's coefficients were >0.850. Among them, the greatest Pearson's coefficient was observed in comparison A for glucose, which was 0.883, while the lowest coefficient was found in comparison A for sodium, which was 0.883. The scatter plots of the abovementioned parameters are displayed in Figures 3 and 4 with 95% CIs.
Figure 1.
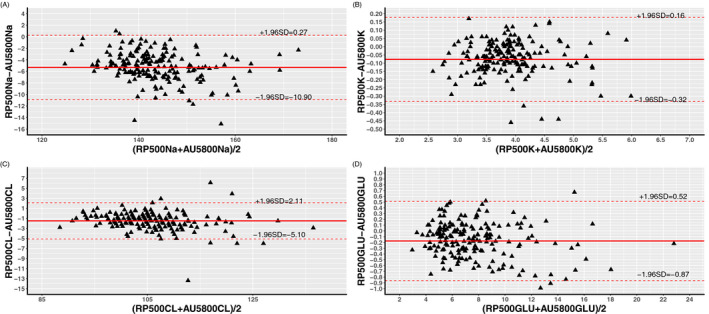
Bland‐Altman analysis of Sodium (A), Potassium (B), Chloride (C), and Glucose (D) between arterial blood samples by RAPIDPOINT 500 and arterial plasma samples by AU5800 showing the 95% LoA
Figure 2.
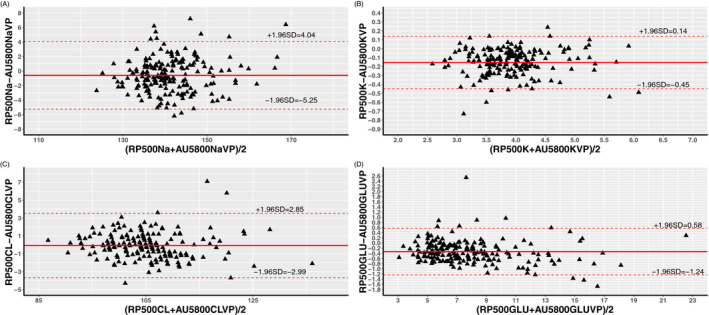
Bland‐Altman analysis of Sodium (A), Potassium (B), Chloride (C), and Glucose (D) between arterial blood samples by RAPIDPOINT 500 and venous plasma samples by AU5800 showing the 95% LoA
Table 3.
Agreement analysis between RAPIDPOINT 500 and AU5800
| Parameter | Bias | Limits of agreement | No. of pairs outside LoA | Pearson's coefficient | ||||
|---|---|---|---|---|---|---|---|---|
| A a | B b | A a | B b | A a | B b | A a | B b | |
| Sodium (mmol/L) | −5.32 | −0.60 | −10.90 to 0.27 | −5.25 to 4.04 | 6 (3%) | 10 (5%) | 0.883 | 0.904 |
| Potassium (mmol/L) | −0.08 | −0.15 | −0.32 to 0.16 | −0.45 to 0.14 | 4 (2%) | 7 (3.5%) | 0.960 | 0.971 |
| Chloride (mmol/L) | −1.50 | −0.07 | −5.10 to 2.11 | −0.87 to 0.52 | 7 (3.5%) | 6 (3%) | 0.941 | 0.959 |
| Glucose (mmol/L) | −0.17 | −0.33 | −0.87 to 0.52 | −1.24 to 0.58 | 3 (1.5%) | 10 (5%) | 0.989 | 0.979 |
Arterial blood by RAPID POINT 500 vs Arterial plasma by AU5800.
Arterial blood by RAPID POINT 500 vs Venous plasma by AU5800.
Figure 3.
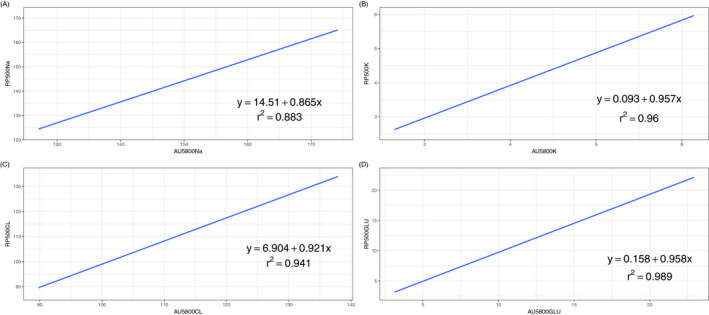
Linear regression of Sodium (A), Potassium (B), Chloride (C), and Glucose (D) between arterial blood samples by RAPIDPOINT 500 and arterial plasma samples by AU5800. The Blue line indicates the linear model established and grey area nearby is the 95% CI. The linear equation and Pearson's coefficient were demonstrated in the figure
Figure 4.
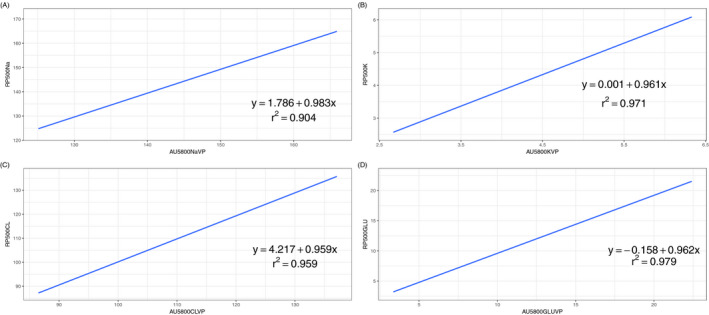
Linear regression of Sodium (A), Potassium (B), Chloride (C), and Glucose (D) between arterial blood samples by RAPIDPOINT 500 and venous plasma samples by AU5800. The Blue line indicates the linear model established and grey area nearby is the 95% CI. The linear equation and Pearson's coefficient were demonstrated in the figure
To assess the diagnostic performance of RAPIDPOINT 500 in electrolyte and glucose measurements, we applied the reference interval demonstrated in Table 1 to classify original values into two different statuses, namely, normal and abnormal. Based on this transformation, we calculated the sensitivity, specificity, kappa value, and Youden Index; the data are presented in Table 4. Notably, we only compared arterial blood samples (RAPIDPOINT 500) and venous blood samples (AU5800), and the latter was used as the gold standard. The reason we did not calculate the diagnostic performance of arterial plasma samples (AU5800) is that, in clinical practice, we do not use this approach to analyze samples. The sensitivity of the RAPIDPOINT 500 is relatively satisfying, as it ranges from 0.780 to 1.00 for different parameters; however, the specificity was lower, ranging from 0.502 to 0.711.
Table 4.
Sensitivity and specificity of RAPIDPOINT 500
| Parameter | Sensitivity | Specificity | Kappa | Youden index |
|---|---|---|---|---|
| Sodium (mmol/L) | 0.800 | 0.821 | 0.617 | 1.621 |
| Potassium (mmol/L) | 0.974 | 0.733 | 0.502 | 1.707 |
| Chloride (mmol/L) | 0.780 | 0.820 | 0.599 | 1.600 |
| Glucose (mmol/L) | 1.000 | 0.639 | 0.711 | 1.639 |
The Youden index of potassium was the highest, which reached 1.707, and that of chloride was the lowest, which was only 1.600. The kappa value is an important indicator for evaluating the consistency between the two methods, and as shown in Table 4, none of the kappa values for each parameter were higher than 0.750, indicating inconsistency between the two analyzers.
4. DISCUSSION
In summary, we conducted electrolyte and glucose measurements in arterial blood samples by using different analyzers, and venous blood samples were used as a reference for comparison to gain insight into whether blood gas analyzers can provide accurate results for these parameters. The blood gas analyzer we used in the present study was a RAPIDPOINT 500, which is capable of delivering results within approximately 60 seconds. This specific analyzer employs a potentiometric method using standard ion‐selective electrode (ISE) technology to measure electrolytes and an amperometric method using an enzyme electrode that contains glucose oxidase to quantify glucose levels. In addition to the rapid analysis procedure, the RAPIDPOINT 500 also has an advantage in that it does not require a large blood sample for analysis, which would significantly reduce the present difficulties nurses experience when preparing eligible samples. That being said, the reliability of blood gas analyzers must be compared with automated chemistry analyzers, which are widely accepted by clinicians. Hence, we collected 200 arterial blood samples and matched venous blood samples from hospitalized patients at exactly the same time to improve the comparability of these samples.
The major finding of the present study is that we observed significant differences between the two analyzers in measuring arterial blood samples by using paired t tests, except for glucose levels, indicating the presence of inter‐device variability. We further compared the results of arterial blood samples from the RAPIDPOINT 500 and matched venous blood sample data from the AU5800, and all parameters showed significant differences, which means that the rapid test results may not be interchangeable with the gold standard used by clinicians. A similar study conducted by Solak9 suggested that blood gas analyzers tend to underestimate the sodium level when compared with chemistry analyzers, regardless of their absolute sodium level in the blood. Gavala et al7 conducted a similar comparison in a small sample size, and both sodium and potassium showed a significant reduction in the blood gas analyzer data, judging from biases calculated and statistical analysis. Our results for sodium, potassium, and chloride levels were completely consistent with the abovementioned publications. The bias we found in comparison A can be attributed to the different methods that these two analyzers employed, especially regarding the electrolytes. The direct ISE method is universally used with blood gas analyzers, while the indirect ISE method is applied in chemistry analyzers. It is believed that plasma protein concentrations are the major confounding factors that lead to this discrepancy between the two methods. Dimeski et al10 conducted a retrospective study reviewing clinical laboratory data from 3 months and observed that hypoproteinemia is mainly associated with the overestimation of sodium levels when using the indirect ISE method, although 50% of samples with a protein level >100 g/L also violated the USCLIA 88 rule by comparing two methods. Therefore, it can be concluded that either hypoproteinemia or hyperproteinemia can lead to the disagreement we have experienced in clinical practice. Hypoproteinemia, in particular, is very common among aged hospitalized patients, and it can be caused by a wide range of diseases, including cancers,11 infections,12 and other diseases related to protein synthesis. Given the high prevalence of hypoproteinemia among hospitalized patients and critically ill patients, the use of two methods to measure electrolytes can potentially lead to confusion and erroneous clinical management if the sodium level is poorly evaluated.13 In comparison B, which compares arterial blood samples and venous blood samples, the biases of electrolytes are smaller, except for potassium. It is worth mentioning that this comparison has a more realistic meaning in real‐world circumstances. However, the significant reduction in electrolytes indicates that the results obtained by blood gas analyzers should be interpreted with caution.
In the linear regression, we obtained a high Pearson's coefficient regardless of the comparison we conducted or which parameter we tested. Although all coefficients were >0.800, this strong correlation can only be considered from a statistical perspective. If we take the intercepts into account, it would affect the results and may lead to a different conclusion. Our data are inconsistent with the correlation analysis conducted by Uyanik et al,14 which suggested that two kinds of analyzers have no strong correlation regarding test results. This may be explained by the small sample size they used since establishing a linear model is very sensitive to sample size.
For the glucose level measurements, we employed the hexokinase method, which is widely recommended worldwide.15 Compared with the results obtained from the chemistry analyzer, the blood gas analyzer consistently demonstrated significant reductions. The deviation between the two methods can be explained from two perspectives: the methodology and the preanalytical process. The blood gas analyzer, as previously mentioned, uses an enzyme electrode combined with glucose oxidase to measure glucose levels. In other words, this method is based on the interaction between glucose oxidase and glucose.16 This method, however, has several deficits that need to be noted. A wide range of substances in human blood samples can interact with H2O2 and consequently inhibit chromogenic reactions, including high levels of vitamin C, creatine, bilirubin,17 and uric acid.18 However, we carefully compared the glucose difference in arterial blood samples between subjects with high levels of vitamin C, creatine, bilirubin, and uric acid and those with normal levels, and the results showed that the glucose differences were not significantly different between these groups (see Table S1). Apart from the interferences of this particular method, red blood cells would consume glucose if the sample is placed at room temperature for a long period of time. However, the reduction we observed in the present study is unlikely to be caused by these reasons because we initiated the analysis as soon as we received the samples. In addition, we compared the glucose differences between patients with reduced levels of red blood cells and patients with normal levels of red blood cells, and the results were not significant (see Table S2). The bias for glucose in our analysis is similar to the study that employed analyzers of the same brands that we used, suggesting that blood gas analyzers tend to underestimate the glucose level.14 Nevertheless, controversy remains regarding whether blood gas analyzers underestimate glucose levels. A study involving 31 060 patients indicated an opposite trend.19 The inconsistency between our study and this large‐scale study is possibly because the latter one compared the data simply collected from a laboratory information system instead of testing the same samples by using two methods simultaneously, which would include many confounding factors in the comparison.
According to the results of the diagnostic performance evaluation, we found inconsistency in every parameter we investigated. It is worth noting that patients who are subject to BGA are normally critical. With our study participants, for example, most were hospitalized due to respiratory and cardiovascular diseases and renal failure. Therefore, it is of critical importance to rectify the acid‐base imbalance. However, our data showed that a number of participants would be misclassified due to the inconsistency between the two kinds of analyzers. If the corresponding treatment, such as calcium gluconate, was not given to a patient with a high potassium level or this treatment was applied to a patient who actually did not have a high potassium level, the consequence would be lethal.
In conclusion, we intensively investigated the agreement of electrolyte and glucose levels between blood gas analyzers and chemistry analyzers. All measurements were conducted simultaneously to eliminate confounding factors and to improve comparability. The results suggested that blood gas analyzers tend to present lower results when compared with chemistry analyzers, and inconsistency was observed if we applied the reference intervals to determine if the samples were normal. These findings suggested that the results from these two different kinds of analyzers cannot be used interchangeably. Given the systematic underestimation, we found in blood gas analyzers, the reference interval should be adjusted accordingly.
AUTHOR CONTRIBUTIONS
All authors have contributed to the present study. YL conceptualized the study and revised the article. HCY, WSS, and YHZ conducted the experimental process and drafted the article. XZZ, YY, and WXX were in charge of analyzers’ quality control and calibration and data acquisition. DZ conducted the data analysis.
Supporting information
Table S1
Table S2
ACKNOWLEDGMENT
The authors would like to express gratitude to all participants for their cooperation.
Yi H‐C, Shi W‐S, Zhang Y‐H, et al. Comparison of electrolyte and glucose levels measured by a blood gas analyzer and an automated biochemistry analyzer among hospitalized patients. J Clin Lab Anal. 2020;34:e23291 10.1002/jcla.23291
Huo‐Chun Yi, Wen‐Sheng Shi and Yin‐Hui Zhang equally contributed to this work.
REFERENCES
- 1. Davis MD, Walsh BK, Sittig SE, Restrepo RD. AARC clinical practice guideline: blood gas analysis and hemoximetry: 2013. Respir Care. 2013;58(10):1694‐1703. [DOI] [PubMed] [Google Scholar]
- 2. Mikkelsen S, Wolsing‐Hansen J, Nybo M, Maegaard CU, Jepsen S. Implementation of the ABL‐90 blood gas analyzer in a ground‐based mobile emergency care unit. Scand J Trauma Resusc Emerg Med. 2015;23:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khattak HK, Khalid S, Manzoor K, Stein PK. Recurrent life‐threatening hyperkalemia without typical electrocardiographic changes. J Electrocardiol. 2014;47(1):95‐97. [DOI] [PubMed] [Google Scholar]
- 4. Filippatos T, Tzavella E, Rizos C, Elisaf M, Liamis G. Acid‐base and electrolyte disorders associated with the use of antidiabetic drugs. Expert Opin Drug Saf. 2017;16(10):1121‐1132. [DOI] [PubMed] [Google Scholar]
- 5. Oronsky B, Caroen S, Oronsky A, et al. Electrolyte disorders with platinum‐based chemotherapy: mechanisms, manifestations and management. Cancer Chemother Pharmacol. 2017;80(5):895‐907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cervera J, Mansouri S, Pamidi PVA. Analytical performance assessment of a novel cartridge‐based blood gas analyzer. Clin Biochem. 2019;63:154‐155. [DOI] [PubMed] [Google Scholar]
- 7. Gavala A, Myrianthefs P. Comparison of point‐of‐care versus central laboratory measurement of hematocrit, hemoglobin, and electrolyte concentrations. Heart Lung. 2017;46(4):246‐250. [DOI] [PubMed] [Google Scholar]
- 8. Zhang JB, Lin J, Zhao XD. Analysis of bias in measurements of potassium, sodium and hemoglobin by an emergency department‐based blood gas analyzer relative to hospital laboratory autoanalyzer results. PLoS One. 2015;10(4):e0122383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Solak Y. Comparison of serum sodium levels measured by blood gas analyzer and biochemistry autoanalyzer in patients with hyponatremia, eunatremia, and hypernatremia. Am J Emerg Med. 2016;34(8):1473‐1479. [DOI] [PubMed] [Google Scholar]
- 10. Dimeski G, Morgan TJ, Presneill JJ, Venkatesh B. Disagreement between ion selective electrode direct and indirect sodium measurements: estimation of the problem in a tertiary referral hospital. J Crit Care. 2012;27(3):326.e9‐326.e16. [DOI] [PubMed] [Google Scholar]
- 11. Critselis E, Panagiotakos DB, Machairas A, Zampelas A, Critselis AN, Polychronopoulos E. Postoperative hypoproteinemia in cancer patients following extensive abdominal surgery despite parenteral nutritional support. Nutr Cancer. 2011;63(7):1021‐1028. [DOI] [PubMed] [Google Scholar]
- 12. Li F, Yuan MZ, Wang L, Wang XF, Liu GW. Characteristics and prognosis of pulmonary infection in patients with neurologic disease and hypoproteinemia. Expert Rev Anti Infect Ther. 2015;13(4):521‐526. [DOI] [PubMed] [Google Scholar]
- 13. Lang T, Prinsloo P, Broughton AF, Lawson N, Marenah CB. Effect of low protein concentration on serum sodium measurement: pseudohypernatraemia and pseudonormonatraemia!. Ann Clin Biochem. 2002;39(Pt 1):66‐67. [DOI] [PubMed] [Google Scholar]
- 14. Uyanik M, Sertoglu E, Kayadibi H, et al. Comparison of blood gas, electrolyte and metabolite results measured with two different blood gas analyzers and a core laboratory analyzer. Scand J Clin Lab Invest. 2015;75(2):97‐105. [DOI] [PubMed] [Google Scholar]
- 15. Bondar RJ, Mead DC. Evaluation of glucose‐6‐phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem. 1974;20(5):586‐590. [PubMed] [Google Scholar]
- 16. Rosevear JW, Pfaff KJ, Service FJ, Molnar GD, Ackerman E. Glucose oxidase method for continuous automated blood glucose determination. Clin Chem. 1969;15(8):680‐698. [PubMed] [Google Scholar]
- 17. Chaudhuri RK, Mukherjee M, Sengupta D, Mazumder S. Limitation of glucose oxidase method of glucose estimation in jaundiced neonates. Indian J Exp Biol. 2006;44(3):254‐255. [PubMed] [Google Scholar]
- 18. Chinh NH. Mechanism of interference by uric acid in the glucose oxidase‐peroxidase method for serum glucose. Clin Chem. 1974;20(4):499‐501. [PubMed] [Google Scholar]
- 19. Altunok İ, Aksel G, Eroğlu SE. Correlation between sodium, potassium, hemoglobin, hematocrit, and glucose values as measured by a laboratory autoanalyzer and a blood gas analyzer. Am J Emerg Med. 2019;37(6):1048‐1053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


